Abstract
Background:
Allosensitization has been reported after discontinuation of immunosuppression following graft failure in islet transplantation (ITx) recipients, though duration of its persistence is unknown.
Methods:
We evaluated 35 patients with type 1 diabetes who received ITx, including 17 who developed graft failure (ITx alone, n=13; ITx plus bone marrow-derived hematopoietic stem cells, n=4) and 18 with persistent graft function. Panel reactive antibody (PRA) was measured yearly for the duration of graft function within 1 year after graft failure at enrollment and yearly thereafter.
Results:
In ITx alone graft failure patients, 61% (8/13) were PRA-positive at 6 years postgraft failure, and 46% (6/13) developed donor-specific anti-HLA antibodies (DSA to 2 ± 1 donors) during follow up. The degree of sensitization was variable (cPRA ranging between 22% and 100% after graft failure). Allosensitization persisted for 7 to 15 years. Three subjects (3/13) were not allosensitized. In ITx plus bone marrow-derived hematopoietic stem cell recipients, cPRA-positivity (88% to 98%) and DSA-positivity persisted for 15 years in 75% (3/4) of subjects.
Conclusions:
Allosensitization was minimal while subjects remained on immunosuppression but after discontinuation of immunosuppressive therapy the majority of subjects (77%) became allosensitized with persistence of PRA positivity for up to 15 years. Persistence of allosensitization in this patient population is of clinical importance as it may result in longer transplant waiting-list times for identification of a suitable donor in case of requiring a subsequent transplant.
Introduction
Improved metabolic control, hypoglycemia awareness, quality of life and prevention of severe hypoglycemia can be consistently obtained after islet transplantation (ITx) in patients with type 1 diabetes (T1D), even with partial graft function requiring exogenous insulin to maintain glycated hemoglobin (HbA1c) in the target range.1–6 Although islet allograft survival at 1 year is approximately 80%, graft function decreases progressively with time.7 Allosensitization develops in approximately 40–70% of ITx recipients following discontinuation of immunosuppression after graft failure.8–11 Allosensitization is defined as the presence of circulating antibodies against HLAs and can be measured by different techniques and expressed as percentage reactivity of the patient’s serum to a panel of lymphocytes with known HLA phenotypes or purified HLA antigens in solid phase assays (% panel reactive antibody; % PRA).12 The calculated PRA (cPRA) is obtained using a calculator by the United Network for Organ Sharing (UNOS).13 The cPRA provides a more accurate estimate of allosensitization since it includes both class I and class II HLA specificities. Use of traditional PRA (% of class I and class II HLA) underestimates the degree of allosensitization.14 In fact, there is 50% concordance in the group with PRA values of 1% up to 20%, while there is 90% concordance in the group with PRA values equal or greater than 80%.14 Allosensitization after transplantation of solid organs and cellular grafts is a common problem, potentially decreasing the donor pool for a given transplant candidate or prolonging the wait time for subsequent organ transplantations. In the case of pancreatic islets, the need for a relatively large number of beta cells to achieve adequate metabolic control has led to the use of multiple donors per recipient in most cases. This may increase the risk for allosensitization to multiple HLAs and represents a significant concern after graft failure as those patients who become allosensitized and subsequently require another transplant will likely experience increased wait-time for identification of a suitable donor limiting the chances of future transplantation.
The duration of persistence of PRA-positivity after discontinuation of immunosuppression in ITx recipients still remains unknown. Therefore, the purpose of this study was to evaluate the frequency and persistence of allosensitization postgraft failure and upon discontinuation of immunosuppression in ITx recipients and to identify associated factors which may predict the development of allosensitization.
Materials and Methods
Study population
Fifty-six subjects received islet transplants at our institution from 2000–2017. Nine subjects were lost to follow-up and 1 subject died. Of the remaining 46 subjects, 28 subjects had graft failure (22 IA and 6 IA+BM). Of the 22 IA, 13 subjects (IA group) signed consent to enroll in the follow-up post graft failure protocol. Nine did not agree to participate. Of the 6 subjects receiving ITx+BM group, 4 subjects (IA+BM group) signed the consent for the follow-up after graft failure. Two subjects did not sign consent. The remaining 18 subjects constitute the graft function group. In total, 35 subjects were included in this analysis (Figure 1).
Figure 1 -. Study population diagram representing our retrospective study design on a cohort of patients who received islet transplantation.
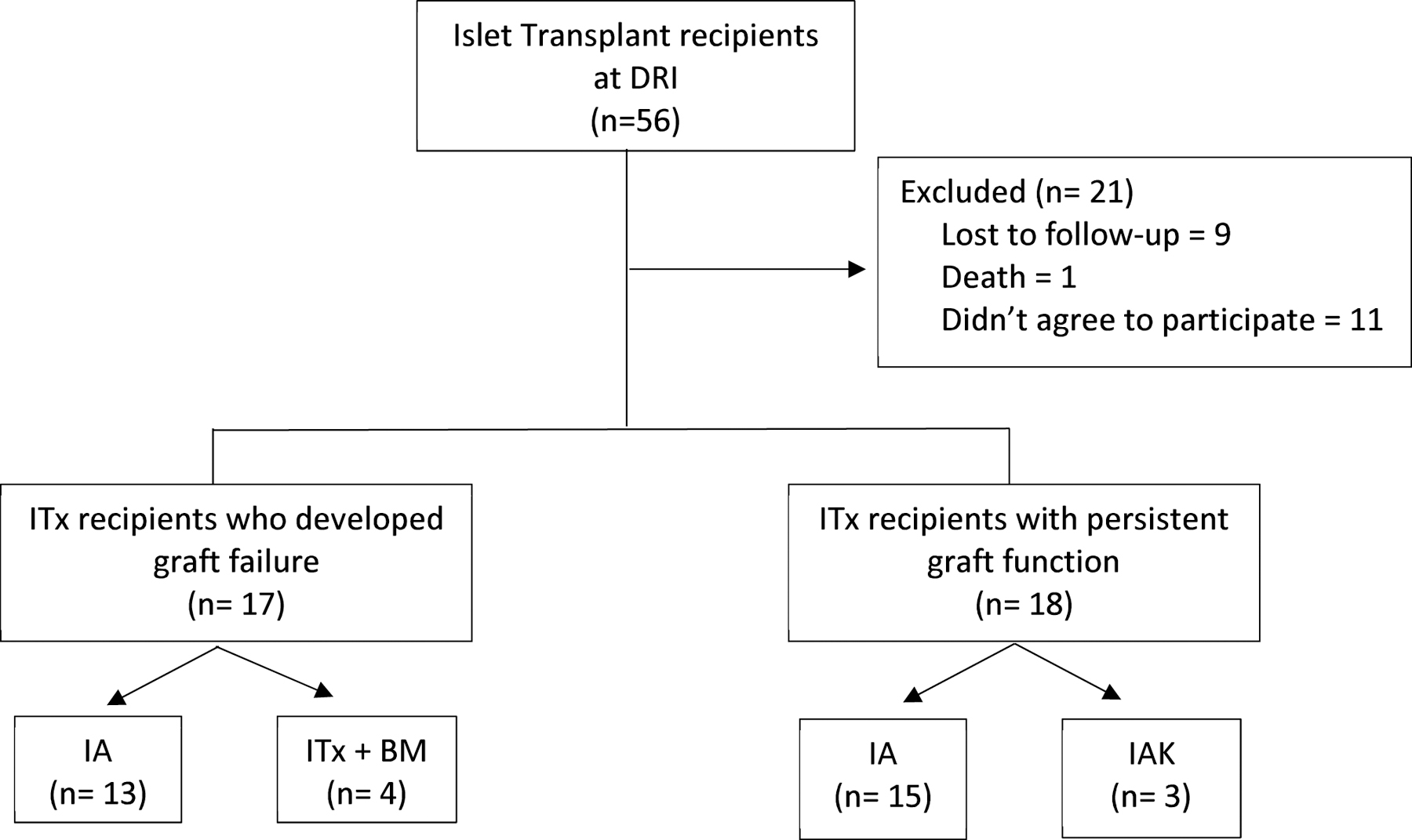
Abbreviations: IA, islet transplantation alone; IAK, islet after kidney transplantation; ITx, islet transplantation; IA + BM, islet transplantation plus bone marrow-derived CD34+ hematopoietic stem cells.
