Graphic abstract
Lagerstroemia speciosa (L.) Pers., (Lythraceae), commonly called Banaba, is a native plant of Southeast Asia and is widely used in the treatment of diabetics, obesity, kidney diseases, and other inflammatory disorders. L. speciosa consists of several phytoconstituents like glycosides, flavones, corosolic acid, ellagic acids, triterpenes, tannins, which are reported to be present in leaves, stem, flowers, fruit, bark, and roots. This paper presents an investigation on the binding interaction of phytosterols derivatives identified from the ethanolic extract of Lagerstroemia speciosa seeds against breast cancer target protein. The ethanolic extracts Lagerstroemia speciosa seeds were analyzed via GC–MS for the identification of their chemical constituent. In silico methods are adopted to predict ADME parameters, pharmacokinetic properties, drug-likeliness, and acute toxicity of the identified phytosterols molecules. Molecular docking analysis of the phytosterols was performed against three breast cancer targets. A total of 29 compounds were identified from the extract by GC–MS analysis, among which four phytosterols derivatives namely cholesterol margarate, 7-dehydrodiosgenin, Stigmastan-3,5-diene, and γ-sitosterol have been considered for the present study. These phytosterols are identified as non-toxic, non-carcinogenic, and non-mutagenic. Molecular docking studies reveal the extent of molecular interaction with breast cancer targets. The outcomes of the investigation suggest that the phytosterols obtained from the ethanolic seed extract of Lagerstroemia speciosa could act as a promising candidate against breast cancer.
Keywords: Lagerstroemia speciosa, Phytosterols, Molecular docking, In silico ADMET, Breast cancer
Introduction
Lagerstroemia speciose plant, a primary native of tropical Southeast Asia, is popular for its values in Ayurvedic and folklore medicines (Thakur and Devaraj 2020; Agarwal et al. 2018). It is widely used in the treatment of diabetics, obesity, kidney diseases, and other inflammatory disorders (Sharmin et al. 2018). The Lagerstroemia speciosa plant, commonly called Banaba, extracts from various parts of the plant have been studied for the rich mélange of phytochemicals it had retained in it (Mousa et al. 2019). These phytochemical compositions differ with respect to the part of the plant and the solvent of extraction (Sirikhansaeng et al. 2017). The therapeutic effects of Lagerstroemia speciosa are often related to the presence of phytochemicals such as corosolic acid, lagerstroemin, and ellagitannins (Andrade et al. 2020). The most commonly isolated phytochemicals are of the leaf extracts of Lagerstroemia speciosa, which are investigated for their anti-diabetic, anti-obesity, antimicrobial and anti-inflammatory activities (Amresh et al. 2018). The Lagerstroemia speciosa seeds have been previously studied for the estimation of total phenolic content, keto-fatty acids, and their antioxidant activity (Junaid et al. 2013; Jehan et al. 1990). The phytochemical profile of the seed extract has not been well explored. The extracts are rich in organic compounds such as long-chain fatty acids, hydrocarbons, esters, vitamins, and phytosterols which are responsible for their superior medicinal significance.
Phytosterols are phytochemicals with similarity in structure and biological activity to that of cholesterol. Over the years, more than 200 phytosterols have been isolated and characterized from seeds and nuts of various plants (López-García et al. 2019). Phytosterols are known for their protection against chronic ailments like cardiovascular diseases, diabetics, and cancer (Ms et al. 2018). They have been reported to alleviate cancers of breast, prostate, lung, liver, stomach, and ovary. Phytosterols have been shown to inhibit breast cancer and recuperate the altered lipid levels caused by cancer. Breast cancer drug targets the function of receptors such as ERα (estrogen receptor alpha), PR (progesterone receptor), EGFR (epidermal growth factor receptor) etc. ERα plays an vital role in breast cancer initiation and propagation, while over expression of PR is widely observed in breast cancer cells. EGFR has been reported to play an important role in triple negative breast cancer (Acharya et al. 2019). Recently, Lagerstroemia speciosa leaf extract was studied for cytotoxicity activity against breast cancer cell lines-MCF-7 (Saraswathi and Santhakumar 2017).
