Abstract
People with HIV (PWH) have reduced cardiorespiratory fitness, but a high intensity, easily disseminated exercise program has not yet been successfully developed in older PWH. The purpose of this article is to describe a synchronous telehealth exercise intervention in older PWH, delivered from one medical center to two other centers. Eighty older PWH (≥50 years) on antiretroviral therapy will be randomized to exercise or delayed entry control groups. Functional circuit exercise training, which does not entail stationary equipment, will be provided by real-time videoconferencing, 3 times weekly for 12 weeks, to small groups. Continuous remote telemonitoring of heart rate will ensure high exercise intensity. We hypothesize that telehealth exercise will be feasible and increase cardiorespiratory fitness and reduce sarcopenia and frailty. Findings will provide new insight to target successful aging in older PWH and can also be widely disseminated to PWH of any age or other patient populations.
Keywords: aerobic exercise, circuit exercise, functional exercise, resistance training, telehealth, videoconferencing
Contemporary antiretroviral therapy (ART) has changed infection with HIV into a chronic disease (Deeks et al., 2013). People with HIV (PWH) on ART have a life expectancy close to the general population (Samji et al., 2013). However, despite decreased mortality, chronic HIV infection advances the aging process (Deeks, 2010; John et al., 2016; Pathai et al., 2014). The threshold of 50 years was established to define “older” PWH due to this increased risk of age-related disease and geriatric syndromes, including sarcopenia (Yarasheski et al., 2011) and frailty (Althoff et al., 2014).
Cardiorespiratory fitness (CRF) is a physiological marker of aging which declines 10%–26% per decade after age 40 years in healthy adults (Fleg et al., 2005).PWH with viral suppression have a 30%–40% reduced CRF compared with age-matched healthy controls (Oursler et al., 2006; Vancampfort et al., 2016). Like sarcopenia and frailty, the etiology of low CRF in PWH is multifactorial and includes advanced biological aging processes and lifestyle and disease factors (Oursler & Sorkin, 2016). Half of PWH in the United States are 50 years and older, which will continue to rise (Centers for Disease Control and Prevention, 2020). Thus, there is an urgent need for effective exercise programs in PWH that can improve CRF, sarcopenia, and frailty to prevent widespread disability and early mortality for older PWH (Yahiaoui et al., 2012)
The challenge still remains to develop sustainable strategies for exercise training in older adults (Centers for Disease Control and Prevention, 2013). For older PWH, the common barriers (e.g., fatigue, poly-pharmacy, transportation) may be accentuated (Montoya et al., 2015). Furthermore, high-intensity aerobic exercise (AEX) has a greater positive effect on the biologic processes of aging, including CRF, and therefore should be a priority in PWH despite its additional difficulties (Balducci et al., 2010; Chodzko-Zajko et al., 2009; Oursler et al., 2018). Recent work shows that high-intensity AEX, performed as high-intensity interval training (HIIT), significantly increased CRF in older PWH and involved less time per session in the high-intensity zone compared with AEX performed as a continuous uninterrupted session (mean minutes/session [SE]; 12.3 [0.7] vs. 31.4 [1.3]; p < .001; Briggs et al., 2020). However, wide-spread dissemination is hindered by requirements for equipment and individual-level supervision.
Circuit exercise training is a strategy that can address these challenges and is designed to provide both cardiovascular and strength benefits by performing exercises quickly with minimal rest between each set and exercise station. In adults of 65 years and older without HIV, high-intensity circuit exercise training performed in an exercise facility increased CRF (Brentano et al., 2008; Takeshima et al., 2004). But, gains in strength with circuit exercise can be variable and seem to be dependent on intensity (Paoli et al., 2010; Romero-Arenas et al., 2013). Unfortunately, stationary equipment often employed in these studies is not always available; additionally, the stationary equipment used can be challenging for some older adults.
