Abstract
Ankyrins are scaffolding proteins widely expressed throughout the nervous system. Ankyrins recruit diverse membrane proteins, including ion channels and cell adhesion molecules, into specialized subcellular membrane domains. These domains are stabilized by ankyrins interacting with the spectrin cytoskeleton. Ankyrin genes are highly associated with a number of neurological disorders, including Alzheimer’s disease, schizophrenia, autism spectrum disorders, and bipolar disorder. Here, we discuss ankyrin function and their role in neurological disease. We propose mutations in ankyrins contribute to disease through two primary mechanisms: 1) altered neuronal excitability by disrupting ion channel clustering at key excitable domains, and 2) altered neuronal connectivity via impaired stabilization of membrane proteins.
Keywords: ankyrin, spectrin, scaffold, neurological disease, neuropsychiatric disease
Introduction
Ankyrin scaffolding proteins are found throughout the body and act as the primary link between the spectrin cytoskeleton and the cytoplasmic domain of many membrane proteins [1] (Figure 1A). Ankyrins use their 24 ANK repeats to bind with a variety of structurally diverse membrane and membrane-associated proteins, including ion channels, ion pumps, cell adhesion molecules (CAMs), and signaling proteins [1–3] (Figure 1B). Their restricted expression patterns within various cells and tissues create localized concentrations of membrane proteins and give rise to specialized subcellular membrane domains (Figure 1C). Ankyrins interact with b spectrins to anchor these membrane proteins to the actin cytoskeleton [4–8]. Super-resolution microscopy revealed a periodic organization of the spectrin/actin cytoskeleton that allows membranes to flex and resist mechanical disruption [8–11]. Thus, ankyrin scaffolds are uniquely positioned to modulate cellular excitability and resist mechanical forces including tension, torsion, and compression.
Figure 1.
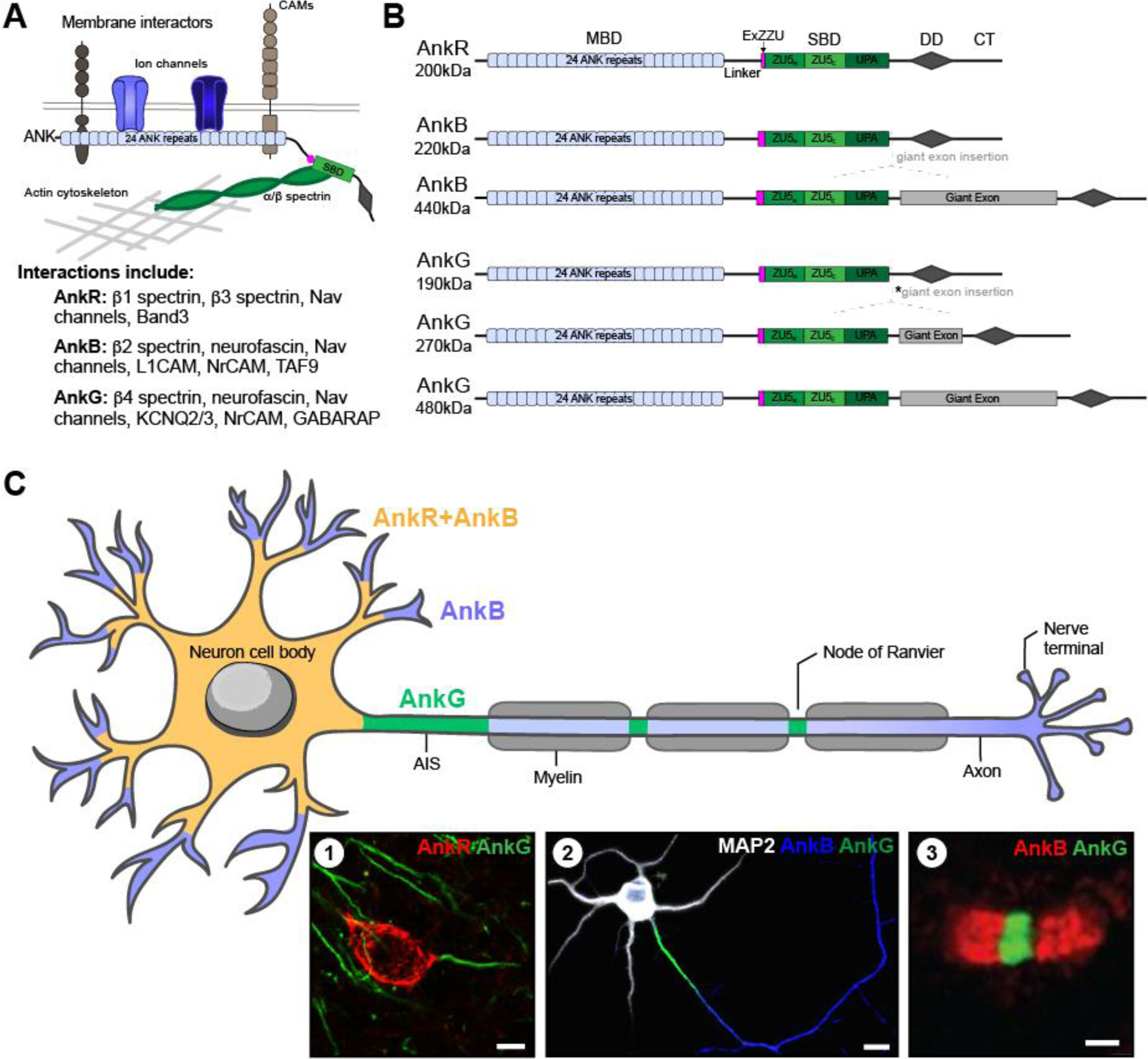
(A) Schematic of subcellular ankyrins with domains and interactions; and list of key interactors for each ankyrin. (B) Primary ankyrin isoforms. AnkR has one main isoform, 200kDa. However, alternative splicing of AnkB results in two main isoforms: 220kDa, and 440kDa. Similarly, alternative splicing of AnkG results in three main isoforms: 190kDa, 270kDa, and 480kDa. The 270kDa isoform results from an in-frame splicing of the giant exon eliminating 1,900 amino acids (*asterisk). Apart from the giant exon present in some isoforms, all three ankyrins have a similar structure. A N-terminal membrane binding domain (MBD) consisting of 24 ANK repeats, a linker domain, a recently discovered extension of the spectrin binding domain (SBD) termed ExZZU thought to enhance ankyrin-spectrin binding affinities, the SBD also known as the ZZU domain due to its three parts (ZU5N, ZU5C, and UPA), the DEATH domain (DD), and a regulatory C-terminal tail. (C) Schematic of ankyrin localization in neurons. All three ankyrins are expressed in neurons; however, each one has a unique pattern of localization. AnkB (blue) is expressed throughout neurons, however in some, AnkR is co-localized with AnkB at the soma and proximal dendrites (yellow). Whereas AnkG (green) is found at the AIS and nodes of Ranvier. Insets show (1) immunolabeling of AnkR and AnkG in adult mouse cortical neurons; scale bar, 10µm; (2) MAP2 (white) labeling the somatodendritic domain, AnkB (blue) co-localized with MAP2 in the somatodendritic domain but is also present in the distal axon, and AnkG (green) labeling the AIS in vitro rat hippocampal neurons at DIV10 [modified from 12]; scale bar, 10µm; and (3) Schwann cell derived paranodal AnkB (red), and AnkG (green) at a node of Ranvier in mouse sciatic nerve [modified from 11]; scale bar, 1µm.
