Abstract
Study objectives
Obesity is often considered mandatory for the diagnosis of Metabolic Syndrome (MS). Data on the prevalence of MS in non-obese patients with Obstructive Sleep Apnea (OSA) is scarce. This study was aimed to determine the prevalence of MS in non-obese patients with OSA.
Methodology
All consecutively diagnosed patients with OSA between October 2018 and November 2019 were screened for metabolic syndrome. Patients with OSA and BMI < 25 kg/m2 (NOOSA) vs BMI > 25 kg/m2 (obese OSA) were compared. Lean waist NOOSA was defined as BMI < 25 kg/m2 and WC < 80 cm (32 in.) for women or < 90 cm (36 in.) for men.
Results
During the study period, 502 patients were diagnosed with OSA. MS was observed in 35% of patients with NOOSA compared to obese patients with OSA (79%). In the NOOSA group, hypertension, impaired fasting glucose, diabetes mellitus and dyslipidemia were observed in 65, 48, 14 and 61% respectively and all of these parameters were significantly more common in the obese group (p < 0.001). Parameters of OSA severity (apnea-hypopnea index or AHI, time spent below 90% saturated or T90, and nadir oxygen) were significantly more severe in the obese group with OSA. Approximately 83% of patients in the NOOSA group had at least two metabolic risk factors, compared to the obese OSA group, in which 95% had two or more metabolic risk factors. Sixty-four percent of patients with NOOSA with lean waist had at least two metabolic risk factors. At BMI cut-offs of < 25, < 27 and < 30 kg/m2; 35, 46 and 57% of patients with OSA respectively had metabolic syndrome.
Conclusion
Metabolic syndrome was observed in approximately one in three patients with OSA and BMI < 25 kg/m2. Approximately two of every three lean waist non-obese patients with OSA had at least two markers of metabolic syndrome. The role of OSA in the development of metabolic syndrome in non-obese individuals needs further exploration.
Keywords: Obstructive Sleep Apnea, Metabolic syndrome, Nonobese, Continuous positive airway pressure
Introduction
Obstructive Sleep Apnea (OSA) is a highly prevalent disorder that leads to significant cardiovascular complications, insulin resistance, hypertension (HTN) and dyslipidaemia [8]. OSA has striking similarities with metabolic syndrome (MS), which is a cluster of metabolic risk factors [22]. The high prevalence of MS in patients with OSA (43–78%) shows the close association between these two disorders [1, 6, 41, 44]. The presence of both of these disorders in the same patient is also known as Syndrome Z.
With changing lifestyles and eating habits, obesity and associated diseases have taken the shape of a pandemic. OSA and MS are both known to be associated with obesity. Asians are known to develop abdominal obesity or apple-shaped obesity, where the abdominal girth is more than the hip circumference [33]. Despite lower weight and BMI, Asians are at greater risk of developing cardiovascular complications than Caucasians matched for BMI [33]. Therefore, the cut-off for obesity (BMI) has been lowered from 30 kg/m2 to 25 kg/m2 in Asians. Similarly the waist circumference cut offs for defining MS has also been lowered for Asians [33].
One of the most important features of MS is obesity. Therefore, in non-obese individuals, MS is often not suspected and is neglected. It has been shown that non-obese individuals may also be prone to developing MS. These patients have been labelled nonobese MS or MONO (Metabolically Obese Non Obese) [30]. There is scarce information on MS in lean Indians; the prevalence of MS in lean individuals (BMI < 25 kg/m2) was found to be 12.6 and 20.4% in two studies from India [12, 34]. In the Indian population, using a BMI < 25 kg/m2 cut off was associated with increased sensitivity of OSA questionnaires [38]. Non-obese OSA is now a known entity; 25% of OSA cases were non-obese in a study from the USA [18]. Data on the prevalence of MS in non-obese OSA are scarce. In a few of the earlier studies from China and Japan, 19–39.6% of non-obese OSA had at least two components of MS [2, 25, 30].
The present studys was aimed to determine the prevalence of MS in patients with non-obese OSA (NOOSA).