Functional training is a modality that emphasizes multijoint body weight movements and can be performed without stationary equipment. Among athletes and the general population, high-intensity functional circuit training increases CRF and exercise adherence (Feito et al., 2018) but is understudied in older adults. In frail, elderly individuals, lower intensity functional circuit interventions improved mobility (Gine-Garriga et al., 2010; Whitehurst et al., 2005). We demonstrated the feasibility of low-moderate intensity (rating of perceived exertion [RPE] 3–6) functional circuit exercise delivered by videoconferencing in adults of 65 years and older without HIV (Briggs et al., 2018). In a recent pilot study of six men older than 65 years without HIV, we found that 12 weeks of moderate-to-high intensity (RPE 4–7) functional circuit exercise performed as a small class improved performance on the timed arm curl (26.8%) and chair stand tests (30.3%; all p < .03). A meta-analysis of randomized trials in cardiac rehabilitation showed that home telerehabilitation had similar increases in CRF compared with center-based exercise training (Rawstorn et al., 2016). In addition, the standard mean difference of exercise adherence was significantly higher in telerehabilitation (standard mean difference = 0.75; 95% confidence interval, 0.52–0.98). Yet, to our knowledge, none of these training strategies have been tested in PWH.
To address these gaps in knowledge, we designed a high-intensity functional circuit exercise intervention to be delivered by live videoconferencing (telehealth exercise [Tele-EX]) and tailored to older PWH. We hypothesized that Tele-EX in older PWH would increase CRF and reduce sarcopenia and frailty. We adapted our center-based high-intensity exercise training strategies in older PWH to a functional modality, which does not require stationary equipment but retains continuous heart rate (HR) monitoring and individualized exercise intensity based on target HR (THR; Briggs et al., 2020; Oursler et al., 2018). We also built upon our experience with Gerofit, a center-based individualized exercise program delivered in a group setting in veterans of 65 years and older (Morey et al., 2018). We adapted Gerofit to videoconferencing delivery that included instructor-led exercise classes, which were broadcast to other Veterans Affairs (VA) locations (Briggs et al., 2018). Based on our observation of the importance of social support in the group setting, we chose to retain the small exercise group design in this trial. In addition, this facilitated continuous telemonitoring of HR, an important safety and treatment fidelity component for high-intensity exercise. In tele-Gerofit, we conducted functional performance testing by videoconferencing. In addition to the traditional measure of CRF, these outcomes offer the potential for large-scale implementation with minimal equipment and therefore will be performed in this trial. We chose the VA as the setting because it has a well-established telehealth infrastructure (Wennergren et al., 2014). The purpose of this article is to describe the details of the study protocol.
Methods
Study Design
The study is a two-group, parallel, multisite, randomized trial of high-intensity functional circuit exercise training to be delivered by live videoconferencing (Tele-EX) in older (≥50 years) PWH. Veterans receiving HIV care at the Baltimore VA Medical Center (VAMC; N = 40) and the Atlanta VAMC (N = 40) will be randomized 1:1 to either an exercise or a standard-of-care sedentary control group. The 12-week exercise training will be broadcast three-times weekly from the Salem VAMC to small groups of participants at the Atlanta and Baltimore VAMCs using existing VA telehealth infrastructure. All outcomes will be measured at baseline and 12-week follow-up visits.
Participants and Randomization
Sedentary PWH, 50 years and older, will be recruited from their respective infectious disease clinics at the Atlanta and Baltimore VAMCs using informational pamphlets and provider referrals. To minimize confounding by other physical activities, sedentary was defined as participating in no more than one structured physical activity session per week. To be eligible for the trial, participants must have HIV infection with viral suppression (reverse transcription polymerase chain reaction <20 copies/mL) and be receiving ART within the prior 3 months. Exclusion criteria are based on our prior exercise research in PWH and recommendations of the American College of Sports Medicine guidelines (American College of Sports Medicine, 2010). Specific exclusion criteria are included to maximize safety but retain generalizability of study results and limit confounding details (ClinicalTrials.gov NCT04103593). In brief, existing cardiometabolic disease must be clinically controlled. Preexisting lung disease cannot limit activities of daily living (Modified Medical Research Council dyspnea score = 4). Clinical factors commonly found in older patients that may affect baseline exercise capacity and biomarkers of aging, such as current smoking or use of hydroxymethylglutaryl-coenzyme A reductase inhibitors, are not exclusion criteria but will be recorded for post hoc analysis. We expect that potential confounding factors will be evenly distributed between the two groups, and this approach will minimize selection bias and increase generalizability of findings. Eligibility will be determined based on a history and physical examination conducted by a clinician investigator at the first research visit after informed consent is obtained. This visit includes a review of laboratory results and electrocardiogram and performance of a targeted history and physical examination specific to this protocol, which confirms each eligibility criterion.