There are three members in the ankyrin gene family: Ankyrin-R (ANK1), Ankyrin-B (ANK2), and Ankyrin-G (ANK3), commonly referred to as AnkR, AnkB, and AnkG, respectively. AnkR plays a critical role in red blood cells where it maintains the cell’s structural integrity via its link between b1 spectrin and anion exchangers [3]. AnkB functions to localize and stabilize CAMs and ion channels along the axon, and assists in the assembly of the axon initial segment (AIS) and paranodal junctions of myelinating axons [12,13]. AnkG binds to the CAM neurofascin-186 and recruits high densities of voltage-gated sodium (Nav) and potassium (Kv) channels to the AIS and nodes of Ranvier, resulting in the fast and efficient propagation of action potentials in neurons [14]. AnkG is also required to maintain neuronal polarity, i.e., the distinction between axonal and somatodendritic domains [15]. Disruption to the ankyrin/spectrin complex leads to dysfunctions in neuronal membrane microdomains, causing several types of human disease.
In recent years, many studies, including large genome-wide association studies (GWAS), consistently link ankyrins to neurological and neuropsychiatric disease (Table 1). These studies suggest the molecular role of ankyrins in the nervous system may be more complex and have broader impacts than previously appreciated. Here, we review these recent works and highlight potential research avenues to further our understanding of these important scaffolding proteins.
Table 1.
Neurological diseases and disorders associated with ankyrins.
| Ankyrin | Associated Neurological Diseases | References |
|---|---|---|
| ANK1 | Motor Dysfunction | 16, 18–21 |
| Alzheimer’s Disease (AD) | 22–27 | |
| Schizophrenia (SCZ) | 28 | |
| ANK2 | Autism Spectrum Disorders (ASD) | 30–37 |
| Schizophrenia | 34, 38 | |
| Parkinson’s Disease (PD) | 40 | |
| ANK3 | Bipolar Disorder (BD) | 44–50 |
| Schizophrenia | 47, 50–53 | |
| Post-traumatic Stress Disorder (PTSD) | 54 | |
| Attention-deficit Hyperactivity Disorder (ADHD) | 55 | |
| Autism Spectrum Disorders | 56 | |
| Intellectual Disability (ID) | 57 |
Diseases linked to Ankyrin-R
AnkR is highly expressed at the soma and proximal dendrites in a sparse population of neurons in the brain (Figure 1C), including cerebellar Purkinje cells [16]. Moreover, it has been linked to multiple neurological diseases; however, compared to the other ankyrin proteins it has been relatively unstudied in the brain and its functions in the brain remain poorly understood. AnkR has been most widely studied in red blood cells as most cases of hereditary spherocytosis (HS), one of the most common hemolytic anemias, are caused by mutations in AnkR. Other causes of HS involve mutations in AnkR binding partners including: a1 spectrin, b1 spectrin, band 3, and band 4.2 [3,17]. Functionally the outcome of these mutations is reduced mechanical resilience of the red blood cell membrane which results in erythrocyte fragmentation due to the high shear environment of the vascular system [3]. While HS is not a neurological disease, case studies of patients with hereditary spherocytic anemia, caused by mutations in AnkR, describe various neurological disturbances including cerebellar defects and spinal cord disease [18–20].