These 35 subjects with T1D enrolled in ITx protocols at the University of Miami were retrospectively evaluated. Study procedures were reviewed and approved by the Institutional Review Board (IRB) at the University of Miami. All participants provided written informed consent and were enrolled in different protocols, namely: i) follow-up of ITx recipients postgraft failure (ClinicalTrials.gov Identifier: NCT02000687, NCT01999361; IRB Protocol Numbers: 20080127, 20071058), and ii) follow-up of ITx recipients with persistent islet graft function (ClinicalTrials.gov Identifier: NCT01999374, NCT00306098; IRB Protocol Numbers: 20130034, 20000196). The study population and participant demographics are detailed in Figure 1 and Table 1, respectively.
Table 1 –
Demographic characteristics of the participants with failed or functioning islet grafts.
| Demographic characteristics | ITx recipients who developed graft failure (n=17) | ITx recipients with current graft function [n=18 (IA, n=15; IAK, n=3] | P-valuea | |
|---|---|---|---|---|
| IA group (n=13) | ITx+BM group (n=4) | |||
| Female, n (%) | 7 (54 ) | 3 (75) | 12 (67) | 0.663 |
| Age at first transplant, years | 44±10.5 | 41±1.9 | 44.6±5.9 | 0.697 |
| Duration of T1D at transplant, years | 17 (14 – 37) | 37 (25 – 40) | 38 (29 – 43) | 0.114 |
| Number of islet infusions | 2 (1 – 3) | 1 | 2 (1 – 2) | 0.059 |
| Total IEQ/Kg body weight | 13 185 (6311 – 15 590) | 7289 (7042 – 10 009) | 14 067 (11 291 – 17 179) | 0.181 |
| Body weight, Kg | 68.6 (60 – 74) | 59 (55.8 – 76) | 60 (54 – 67) | 0.188 |
| Immunosuppression Edmonton Protocol | 11 (85) | 4 (100) | 9 (50) | 0.024 |
| Immunosuppression T cell depletion | 2 (15) | 0 | 9 (50) | 0.024 |
| Duration of graft function (years) | 3.3 (1.9 – 5.0)c | 1.25 (0.8 – 1.3)c | 12.35 (7.5 – 14.8) | <0.001 |
| Years of graft failure at enrolment | 4 (2 – 5) | 8 (8 – 9) | n/a | 0.001 |
| Years of graft failure at last follow-up | 9.85±2.6 | 15±0.81 | n/a | <0.001 |
| Years to graft failure after first infusion | 3 (1– 5) | 0.5 (0 – 1) | n/a | 0.023 |
| Years to graft failure after last infusion | 1 (1 – 4) | 0.5 (0 – 1) | n/a | 0.079 |
| CMV IgG-positivity, n (%) | 8 (61.5) | 3 (75) | 5 (27.8) | 0.075 |
| DSA, n (%) | 6 (46.2) | 3 (75) | 2 (11.1)d | 0.014 |
| DSA toward how many donors | 2 (1 – 3) | 1 | 1 | 0.104 |
| DSA toward HLA I, n (%) | 6 (46.2)c,d | 3 (75)c,d | 1 (5.6) | 0.003 |
| DSA toward HLA II, n (%) | 3 (23.1)c,d | 3 (75)c,d | 1 (5.6) | 0.013 |
| HLA I mismatches | 8 (6 – 10) | 5 (4 – 6)b | 6 (5 – 7)b | 0.035 |
| HLA II mismatches | 3 (2 – 4) | 3 (3 – 4) | 2 (2 – 5) | 0.789 |
| Autoantibodies 0/1/2/converter,e n (%) | n=5/5/1/1 (42/42/8/8) # | n=1/1/1/1 (25/25/25/25) | n=6/3/2/3 (43/21/14/21) § | 0.848 |
Abbreviations: CMV, cytomegalovirus; DSA, donor-specific anti-HLA antibodies; IA, islet transplantation alone; ITx + BM, islet transplantation plus bone marrow- derived CD34+ hematopoietic stem cells; IAK, islet after kidney transplantation; IEQ, islet equivalents; IgG, immunoglobulin G; ITx, islet transplantation; n/a, not applicable; T1D, type 1 diabetes.
Indicates P-value between groups and are according to χ2 test, ANOVA, independent t-test, U Mann-Whitney or Kruskal-Wallis test as appropriate.
Indicates significant difference compared to IA group (P-value <0.05)
indicates significant difference compared to ITx recipients with current graft function (P-value <0.05)
indicates chi-square with significant difference after Bonferroni correction.
Autoantibodies: 0= no antibodies; 1, 1 antibody developed; 2, 2 antibodies developed; converter, individual who became autoantibody-positive during follow up.
Autoantibody assessment was performed only in 11 out of 13 subjects.
Autoantibody assessment was performed only in 14 out of 18 subjects.