The sites at the Atlanta and Baltimore VAMCs will each randomize 40 participants 1:1 to exercise or standard-of-care control groups for a study total of 80 participants. Randomization using a computer algorithm will occur in blocks of 10 participants at each site (5 exercise and 5 control). The block randomization will help ensure balanced assignment to the two groups at each site and will generate a cohort of 10 exercising participants who will constitute one Tele-EX class (5 from each site).
Written informed consent will be obtained from each individual. The protocol will be approved by each site’s respective institutional review board and Research and Development Commitees. The Salem VAMC will function as the coordinating center and will ensure protocol fidelity across sites using the approach advocated by The Treatment Fidelity Workgroup of the NIH Behavior Change Consortium for study design, research staff training, and treatment delivery and receipt (Bellg et al., 2004).
Outcomes and Research Procedures
Testing will be performed at baseline and at 12 weeks and will occur at least 48 hr after an exercise test or training. All procedures will use standardized protocols and experienced research staff who will receive protocol-specific education by the coordinating center. A summary of primary outcomes by domain and testing procedure is available in Table 1.
Table 1.
Summary of Study Outcomes and Procedures by Domain
| Domain | Outcome | Procedure |
|---|---|---|
| Cardiorespiratory fitness | Peak oxygen consumption O2 pulse |
Cardiopulmonary exercise testing |
| Sarcopenia | Sarcopenia Per European Working Group on Sarcopenia (Cruz-Jentoft et al., 2010) | DXA + grip strength; DXA + gait speed |
| Frailty | Frailty phenotype (Schmaltz et al., 2005) VACS index (Tate et al., 2019) |
Gait speed + grip strength Clinical laboratory values |
| Health-related QoL | Symptoms, including pain and fatigue Social inclusion Mobility and working capacity |
WHOQOL-HIV (O’Connell & Skevington, 2012) |
| Social factors | Social support Self-efficacy |
Exercise Social Provisions Scale (Courneya & McAuley, 2016) Barriers-Specific Efficacy Questionnaire (McAuley et al., 2003) |
| Evaluation | Video technology Personal barriers Intervention fidelity |
System Usability Scale (Bangor et al., 2008) Reasons for attrition/missed visits % AEX time at target heart rate |
Note. AEX = aerobic exercise; DXA = dual-energy X-ray absorptiometry; QoL = quality of life.
Cardiorespiratory fitness.
Participants will be asked to exercise until voluntary exhaustion during a graded treadmill test using the modified Bruce protocol and continuous electrocardiogram monitoring (American College of Sports Medicine, 2010). Participants will be considered at moderate to high risk for coronary artery disease (CAD); therefore, cardiopulmonary exercise testing will be monitored by a qualified clinician. Oxygen (O2) consumption, CO2 production, and minute ventilation will be measured continuously using a Quark cardiopulmonary exercise testing (Cosmed) metabolic cart. Oxygen consumption at peak exercise effort, VO2peak, is used as the standard measure of CRF in sedentary older adults because the majority are not able to reach maximal aerobic capacity defined as a plateau in oxygen consumption (Noakes, 1988). VO2peak will be measured as the average of the final 30 s values of oxygen consumption at peak exercise. Oxygen pulse is a measure of the efficiency of oxygen delivery and will be calculated as the milliliters of oxygen consumed per heartbeat (VO2/HR). Participants with results consistent with active CAD, such as ST-segment changes, will be excluded from the study and referred for clinical evaluation.
Skeletal muscle and functional performance.