Interestingly, aged AnkR hypomorphic mutant mice have a severe psychomotor disorder and cerebellar Purkinje cell degeneration [16]. Loss-of-function mutations in b3 spectrin underlie spinocerebellar ataxia type 5 (SCA5), which is a subtype of a neurodegenerative disorder involving progressive gait ataxia and cerebellar atrophy. AnkR is thought to be the binding partner for b3 spectrin in these neurons, and together this complex modulates Purkinje cell excitability [21]. Nevertheless, additional studies are needed to elucidate the precise mechanism by which mutations in b3 spectrin cause SCA5, and the role of AnkR in this disease.
Alzheimer’s disease (AD) is the most common form of dementia with varied and poorly understood mechanisms. One leading hypothesis is that epigenetic changes underlie the pathophysiology of AD. Multiple recent large independent epigenome-wide association studies (EWAS) have consistently highlighted differentially methylated regions of the ANK1 gene, in which hypermethylation was correlated with onset and neuropathology of AD [22–26]. Additionally, a single-nucleotide polymorphism (SNP) in ANK1 is associated with increased vulnerability to late-onset AD [27]. However, how these changes in ANK1 affect AnkR protein remains unknown. De novo mutations in AnkR are also linked to schizophrenia (SCZ) [28], although how they affect AnkR function is unknown.
Diseases linked to Ankyrin-B
In contrast to AnkR, the function of neuronal AnkB is much better understood. AnkB, first characterized in the brain, is present in axons where it promotes the assembly of the AIS by creating an intra-axonal boundary limiting AnkG’s distribution [13] (Figure 1C). A 220 kDa form of AnkB is also found at paranodes of myelinating Schwann cells (a 190 kDa form of AnkG is found at paranodes of oligodendrocytes) and participates in axon-glia interactions through the CAM neurofascin-155 [12]. Moreover, giant AnkB (AnkB-440 kDa) interacts with L1CAM and is essential for maintenance of premyelinated axons [29] (Figure 1B). AnkB knockout mice have reduced L1CAM, optic nerve degeneration, hypoplasia of the corpus collosum, and die by postnatal day 21 [29]. Additionally, alterations in AnkB are known risk factors for autism spectrum disorders (ASD), SCZ, and Parkinson’s disease (PD).
ASD are developmental neuropsychiatric disorders that are characterized as either syndromic or non-syndromic. Syndromic ASD occurs in individuals with neurological disorders, e.g. seizure, has a defined set of phenotypes, and is fully attributed to a mutation in a specific gene or set of genes. Syndromic ASDs include Fragile X Syndrome and Rett Syndrome, caused by the mutation of FMR1 or MECP2, respectively [30]. However, the majority of ASD cases are non-syndromic, heritable but not linked to other neurological diseases. GWAS and other genetic analyses have identified hundreds of genetic risk factors linked to non-syndromic ASD, including ANK2 [30–36].
The mutation of AnkB-440 kDa, a neurospecific alternatively spliced ANK2 variant, is sufficient to cause nonsyndromic ASD [33]. Similarly, its binding partner, L1CAM, has also been implicated in ASD [33]. Mouse models with mutated/deficient AnkB-440 kDa have increased axonal branching yielding increased cortical connectivity and an increased number of excitatory synapses during postnatal development [33]. Another ASD-linked AnkB variant is the alternatively spliced isoform ANK2–013. ANK2–013 contains the membrane binding domain (MBD) which allows ankyrins to bind to diverse and structurally unrelated membrane proteins; this AnkB isoform can bind to the neuron-glia related CAM (NrCAM), the transcriptional activation factor TAF9, and the b-subunit of sodium channels, Navβ4 which can alter channel kinetics [34]. Interestingly, ANK2–013 is decreased in ASD [34]. Additionally, immunoprecipitation experiments targeting AnkB and other ASD-associated genes found that among the latter, the protein Acot7 interacts with AnkB [37]. Taken together, these data suggest that differential regulation of AnkB isoforms change its binding capacity thereby altering the clustering of protein complexes.