Methods
Study setting and participants
This cross-sectional study was done in consecutive patients with OSA diagnosed in our sleep laboratory (October 2018–November 2019). An article comparing the prevalence of syndrome Z in men and women has already been published from this Study [10]. All patients were evaluated for MS according to the NCEP-ATP III [22]. MS was defined as presence of three or more of following features:
Large waist circumference (Asian cut-off was used) [3]: Waist Circumference (WC) ≥ 80 cm (32 in.) for women or ≥ 90 cm (36 in.) for men.
High triglyceride level: ≥ 150 mg/dL
Reduced high-density lipoprotein (HDL) cholesterol — < 40 mg/dL in men or < 50 mg/dL in women
Increased blood pressure — systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg or current use of antihypertensive drugs
Elevated fasting blood sugar — ≥ 100 mg/dL or patients already on oral hypoglycemics.
Metabolic scores were counted as the sum of number of the aforementioned factors found positive. Dyslipidemia was defined as either raised TG or Low HDL level (as mentioned in the foregoing definitions).
-
Inclusion Criteria: a) All patients diagnosed with OSA by level I PSG.
b) Age ≥ 18 years.
c) Patients willing to participate in the study.
Exclusion Criteria: a) People not giving consent to draw blood sample b) AHI < 5 on level I PSG c) Body Mass Index > 25 kg/m2.
Procedures
All patients underwent level I PSG (Philips Respironics Alice 6). Apneas and Hypopneas were scored according to AASM scoring manual 2012 [7]. If a patient was diagnosed as having moderate or severe OSA (AHI > 15), then the patient was manually titrated in the sleep lab with PAP according to AASM protocol [27]. Each sleep study was manually scored and cross checked by a sleep consultant. The severity of OSA was determined by apnea-hypopnea index (AHI). OSA was defined as mild (5–14.9), moderate (15–29.9) or severe (≥ 30) on the basis of AHI. Nadir oxygen levels (minimum oxygen saturation during sleep) and percentage of total time with oxygen saturation level lower than 90% during sleep (T90) were also compared between non-obese and obese patients with OSA. Sleepiness was defined if the Epworth sleepiness score (ESS) was more than 10.
Blood investigations
All patients diagnosed with OSA underwent blood testing in the morning after overnight fasting for fasting blood sugar (FBS) and lipid profile (total cholesterol, triglyceride, low-density lipid, very low-density lipid and high density lipid) by fully automated chemistry analyser (AU680, Beckman Coulter).
Anthropometry and general examination
Waist was defined at the point midway between iliac crest and lower costal margin and waist measurement was performed during normal breath out with tape snugly fitted and horizontal to floor while the subject stood. Hip circumference was measured around the widest portion of the hips with tape snugly fitted and horizontal to floor while the subject stood with arms at the sides, feet close together. Neck circumference was measured at the level of the cricothyroid membrane with tape while patient in standing position. Blood Pressure was measured after 10 min in the sitting position; first reading was discarded. Average of second and third reading of blood pressure was taken. Weight was measured by Seca® weighing machine and height was measured by SECA® stadiometer. BMI was calculated as {weight (kg)/height (m)2}. Non-obese OSA (NOOSA) was defined as patients having BMI < 25 kg/m2 and obese was defined as patients with BMI ≥ 25 kg/m2. Lean waist NOOSA was defined as BMI < 25 kg/m2 and WC < 80 cm (32 in.) for women or < 90 cm (36 in.) for men.
Ethics and permissions
Protocol of this study was reviewed and approved by Institutional Human Ethics Committee (IHEC) of AIIMS Bhopal (IHEC-LOP/2018/MD0014 dated 17 October 2018). A participant information sheet in the Hindi language was given to eligible participants and their queries if any were resolved. Written informed consent was obtained from those who were willing to participate.
Data analysis
Data analysis was done using R software [39], ggplot2 [43] and gtsummary [42] packages. We have summarized nominal variables with count and percentage and numerical variables with mean and its standard deviation. We have stratified our data across those who had BMI > = 25 and BMI < 25 as well as those who were thin and obese (based on waist circumference). Then we tested differences in distribution of numerical and categorical variables across these groups by using t-test and Chi-square test respectively. A p-value less than 0.05 was considered to be statistically significant. Ihe prevalence of MS was estimated as q percentage and its 95% confidence interval was calculated.