Total and regional lean tissue mass will be measured by dual-energy X-ray absorptiometry and used to estimate skeletal muscle mass. Appendicular skeletal mass index predicts clinical outcomes in older patient populations (Baumgartner et al., 1998; Fielding et al., 2011), including PWH (Hawkins et al., 2018; Oursler et al., 2019), and will be calculated as lean appendicular mass/height2. Established cutoffs for appendicular skeletal mass index will be used to define low muscle mass (Cawthon et al., 2014). Sarcopenia will be defined as low skeletal muscle mass with low muscle function (strength or performance; Cruz-Jentoft et al., 2010). Lower extremity strength will be measured by the 30-s chair stand test, which has a test–retest reliability of 0.89 (Rikli & Jones, 1997). The 30-s chair stand correlates with one repetition maximum (1RM) leg press performance in older men (r = 0.78; Jones et al., 1999); it improves with resistance training (RT) (Bottaro et al., 2007) and predicts mobility disability (Rikli & Jones, 1997). Upper extremity strength will be measured by the timed arm curl, which has test–retest reliability of 0.80 (Rikli & Jones, 2013). The 30-s arm curl test correlates with 1RM bicep strength (r = 0.62; Rikli & Jones, 1997) and improves with RT (Bottaro et al., 2007). Hand grip strength is a widely used measure of functional performance that declines greater with age in PWH (Schrack et al., 2016) and will be measured with a dynamometer (Rikli & Jones, 1997). The 4-m walk test will be used to measure normal gait speed because it is easier to conduct by videoconferencing than longer walk tests. The 4-m walk test has excellent test–retest reliability with an intraclass correlation coefficient of 0.93 (Peters et al., 2013). As a substitute for a long-distance walk test, the 2-min step test will be performed as an established surrogate measure in space limited settings with a test–retest reliability of 0.88 (Rikli & Jones, 2013). All testing, apart from grip strength, will be performed by videoconferencing using the approach previously described (Briggs et al., 2018) and the same video equipment used in exercise training, but an exercise physiologist who is blinded to the participant’s randomized group allocation will perform the testing.
Frailty.
Frailty is an established geriatric construct to convey the loss of resilience and increased risk for disability (Walston et al., 2006). As a clinical syndrome, frailty can be characterized by a phenotype of signs and symptoms or an index based on comorbidity and laboratory values. The frailty phenotype developed by Fried et al. (Schmaltz et al., 2005) has been widely studied in PWH (Piggott et al., 2016) and will be used to classify participants as frail or prefrail using the five domains of the phenotype (slowness, weakness, low activity, weight loss, and exhaustion). An additional measure, the Veterans Aging Cohort Study (VACS) index will be calculated. The VACS index is a validated, generalizable risk index based on routine clinical data that independently predicts mortality (Trickey et al., 2017) and has been used as a measure of frailty for PWH (Justice & Tate, 2019). The VACS index is associated with several manifestations of aging, including bone fractures, cognitive decline, and chronic inflammation (Justice et al., 2012). The VACS index 2.0 will be calculated based on clinical laboratory values, age, and BMI (Tate et al., 2019).
Questionnaires.
Health-related quality of life will be assessed with the WHOQOL-HIV BREF, which asks individuals to rate 6 domains (physical, psychological, independence, social relationships, environment, spiritual aspects/religion/personal beliefs) in the prior 2 weeks. The scales show internal consistency (scale reliability) by the Cronbach’s alpha coefficients (α ≥ 0.69; O’Connell & Skevington, 2012) and sensitivity to change with exercise training (DE Medeiros Guerra et al., 2016). Social support related to group exercise will be measured by the exercise social provisions scale, a validated questionnaire for social support in the exercise setting (Courneya & McAuley, 2016). The questionnaire consists of 24 items on a 4-point Likert scale that evaluates six distinct components of social support: attachment, social integration, reassurance of worth, reliable alliance, guidance, and opportunity for nurturance. The Cronbach’s alpha coefficient across the six scales is 0.66–0.92; good test–retest reliability also was reported across three test periods (Courneya & McAuley, 2016). Exercise self-efficacy will be measured using the Barriers-Specific Efficacy Questionnaire, a 13-item scale with high internal consistency (mean α = 0.92), which assesses individual beliefs about participating in exercise despite commonly reported barriers to exercise participation (McAuley et al., 2003).
Evaluation outcomes.
The feasibility of Tele-EX will be assessed within multiple domains. Enrollment, attendance, and retention rates will be calculated. Acceptability and demand will be evaluated through aggregating the reasons for missed sessions and attrition. This will include adverse events and exercise tolerance. The capacity to deliver high-intensity exercise will be assessed by intervention fidelity measures. The system usability scale is a 10-item assessment tool (Bangor et al., 2008), which will be adapted for the Tele-EX system by inserting the terms “exercise” and “videoconferencing” and will measure participant satisfaction with the videoconferencing delivery. In addition, technical issues with equipment will be recorded.
Exercise Intervention
Overview.