Maintenance immunosuppression
In IA and IA+BM subjects, induction immunosuppression consisted of anti-IL2 blockade with either daclizumab or basiliximab and anti-TNF blockade with either infliximab or etanercept (Edmonton like protocol).2,15–17 Maintenance immunosuppression consisted of a dual combination strategy with either sirolimus, tacrolimus or mycophenolate mofetil (MMF). MMF was primarily used in case of intolerance/adverse events to either sirolimus or tacrolimus. Target trough levels for sirolimus were 12–15 ng/mL for 3 months then 7–10 ng/mL thereafter; tacrolimus trough levels were kept at 3–6 ng/mL (or 8–10ng/mL if on Tacrolimus-MMF). MMF target dose was 1000 mg PO twice daily with the dose adjusted depending on subject tolerance. In the group of subjects with graft function, 50% underwent induction with T-cell depleting agents (alemtuzumab or antithymocyte globulin).1,18,19
Subjects with Islet Graft Failure
Seventeen ITx recipients who ultimately experienced graft failure were enrolled. Among them, 13 patients received ITx alone (IA group) and 4 received same-donor CD34+ bone marrow (BM)-derived hematopoietic stem cells (HSC; a minimum of 2×106 HSC/Kg of recipient body weight) in combination with ITx (ITx+BM group). In the ITx+BM group, bone marrow cells were used to induce hematopoietic chimerism. As per the study design, all subjects were required to stop immunosuppression at 1 year independent of graft function status. Therefore, no subject remained on immunosuppression after 1 year. Apart from 1 subject who lost function 5 months after islet transplantation, all other subjects maintained graft function at the 1 year time point when immunosuppression was discontinued. After discontinuation of immunosuppression all grafts failed. Immunosuppression dosing was managed by the senior author of this study to ensure that all patients remained on full immunosuppression for the duration of the study. No subject was on monotherapy at any given time. It is expected that immunosuppression levels would had been undetectable after graft failure as cessation of immunosuppression preceded graft failure.
Subjects in the IA group and ITx+BM group were enrolled into the follow-up protocol 3.6±1.9 years and 8±0.7 years after graft failure, respectively (Table 1). Laboratory workup was performed every year following graft failure. Blood samples were collected for determination of alloantibodies and cPRA calculated. For the purpose of data analysis, we established the following time points: i) pre-ITx; ii) half-life graft survival (1/2 GS), defined as the midpoint between first islet infusion and occurrence of graft failure; iii) graft failure, defined as the time at which serum levels of stimulated C-peptide (assessed by Mixed Meal Tolerance Test; MMTT) were less than 0.3 ng/mL15; and iv) yearly follow-up postgraft failure.20
Subjects with Persistent Islet Graft Function
Eighteen subjects with persistent islet graft function at 10.6±4.8 years follow-up were included in the study for comparison analysis. Among them, 15 received IA and 3 received islet after kidney transplantation (IAK). Alloantibody data for these patients were evaluated pre-ITx and at 1, 5, 7, and 10 years posttransplant.
Alloantibody Determination
Subjects belonging to IA and IA+BM groups were tested for alloantibodies using single antigen bead solid phase assays for HLA class I and II (LabScreen Single Antigen Class I and LabScreen Single Antigen Class II from One Lambda [Thermofisher]). Values of cPRA were calculated using the cPRA calculator by UNOS.13,14 A mean fluorescence intensity (MFI) exceeding 3000 was reported as positive and subsequently compared with donor HLA to determine donor-specific anti-HLA antibodies (DSA)-positivity in the recipient’s serum. cPRA was used as a continuous measure of allosensitization.13,14,21 HLA-DP was not included in either the cPRA calculations and DSA analyses. In the subjects with graft function, alloantibodies were evaluated pre-ITx and at 1 year, 5, 7, and 10 years posttransplant. HLA mismatches were evaluated between donor and recipient using HLA-A, -B, -DR and DQ loci.
Autoantibody Determination
Autoantibodies to 65-kilodalton isoform of glutamic acid decarboxylase (GAD65) and islet antigen-2 (IA-2) were measured using radioimmunoassays validated in the proficiency workshops of the Immunology of Diabetes Society and Centers for Disease Control and Prevention.22,23 Autoantibody levels are expressed as the ratio of the autoantibody index levels of the patient over the cutoff index of each assay. A ratio of ≥1 indicates a positive result.
Statistical Analysis
Results are expressed as mean ± SD, median (25th percentile; 75th percentile) or percentages. Descriptive characteristics were analyzed between groups through 1-way Analysis of Variance (ANOVA), unpaired 2 tailed T-test with Welch correction, Kruskal Wallis 1-Way, U Mann-Whitney, or chi-square test, as appropriate. Post hoc analyses were performed with Tukey and Bonferroni correction. To determine differences of cPRA within IA graft failure group, we chose to compare the following time points: i) pre-ITx, ii) graft failure, and iii) 6-year follow-up. For this comparison, it was used related-samples Friedman’s with Dunn-Bonferroni post hoc tests. Statistical analysis was not performed for evaluating time-points in ITx+BM group due to the small sample size. Data were analyzed using IBM SPSS v22.0 (New York, USA). A P value <0.05 was considered to indicate statistical significance.
Results
Recipients with Islet Graft Failure
IA group (n=13):
Among the 13 subjects enrolled, 9 completed more than 6 years of follow-up (range: 6–15 years) and 4 were lost to follow-up. Patient demographics and baseline characteristics are detailed in Table 1. Subjects were transplanted between 2001 and 2006. Immunosuppression induction included daclizumab, along with an anti-TNF-alpha agent (infliximab, n=2; etanercept, n=11). Table 2 shows the demographic characteristics of DSA-positive and DSA-negative recipients in IA group subjects with graft failure. There was no significant association between number of HLA-I and HLA-II mismatches, development of DSA and number of donors (Table 3). Regarding islet autoantibodies, 42% of subjects had 1 islet autoantibody (GAD65 or IA-2), 42% were negative for autoantibodies, 8% had both autoantibodies, and the remaining 8% became positive during follow-up (“converter” subjects) (Table 1). Graft failure in these subjects occurred at 3 (1 – 5) years after ITx and 1 (1 – 4) years after last ITx. Nondonor specific alloantibodies were also detected in 10/13 (77%) subjects (Table S1).
Table 2 –
Demographic characteristics of DSA-positive and DSA-negative recipients in IA group subjects with graft failure (n=13).
| DSA-positive recipients (n=6) | DSA-negative recipients (n=7) | p-value | |
|---|---|---|---|
| Female | 3 (50) | 4 (57.1) | 0.999 |
| Recipient Age at first transplant (years) | 43.7±6.9 | 45.8±13.8 | 0.733 |
| Duration of T1D (years) | 26.5±16.5 | 22.5±13.6 | 0.639 |
| Number of donors | 3±1 | 2±1 | 0.629 |
| HLA I mismatches d | 8 (7 – 9) | 8 (5 – 11) | 0.731 |
| HLA II mismatches d | 4 (2 – 5) | 2 (1 – 4) | 0.295 |
| Total IEQ/Kg body weight | 13 382 (12 956 – 29 593) | 8681 (5261 – 14 144) | 0.534 |
| Body weight (Kg) | 67.4±12.8 | 68.4±10.8 | 0.877 |
| Immunosuppression Edmonton Protocol,a n (%) | 6 (100) | 5 (71.4) | 0.462 |
| Immunosuppression T cell depletion,b n (%) | 0 | 2 (28.6) | 0.462 |
| Myfortic protocol,c n (%) | 2 (33.3) | 5 (71.4) | 0.286 |
| On Exenatide | 3 (50) | 4 (57.1) | 0.999 |
| Duration of graft function (years) | 3.3±1.5 | 3.1±2.1 | 0.851 |
| Total pregnancies | 0 (0 – 3; n=3) | 0 (0 – 3; n=4) | NA |
| CMV IgG-positivity | 4 (66.7) | 4 (57.1) | 0.999 |
| Autoantibody positivity # | 4 (66.7) | 3 (60.0) | 0.999 |
Abbreviations: CMV, cytomegalovirus; DSA, donor-specific anti-HLA antibodies; IA, islet transplantation alone;
IEQ, islet equivalents; IgG, immunoglobulin G; T1D, type 1 diabetes.