SCZ and ASD share many genetic risk factors, including ANK2, and ANK2 is significantly upregulated in SCZ brains [38]. Moreover, ANK2–006, an alternatively spliced isoform of ANK2 containing the ZU5 and DEATH domain (DD), is also increased in SCZ [34] (the DD intramolecularly interacts with the MBD while the ZU5 domain is necessary for spectrin binding [7,39]; (Figure 1B)). Interestingly, AnkB is increased in SCZ, while there is a decrease in ASD-associated AnkB variants. The functional implications of these differences and how they impact AnkB’s interactions remain unknown. Lastly, a recent GWAS study screening PD patients identified two disease-related variants of ANK2 that were found in 6/62 patients with idiopathic PD but not in healthy controls [40]. PD is the second most common neurodegenerative disease, and to date only 5–10% of cases have identified monogenic causes, suggesting that many additional genetic components remain unknown [40].
Diseases linked to Ankyrin-G
AnkG is a critical organizer of the AIS and nodes of Raniver (Figure 1C). At these regions, AnkG interacts with the CAMs neurofascin-186 and NrCAM, KCNQ2/3 potassium channels, and Nav channels [14,41,42]. However, contrary to previous models where AnkG was thought to be necessary for Nav channel clustering at nodes of Ranvier, AnkG is not essential due to a hierarchy of ankyrin-spectrin interactions, whereby AnkR and β1 spectrin act as a secondary clustering mechanism [41]. Conversely, AnkG is required for Nav channel clustering at the AIS because AnkR lacks the necessary domain for AIS localization [5]. AnkG is also required to maintain neuronal polarity, and more recently has been revealed to play a role in the regulation of synapses [43–46]. Many studies have found genetic alterations in AnkG in neurological disorders including, but not limited to, bipolar disorder (BD), SCZ, post-traumatic stress disorder (PTSD), intellectual disability (ID), attention-deficit hyperactivity disorder (ADHD), and ASD.
AnkG is one of the strongest and most replicated genes linked to BD and multiple genetic variants in ANK3 have reliably been associated with BD [47–49]. While some of these variants are associated with lower AnkG expression and increased risk of BD [47,48], others, including a loss-of-function variant occurring in a minor AnkG isoform (AnkG-Alt2) expressed in oligodendrocytes, are protective against BD. Conversely, while loss of AnkG-Alt2 is thought to be protective, increased expression of AnkG-Alt2 has been identified in patients with BD [50].
Various SNPs identified in AnkG have also been associated with either reduced or increased SCZ risk [47,51,52], and microarray-based gene expression analyses show AnkG as well as the nodal proteins contactin and neurofascin are downregulated in SCZ [47]. Additionally, the same mutation resulting in reduced AnkG-Alt2, identified as protective for BD, is also protective for SCZ; moreover, elevated expression of AnkG-Alt2, found in BD, has been found in patients with SCZ [50]. While psychotic experiences are normally genetically correlated with disorders such as SCZ, ASD, and ADHD, they also occur in the general population [53]. A recent GWAS study investigating participants from these cohorts found an association between psychotic experiences, i.e. hallucinations and delusions, and AnkG genetic variants identified in SCZ [53].
Genetic variants associated with high AnkG expression and lower BD and SCZ risk have also been associated with lower risk of PTSD; conversely, variants linked to reduced AnkG expression have been associated with an increased risk of PTSD [54]. Additionally, disruption of AnkG has been linked to ID and ADHD often occurring as co-morbidities with ASD [55]. Though AnkG does not have as robust a link as AnkB, three different missense mutations in AnkG have been linked to increased ASD susceptibility, suggesting there may be common etiology for AnkG contributing to these disorders [56].
Ankyrins modulate neuronal excitability
It is well established that ankyrins have essential roles in the formation and maintenance of excitable domains including the AIS and nodes of Ranvier [12–14,41,42]. Thus, mutations in ankyrins could impact neuronal excitability and contribute to the pathophysiology of disease.
Similar to AnkB, AnkG also has a giant isoform, AnkG-480 kDa. AnkG-480 kDa is necessary for many of AnkG’s interactions at the AIS, including recruitment of b4 spectrin, and thus is critical for AIS assembly and maintenance [57] (Figure 1B). Recent mutations in AnkG-480 kDa have been identified in patients that present with neurodevelopmental disorders and ID, and these mutations are thought to alter neuronal excitability by disrupting the assembly of the AIS [57]. Moreover, AnkG has alternative first exons 1a/1a’, 1e, and 1b and the resulting AnkG isoforms are thought to have different localization and function [49]. For example, parvalbumin-expressing interneurons, one of the primary GABAergic neuron subtypes in the brain that are important for maintaining the balance of excitation and inhibition, only express ANK3 exon1b, and mice without ANK3 exon1b lack Nav channels at the AIS of these neurons resulting in altered excitability [49]. Moreover, increased BD susceptibility has been linked to decreased expression of ANK3 exon 1b, whereas decreased ANK3 exon 1e is thought to be protective, suggesting genetic variations resulting in the expression changes of AnkG’s first exons may contribute to BD pathology [47–49]. Interestingly, many BD-associated ANK3 SNPs are located within a cis-regulatory region between exons 1e and 1b [49].