The intervention consists of high-intensity, circuit functional exercise training, which will be performed three times weekly for 12 weeks in a group setting (Tele-EX). High-intensity functional training (HIFT) has been defined as “a training style that incorporates a variety of functional movements, performed at high-intensity, and designed to improve parameters of general physical fitness” (Feito et al., 2018, p. 2). Tele-EX consists of supervised HIFT performed as a group in a circuit format with timed periods of exercise and rest. Figure 1 shows an overview of the circuit design of Tele-EX. Exercise training will be delivered by a certified exercise physiologist through real-time videoconferencing, which is broadcast within the VA network from Salem to participants in Atlanta and Baltimore. These synchronous telemedicine exercise classes will use high-resolution VA cameras and high-speed, encrypted, and Health Insurance Portability and Accountability Act–compliant VA network connections. Rooms will be equipped with steps, hand and ankle weights, dumbbells, chairs, and bands; no stationary exercise equipment will be used. A research coordinator will be in the room with the participants to take an initial blood pressure and help with HR monitors and the portable exercise equipment.
Figure 1.
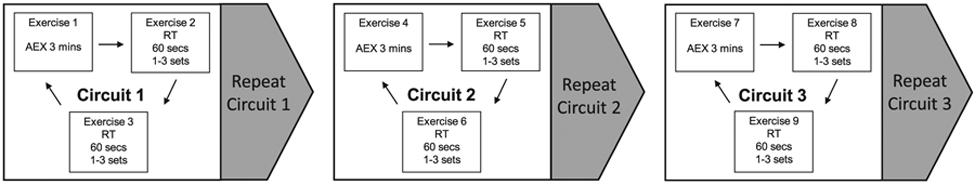
Overview of the Tele-EX design: high-intensity functional circuit exercise training delivered by live videoconferencing. The group exercise training sessions will be approximately an hour, including warm up (8-10 min), functional circuit training (48 min), and cool-down/stretching (8-10 min). An instructor will lead each exercise in order as a group through three unique circuits. Although functional exercise involves multiple muscle groups and dynamic motion, each exercise is classified as either aerobic exercise (AEX) or resistance training (RT) based on its primary purpose. The duration of each exercise is dependent on the individual’s capacity, but the goal is 3 minutes of AEX and 2 minutes of RT per circuit. Participants will be progressed through each circuit with minimal rest between AEX and RT exercises. The number of circuits performed per session and the inter-circuit rest period will be adjusted based on the exercise progression protocol.
Functional circuit training.
The group exercise training sessions will be performed 3 times weekly and last approximately 1 hr, including warm-up (8–10 min), functional circuit training (48 min), and cooldown/stretching (8–10 min). Functional exercises use multiple muscle groups and dynamic motion. However, for the purpose of this study, we will classify each exercise as either AEX or RT based on its primary purpose to increase CRF or muscle strength and size. The exercise class will contain three unique circuits, which can be repeated one to two times (Figure 1). Each circuit will consist of one type of AEX and two types of RT. In a single circuit, the AEX will last three minutes, and each RT will be performed with as many repetitions as possible in 1 min (total 5 min/circuit). Participants will be instructed and progressed through each circuit with minimal rest between AEX and RT exercises.
Exercise intensity and progression.
Intensity of the functional exercises will be moderated by the difficulty of AEX and the number of repetitions and weight of dumbbells or hand/ankle weights (RT). To evaluate AEX intensity, participants will wear HR chest straps with Bluetooth capacity (Polar, Lake Success, NY). The remote exercise physiologist will monitor the continuous HR of all participants during the exercise session using the Polar Club app loaded on an iPad located at each of the sites. The THR range will be set for each participant and will be adjusted as intensity is progressed. The AEX training protocol is built upon our prior strategies that have been successful in older PWH and uses data from the baseline treadmill test to derive target HR ranges (Oursler et al., 2018). For the first 4 weeks (Sessions 1–12), participants will perform AEX at a THR of 64%–76% HRmax with a goal of 18 min in THR per session. In Weeks 5–12 (Sessions 13–36), THR will transition to intensity of 77%–95% HRmax (Table 2). Because each AEX circuit lasts 3 min at a high intensity, this will be analogous to HIIT where the RT circuit serves as the recovery segment. However, unlike HIIT, as-needed breaks between circuits can be used to gradually progress the participants, and therefore, it would be characterized more precisely as HIFT (Feito et al., 2018). For RT, the intensity cannot be gauged by the traditional percentage of 1-RM measured with equipment because functional RT exercises use multiple muscle groups. Participants will be instructed to perform RT exercise based on their perceived exertion intensity on the modified Borg RPE scale (RPE 0–10). RT exercises will be progressed from moderate intensity (RPE 4–6) to vigorous intensity (RPE 7–8). Progression of RT intensity is achieved by increasing the number of repetitions and weight of dumbbells within the time allowance. Therefore, the number of repetitions for each functional RT exercise serves as the target (Rikli & Jones, 2013). In addition, more complex exercises with multiple muscles engaged will be used to provide higher-intensity RT.