The Edmonton Protocol for islet transplantation consists of a glucocorticoid-free immunosuppressive regimen (sirolimus, tacrolimus, daclizumab).
Alemtuzumab or thymoglobulin.
Myfortic=mycophenolic acid.
Autoantibody assessment was performed in 12 out of 13 subjects.
Maximum number of mismatches per donor = 4.
Table 3 -.
Values of cPRA in IA group subjects (n=13) before transplantation, during transplant follow-up and after graft failure.
| Subject | cPRA (%) Pre-ITx | cPRA (%) 1/2 GS | cPRA (%) GF | cPRA (%) 1 year post-GF | cPRA (%) 2 years post-GF | cPRA (%) 3 years post-GF | cPRA (%) 4 years post-GF | cPRA (%) 5 years post-GF | cPRA (%) 6 years post-GF | cPRA (%) 7 years post-GF | cPRA (%) 8 years post-GF | cPRA (%) 9 years post-GF | cPRA (%) 10 years post-GF | cPRA (%) 11 years post-GF | cPRA (%) 12 years post-GF | cPRA (%) 13 years post-GF | cPRA (%) 14 years post-GF | cPRA (%) 15 years post-GF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 0 | 0 | 89 | 89 | n/a | n/a | n/a | n/a | 90 | 78 | 27 | n/a | 79 | 82 | 83 | 82 | 82 | 85 |
| #2 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | n/a | n/a | 63 | 59 | 8 | 0b | 56 | 56 | 56 | 56 | 83 |
| #3 | 22 | 22 | 24 | n/a | n/a | n/a | n/a | 75 | 84 | 0 | 0 | 0 | 0 | 0 | 0 | Enrolleda | Enrolleda | Enrolleda |
| #4 | 0 | 0 | 99 | 99 | n/a | n/a | 92 | 96 | 23 | 23 | 23 | 23 | 64 | 64 | 0 | 0 | 0 | Enrolleda |
| #5 | 0 | 0 | 0 | n/a | n/a | 0 | 0 | 0 | 0 | 0 | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP |
| #6 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | 99 | 98 | 81 | 81 | 82 | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LFTUP |
| #7 | 0 | 0 | 96 | n/a | n/a | n/a | n/a | n/a | 100 | 0b | 86 | 93 | 95 | 89 | 89 | 91 | 79 | 89 |
| #8 | 0 | 0 | 0 | n/a | n/a | n/a | 0 | 0 | 0 | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda |
| #9 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 24 | 3 | 8 | 0 | 0 | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda | Enrolleda |
| #10 | 0 | 0 | 0 | 24 | 11 | 4 | 4 | 11 | n/a | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP |
| #11 | 0 | 0 | 22 | n/a | n/a | n/a | n/a | n/a | 22 | 30 | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP | LTFUP |
| #12 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | n/a | n/a | 0 | 0 | 0 | 0 | n/a | 0 | 0 | 0 | Enrolleda |
| #13 | n/a | 0 | 0 | n/a | n/a | 70 | 24 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Enrolleda | Enrolleda | Enrolleda |
Abbreviations: IA, islet transplantation alone; ITx, islet transplant; ½ GS, half-life graft survival; GF, graft failure; cPRA, calculated panel-reactive antibody; LTFUP lost to follow-up; n/a data not available.
Enrolled: patients have not reached this evaluation point (still enrolled in the protocol).
Presence of antigen specific antibodies and values of MFI below 3000, resulting in cPRA “0”.
The values of cPRA in IA group subjects (n=13) before transplantation, during transplant follow-up and after graft failure are listed in Table 3 and are depicted in Figure 2. Before ITx and at the 1/2 GS time point only 1 recipient was cPRA-positive (Subject #3; cPRA: 22 %). At graft failure, 5 subjects (subjects #1, #3, #4, #7, #11; cPRA: 89, 24, 99, 96, and 22%, respectively). In this group, immunosuppression was tapered and discontinued at 73.5 ± 69.4 days after graft failure. At 6 years postgraft failure, 55% of subjects (6 out of 11 patients) had a cPRA>20% (cPRA: 22%−100%). Collectively, 10 out of 13 subjects (77%) became allosensitized at any time point after graft failure, among which 6 out of 13 subjects (46.2%) developed DSA-positivity (DSA to 2 [1 – 3] donors). The degree of sensitization was variable (cPRA ranging between 22% and 100% during the follow-up after graft failure). Allosensitization persisted for up to 15 years in subject #2 (cPRA: 83%; Table 3).
Figure 2 -. Values of cPRA (%) in IA group subjects (n=13) before transplantation, during transplant follow-up and after graft failure. Box plot shows cPRA distribution mean, interquartile range and minimum and maximum values. The lower bar shows the number of patients evaluated at each time point during the follow-up.