Results
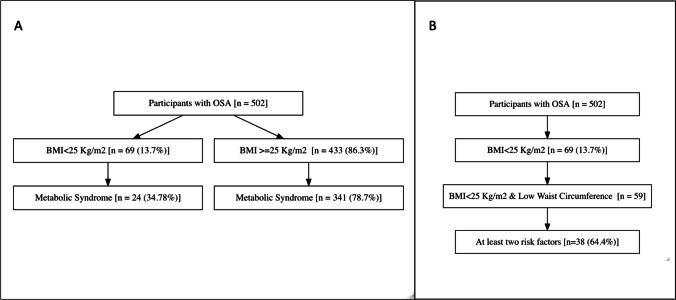
During the study period, 502 patients (357 men; 145 women) were diagnosed with OSA (AHI > 5) [10]. Mean BMI was 31.6 ± 11.1 kg/m2 (women: 35.3 ± 7.2 and men: 30.1 ± 12.0 kg/m2) (p < 0.001). Of 502 patients with OSA, 69 (14%) had BMI < 25 kg/m2 (NOOSA). Of these 69 patients in the NOOSA group, 24 met the definition of metabolic syndrome and thus the prevalence of metabolic syndrome in OSA patients with BMI < 25 kg/m2 was 35% (95% CI 25 to 47) (Fig. 1). Various clinical parameters across OSA patients with BMI < 25 kg/m2 and BMI > 25 kg/m2 (obese OSA) were compared (Table 1). MS was seen in 35% of patients with NOOSA compared to 79% in obese patients with OSA. Metabolic scores were higher in the obese group (3.4 ± 1.1) compared to 2.2 ± 1.0 in the NOOSA group (p < 0.001).
Fig. 1 .
A Metabolic syndrome prevalence in patients with OSA (< 25 kg/m2—Non-Obese OSA) and those with BMI ≥ 25 kg/m2. B Showing presence of at least two metabolic factors in patients with lean waist non-obese patients with OSA (BMI < 25 kg/m2 and lean waist)
Table 1.
Comparison of non-obese OSA patients (BMI < 25 kg/m2) with obese OSA (BMI > 25 kg/m2) patients
| Characteristic | Overall, N = 502 | BMI in kg/m2 | p-value | |
|---|---|---|---|---|
| < 25, N = 69 (14%) | 25 + , N = 433 (86%) | |||
| Age | 51.9 (12.2) | 49.5 (15.4) | 52.2 (11.6) | 0.162 |
| Gender | 0.001 | |||
| Female | 145 (29%) | 8 (12%) | 137 (32%) | |
| Male | 357 (71%) | 61 (88%) | 296 (68%) | |
| Metabolic Syndrome | < 0.001 | |||
| No MS | 137 (27%) | 45 (65%) | 92 (21%) | |
| MS | 365 (73%) | 24 (35%) | 341 (79%) | |
| Metabolic scores | 3.2 (1.1) | 2.2 (1.0) | 3.4 (1.1) | < 0.001 |
| Blood Pressure (mean (SD)) | ||||
| Systolic Blood Pressure | 133.4 (19.4) | 131.6 (21.5) | 133.7 (19.0) | 0.437 |
| Diastolic Blood Pressure | 83.6 (12.3) | 84.2 (13.5) | 83.5 (12.1) | 0.662 |
| Fasting Blood Sugar (mean (SD)) | 110.4 (33.0) | 100.7 (24.9) | 111.9 (33.9) | 0.001 |
| Impaired fasting glucose | 306 (61%) | 33 (48%) | 273 (63%) | 0.023 |
| Triglyceride (mean (SD)) | 148.2 (70.0) | 146.5 (74.4) | 148.5 (69.3) | 0.834 |
| Raised Triglyceride | 176 (35%) | 20 (29%) | 156 (36%) | 0.316 |
| HDL (mean (SD)) | 41.6 (10.4) | 41.8 (10.6) | 41.6 (10.4) | 0.864 |
| Low HDL | 281 (56%) | 31 (45%) | 250 (58%) | 0.063 |
| Heart disease | 71 (14%) | 9 (13%) | 62 (14%) | 0.918 |
| Hypertension | 395 (79%) | 45 (65%) | 350 (81%) | 0.005 |
| Diabetes Mellitus | 145 (29%) | 10 (14%) | 135 (31%) | 0.007 |
| High Waist circumference | 373 (74%) | 10 (14%) | 363 (84%) | < 0.001 |
| Dyslipidemia | 354 (71%) | 42 (61%) | 312 (72%) | 0.080 |
| AHI | 62.8 (35.2) | 35.9 (22.2) | 67.0 (35.1) | < 0.001 |
| Lowest oxygen (mean (SD)) | 77.0 (16.3) | 83.2 (14.0) | 76.0 (16.5) | < 0.001 |