Inspired by the importance of Lagerstroemia speciosa and its potential role against breast cancer, we investigate the interaction of phytosterols obtained from the unexplored ethanolic seed extract of Lagerstroemia speciosa with breast cancer targets using in silico approaches. The phytochemical constitution of the extract is determined by GC–MS analysis and the identified phytosterols are computed to predict their ADMET, pharmacokinetic properties, and drug-likeness properties. Further to understand the interaction of the phytosterols against breast cancer targets, molecular docking analysis was carried out. This present work signifies the broadening of applications of phytosterols against various cancer targets.
Methods and materials
Collection of plant material
The Lagerstroemia speciosa plant seeds were collected from Madras Christian College Campus, Chennai, India, and the plant seed specimen were identified and authenticated at the Department of Plant Biology and Plant Biotechnology, Madras Christian College, Chennai, India. Lagerstroemia speciosa (L.) Pers. is an accepted name contained in the plant list database (http://theplantlist.org; accession date 05/09/2020). The seeds were washed thoroughly with distilled water to remove dust and dried in shade for a few days. The dry seeds were later ground into a fine powder.
Preparation of plant seed extract
5 g powdered seeds were extracted in 50 mL ethanol using a soxhlet apparatus. The obtained extracts were filtered and stored in amber bottles under refrigeration to avoid any possible degradation.
GC–MS analysis
The chemical composition of the ethanolic extracts of Lagerstroemia speciosa seeds was analyzed using GC–MS, on Agilent technologies (GC-7890B: MS-5977A MSD) using Column HP-5MS (5% phenyl methyl siloxane) 30 m × 205 µm × 250 µm. Helium was used as a carrier gas with a constant flow of 1.0 ml/min with 1 μl injection volume. An injector temperature was maintained at 250 °C. The oven temperature program used was maintained a temperature of 40 °C for 2 min, the heating is increased up to 270 °C at 5 °C/min and maintaining the temperature constant at 270 °C for 15 min. The mass spectrometer scan parameters included electron impact ionization voltage of 70 eV, a mass range of 40–700 m/z. The chemical constituents were characterized by comparing their mass spectra with those of the NIST library Version-2011.
Molecular docking
3D structures of the phytosterols studied were drawn using draw by PubChem Sketcher V2.4 (https://pubchem.ncbi.nlm.nih.gov/edit3/index.html) in mol file format and later converted into the.pdb format using Open Babel GUI software (Oboyle et al. 2011). The X-ray crystal structure of the three molecular targets estrogen receptor alpha (PDB ID: 3ERT), progesterone receptor (PDB ID: 4OAR), and EGFR (epidermal growth factor receptor (PDB ID: 2JRM), were retrieved as a PDB file from the Protein Data Bank for docking (Acharya et al. 2019). A grid box with the sizes 60, 60, and 60 along the X-, Y-, and Z-axes respectively were set during docking. The docking analysis was carried out using AutoDockVina software (Forli et al. 2012). The 3D docked protein–ligand complex poses were visualized using PyMolmolecular visualization software program (DeLano 2002). The 2D view of protein-inhibitor interactions was generated using Ligplot software (Wallace et al. 1995).
Pharmacokinetics and drug-likeness
The ADME (Adsorption, Distribution, Metabolism, Excretion) and drug-likeness and properties of the phytosterols were evaluated by SWISSADME and admetSAR webservers (Daina et al. 2017; Cheng et al. 2012). To understand the drug likeliness properties of the phytosterols Lipinski’s “rule of 5” and the number of free rotatable bonds were evaluated. According to the rule of 5, if a molecule follows 2 or more of the following rules such as lipophilicity (expressed as Log P) must be less than 5, the number of hydrogen bond acceptors should be less than 10, molecular weight must be less than 500 Dalton, the number of hydrogen donors should be less than 5, is said to be a drug-like molecule (Lipinski 2004). Insilco prediction of LD50 values and the class of toxicity were obtained from admetSAR results. Some of the toxicity parameters like mutagenicity, tumorigenicity, etc. were computed using OSIRIS Property Explorer (Sander 2001).