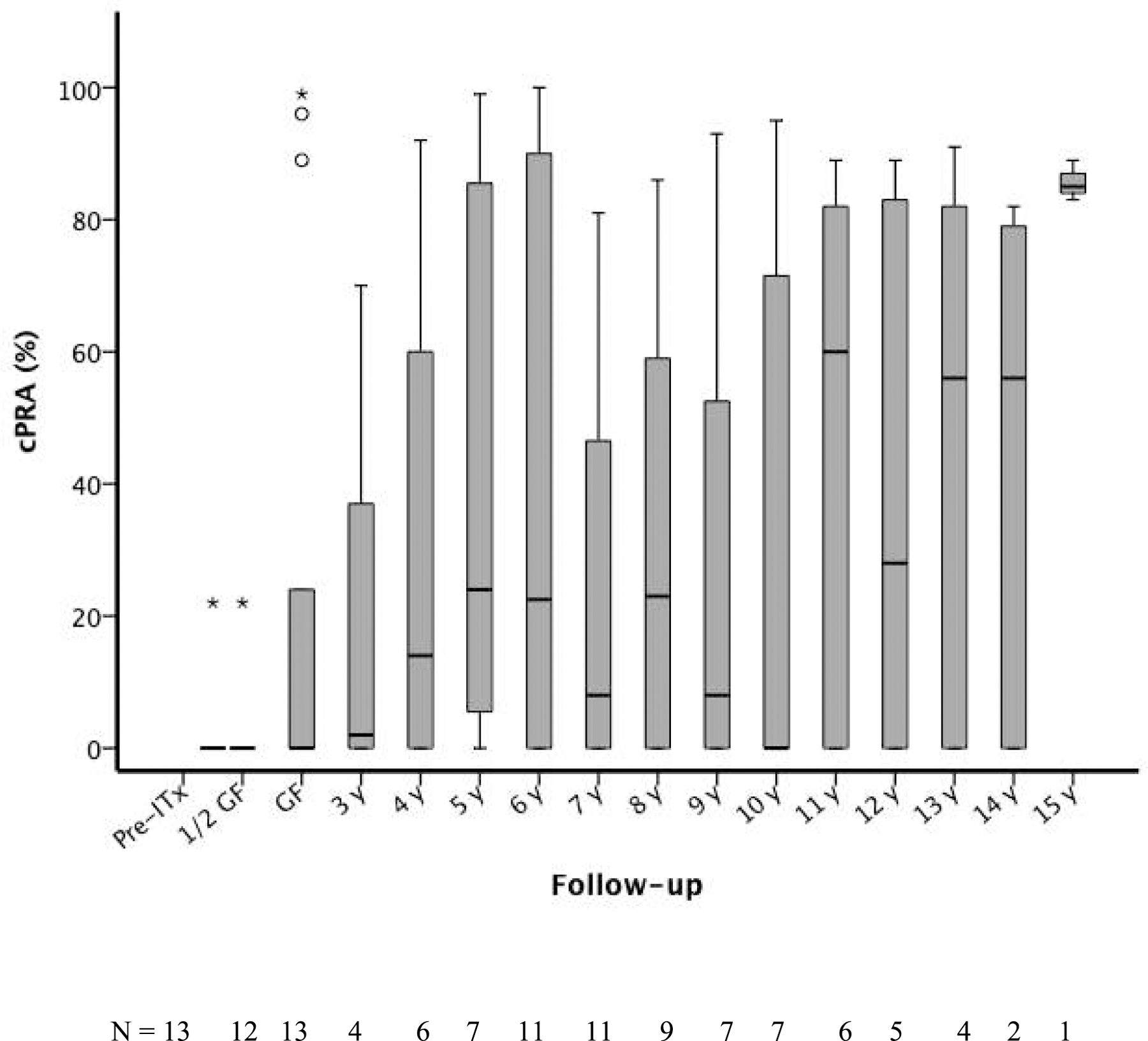
Abbreviations: 1/2 GS, half-life graft survival; cPRA, calculated panel-reactive antibody; GF, graft failure; IA, islet transplantation alone; ITx, islet transplantation.
Three subjects (#5, #8 and #13) were enrolled in a protocol to prevent allosensitization after discontinuation of immunosuppression following graft failure (ClinicalTrials.gov Identifier: NCT01999361; IRB Protocol Number: 20071058), which consisted of mycophenolate mofetil (MMF) monotherapy for 2 years. Two of these subjects (#5 and #8) were not allosensitized (cPRA-negative) after graft failure. The third subject (#13) was cPRA-positive 3 years postgraft failure (cPRA: 70%; DSA-negative), became cPRA-negative at 6 years postgraft failure (cPRA: 0%) and has remained negative since then (last follow-up 11 years postgraft failure) (Table 3). One subject (#12) required IVIG prior to graft failure for treatment of parvovirus infection, at which time immunosuppression had to be discontinued. This subject had an initial cPRA negative prior to transplantation and subsequent cPRA measurements remained negative for the following 14 years (Table 3).
We compared the percentage values of cPRA before ITx, at the time of graft failure, and at 6 years postgraft failure in order to detect changes according to the time-points. We found a positive direction change among groups (P = 0.01), indicating significantly higher levels of cPRA at 6 years postgraft failure as compared to those observed before ITx (Dunn-Bonferroni adjustment, P = 0.042) (Figure 3a). Due to our small sample size, we were unable to evaluate the association between cPRA values and numerous variables of interest, including age, transplanted IEQ/Kg of recipient body weight, cytomegalovirus (CMV) serologic status, immunosuppressive regimen, islet autoantibody-positivity and T1D duration.
Figure 3 -. Values of cPRA (%) in IA group subjects (n = 13) and ITX + BM subjects (n = 4). Figure 3a shows the change in cPRA (%) before transplantation, ½ time between transplant and graft failure, at graft failure, and 6 years after graft failure in the IA group (n = 13); * Dunn-Bonferroni Post hoc analysis between Pre-ITX and 6 years post GF, P = 0.042. Figure 3b shows the change in cPRA (%) before transplantation, at the time of graft failure, and 15 years after graft failure in the ITx + BM group (n = 4); α cPRA was performed in 2 out of 4 patients before transplant.
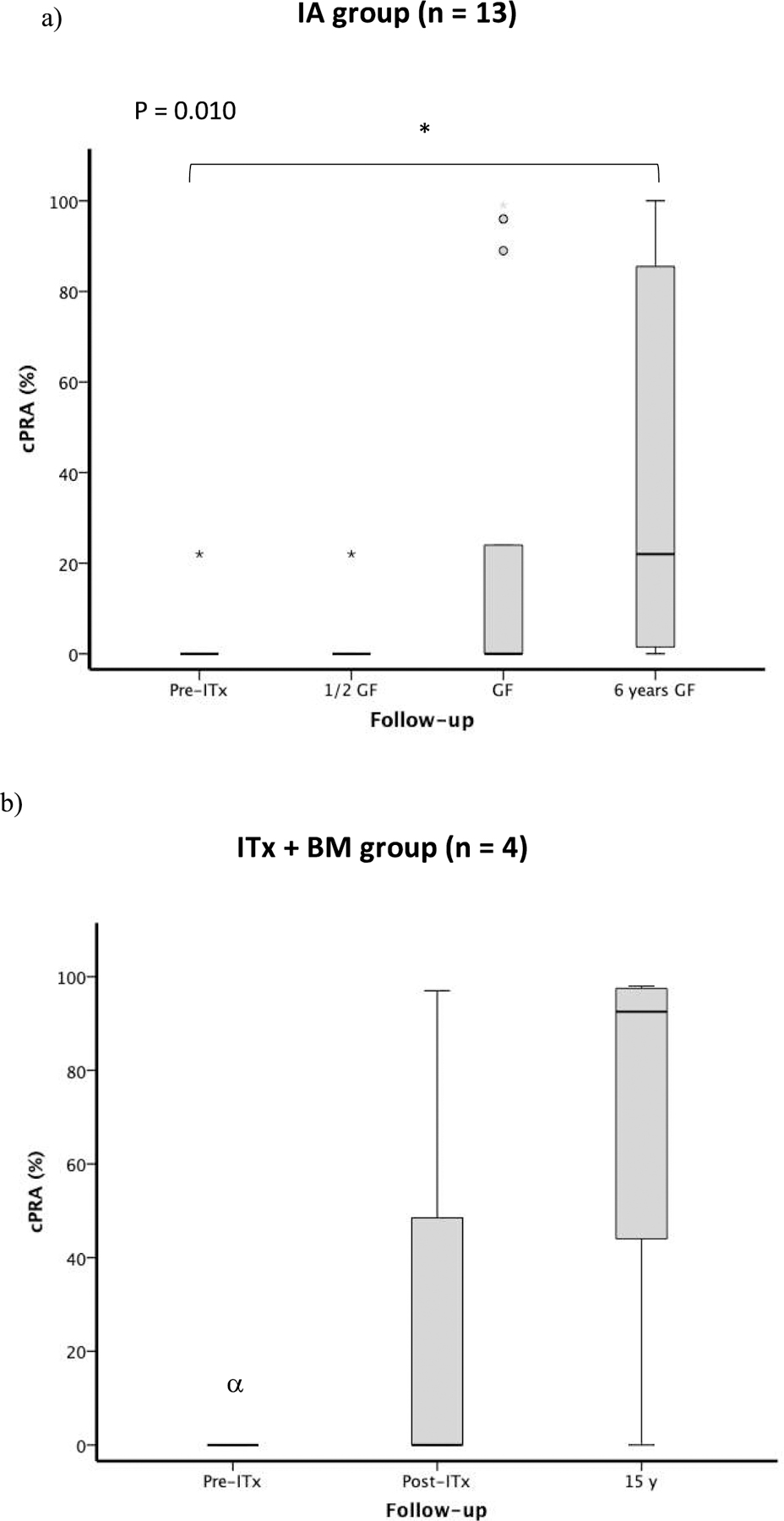
Abbreviations: cPRA, calculated panel-reactive antibody; GF, graft failure; IA, islet transplantation alone; ITx + BM, islet transplantation plus bone marrow-derived CD34 + hematopoietic stem cells.