Table 2.
Progression of Aerobic Exercise Training
| Intervention Period | Session No. | Target Heart Rate |
|---|---|---|
| 1–4 week | 1–12 | 64%–76% HRmax |
| 5–12 weeks | 13–36 | 77%–95% HRmax |
Note. Heart rate is continuously measured and recorded during exercise by a chest strap sensor. Target heart rate is based on percentage of maximum heart rate (HRmax) at baseline treadmill testing.
Telemonitoring.
A challenge to clinical cardiac telerehabilitation is telemonitoring, which is especially important in higher-intensity exercise for both safety and intervention fidelity reasons. We derived an innovative strategy to continuously monitor HR at a remote site using existing hardware and applications. Polar Club (www.polar.com/us-en/club) is an Apple-based iPad application that provides a group exercise HR monitoring solution. Polar Club uses a Polar H10 HR sensor with Bluetooth connection (chest strap) that allows multiple users to link with an iPad located in the room with the participants. The H10 sensor transmits HR data in real time to the Polar Club application, which displays actual HR and the THR zone for each individual.
For the purpose of this study, the exercise physiologist will set the THR zone by logging into Polar Club prior to the exercise session. These metrics are displayed on the iPad during the exercise training session. A web camera is focused on the iPad to allow the exercise physiologist, who is located at another site, to see the iPad display. This system requires the research coordinator to initiate a video chat using a VA computer and messaging system with the exercise physiologist and arrange the web camera and iPad. In addition to real-time monitoring, this system also allows recording of the HR for further analysis. The iPad uses an internet connection to store the HR data on the Polar server that is then downloaded and analyzed for treatment fidelity. As a backup in case of loss of internet connection, HR data are also stored in the sensor and can be uploaded to the Polar server manually.
Treatment fidelity.
As detailed above, HR data will be recorded continuously and then downloaded from the Polar server to a computer to evaluate the AEX duration and intensity for each individual. The participant’s percent of time in THR will be calculated for each session. The research coordinator will maintain a paper log of the RT intensity (i.e., RPE) and the number of exercise repetitions under the direction of the exercise physiologist during the exercise session. Exercise progression and any medical issues will be reviewed weekly to ensure that interim goals of sessions are met for each participant.
Safety.
We have developed the exclusion criteria to minimize the risk of adverse events in older PWH. In addition, the baseline treadmill test also serves to screen for preclinical CAD. During exercise training, the HRmax from the treadmill will not be exceeded. Exercise training and functional performance testing will take place in rooms located with emergency equipment and specific safety procedures developed for exercise telehealth. The research coordinator at each site will measure blood pressure before every session and will remain in the room with the participant(s). Risk of minor muscle soreness and joint pain, the most common adverse events, is minimized by warm-up and cooldown/stretching periods for each session and by gradual exercise progression. In addition, participants who miss six consecutive exercise sessions will be disqualified, based on our experience that exercise progression in a shorter time frame is not well tolerated.
Analysis
Data management.
Data will be collected using forms coded only with a participant’s study ID number. Data will be entered into a REDCap relational database stored on a VA REDCap server, which is housed in the VA Informatics and Computing Infrastructure (vaww.vinci.med.va.gov). Source data that are generated as an electronic file or print out will be stored in a shared VA network folder with access limited to authorized research staff. All electronic files or scanned documents with study data will be coded with participant study IDs only. A separate file with PHI and the study key code will be kept in a different location in a password protected file. No publications or presentations will use information with PHI.
Data analysis.