Results and discussion
GC–MS profiling of phytochemicals from Lagerstroemia speciosa
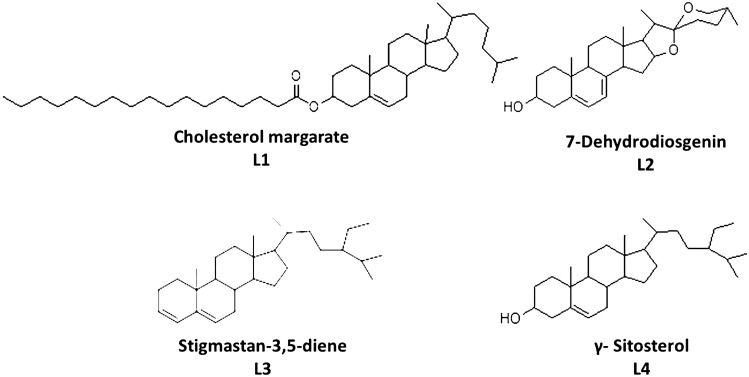
The ethanolic extracts of the Lagerstroemia speciosa seeds were analyzed for the chemical constituents using gas chromatography and Mass spectrometry (GC–MS). The characterization of the ethanolic extract through GC–MS revealed the presence of twenty-nine (29) compounds accounting for total components present in it. The phytochemical compounds identified with their respective molecular masses, relative percentage, and retention time have been tabulated in Table 1, From the GC–MS, it is evident that Lagerstroemia speciosa seed is a rich combination of organic compounds ranging from long-chain fatty acids, alcohols, esters, hydrocarbons, and phytosterols. Four phytosterols derivatives were identified in the ethanolic seed extract which are Cholesterol margarate(L1), 7-Dehydrodiosgenin (L2), Stigmastan-3,5-diene (L3), and γ-Sitosterol (L4). It must be noted that L2 is a derivative of diosgenin and it is one among the highly studied phytosterols for its utility as an anti-breast cancer agent (Shi et al. 2018). Similarly, L3 and L4 are derivatives of phytosterols stigmasterol and β-sitosterol respectively, which are known for their activity against breast cancer (Kangsamaksin et al. 2017; Ju et al. 2004).
Table 1.
Major constituents of ethanolic extracts of the Lagerstroemia speciosa as determined by GC–MS
| Peak # | Compounds | Retention time | Percentage of total |
|---|---|---|---|
| 1 | 3-Methyl-5-furandione | 7.703 | 5.87 |
| 2 | 3-Methylenedihydro-2,5-furandione | 9.954 | 1.05 |
| 3 | Naphthalene | 13.787 | 0.15 |
| 4 | Dodecane | 14.218 | 0.21 |
| 5 | Octan-2-yl 3-chlorobenzoate | 18.846 | 0.20 |
| 6 | cis-3-Tetradecene | 19.44 | 0.08 |
| 7 | Tetradecane | 19.671 | 0.94 |
| 8 | 1-Dodecanol | 21.58 | 0.15 |
| 9 | Dodecanoic acid | 23.964 | 0.57 |
| 10 | 1-Hexadecanol | 24.366 | 0.10 |
| 11 | Hexadecane | 24.566 | 1.14 |
| 12 | Dodecyl acrylate | 26.691 | 0.28 |
| 13 | Tetradecanoic acid | 28.347 | 0.29 |
| 14 | Octadecane | 28.964 | 0.75 |
| 15 | n-Hexadecanoic acid | 32.582 | 5.04 |
| 16 | cis-9,cis-12-Octadecadienoic acid | 37.269 | 69.91 |
| 17 | cis-13,16-Docasadienoic acid | 39.468 | 6.08 |
| 18 | Eicosanoic acid | 39.78 | 1.05 |
| 19 | Docosanoic acid | 42.729 | 0.36 |
| 20 | Tricosanoic acid | 44.2 | 0.13 |
| 21 | Tetracosanoic acid | 45.641 | 0.12 |
| 22 | Squalene | 46.451 | 0.19 |
| 23 | Cholesterol margarate | 47.513 | 0.12 |
| 24 | Stigmastan-3,5-diene | 48.643 | 0.23 |
| 25 | γ-Tocopherol | 50.5 | 0.12 |
| 26 | 7-Dehydrodiosgenin | 50.886 | 0.33 |
| 27 | Stigmastan-3,5-diene | 51.391 | 0.86 |
| 28 | α-Tocopherol | 52.416 | 0.10 |
| 29 | γ-Sitosterol | 57.758 | 0.71 |
The drug likeness, ADMET analysis, and molecular docking analysis against three breast cancer target proteins were done for the four phytosterols L1, L2, L3, and L4 for the evaluation of their efficacy as anti-cancer agents (Fig. 1).