Ankyrins modulate connectivity by synaptic regulation
Synapses and spine morphology have been implicated as key sites of pathogenesis in neurological disorders, including BD, SCZ, and ASD, yet the mechanisms underlying the subcellular organization of synapses remain poorly understood. All three ankyrins have been detected by proteomics in postsynaptic density preparations, and increasing evidence suggests both AnkB and AnkG regulate connectivity, and may contribute to disease pathogenesis, by regulating the structure and function of synapses [44–46,58,59]. This possibility has been explored in C. elegans, where orthologs of AnkB and L1CAM have been implicated as regulators of dendritic arborization [58]. In Drosophila, the two giant AnkB isoforms, together with the homolog of MAP1B, control microtubule organization and the distribution and organization of synaptic CAMs including L1CAM, thereby regulating synapse size and stability [59]. Moreover, AnkG is thought to stabilize cell surface GABAA receptors, important for fast inhibitory synaptic transmission, by directly interacting with the GABAA receptor associated protein, GABARAP [46]. Mice with a mutation preventing the AnkG/GABARAP interaction have reduced GABAergic synapses in the forebrain resulting in hyperexcitability of pyramidal neurons and altered network synchronization; furthermore, this mutation was identified in a family with BD [46]. AnkG may also modulate AMPAR-mediated synaptic transmission by forming nanodomain structures that act as a perisynaptic scaffold and barrier in the head and neck of spines [44]. Taken together, ankyrin disruption, leading to altered connectivity, may be a common pathomechanism of these disorders.
Conclusion
Differences in gene expression underpin much of human diversity. Not surprisingly, some differences can increase the risk for neurological and neuropsychiatric disorders. In recent years many studies have sought to identify genetic risk factors for various disorders by using differential expression analyses as well as genome- and epigenome-wide association studies. These studies have yielded hundreds of promising candidates, but the need to develop effective treatments remains a major health priority. In order to develop treatments, we first need to understand the functional implications of these genetic variations and how they impart risk. As described in this review, understanding the function of ankyrins in the nervous system may lead to key insights into the pathophysiology of complex neurological diseases and may even lead to insights that suggest treatments.
However, much remains unknown about ankyrin biology. For example, new structural evidence shows that despite direct and strong interactions between ankyrins and b spectrins, these interactions do not dictate their preferential interactions (e.g. AnkG/β4 spectrin, AnkB/β2 spectrin, and AnkR/β1 spectrin) in neurons [7]. In fact, ankyrins have high binding affinities with multiple b spectrins, not just their classical partner [7]. These surprising observations prompt the questions, what are the mechanisms that modulate these interactions? Are they altered by genetic variations in ankyrins? And does this contribute to disease?
Ankyrins are the bridge between numerous membrane proteins and the cytoskeleton. They are well characterized as membrane organizers and are necessary for the formation and maintenance of the subcellular membrane domains required for proper neuronal structure and function. Here, we have proposed mutations in ankyrins or changes in their expression levels primarily lead to 1) reduced ion channel/membrane protein expression and 2) altered neural connectivity. However, in many instances, apart from identification as a candidate molecule, little is currently known about their contribution to neurological disease, therefore additional studies are required to elucidate these mechanisms.
Highlights.
Ankyrins link membrane proteins to the cytoskeleton to form and maintain membrane micro-domains.
Ankyrins cluster and organize protein complexes that modulate connectivity and excitability.
Ankyrins, coupled to spectrins, render membranes resilient to external mechanical forces.
Large genomic and epigenetic studies have linked ankyrin proteins to neurological disease.
The underlying molecular mechanisms linking ankyrin proteins to disease remain largely unknown.
Funding Sources
Supported by NIH grant NS044916, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and a Baylor College of Medicine/Houston Methodist Hospital Alzheimer’s research award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
The authors declare no competing financial interests
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Michaely P, Bennett V: Mechanism for Binding Site Diversity on Ankyrin: J Biol Chem 1995, 270:31298–31302. [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick SG, Smerdon SJ: The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem Sci 1999, 24:311–316. [DOI] [PubMed] [Google Scholar]
- 3.Bennett V, Healy J: Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med 2008, 14:28–36. [DOI] [PubMed] [Google Scholar]
- 4.Ho TSY, Zollinger DR, Chang KJ, Xu M, Cooper EC, Stankewich MC, Bennett V, Rasband MN: A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosci 2014, 17:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CH, Seo R, Ho TSY, Stankewich M, Mohler PJ, Hund TJ, Noebels JL, Rasband MN: β spectrin-dependent and domain specific mechanisms for Na+ channel clustering. Elife 2020, 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CH, Stevens SR, Teliska LH, Stankewich M, Mohler PJ, Hund TJ, Rasband MN: Nodal β spectrins are required to maintain Na+ channel clustering and axon integrity. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Chen K, Zhu R, Zhang M: Structural Basis Underlying Strong Interactions between Ankyrins and Spectrins. J Mol Biol 2020, 432:3838–3850. • The authors show all ankyrins have a sequence in the linker domain adjacent to the spectrin binding domain that enhances ankyrin-spectrin interactions. Furthermore, they find that the binding affinity of ankyrins and spectrins does not explain why ankyrins have preferential spectrin binding partners, thus raising questions about the mechanism underlying specific ankyrin-spectrin interations.