We will use regression to determine if the intervention (exercise vs. control) affects outcome, where change (post value-pre value) will be the dependent variable and group (exercise vs. standard of care) will be the independent variable. If, despite randomization, there are differences in the outcome measures at baseline between the groups, the prevalue of the outcome measures will be added to the model. Similarly, if demographic or clinical characteristics differ between the groups, these factors will be added to the models. For future planning, feasibility analyses will include calculation of rates of enrollment, retention, and attendance overall and by site. Treatment fidelity will be measured as percent of AEX time at THR. Aggregate data will be collected on adverse events and reasons for loss to follow-up.
Analyses will be performed following an intention-to-treat paradigm using multiple imputation. If the pattern of missing data is missing completely at random, or at random, multiple imputation should eliminate bias due to differential loss to follow-up. Because the participants will be recruited from VA clinics, we will be able to obtain clinical data from participants who dropout of the study but remain in clinical care, to determine if dropout was related to medical conditions that may influence outcomes. If there is sufficient information, we may be able to model the dropout process and adjust our analyses for loss to follow-up even if the missing data pattern is nonignorable.
Sample size.
Sample size calculations for the primary outcome CRF are based on results from our high-intensity AEX intervention in older PWH that showed a M (SD) within person change in VO2peak mL/kg/min of 3.59 (3.61), which is a clinically meaningful increase (Oursler et al., 2018). We assumed a two-tailed α of 0.05, 80% power, and two samples with equal variances. Given these assumptions and allowing for a 0.75 mL/mg/kg increase in the control group due to test learning effect, 26 participants will be needed in each group to detect a significant between-group difference in VO2peak. Therefore, based on the plan to randomize 80 participants, we expect to have adequate sample size even with attrition as high as 35%.
Discussion
Our long-term goal is to derive sustainable exercise strategies that ameliorate advanced aging of PWH. The objective of this randomized trial is to determine the feasibility and efficacy of a high-intensity, center-based, functional circuit exercise intervention to be delivered by live videoconferencing (Tele-EX) in older PWH. The high-intensity exercise is designed to optimally reduce biologic aging but includes the safety of continuous HR telemonitoring. The functional circuit modality removes the limitations for stationary equipment and fully equipped exercise facilities. The telehealth delivery further promotes dissemination and wide-spread implementation in difficult to reach communities. In light of the COVID-19 pandemic, future work could adapt Tele-EX to home delivery to provide exercise telerehabilitation that is compliant with social distancing yet maintains group support. The primary barriers to home-based exercise telerehabilitation are available technology, including patients’ computer devices and internet accessibility, as well as provisions for safety given the high-intensity of Tele-EX. Therefore, we plan to implement this protocol as originally designed but will adjust the size of the exercise class to allow for appropriate physical distancing. Although telerehabilitation is an established exercise strategy for cardiac patients (Frederix et al., 2015; Rawstorn et al., 2016), it is relatively unexplored in PWH and is limited to recorded video exercise education and instruction (Piraux et al., 2019). Results from our study will be novel and applicable to PWH of any age but will specifically address the challenge to prevent and minimize disability in older PWH. In addition, Tele-EX is designed as a long-term exercise program for successful aging rather than telerehabilitation for a specific condition or event and as such can be applied to all older adults.
Potential challenges include retention of participants given that exercise interventions in both older adults and PWH historically have high attrition rates. We will explore the effect of peer support in a group exercise setting to address this difficulty. Conversely, the exercise class design may be a barrier for enrollment if patients are concerned about stigma or have low self-efficacy. Although our work supports the feasibility of exercise delivery via videoconferencing, technical issues can occur and may amplify the difficulty of high-intensity exercise training in sedentary older adults. The study uses the well-established telehealth infrastructure of the VA but will be applicable as other health care systems further develop telehealth.
Key Considerations.
Tele-EX is a proposed telehealth exercise strategy that will provide high-intensity exercise under supervision but with the flexibility of videoconferencing and functional circuit training.
In contrast to telerehabilitation whose focus is treatment of a condition, Tele-EX is designed to provide long-term, sustained exercise training for the goal of healthy aging with HIV.
Tele-EX, if proven feasible and efficacious, can be studied in larger implementation trials among PWH of any age and other patient populations with the potential for wide dissemination using telehealth.