Fig. 1.
Chemical Structures of the phytosterols studied
Drug likeliness
The selected four phytosterols are evaluated for their drug likeliness properties with the help of the Lipinski rule of five filters. This analysis helps to distinguish between drug-like and non-drug-like molecules. From Table 2, it can be noted that out of four molecules, only one compound violates Lipinski’s rule of five with two violations (L1, MW > 500 and LogP = 9.01). The molecular mass of L1, L2, L3, and L4 are 641.1, 400.64, 396.69 and 414.71 g/mol respectively. L1 and L3 violate the LogP values by acquiring a value greater than 5. For instance, the Log P values of L1, L2, L3 and L4 are 9.01, 4.75, 5.11, and 4.79, respectively. L1 and L3 violate the LogP values by being assigned a value greater than 5 which indicates its hydrophobic nature. The high LogP value of L1 is because of the long alkyl chain and for L3, it is because of the absence of polar groups, whereas in L2 and L4 the presence of hydrophilic hydroxyl groups supports the low LogP value. It must be noted that at times in the case of naturals products Lipophilicity does not directly relate to its physicochemical profile violating the rule of five.(Lipinski 2016) The number of hydrogen bond acceptors for L1, L2, L3 and L4 are 2, 2, 0, and 1, respectively. Hydrogen bond donors of L1, L2, L3, and L4 are 0, 0, 0, and 1, respectively. The number of rotatable bonds of the four molecules varies from 0 to 22, indicating the flexibility of some molecules compared to others.
Table 2.
Drug likeliness properties of phytosterols from ethanolic extracts of the Lagerstroemia speciosa
| L1 | L2 | L3 | L4 | |
|---|---|---|---|---|
| Molecular weight (g/mol) | 641.1 | 400.64 | 414.71 | 396.69 |
| Num. of heavy atoms | 46 | 29 | 30 | 29 |
| Num. of rotatable bonds | 22 | 0 | 6 | 6 |
| Num. of H bond acceptor | 2 | 2 | 1 | 0 |
| Num. of H bond donor | 0 | 0 | 1 | 0 |
| Log Po/w | 9.0 | 4.7 | 4.79 | 5.11 |
| Num. of violations | 2 | 1 | 1 | 1 |
ADMET analysis
To understand the pharmacokinetic properties of the molecules, ADMET analysis was carried out. Solubility expressed as Log S is an important parameter of a drug-like molecule at its value ideally vary from − 0.5 to − 5.5.(Joshi et al. 2020) The solubility of all molecules, except L2 (− 6.9) falls in the above-mentioned range indicating the less/insoluble nature of L2. The maximum solubility is shown by L4 (− 4.7) and the least solubility for L2 (− 6.9). The drug once administered orally gets absorbed in the intestine to reach the specific targets. All molecules have shown a positive result with respect to human intestinal absorption (HIA). The parameters such as blood–brain barrier (BBB) and colorectal carcinoma (Caco-2) have been studied to access the permeability of the membrane (Singh et al. 2020). The BBB and Caco-2 values for all molecules show positive values hence it can cross the barriers with ease. The studied molecules are found to be non-inhibitors of Renal Organic Cation Transporter (ROCT) and also shown to be a substrate and inhibitor of P-glycoprotein signifying the distribution ability of the drugs (Joshi et al. 2020). Due to the role of cytochrome P450 (CYP450) in Phase I drug metabolism, it is considered as the main parameter to examine ADME of the drugs (Paramashivam et al. 2015). From Table 3 it can be seen that all molecules are shown to be non-substrates and non-inhibitors of CYP450. None of the molecules were found to show toxicity risks such as carcinogenic, mutagenic, etc. All molecules were AMES negative, i.e., non-toxic. The results of LD50 values and other toxicity risks are listed in Table 4.
Table 3.