- 8.Leterrier C: Putting the axonal periodic scaffold in order. Curr Opin Neurobiol 2021 [DOI] [PubMed]
- 9. Leterrier C: The axon initial segment: An updated viewpoint. J Neurosci 2018, 38:2135–2145. • We highly recommend the above two reviews, [8] and [9], by Christophe Leterrier to interested readers as they provide a recent and in-depth discussion about AIS and axon structure and function beyond the scope of the present review.
- 10.Zhang Y, Abiraman K, Li H, Pierce DM, Tzingounis AV., Lykotrafitis G: Modeling of the axon membrane skeleton structure and implications for its mechanical properties. PLoS Comput Biol 2017, 13:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leterrier C, Potier J, Caillol G, Debarnot C, Rueda Boroni F, Dargent B: Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Rep 2015, 13:2781–2793. [DOI] [PubMed] [Google Scholar]
- 12.Chang KJ, Zollinger DR, Susuki K, Sherman DL, Makara MA, Brophy PJ, Cooper EC, Bennett V, Mohler PJ, Rasband MN: Glial ankyrins facilitate paranodal axoglial junction assembly. Nat Neurosci 2014, 17:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galiano MR, Jha S, Ho TSY, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN: A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 2012, 149:1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett V, Lorenzo DN: An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues. Elsevier Ltd; 2016. [DOI] [PubMed] [Google Scholar]
- 15.Hedstrom KL, Ogawa Y, Rasband MN: AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 2008, 183:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters LL, Birkenmeier CS, Bronson RT, White RA, Lux SE, Otto E, Bennett V, Higgins A, Barker JE: Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nb mice. J Cell Biol 1991, 114:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eber S, Lux SE: Hereditary Spherocytosis - Defects in Proteins That Connect the Membrane Skeleton to the Lipid Bilayer. Semin Hematol 2004, 41:118–141. [DOI] [PubMed] [Google Scholar]
- 18.McCann SR, Jacob HS: Spinal Cord Disease in Hereditary Spherocytosis: Report of Two Cases With a Hypothesized Common Mechanism for Neurologic and Red Cell Abnormalities. Blood 1976, 48:259–263. [PubMed] [Google Scholar]
- 19.Coetzer TL, Lawler J, Liu S-C, Prchal JT, Gualtieri RJ, Brain MC, Dacie JV., Palek J: Partial Ankyrin and Spectrin Deficiency in Severe, Atypical Hereditary Spherocytosis. N Engl J Med 1988, 318:230–234. [DOI] [PubMed] [Google Scholar]
- 20.Miya K, Shimojima K, Sugawara M, Shimada S, Tsuri H, Harai-Tanaka T, Nakaoka S, Kanegane H, Miyawaki T, Yamamoto T: A de novo interstitial deletion of 8p11.2 including ANK1 identified in a patient with spherocytosis, psychomotor developmental delay, and distinctive facial features. Gene 2012, 506:146–149. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson YL, Perkins EM, Cairncross CJ, Lyndon AR, Skehel PA, Jackson M: β-III spectrinunderpins ankyrin R function in purkinje cell dendritic trees: Protein complex critical for sodium channel activity is impaired by SCA5-associated mutations. Hum Mol Genet 2014, 23:3875–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith AR, Smith RG, Burrage J, Troakes C, Al-Sarraj S, Kalaria RN, Sloan C, Robinson AC, Mill J, Lunnon K: A cross-brain regions study of ANK1 DNA methylation in different neurodegenerative diseases. Neurobiol Aging 2019, 74:70–76. • This paper seeks to understand the epigenetic changes in ANK1 by quantifying DNA methylation levels in Alzheimer’s disease and comparing them to levels in other age-related neurological disorders. They confirm previous ANK1 hypermethylation findings and discover differential DNA methylation that is both disease and region specific.