| T90 (mean (SD)) | 13.4 (24.1) | 6.3 (18.2) | 14.6 (24.8) | 0.001 |
| ESS Total (mean (SD)) | 8.2 (5.0) | 6.3 (4.2) | 8.5 (5.1) | < 0.001 |
| ESS < 10 | 308 (61%) | 53 (77%) | 255 (59%) | 0.007 |
| ESS 10 + | 194 (39%) | 16 (23%) | 178 (41%) | |
AHI, apnea hypopnea index; ESS, Epworth sleepiness scale; HDL, high density lipoprotein; OSA, obstructive sleep apnea; T90, percent sleep time spent with sPO2 ≤ 90
In the NOOSA group, hypertension, impaired fasting glucose, diabetes mellitus and dyslipidemia was seen in 65, 48, 14 and 61% respectively and all these parameters were significantly more common in the obese group. Parameters of OSA severity (AHI, T90 and nadir oxygen) were significantly more severe in the obese OSA group. Sleepiness (Epworth sleepiness scale (ESS) > 10) was more commonly seen in the obese group (41 vs 23%) and mean ESS was also higher in the obese group.
Of patients with NOOSA with lean waist, 64% had at least two metabolic risk factors (Table 2). In the lean waist NOOSA group, HTN, impaired fasting glucose, diabetes mellitus and dyslipidemia was seen in 64, 51, 15 and 61% respectively and all these parameters were significantly more common in the obese group.
Table 2.
Comparison of lean waist NOOSA patients (BMI < 25 kg/m2 and lean waist#) with other subjects (include obese as well as non-obese but non lean waist)
| Characteristic | Lean Waist NOOSA N = 59(12%) | Others N = 443 (88%) | p-value |
|---|---|---|---|
| Age | 47.9 (15.6) | 52.4 (11.6) | 0.038 |
| Gender | |||
| Women | 6 (10%) | 139 (31%) | 0.001 |
| Men | 53 (90%) | 304 (69%) | |
| Blood Pressure {mean (SD)} | |||
| Systolic Blood Pressure | 131.1 (22.7) | 133.7 (18.9) | 0.396 |
| Diastolic Blood Pressure | 84.4 (14.4) | 83.5 (12.0) | 0.647 |
| Fasting Blood sugar {mean (SD)} | 100.8 (26.2) | 111.7 (33.6) | 0.005 |
| Impaired Fasting Glucose | 30 (51%) | 276 (62%) | 0.121 |
| Triglyceride {mean (SD)} | 149.8 (79.6) | 148.0 (68.7) | 0.870 |
| High Triglyceride | 19 (32%) | 157 (35%) | 0.731 |
| High density Lipoprotein (HDL) {mean (SD)} | 42.1 (10.9) | 41.5 (10.4) | 0.696 |
| Low HDL | 26 (44%) | 255 (58%) | 0.068 |
| Heart disease | 8 (14%) | 63 (14%) | N.S |
| Hypertension | 38 (64%) | 357 (81%) | 0.007 |
| Diabetes mellitus | 9 (15%) | 136 (31%) | 0.021 |
| Dyslipidemia | 36 (61%) | 315 (71%) | 0.151 |
| Two of three parameters present* | 38 (64%) | 349 (79%) | 0.021 |
#Waist circumference < 80 cm in females and < 90 cm in males
* Hypertension or Impaired Fasting Glucose or Dyslipidemia
NOOSA, non-obese obstructive sleep apnea; OSA, obstructive sleep apnea
At different cut-off values of BMI (< 25, < 27 and < 30 kg/m2) were used: 34.8, 45.5 and 57.4% had MS respectively. (Table 3).