ITx+BM group (n=4):
As per transplant protocol design, immunosuppression was discontinued in 3 patients 1 year after ITx.16 The remaining subject discontinued immunosuppression at the time of graft failure (178 days post-ITx). Subjects have been followed for up to 17 years postgraft failure. Subjects in this group were enrolled in the study protocol 8 (8 – 9) years after graft failure. Patient demographics and baseline characteristics are detailed in Table 1. Induction immunosuppression consisted of anti-IL-2 (daclizumab) and anti-TNF-alpha (infliximab) agents, while maintenance immunosuppression included sirolimus and tacrolimus. The remaining subject became positive (for IA-2 only) during follow-up. Median duration of graft function in this group was 1.25 (0.8 – 1.3) years (Table 1). Peripheral chimerism was maximal at 1 month (5.92±0.48%), highly reduced at 1 year (0.20±0.08%) and was undetectable at graft failure.16
The values of cPRA (%) in ITx+BM group subjects before transplantation, during transplant follow-up and after graft failure are listed in Table S2. Prior to transplantation, subjects #1 and #2 were cPRA-negative, and remained negative while on immunosuppression. For subjects #3 and #4 the PRA tested by single antigen beds were not available for the pretransplant time. At the time of graft failure, subjects #1, #2, and #4 remained cPRA-negative, subject #3 was cPRA-positive (PRA: 97%, DSA-negative). After discontinuation of immunosuppression (as per protocol), subjects #1, #2 and #3 became DSA-positive and showed cPRA-positivity during the follow-up (Table S1). Subject #3 presented cPRA 97% after transplant and remained cPRA positive for 16 years (DSA-negative). Subject #4 was negative at the time of graft failure and did not undergo further testing until 9 years postgraft failure, where results showed cPRA-positivity (cPRA: 51%) and lack of DSA development. Repeat testing of subject #4 yearly thereafter was consistently negative until 17 years postgraft failure (cPRA: 41%; DSA-negative). Overall, allosensitization persisted for 15 years postgraft failure in 3 out of 4 subjects (cPRA in subjects #1, #2 and #3: 98%, 88%, and 97%, respectively; see Table S1). Percentage of cPRA before ITx, at the time of graft failure, and at 15 years postgraft failure is displayed in Figure 3b.
Recipients with Persistent Islet Graft Function
We included 18 subjects with persistent graft function for comparison in this analysis. Patient demographics and baseline characteristics are detailed in Table 1. ITx occurred between 2000 and 2017 and the majority were transplanted before 2011. Induction immunosuppression included daclizumab (50%) or T-cell depleting agents (50%) in combination with an anti-TNF-alpha agent (infliximab, n=1; etanercept, n=17). Sirolimus and tacrolimus were used for maintenance immunosuppression. Four out of 18 subjects (#4 [IAK], #5 [IAK], #8[IA], and #10 [IA]) became allosensitized (cPRA: 71%, 23%, 54%, and 46%, respectively) at 10 years post-ITx while on immunosuppression, and 2 of them (#8 [IA] and #10 [IA]) developed DSA-positivity.
Discussion
After graft failure, immunosuppression is often discontinued in subjects receiving IA since there is no longer need for immunosuppressive therapy. PRA-positivity subsequently develops in almost all subjects.8–11 In our study, allosensitization has persisted in the majority of subjects after discontinuation of immunosuppression for up to 15 years of follow-up. Three out of 4 subjects who received ITx+BM developed allosensitization and DSA-positivity after discontinuation of immunosuppression, with PRA-positivity persisting for 17 years after graft failure in this group. The persistently high cPRA levels in these patients are most likely secondary to the BM cellular load. We hypothesize that DSA-positivity was high likely due to the infusion of CD34-enriched cells, which contain a mixture of hematopoietic stem cells, hematopoietic progenitors and various contaminants.16
At time of graft failure, there was minimal allosensitization. At 6 years postgraft failure, 55% of subjects (6/11; data not available in 2 subjects) in the IA group had cPRA>20% (cPRA: 22%−100%), and 77% (10/13) became allosensitized at any time after graft failure. DSA-positivity to multiple donors was observed in (6/13) 46% of subjects. Although the degree of sensitization was variable (cPRA ranging between 22% and 100%), it has persisted for up to 15 years of follow-up after discontinuation of immunosuppression. Among the 3 subjects in the IA group who experienced graft failure (18%) and did not develop allosensitization, 2 were treated with MMF for 2 years after discontinuation of protocol-specific immunosuppression in an attempt to specifically prevent the occurrence of allosensitization. Another subject received IVIG prior to graft failure due to parvovirus infection. This treatment is usually employed as part of desensitization protocols24 and may account for the prevention of allosensitization in this subject.