- 23.Smith AR, Smith RG, Pishva E, Hannon E, Roubroeks JAY, Burrage J, Troakes C, Al-Sarraj S, Sloan C, Mill J, et al. : Parallel profiling of DNA methylation and hydroxymethylation highlights neuropathology-associated epigenetic variation in Alzheimer’s disease. Clin Epigenetics 2019, 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT, Ernst J, McCabe C, et al. : Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 2014, 17:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunnon K, Smith R, Hannon E, De Jager PL, Srivastava G, Volta M, Troakes C, Al-Sarraj S, Burrage J, Macdonald R, et al. : Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat Neurosci 2014, 17:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasparoni G, Bultmann S, Lutsik P, Kraus TFJ, Sordon S, Vlcek J, Dietinger V, Steinmaurer M, Haider M, Mulholland CB, et al. : DNA methylation analysis on purified neurons and glia dissects age and Alzheimer’s disease-specific changes in the human cortex. Epigenetics and Chromatin 2018, 11:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi S, Song JH, Tan MS, Zhang W, Wang ZX, Jiang T, Tan L, Yu JT: Association of Single-Nucleotide Polymorphism in ANK1 with Late-Onset Alzheimer’s Disease in Han Chinese. Mol Neurobiol 2016, 53:6476–6481. [DOI] [PubMed] [Google Scholar]
- 28.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, et al. : De novo mutations in schizophrenia implicate synaptic networks. Nature 2014, 506:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scotland P, Zhou D, Benveniste H, Bennett V: Nervous System Defects of Ankyrin. J Cell Biol 1998, 143:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez BA, Scherer SW: Syndromic autism spectrum disorders: moving from a clinically defined to a molecularly defined approach. Dialogues Clin Neurosci 2017, 19:353–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Gonzalez A, Rodriguez-Fontenla C, Carracedo A: De novo mutations (DNMs) in autism spectrum disorder (ASD): Pathway and network analysis. Front Genet 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Wang J, Cicek E, Roeder K, Yu H, Devlin B: De novo missense variants disrupting protein-protein interactions affect risk for autism through gene co-expression and protein networks in neuronal cell types. Mol Autism 2020, 11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang R, Walder-Christensen KK, Kim N, Wu D, Lorenzo DN, Badea A, Jiang YH, Yin HH, Wetsel WC, Bennett V: ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proc Natl Acad Sci U S A 2019, 116:15262–15271. •• These authors show that AnkB-440 mutant mice have increase axonal branching and altered structural connectivity, suggesting disrupted connectivity as a potential disease mechanism for AnkB.
- 34. Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, Won H, van Bakel H, Varghese M, Wang Y, et al. : Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science (80- ) 2018, 362. •• The authors show two alternatively spliced isoforms of AnkB are differentially regulated in schizophrenia and autism spectrum disorders altering disease risk, highlighting that isoform dysregulation in AnkB and other genes is a mechanism for disease pathophysiology.
- 35.Kumar S, Reynolds K, Ji Y, Gu R, Rai S, Zhou CJ: Impaired neurodevelopmental pathways in autism spectrum disorder: A review of signaling mechanisms and crosstalk. J Neurodev Disord 2019, 11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppa VMM, Kravitz SNN, Martin CLL, Andrieux J, Le Caignec C, Martin-Coignard D, DyBuncio C, Sanders SJJ, Lowe JKK, Cantor RMM, et al. : Rare Inherited and De Novo CNVs Reveal Complex Contributions to ASD Risk in Multiplex Families. Am J Hum Genet 2016, 99:540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Ma Z, Shi M, Malty RH, Aoki H, Minic Z, Phanse S, Jin K, Wall DP, Zhang Z, et al. : Identification of Human Neuronal Protein Complexes Reveals Biochemical Activities and Convergent Mechanisms of Action in Autism Spectrum Disorders. Cell Syst 2015, 1:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai L, Huang T, Su J, Zhang X, Chen W, Zhang F, He L, Chou KC: Implications of Newly Identified Brain eQTL Genes and Their Interactors in Schizophrenia. Mol Ther - Nucleic Acids 2018, 12:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett V, Healy J: Membrane Domains Based on Ankyrin and Spectrin Associated with Cell-Cell Interactions. Cold Spring Harb Perspect Biol 2009, 1:a003012–a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germer EL, Imhoff S, Vilariño-Güell C, Kasten M, Seibler P, Brüggemann N, Klein C, Trinh J: The Role of Rare Coding Variants in Parkinson’s Disease GWAS Loci. Front Neurol 2019, 10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho TSY, Zollinger DR, Chang KJ, Xu M, Cooper EC, Stankewich MC, Bennett V, Rasband MN: A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosci 2014, 17:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rasband MN, Peles E: Mechanisms of node of Ranvier assembly. Nat Rev Neurosci 2020, doi: 10.1038/s41583-020-00406-8. • Disruption of ankyrins, particularly AnkG, is known to alter excitability by disrupting ion channel clustering at the AIS and nodes of Ranvier. While the present review does not delve into the mechanisms of node assembly and how ankyrins are involved, we highly recommend this review which does.