Table 3.
Proportion of MS in OSA patients at different cut off of obesity
| BMI Cut-off (kg/m2) | No MS | MS | p-value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| < 25 | 45 | 65.2% | 24 | 34.8% | < 0.001 | |
| 25 + | 92 | 21.2% | 341 | 78.8% | ||
| < 27 | 78 | 54.5% | 65 | 45.5% | < 0.001 | |
| 27 + | 59 | 16.4% | 300 | 83.6% | ||
| < 30 | 103 | 42.6% | 139 | 57.4% | < 0.001 | |
| 30 + | 34 | 13.1% | 226 | 86.9% | ||
BMI, body mass index; MS, metabolic syndrome; OSA, obstructive sleep apnea
Discussion
In this prospective cross-sectional study, MS was seen in approximately one in every three patients with OSA and BMI < 25 kg/m2. Also, approximately two of every three (64%) patients with lean waist non-obese OSA had at-least two markers of MS. Nonobese Indians with OSA are at much higher risk than other ethnicities for complications. OSA may be a neglected reason for development of MS in non-obese Indians. Our study highlights the necessity of evaluating MS, even in non-obese patients with OSA, so that early intervention can be implemented in these patients to prevent cardiovascular complications.
Over the last few decades, an increased prevalence of obesity has been reflected in a simultaneous increase in the prevalence of OSA. A four-year follow-up study of the Wisconsin sleep cohort reported that a 10% increase in weight was associated with a 32% increase in AHI and a 10% weight reduction was associated with a 26% decrease in AHI [36]. There are multiple definitions of MS by different societies using various cut-offs for systolic and diastolic blood pressures, HDL, triglyceride and fasting blood sugar. But the presence of abdominal obesity is central to all definitions [23]. Both OSA and MS are known to be commonly present in obese individuals and often OSA and MS are overlooked in non-obese individuals. Data from the Wisconsin sleep cohort showed almost 14% and 5% of nonobese men and women (aged 50–70 years) respectively had moderate to severe OSA [37]. The community based HypnoLaus study from Switzerland (mean BMI of 25.6 kg/m2) showed very high rates of moderate to severe OSA (approximately 23% in women and 50% in men) [20]. In our current study done at a referral sleep centre, 14% of the OSA population had BMI < 25 kg/m2. Non-obese men with OSA have been shown to have higher levels of visceral fat compared to subjects without OSA [26]. Metabolic parameters including triglycerides, cholesterol and inflammatory markers are elevated in non-obese men with OSA compared to controls [26].
Expectedly NOOSA had less severe OSA (in terms of AHI, T90 and nadir oxygen) compared to obese patients with OSA. NOOSA also had less severe metabolic factors than obese patients with OSA. But the prevalence of markers of MS was significantly higher than seen in non-obese individuals from the general population.
MS in non-obese general population
In an Indian study done in general population, 1710 out of 2350 people had BMI < 25 kg/m2 and 355/1710 (20.4%) had MS [12]. These were called Metabolically Obese No Obese (MONO). In a multi-ethnic study done in Malaysia, prevalence of MONO was 11.7% and it was highest in people from Indian origin (39.3%). The prevalence of MONO is reported from 4.6–12.7% from big community based studies from the USA, Korea, and Iran [19, 28, 32, 35] Compared to 4.6–20.4% in the community population; 35% of nonobese patients with OSA in our current study had MS.
OSA and MS have a bidirectional relationship and they fuel each other. MS is linked to increased cardiovascular complications. OSA may promote the onset of MS through several mechanisms: increased sympathetic activity, intermittent hypoxemia, hypothalamic–pituitary–adrenal axis dysfunction, impaired endothelial function, and abnormal production of inflammatory cytokines and adipokines. In a study of follow-up of two population-based cohorts (from Brazil and Switzerland) association between the presence of OSA and the incidence of MS was assessed [21]. Approximately 17% participants without baseline MS developed MS after follow up of 6 years. In this study, moderate-to-severe OSA was independently associated with the development of MS (OR 2.58, 95% CI 1.61–4.11).