Although guidelines to prevent or decrease PRA-positivity after graft failure in ITx have not yet been established, preliminary data from an ongoing study (ClinicalTrials.gov Identifier: NCT01999361) suggest that administration of MMF for 2 years after graft failure may be a valid option to prevent, decrease or reverse PRA-positivity. Similarly, Campbell et al recommend avoiding discontinuation of immunosuppression until patients are evaluated for the need for subsequent transplant; during this evaluation process, subjects are maintained on mycophenolic acid therapy for a 2 year-period.9 A single-center study conducted on kidney transplantation recipients reported that prolonging immunosuppression for more than 3 months after graft failure resulted in decreased allosensitization and lower mean PRA levels, as compared to early discontinuation of immunosuppression.25 Moreover, gradual tapering of immunosuppression after kidney graft failure has been shown to decrease allosensitization compared to abrupt cessation of the immunosuppressive regimen.25
ITx has evolved over time and most current protocols use T-cell depleting agents (alemtuzumab, antithymocyte globulin) for induction. In our study, 85% of subjects in the graft failure group (transplanted between 2000 and 2006) (11/13) and 50% in the islet graft function group (transplanted between 2001 and 2017) did not receive T-cell depleting agents for induction. Therefore, our findings in the graft failure group are only applicable to subjects who received Edmonton like immunosuppression. Brooks et.al evaluated whether alemtuzumab induction affected DSA positivity (de novo) at 12 months post-ITx and found that in subjects who did not develop DSA, 95% received alemtuzumab whereas in subjects who developed DSA, 60% received alemtuzumab.26 Furthermore, the Edmonton group showed that recipients who received alemtuzumab did not developed allosensitization up to 36 months post-ITx.26 Of note, 2 out of 3 subjects (66%) in our study cohort who remained cPRA negative following cessation of immunosuppression underwent induction with T-cell depleting agents; however, 1 of them was also kept on MMF for 2 years post graft failure to prevent allosensitization. Thus, it is unclear whether prevention of allosensitization on this subject was attributed to T-cell depleting induction, MMF therapy postgraft failure or a combination of both. In the ITX+BM group 3 of 4 subjects islet graft failure developed months after discontinuation of immunosuppression (as per protocol), therefore graft failure is the result of immunosuppression discontinuation.
The number of ITx recipients with persistent graft function who became allosensitized while on immunosuppression in our study is comparable to data reported for kidney transplant recipients with stable serum creatinine during maintenance immunosuppression.27 Development of DSA-positivity is generally associated with rapid loss of islet allograft function, although results are controversial across studies.11,26,28 However, development of DSA-positivity within 4 weeks after ITx has been associated with decreased islet allograft function at 3 months and can predict graft failure at 12 months.26 It still remains controversial whether antibodies detected exclusively by solid phase techniques influence long-term graft outcomes. Unlike kidney transplantation, the clinical impact of anti-HLA antibodies on ITx remains unclear. Recently, Chen et al showed that allogeneic islets are resistant to DSA-mediated rejection, with a similar rate of islet graft attrition between patients with or without DSA (n=49, patients who were successfully grafted with allogeneic islet).29
The reason for the persistence of alloantibodies after discontinuation of immunosuppression following allograft failure in subjects receiving ITx is unclear. The mechanism for the variable alloantibody response and persistence in certain subjects is also undetermined. No islet alloantigens should theoretically persist after allograft rejection and discontinuation of immunosuppression, and thus the stimuli for persistent alloantibody production are still unclear. However, factors accounting for persistent alloantibody production may include the persistence of cells of donor origin (microchimerism) or the persistence of alloantigens in dendritic cells30 and the presence of long lived plasma cells generated during the alloimmune response.31–33
Allosensitization to allografts utilized for repair of heart defects has also been described.34–36 In a study of adults receiving homograft aortic graft implantation, anti-HLA antibodies were shown to persist for up to 15 years.34 Pediatric patients receiving implantation of cryopreserved valved and nonvalved allografts showed persistence of anti-HLA antibodies for up to 8 years after implantation, although antibody levels decreased with time.35 Similarly, another study of pediatric surgical patients observed long-term sensitization for up to 18 years.36 Our study is the first to highlight the persistence of allosensitization for such duration specifically in ITx recipients.
Allosensitization after ITx is of concern given that T1D patients may require pancreas, kidney or other organ transplantation in the future. Therefore, a high cPRA will increase wait time to identify a potential HLA compatible donor. Sensitization may impact outcomes although careful matching based upon detailed HLA antibody analysis and current immunosuppression strategies may reduce this risk.
Several studies suggest that pancreas after islet (PAI) transplantation can be a successful strategy to achieve long-term insulin independence in previously failed islet allograft recipients.12,37 However, allosensitization remains of concern given the prolonged wait time for subsequent transplantation.37,38
The main limitations of our study include the small sample size, retrospective design, inclusion of participants from different study protocols, and patients lost to follow-up. In addition, we may have underestimated allosensitization with flow cytometry since the cutoff of MFI was set at 3000.
The high frequency of allosensitization development and persistence after islet allograft failure is a significant finding which argues in favor of minimizing the number of islet infusions (donors) per each recipient. Naziruddin et al showed that the presence of low levels of PRA at first islet infusion significantly increased the development of PRA>20% after transplantation.11 Mohanakumar et al showed that preexisting HLA sensitization may be 1 of the factors that lead to loss of transplanted islets.28 Accurate alloantibody characterization using the same methodology, single antigen solid phase HLA antibody assays is mandatory before transplantation. In many recent trials of islet allograft transplantation, the presence of any level of PRA (using single antigen solid phase assays) above background is listed as an exclusion criteria for the first infusion. For subsequent infusions if alloantibodies are detected before those unacceptable antigens are avoided. T cell depleting agents are now more commonly used in islet allograft transplantation and there is evidence5,39 that DSA formation is much less commonly seen. Therefore, our results need to be interpreted in this context and may not be applicable to the people that have been more recently transplanted on T-cell depleting agents. In the case of sensitization It is possible that combining solid phase HLA antibody identification methodologies with virtual crossmatching will increases the allocation of deceased donor organs to highly sensitized patients for islet transplantation similar to kidney transplantation.40,41 This approach needs to be evaluated in islet allograft transplant trials.
Monitoring for allosensitization should also be considered in study protocols using allogeneic cell products (eg, stem cells). Lastly, strategies to prevent allosensitization prior to discontinuation of immunosuppression after graft failure should be included as part of transplant protocols.
In conclusion, allosensitization is a highly prevalent and persistent feature following islet allograft failure with potential negative implications regarding the need for future organ transplantation (ie, increased wait list times). Treatment with MMF for up to 2 years to prevent allosensitization after islet graft failure may be an effective therapeutic option, although further large-scale studies are required to confirm our findings.
Supplementary Material
Acknowledgments
The authors are grateful to the members of the cGMP Human Cell Processing Facility, the preclinical Human Immunology and Immunogenetics Program, the Clinical Cell Transplant Program (CCTP) at the Diabetes Research Institute (DRI), and the University of Miami CTSI for their support of this work.
Financial Disclosure
This work was supported by NIH grants R01 DK55347, R01 DK056953, R01 DK025802, DK070460, R01 DK55347, U42 RR016603, M01RR16587, UL1TR000460; the Miami Clinical and Translational Science Institute (CTSI) from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities; the Juvenile Diabetes Research Foundation International 4-200-946, 4-2004-361, 17-2012-361, 3-SRA-2017-347-M-B; the State of Florida; and the Diabetes Research Institute Foundation.