ADME profile prediction of the phytosterols from Lagerstroemia speciosa
| L1 | L2 | L3 | L4 | |
|---|---|---|---|---|
| Log S | − 5.42 | − 6.9 | − 5.71 | − 4.7 |
| Solubility class | Insoluble | Poorly soluble | Poorly Soluble | Poorly soluble |
| Absorption | ||||
| BBB | BBB + (0.9676) | BBB + (0.9391) | BBB + (0.9841) | BBB + (0.9743) |
| HIA | HIA + (1.0) | HIA + (0.9961) | HIA + (1.00) | HIA + (1.00) |
| Caco-2 | caco2 + (0.7337) | caco2 + (0.5872) | caco2 + (0.6658) | caco2 + (0.7953) |
| Distribution | ||||
| p-glyco substrate | Substrate | Substrate | Substrate | Substrate |
| p-glyco Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor |
| ROCT | Non-Inh | Non-Inh | Non-Inh | Non-Inh |
| Metabolism | ||||
| 2C9 SUB | Non-sub | Non-sub | Non-sub | Non-sub |
| C2DR SUB | Non-sub | Non-sub | Non-sub | Non-sub |
| 3A4 SUB | Substrate | Substrate | Substrate | Substrate |
| 1A2 INH | Non-Inh | Non-Inh | Non-Inh | Non-Inh |
| 2C9 INH | Non-Inh | Non-Inh | Non-Inh | Non-Inh |
| 2DR INH | Non-Inh | Non-Inh | Non-Inh | Non-Inh |
| 2C19 INH | Non-Inh | Non-inh | Non-Inh | Non-Inh |
| 3A4 INH | Non-Inh | Non-Inh | Non-Inh | Non-Inh |
Table 4.
In silico toxicity predictions of the phytosterols from Lagerstroemia speciosa
| L1 | L2 | L3 | L4 | |
|---|---|---|---|---|
| H ERG inhibitor | Weak inhibitor | Weak inhibitor | Weak inhibitor | Weak inhibitor |
| AMES toxicity | Non toxic | Non toxic | Non toxic | Non toxic |
| Carcinogens | Non carcinogens | Non carcinogens | Non carcinogens | Non carcinogens |
| Rate acute toxicity | 2.0248 | 1.7459 | 1.688 | 2.6561 |
| Toxicity risks | ||||
| Mutagenic | None | None | None | None |
| Tumorigenic | None | None | None | None |
| Irritant | None | None | None | None |
| Reproductive effective | None | None | None | None |
Bio-molecular interaction studies
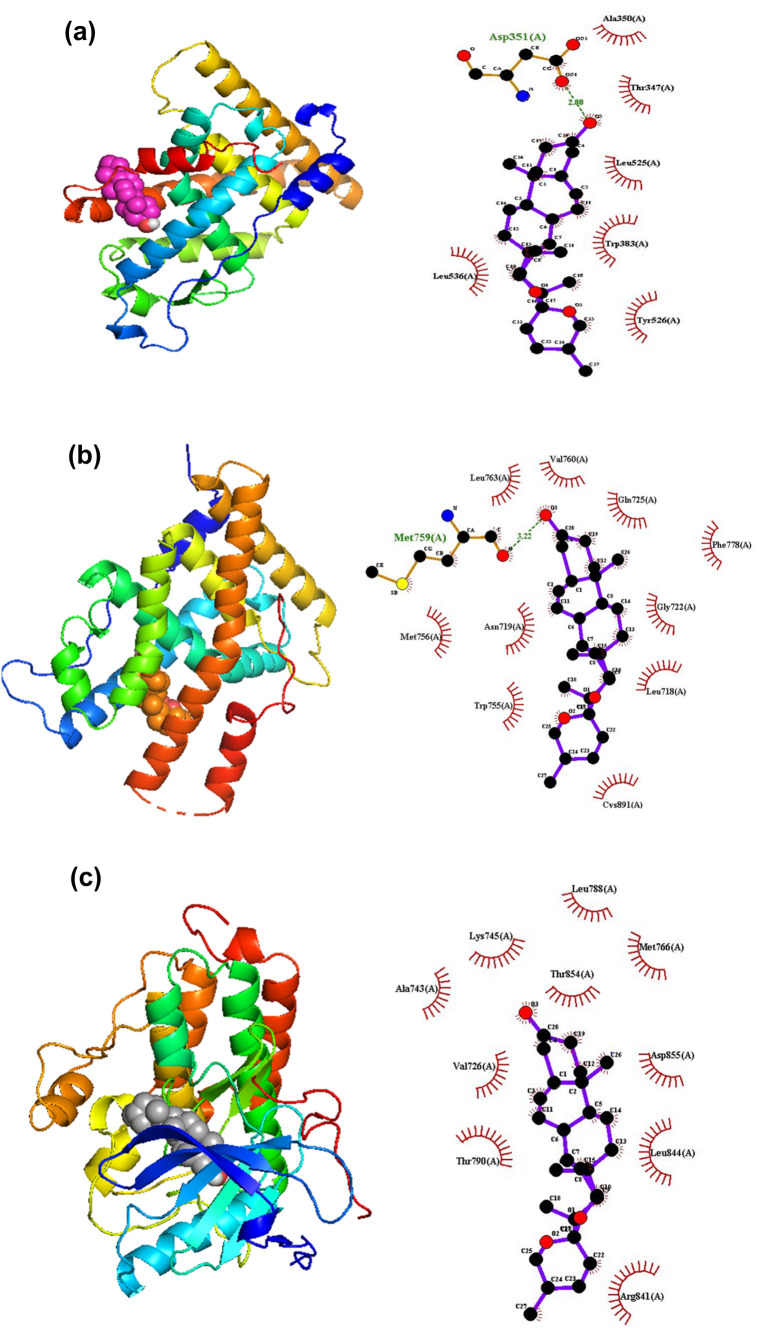