MS in non-obese OSA
Asians are at higher risk of developing cardiovascular complications at a lower BMI and at a younger age compared to Caucasians. So the cut off for both WC to define MS and BMI were lowered, since these lower cut off could better predict patients at risk. Even when a BMI cut off of 25 kg/m2 was used, patients with OSA had a significantly higher proportion of HTN, IFG, DM, dyslipidemia and MS in our study. Approximately half of nonobese patients with OSA in the current study had impaired fasting glucose and two thirds had hypertension, which is significantly higher than that seen in general Indian population [4, 5].
We could find only three studies in the English literature, in which components of MS were assessed in nonobese patients with OSA. Two studies from Japan used Japanese Diagnostic Standards for MS and excluded patients with WC > 85 cm [2, 25]. Similarly, a Chinese study used NCEP ATP III criteria, but excluded patients with WC > 80 and 90 in women and men respectively [30]. All these studies assessed the presence of at-least two of three metabolic parameters (hypertension, impaired fasting glucose and dyslipidemia):19–39.6% of non-obese OSA had at least two of three components [2, 25, 30]. We used similar criteria as the Chinese study and found 64% of lean waist non-obese patients had two of three metabolic parameters present, which was significantly higher than found in previous studies (Table 4).
Table 4.
Summary results of studies on metabolic syndrome among non-obese OSA
| Author | Country | Sample size | Prevalence | Criteria |
|---|---|---|---|---|
| Kono M. et al | Japan | 42 | 8 (19%) | BMI < 30 kg/m2 and Visceral Fat A was 90 cm |
| Akahoshi et al | Japan | 101 | 40 (39.6%) | Subjects who had BMI ≥ 25 and waist circumference at ≥ 85 were excluded |
|
Qi-Chang Lin et al |
China | 113 | 44 (38.9%) | Subjects who had BMI ≥ 25 and waist circumference at ≥ 90 cm in men and ≥ 80 cm in women were excluded |
| Present study | India | 59 | 38 (64%) |
Subjects with at least two of the following: hypertension, hyperglycemia and dyslipidemia
BMI, Body Mass Index
South Asians living in the UK were found to have a higher prevalence and more severe OSA than BMI-matched Europeans [29]. As Asians are probably at higher risk of severe OSA; higher prevalence of parameters of MS in lean waist NOOSA seen in our study also hints that probably non-obese Asians with OSA are at greater risk to develop MS compared to other ethnicities and this aspect needs further exploration.
Continuous positive airway pressure (CPAP) has been shown to have some beneficial effects on individual metabolic components of MS [11]. Treatment of OSA has been shown to improve symptoms of sleep apnea, particularly excessive daytime sleepiness, and lowers blood pressure. CPAP can reduce cardiovascular disease risk and may improve diabetes control [31]. Non-obese patients with OSA were found to have lower CPAP adherence than obese patients with OSA possibly due to a low respiratory arousal threshold [18]. Comprehensive management including weight reduction, active life style, dietary weight loss, and regular CPAP usage are keys for management of this syndrome Z (OSA and MS).
OSA has been associated with cardiovascular, neurological complications and poor quality of life [9, 15–17]. MS, DM, HTN, fatty liver disease, OSA and Coronary artery disease are inter-connected in many ways. With changing lifestyle and dietary habits, obesity and associated diseases are increasing significantly. Both MS and OSA have been associated with increased risk of COVID-19 related infection and complications [24, 40]. We believe that even in non-obese patients with OSA, MS should be screened and if present they should be aggressively managed with both life style modification and CPAP.
OSA is much neglected disease worldwide and this is even more true for non-obese individuals, where OSA is often not even considered in the differential diagnosis [13, 14].