Abbreviations
- ½ GS
half-life graft survival
- ANOVA
Analysis of Variance
- BM
bone marrow
- CMV
Cytomegalovirus
- cPRA
calculated panel reactive antibody
- DSA
donor-specific anti-HLA antibodies
- ELISA
enzyme-linked immunosorbent assay
- GAD65
glutamic acid decarboxylase
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin
- HSC
hematopoietic stem cells
- IA
islet transplant alone
- IA-2
islet antigen-2
- IAK
islet after kidney transplantation
- IEQ
islet equivalents
- IL-2
interleukin-2
- IRB
Institutional Review Board
- ITx
Islet transplantation
- LCT CDC
Lambda Cell Tray – complement-dependent microlymphocytotoxicity techniqu
- MFI
mean fluorescence intensity
- MMF
mycophenolate mofetil
- MMTT
mixed meal tolerance test
- PAI
pancreas after islet transplantation
- PRA
panel reactive antibody
- T1D
type 1 diabetes
- TNF
-alpha tumor necrosis factor-alpha
- UNOS
United Network for Organ Sharing
Footnotes
Disclaimer
The authors declare no conflicts of interest.
References
- 1.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cure P, Pileggi A, Froud T, et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85(6):801–812. [DOI] [PubMed] [Google Scholar]
- 3.Foster ED, Bridges ND, Feurer ID, et al. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41(5):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vantyghem MC, Kerr-Conte J, Arnalsteen L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. August 2009;32(8):1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13(5):268–277. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Islet Transplant Registry (CITR): 2016 (Ninth) Annual Report. Collaborative Islet Transplant Registry website. https://citregistry.org/content/citr-9th-annual-report. Accessed January 10, 2020
- 8.Cardani R, Pileggi A, Ricordi C, et al. Allosensitization of islet allograft recipients. Transplantation. 2007;84(11):1413–1427. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PM, Senior PA, Salam A, et al. High risk of sensitization after failed islet transplantation. Am J Transplant 2007;7(10):2311–2317. [DOI] [PubMed] [Google Scholar]
- 10.Pouliquen E, Baltzinger P, Lemle A, et al. Anti-donor HLA antibody response after pancreatic islet grafting: characteristics, risk factors, and impact on graft function. Am J Transplant 2017;17(2):462–473. [DOI] [PubMed] [Google Scholar]
- 11.Naziruddin B, Wease S, Stablein D, et al. HLA class I sensitization in islet transplant recipients: report from the Collaborative Islet Transplant Registry. Cell Transplant 2012;21(5):901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisel SA, Gardner JM, Roll GR, et al. Pancreas-after-islet transplantation in nonuremic type 1 diabetes: a strategy for restoring durable insulin independence. Am J Transplant 2017;17(9):2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.cPRA Calculator. U.S. Department of Health & Human Services. 2019. https://optn.transplant.hrsa.gov/resources/allocation-calculators/cpra-calculator/. Acessed October 12, 2020.
- 14.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant 2010;10(1):26–29. [DOI] [PubMed] [Google Scholar]
- 15.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005;5(8):2037–2046. [DOI] [PubMed] [Google Scholar]
- 16.Mineo D, Ricordi C, Xu X, et al. Combined islet and hematopoietic stem cell allotransplantation: a clinical pilot trial to induce chimerism and graft tolerance. Am J Transplant 2008;8(6):1262–1274. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 18.Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an intraabdominal endocrine pancreas. N Engl J Med 2017;376(19):1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froud T, Baidal DA, Faradji R, et al. Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes. Transplantation. 2008;86(12):1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peixoto E, Vendrame F, Arnau A, et al. Ten years of preserved kidney function after islet transplant graft failure. Diabetes Care. 2016;39(12):e209–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zachary AA, Braun WE. Calculation of a predictive value for transplantation. Transplantation. 1985;39(3):316–318. [DOI] [PubMed] [Google Scholar]
- 22.Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes. 2003;52(5):1128–1136. [DOI] [PubMed] [Google Scholar]
- 23.Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59(4):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marfo K, Lu A, Ling M, et al. Desensitization protocols and their outcome. Clin J Am Soc Nephrol 2011;6(4):922–936. [DOI] [PubMed] [Google Scholar]
- 25.Casey MJ, Wen X, Kayler LK, et al. Prolonged immunosuppression preserves nonsensitization status after kidney transplant failure. Transplantation. 2014;98(3):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks AM, Carter V, Liew A, et al. De novo donor-specific HLA antibodies are associated with rapid loss of graft function following islet transplantation in type 1 diabetes. Am J Transplant 2015;15(12):3239–3246. [DOI] [PubMed] [Google Scholar]
- 27.Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. The impact of de novo donor-specific anti-human leukocyte antigen antibodies on 5-year renal transplant outcome. Transplant Proc 2013;45(4):1449–1452. [DOI] [PubMed] [Google Scholar]
- 28.Mohanakumar T, Narayanan K, Desai N, et al. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006;82(2):180–187. [DOI] [PubMed] [Google Scholar]
- 29.Chen CC, Pouliquen E, Broisat A, et al. Endothelial chimerism and vascular sequestration protect pancreatic islet grafts from antibody-mediated rejection. J Clin Invest 2018;128(1):219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson AW, Lu L, Murase N, et al. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13(6):622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ionescu L, Urschel S. Memory B cells and long-lived plasma cells. Transplantation. 2019;103(5):890–898. [DOI] [PubMed] [Google Scholar]
- 32.Woodle ES, Tremblay S, Rossi A, et al. Plasma cell targeting to prevent antibody-mediated rejection. Am J Transplant 2020;20(S4):33–41. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Schuster SJ, Lacey SF, et al. Stable HLA antibodies following sustained CD19+ cell depletion implicate a long-lived plasma cell source. Blood Adv 2020;4(18):4292–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JD, Ogino H, Hunt D, et al. Humoral immune response to human aortic valve homografts. Ann Thorac Surg 1995;60(2 Suppl):S127–S130. [DOI] [PubMed] [Google Scholar]
- 35.Hooper DK, Hawkins JA, Fuller TC, et al. Panel-reactive antibodies late after allograft implantation in children. Ann Thorac Surg 2005;79(2):641–644; discussion 645. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor MJ, Lind C, Tang X, et al. Persistence of anti-human leukocyte antibodies in congenital heart disease late after surgery using allografts and whole blood. J Heart Lung Transplant 2013;32(4):390–397. [DOI] [PubMed] [Google Scholar]
- 37.Andres A, Livingstone S, Kin T, et al. Islet-after-failed-pancreas and pancreas-after-failed islet transplantation: Two complementary rescue strategies to control diabetes. Islets 2015;7(6):e1126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruessner AC, Sutherland DE, Gruessner RW. Matching in pancreas transplantation--a registry analysis. Transplant Proc 2001;33(1–2):1665–1666. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro AM, Toso C, Koh A, Calne RY, Kin T, O’Gornan D, et al. Alemtuzumab + Tac/MMF substantially improves long-term insulin-independence, and strongly suppresses auto and alloreactivity after clinical islet transplantation. Transplantation. 2010;90:134. [Google Scholar]
- 40.Bray RA, Gebel HM. Strategies for human leukocyte antigen antibody detection. Curr Opin Organ Transplant 2009;14(4):392–397. [DOI] [PubMed] [Google Scholar]
- 41.Schinstock CA, Gandhi MJ, Stegall MD. Interpreting anti-HLA antibody testing data: A practical guide for physicians. Transplantation. 2016;100(8):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