Molecular docking analysis was carried out to evaluate the possibility to use these molecules against Breast Cancer. Docking was performed over/on the four phytosterols (L1–L4) identified from the ethanolic extract of lagerstroemia speciosa seeds on the binding pocket of breast cancer target proteins (PDB IDs: 3ERT; 4OAR; 2JRM). All four molecules were docked against the three target proteins and listed in Table 5. All molecules possess a binding score of more than − 7.0 with the three proteins, except L1. For instance, the binding score of L1 with 4OAR is − 6.9 whereas with 2JRM is − 6.2. L1 showed a binding score of − 7.5 when bound to the protein 3ERT with hydrophobic interactions with residues such as Met343, Ala350, Asp351, Thr347, Leu525, Leu346, Leu384, Trp383, Val534, Leu536, Met522, Tyr526, Val533, Lys529, and Glu523. It is interesting to note that in all three cases, L2, a derivative of a potent anti-breast cancer agent diosgenin shows the maximum binding score. The binding score of L2 with 3ERT, 4OAR, and 2JRM are − 8.9, − 8.9, and − 10.0 respectively. L2 shows a Hydrogen bonding interaction with 3ERT (Asp351) and 4OAR (Met759) with a bond length of 2.80 and 3.20 Å respectively. Though L2 has maximum interaction with 2JRM compared to all cases, no hydrogen bonding interactions are possessed. L4 bound to 2JRM is found to show a hydrogen bond with the amino acid residue Asp800 (2.99 Å). All hydrogen bonding falls under the mostly electrostatic type of interactions according to Jeffery (Jeffrey 1997). The number of residues taking part in hydrophobic interactions varies from 6 to 15. For instance, L1 bound to 3ERT is shown to have hydrophobic interactions with amino acid residues, Met343, Ala350, Asp351, Thr347, Leu525, Leu346, Leu384, Tr383, Val534, Leu536, Met522, Tyr526, Val533, Lys529, and Glu523. It must be noted that the ligand–protein complexes with the highest binding score show the least number of hydrophobic interactions. The three-dimensional and two-dimensional conformations of the ligand having the highest binding score (L2) with all the three proteins studied are shown in Fig. 2.
Table 5.