Strengths and limitations
Our study had several strengths. (i) To the best of our knowledge, it is first Indian study on the prevalence of MS in nonobese OSA (ii) A level I PSG was done in all subjects iii) The most widely accepted criteria for MS i.e. NCEP-ATP III were used for diagnosis. Limitations of the study were that there were fewer participants in the NOOSA group and therefore the prevalence estimates have a wide confidence interval. Also, being a cross-sectional study, temporality of the presence of MS and OSA or vice-versa could not be established. Participants were selected from patients with OSA in a referral sleep clinic; this selection process may have resulted in an overestimation of the prevalence of MS in this study.
Conclusions
To conclude, metabolic syndrome is highly prevalent in the non-obese population with OSA (35%); 64% of lean waist non obese OSA patients had at-least two markers of metabolic syndrome. All patients with OSA, even if nonobese should be screened for MS so that early intervention can be implemented in these patients to prevent cardiovascular complications.
Acknowledgements
We want to thank Mr Amit Sen for doing scoring and data collection of OSA patients.
Abbreviations
- AHI
Apnea hypopnea index
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- MS
Metabolic syndrome
- NCEP-ATPIII
National cholesterol education program adult treatment panel III
- NOOSA
Non-obese OSA
- OSA
Obstructive sleep apnea
Author contribution
PC: Data collection, analysis.
AG: Conceptualized, data collection, analysis and manuscript writing.
AP: Data collection, analysis.
SG: Conceptualized and manuscript writing.
AK: Conceptualized and manuscript writing.
MAR: Data collection.
VA: Data collection.
Declarations
Ethics approval and consent to participate
This is a retrospective analysis study and waiver of this study was taken from ethical clearance committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agrawal S, Sharma SK, Sreenivas V, Lakshmy R. Prevalence of metabolic syndrome in a north Indian hospital-based population with obstructive sleep apnoea. Indian J Med Res. 2011;134:639–644. doi: 10.4103/0971-5916.90988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akahoshi T, Uematsu A, Akashiba T, et al. Obstructive sleep apnoea is associated with risk factors comprising the metabolic syndrome: Metabolic syndrome and OSA. Respirology. 2010;15:1122–1126. doi: 10.1111/j.1440-1843.2010.01818.x. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association, National Heart, Lung, and Blood Institute. Grundy SM, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement Executive summary. Cardiol Rev. 2005;13:322–327. doi: 10.1097/01.crd.0000380842.14048.7e. [DOI] [PubMed] [Google Scholar]
- 4.Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32:1170–1177. doi: 10.1097/HJH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 6.Barreiro B, Garcia L, Lozano L, et al. Obstructive sleep apnea and metabolic syndrome in spanish population. Open Respir Med J. 2013;7:71–76. doi: 10.2174/1874306401307010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouzerda A. Cardiovascular risk and obstructive sleep apnea. Pan Afr Med J. 2018;29:47. doi: 10.11604/pamj.2018.29.47.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary B, Patil R, Bhatt GC, et al. Association of sleep disordered breathing with mono-symptomatic nocturnal enuresis: a study among school children of Central India. PLoS ONE. 2016;11:e0155808. doi: 10.1371/journal.pone.0155808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary P, Goyal A, Goel SK, et al. Women with OSA have higher chances of having metabolic syndrome than men: effect of gender on syndrome Z in cross sectional study. Sleep Med. 2021;79:83–87. doi: 10.1016/j.sleep.2020.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR, Mawdsley L, Mugarza JA, et al. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 12.Geetha L, Deepa M, Anjana RM, Mohan V. Prevalence and clinical profile of metabolic obesity and phenotypic obesity in Asian Indians. J Diabetes Sci Technol. 2011 doi: 10.1177/193229681100500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal A, Agarwal N, Pakhare A. Barriers to CPAP use in India: An Exploratory Study. J Clin Sleep Med. 2017;13:1385–1394. doi: 10.5664/jcsm.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal A, Aswin P, Pakhare AP. Poor knowledge and attitude regarding Obstructive Sleep Apnea (OSA) among medical students in India: a call for MBBS curriculum change. Sleep Vigilance. 2018;2:45–50. doi: 10.1007/s41782-017-0028-3. [DOI] [Google Scholar]