- 43.Hedstrom KL, Ogawa Y, Rasband MN: AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 2008, 183:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith KR, Kopeikina KJ, Fawcett-Patel JM, Leaderbrand K, Gao R, Schürmann B, Myczek K, Radulovic J, Swanson GT, Penzes P: Psychiatric Risk Factor ANK3/Ankyrin-G Nanodomains Regulate the Structure and Function of Glutamatergic Synapses. Neuron 2014, 84:399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KR, Penzes P: Ankyrins: Roles in synaptic biology and pathology. Mol Cell Neurosci 2018, 91:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson AD, Caballero-Florán RN, Rodríguez Díaz JC, Hull JM, Yuan Y, Li J, Chen K, Walder KK, Lopez-Santiago LF, Bennett V, et al. : Ankyrin-G regulates forebrain connectivity and network synchronization via interaction with GABARAP. Mol Psychiatry 2020, 25:2800–2817. • This paper explores how AnkG, via its interaction with GABARAP, regulates GABAergic circuitry in vivo and establishes a mechanism by which AnkG modulates excitability at GABAergic synapses.
- 47.Roussos P, Katse P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, Rozsnyai K, Collier D, Frangou S, Siever LJ, et al. : Molecular and genetic evidence for abnormalities in the nodes of ranvier in schizophrenia. Arch Gen Psychiatry 2012, 69:7–15. [DOI] [PubMed] [Google Scholar]
- 48.Rueckert EH, Barker D, Ruderfer D, Sarah E, Dushlaine CO, Luce CJ, Ms MS, Sheridan SD, Theriault KM, Chambert K, et al. : Cis-Acting Regulation of Brain-Specific ANK3 Gene Expression by a Genetic Variant Associated with Bipolar Disorder. 2014, 18:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez AY, Wang X, Xu M, Maheshwari A, Curry D, Lam S, Adesina AM, Noebels JL, Sun Q-Q, Cooper EC: Ankyrin-G isoform imbalance and interneuronopathy link epilepsy and bipolar disorder. Mol Psychiatry 2017, 22:1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes T, Sønderby IE, Polushina T, Hansson L, Holmgren A, Athanasiu L, Melbø-Jørgensen C, Hassani S, Hoeffding LK, Herms S, et al. : Elevated expression of a minor isoform of ANK3 is a risk factor for bipolar disorder. Transl Psychiatry 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo X, Zhang Y, Du J, Yang H, Ma Y, Li J, Yan M, Jin T, Liu X: Association analysis of ANK3 gene variants with schizophrenia in a northern Chinese Han population. Oncotarget 2016, 7:85888–85894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong X, Zhang L, Han S, Luo X, An Z, Yi Q: Case control study of association between the ANK3 rs10761482 polymorphism and schizophrenia in persons of Uyghur nationality living in Xinjiang China. Shanghai Arch Psychiatry 2014, 26:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legge SE, Jones HJ, Kendall KM, Pardiñas AF, Menzies G, Bracher-Smith M, Escott-Price V, Rees E, Davis KAS, Hotopf M, et al. : Association of Genetic Liability to Psychotic Experiences with Neuropsychotic Disorders and Traits. JAMA Psychiatry 2019, 76:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Logue MW, Solovieff N, Leussis MP, Wolf EJ, Melista E, Baldwin C, Koenen KC, Petryshen TL, Miller MW: The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology 2013, 38:2249–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iqbal Z, Vandeweyer G, Van der voet M, Waryah AM, Zahoor MY, Besseling JA, Roca LT, Vulto-van silfhout AT, Nijhof B, Kramer JM, et al. : Homozygous and heterozygous disruptions of ANK3: At the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet 2013, 22:1960–1970. [DOI] [PubMed] [Google Scholar]
- 56.Bi C, Wu J, Jiang T, Liu Q, Cai W, Yu P, Cai T, Zhao M, Jiang YH, Sun ZS: Mutations of ANK3 identified by exome sequencing are associated with Autism susceptibility. Hum Mutat 2012, 33:1635–1638. [DOI] [PubMed] [Google Scholar]
- 57. Yang R, Walder-Christensen KK, Lalani S, Yan H, García-Prieto ID, Álvarez S, Fernández-Jaén A, Speltz L, Jiang YH, Bennett V: Neurodevelopmental mutation of giant ankyrin-G disrupts a core mechanism for axon initial segment assembly. Proc Natl Acad Sci U S A 2019, 116:19717–19726. •• Ankyrin proteins stabilize membrane proteins by linking them to the spectrin cytoskeleton. This paper identifies human mutations that disrupt giant AnkG, via altered phoshorylation and confirmational changes; this prevents b4 spectrin recruitment and destabilizes the AIS thereby impacting neuronal excitability.
- 58.Aguirre-Chen C, Stec N, Ramos OM, Kim N, Kramer M, McCarthy S, Gillis J, Richard McCombie W, Hammell CM: A Caenorhabditis elegans model for integrating the functions of neuropsychiatric risk genes identifies components required for normal dendritic morphology. G3 Genes, Genomes, Genet 2020, 10:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephan R, Goellner B, Moreno E, Frank CA, Hugenschmidt T, Genoud C, Aberle H, Pielage J: Hierarchical Microtubule Organization Controls Axon Caliber and Transport and Determines Synaptic Structure and Stability. Dev Cell 2015, 33:5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