Binding affinity (in Kcal/mol) and binding interaction of phytosterols with three breast cancer molecular targets (PDB ID: 3ERT, 4OAR, 2JRM)
| Ligands | 3ERT | 4OAR | 2JRM | |||
|---|---|---|---|---|---|---|
| Residue | Binding affinity | Residue | Binding affinity | Residue | Binding affinity | |
| L1 | Met343, Ala350, Asp351, Thr347, Leu525, Leu346, Leu384, Trp383, Val534, Leu536, Met522, Tyr526, Val533, Lys529, Glu523 | − 7.5 | Glu723, Leu726, Trp755, Met759, Gly722, Met756, Leu718, Cys891, Met801, Leu887, Leu887, Thr894, Leu797, Asn719 | − 6.9 | Arg841, Cys797, Asp800, Gly796, Leu718, Phe795, Pro794,Val726, Leu844, Ala 743, Thr790, Thr854, Asp855, Phe723 | − 6.2 |
| L2 | Asp351(2.80), Ala350, Thr347, Leu525, Trp383, Tyr526, Leu536 | − 8.9 | Met759(3.20), Leu763, Val760, Gln725, Phe778, Gly722, Leu718, Cys891, Trp755,Asn719, Met756 | − 8.9 | Leu788, Met766, Thr854, Asp855, Leu844, Arg841,Thr790, Val726, Ala743, Lys745 | − 10.0 |
| L3 | Lys529, Tyr526, Leu539, Leu536, Trp383, Ala350, Asp351,Leu354, Val534, Leu525, Met522 | − 7.6 | Thr894, Leu797, Leu715,Phe794, Leu718, Leu721, Phe778, Gln725, Met759, Gly722, Val760,Trp755, Met756, Asn719 | − 8.1 | Arg841,Asp855,Gly796, Cys797, Leu844, Ala743, Met793,Lys745, Thr790, Val726, Thr854, Asn842,Phe723 | − 8.3 |
| L4 | Lys529, Trp383, Ala350, Leu 346, Leu384, Met343, Asp 351, Leu 525, Thr347, Met528, Cys530 | − 8.2 | Glu723, Trp755, Cys891, Leu718, Phe778, Arg766,Val760, Met759, Leu763,Gln725, Leu721, Gly722, Asn719 | − 8.3 | Asp800(2.99), Cys797, Gly719, Leu718, Val726, Leu844, Ala743, Leu788, Thr790, Lys745, Thr854, Asp855, Glu762 | − 8.4 |
Fig. 2.
The 3D and 2D diagrams representing the binding pose and protein–ligand interaction respectively of L2 with molecular targets. a 3ERT, b 4OAR and c 2JRM
Conclusion
In the present study, chemical constituents from ethanolic seed extract of the Lagerstroemia speciosa have been determined by GC–MS analysis. The four phytosterols(L1-L4) identified from the extract was predicted using in silico approaches for their inhibiting activities against breast cancer. One of the phytosterols studied violates the “rule of five” which is common in natural products. All molecules are shown to have positive BBB and Caco-2 values indicating their permeability through membranes. The phytosterols are found to no toxic risks such as carcinogenicity, mutagenicity and with a negative AMES value displaying its non-toxic nature. The molecular docking analysis revealed the high binding nature of the selected phytosterols with the three breast cancer targets studied. L2, a derivative of diosgenin, showed the highest inhibitory effect against all the three proteins as evident from its binding scores. The results support that phytosterols obtained from the ethanolic seed extract of Lagerstroemia speciosa could act as potential therapeutics against breast cancer. The current predictions over these phytosterol derivatives will be needed to further investigate in vivo and in vitro conditions to identify the optimum therapeutic efficacy and least toxicity.
Funding
DST-SERB grant in aid.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acharya R, Chacko S, Bose P, Lapenna A, Pattanayak SP. Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-52162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal VK, Amresh G, Chandra P. Pharmacodynamic evaluation of self micro-emulsifying formulation of standardized extract of Lagerstroemia speciosa for antidiabetic activity. J Ayurveda Integr Med. 2018;9(1):38–44. doi: 10.1016/j.jaim.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amresh G, Agarwal VK, Rao CV. Self microemulsifying formulation of Lagerstroemia speciosa against chemically induced hepatotoxicity. J Tradit Complement Med. 2018;8(1):164–169. doi: 10.1016/j.jtcme.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C, Gomes NG, Duangsrisai S, Andrade PB, Pereira DM, Valentão P (2020) Medicinal plants utilized in Thai traditional medicine for diabetes treatment: ethnobotanical surveys, scientific evidence and phytochemicals. J Ethnopharmacol:113177 [DOI] [PubMed]
- Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y. admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. Washington, D.C.: ACS Publications; 2012. [DOI] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL (2002) Pymol: An open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr 40(1):82–92