- 15.Goyal A, Pakhare AP, Bhatt GC, et al. Association of pediatric obstructive sleep apnea with poor academic performance: a school-based study from India. Lung India. 2018;35:132–136. doi: 10.4103/lungindia.lungindia_218_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal A, Pakhare A, Chaudhary P. Nocturic obstructive sleep apnea as a clinical phenotype of severe disease. Lung India. 2019;36:20–27. doi: 10.4103/lungindia.lungindia_153_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal A, Pakhare A, Tiwari IR, et al. Diagnosing obstructive sleep apnea patients with isolated nocturnal hypoventilation and defining obesity hypoventilation syndrome using new European Respiratory Society classification criteria: an Indian perspective. Sleep Med. 2020;66:85–91. doi: 10.1016/j.sleep.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13:81–88. doi: 10.5664/jcsm.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadaegh F, Zabetian A, Harati H, Azizi F. Metabolic syndrome in normal-weight Iranian adults. Ann Saudi Med. 2007;27:18–24. doi: 10.5144/0256-4947.2007.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirotsu C, Haba-Rubio J, Togeiro SM, et al. Obstructive sleep apnoea as a risk factor for incident metabolic syndrome: a joined Episono and HypnoLaus prospective cohorts study. Eur Respir J. 2018 doi: 10.1183/13993003.01150-2018. [DOI] [PubMed] [Google Scholar]
- 22.Howard WJ. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Yearb Endocrinol. 2006;2006:113–114. doi: 10.1016/S0084-3741(08)70316-0. [DOI] [Google Scholar]
- 23.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kar A, Saxena K, Goyal A et al (2020) Association of Obstructive Sleep Apnea and severity of COVID-19: a hospital based observational study. medRxiv:2020.11.12.20230631. 10.1101/2020.11.12.20230631
- 25.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 26.Kritikou I, Basta M, Tappouni R, et al. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41:601–609. doi: 10.1183/09031936.00183411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. doi: 10.5664/jcsm.27133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr. 2009;18:280–284. [PubMed] [Google Scholar]
- 29.Leong WB, Arora T, Jenkinson D, et al. The prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. J Clin Sleep Med. 2013;9:853–858. doi: 10.5664/jcsm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Q-C, Zhang X-B, Chen G-P, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath. 2012;16:571–578. doi: 10.1007/s11325-011-0544-7. [DOI] [PubMed] [Google Scholar]
- 31.Malik JA, Masoodi SR, Shoib S. Obstructive sleep apnea in Type 2 diabetes and impact of continuous positive airway pressure therapy on glycemic control. Indian J Endocrinol Metab. 2017;21:106–112. doi: 10.4103/2230-8210.196005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meigs JB, Wilson PWF, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 33.Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes. 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, Ghosh S, Bhattacharjee K, et al. Lean metabolic syndrome: A concept or a reality? Indian J Endocrinol Metab. 2018;22:303. doi: 10.4103/ijem.IJEM_639_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y-W, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pe P, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000 doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 37.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad K, Sehgal I, Agarwal R, et al. Assessing the likelihood of obstructive sleep apnea: a comparison of nine screening questionnaires. Sleep And Breathing. 2017 doi: 10.1007/s11325-017-1495-4. [DOI] [PubMed] [Google Scholar]
- 39.R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 40.Saxena K, Kar A, Goyal A. COVID 19 and OSA: exploring multiple cross-ways. Sleep Med. 2020 doi: 10.1016/j.sleep.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma SK, Reddy EV, Sharma A, et al. Prevalence and risk factors of syndrome Z in urban Indians. Sleep Med. 2010;11:562–568. doi: 10.1016/j.sleep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Sjoberg DD, Curry M, Hannum M et al (2020) gtsummary: presentation-ready data summary and analytic result tables. https://github.com/ddsjoberg/gtsummary
- 43.Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York. https://ggplot2.tidyverse.org
- 44.Zito A, Steiropoulos P, Barceló A, et al. Obstructive sleep apnoea and metabolic syndrome in Mediterranean countries. Eur Respir J. 2011;37:717–719. doi: 10.1183/09031936.00120510. [DOI] [PubMed] [Google Scholar]