- Forli W, Halliday S, Belew R, Olson AJ (2012) AutoDock Version 4.2. Citeseer, Princeton
- Jeffrey GA. An introduction to hydrogen bonding. New York: Oxford University Press; 1997. [Google Scholar]
- Jehan CM, Daulatabad D, Mirajkar AM. A keto fatty acid from Lagerstroemia speciosa seed oil. Phytochemistry. 1990;29(7):2323–2324. doi: 10.1016/0031-9422(90)83061-5. [DOI] [Google Scholar]
- Joshi T, Joshi T, Sharma P, Pundir H, Chandra S (2020) In silico identification of natural fungicide from Melia azedarach against isocitrate lyase of Fusarium graminearum. J Biomol Struct Dyn:1–19 [DOI] [PubMed]
- Ju YH, Clausen LM, Allred KF, Almada AL, Helferich WG. β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J Nutr. 2004;134(5):1145–1151. doi: 10.1093/jn/134.5.1145. [DOI] [PubMed] [Google Scholar]
- Junaid S, Rakesh KN, Dileep N, Poornima G, Kekuda TP, Mukunda S. Total phenolic content and antioxidant activity of seed extract of Lagerstroemia speciosa L. Chem Sci Trans. 2013;2(1):75–80. doi: 10.7598/cst2012.310. [DOI] [Google Scholar]
- Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C, Svasti J (2017) Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One 12(12):e0189628 [DOI] [PMC free article] [PubMed]
- Lipinski CA. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Rule of five in 2015 and beyond: target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv Drug Deliv Rev. 2016;101:34–41. doi: 10.1016/j.addr.2016.04.029. [DOI] [PubMed] [Google Scholar]
- López‐García G, Alegría A, Barberá R, Cilla A (2019) Antiproliferative effects and mechanism of action of phytosterols derived from bioactive plant extracts. Nutraceuticals Nat Prod Deriv Dis Prev Drug Discov:145–165
- Mousa AM, El-Sammad NM, Abdel-Halim AH, Anwar N, Khalil WK, Nawwar M, Hashim AN, Elsayed EA, Hassan SK (2019) Lagerstroemia speciosa (L.) Pers leaf extract attenuates lung tumorigenesis via alleviating oxidative stress, inflammation and apoptosis. Biomolecules 9(12):871 [DOI] [PMC free article] [PubMed]
- MS U, Ferdosh S, Haque Akanda MJ, Ghafoor K, AH R, Ali ME, Kamaruzzaman B, MB F, Shaarani S, Islam Sarker MZ (2018) Techniques for the extraction of phytosterols and their benefits in human health: a review. Sep Sci Technol 53(14):2206–2223
- Oboyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminformatics. 2011;3(1):33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramashivam SK, Elayaperumal K, bhagavan Natarajan B, devi Ramamoorthy M, Balasubramanian S, Dhiraviam KN, In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases. Bioinformation. 2015;11(2):73. doi: 10.6026/97320630011073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander T (2001) OSIRIS property explorer. Org Chem Portal
- Saraswathi VS, Santhakumar K. Photocatalytic activity against azo dye and cytotoxicity on MCF-7 cell lines of zirconium oxide nanoparticle mediated using leaves of Lagerstroemia speciosa. J Photochem Photobiol B Biol. 2017;169:47–55. doi: 10.1016/j.jphotobiol.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Sharmin T, Rahman MS, Mohammadi H. Investigation of biological activities of the flowers of Lagerstroemia speciosa, the Jarul flower of Bangladesh. BMC Complement Altern Med. 2018;18(1):231. doi: 10.1186/s12906-018-2286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Lu L, Li Y, Hou L, Chen X, Rui H, Wang C, Yue Y (2018) Diosgenin inhibits the proliferation of MCF-7 breast cancer cells through the demethylation of miR-145 gene. AACR [DOI] [PubMed]
- Singh S, Verma E, Mishra AK. In silico molecular docking analysis of cancer biomarkers with GC/MS identified compounds of Scytonema sp. Netw Model Anal Health Inf Bioinform. 2020;9:1–14. doi: 10.1007/s13721-019-0207-3. [DOI] [Google Scholar]
- Sirikhansaeng P, Tanee T, Sudmoon R, Chaveerach A (2017) Major phytochemical as γ-sitosterol disclosing and toxicity testing in Lagerstroemia species. Evid Based Complement Altern Med [DOI] [PMC free article] [PubMed]
- Thakur RS, Devaraj E (2020) Lagerstroemia speciosa (L.) Pers. triggers oxidative stress mediated apoptosis via intrinsic mitochondrial pathway in HepG2 cells. Environ Toxicol 35(11):1225–1233 [DOI] [PubMed]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng Design Sel. 1995;8(2):127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.