Acknowledgments
Disclosures
Vincent C. Marconi has served as a consultant or received research support from Lilly, Gilead, ViiV, and Bayer. The other authors report no financial interests or potential conflicts of interest.
This research was supported by The Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service (RRR&D; I01 RX002790, PIs: Krisann Oursler, Vince Marconi, and Alice Ryan) and VA RR&D Senior Research Career Scientist Award (PI: Alice S. Ryan), the NIA Claude D. Pepper Older Americans Independence Center (P30AG028747; PI: Jay S. Magaziner), and the Emory Center for AIDS Research (P30-AI-050409, PI: Carlos Del Rio).
Appendix
Acknowledgments
The FIT VET Project Team includes the following collaborative authors: Kim Birkett, MPH, Chani Jain, MPH, Theron Clark-Stuart, BS, Katherine L. Meagley, MPH, Ernest L. Tate, and Kristina K. Marcus, MS. Clinical Trials registration number: NCT04103593
Contributor Information
Krisann K. Oursler, Geriatric Research and Education, Salem Veterans Affairs Medical Center, Virginia Tech Carilion School of Medicine, Roanoke, Virginia, USA..
Vincent C. Marconi, Infectious Diseases Research Program, Atlanta Veterans Affairs Medical Center, Emory University School of Medicine and Emory University Rollins School of Public Health, Atlanta, Georgia, USA..
Brandon C. Briggs, Salem Veterans Affairs Medical Center, Concordia University Chicago, Chicago, Illinois, USA..
John D. Sorkin, Biostatistics and Informatics, Veterans Affairs Maryland Health Care System, Baltimore Veterans Affairs Medical Center Geriatric Research, Education, and Clinical Center, University of Maryland School of Medicine, Baltimore, Maryland, USA..
Alice S. Ryan, Rehabilitation Research & Development, Veterans Affairs Maryland Health Care System, Baltimore Veterans Affairs Medical Center Geriatric Research, Education, and Clinical Center, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, & Margolick JB (2014). Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(2), 189–198. 10.1093/gerona/glt148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2010). ACSM’s guidelines for exercise testing and prescription (8th ed.,Vol. 6).Williams and Wilkins. [DOI] [PubMed] [Google Scholar]
- Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, & Pugliese G (2010). Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, Metabolism, and Cardiovascular Diseases, 20(8), 608–617. 10.1016/j.numecd.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Bangor A, Kortum PT, & Miller JT (2008). An empirical evaluation of the system usability scale. International Journal of Human–Computer Interaction, 24(6), 574–594. 10.1080/10447310802205776 [DOI] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, & Lindeman RD (1998). Epidemiology of sarcopenia among the elderly in New Mexico. American Journal of Epidemiology, 147(8), 755–763. 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, & Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Bottaro M, Machado SN, Nogueira W, Scales R, & Veloso J (2007). Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. European Journal of Applied Physiology, 99(3), 257–264. 10.1007/s00421-006-0343-1 [DOI] [PubMed] [Google Scholar]
- Brentano MA, Cadore EL, Da Silva EM, Ambrosini AB, Coertjens M, Petkowicz R, Viero I, & Kruel LF (2008). Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. Journal of Strength & Conditioning.Research, 22(6), 1816–1825. 10.1519/JSC.0b013e31817ae3f1 [DOI] [PubMed] [Google Scholar]
- Briggs BC, Jain C, Morey MC, Blanchard EH, Lee CC, Valencia WM, & Oursler KK (2018). Providing rural veterans with access to exercise through Gerofit. Federal Practitioner, 35(11), 16–23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6366594/pdf/fp-35-11-16.pdf [PMC free article] [PubMed] [Google Scholar]
- Briggs BC, Ryan AS, Sorkin JD, & Oursler KK (2020). Feasibility and effects of high-intensity interval training in older adults living with HIV. Journal of Sports Sciences. Advance online publication. 10.1080/02640414.2020.1818949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam T-TL, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, Ferrucci L, Kritchevsky SB, Vassileva MT, Studenski SA, & Alley DE (2014). Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(5), 567–575. 10.1093/gerona/glu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. Morbidity and Mortality Weekly Report, 62(17), 326–330. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a2.htm [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020, May). Estimated HIV incidence and prevalence in the United States, 2014–2018. HIV Surveillance Supplemental Report (Vol. 25, No. 1). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-1.pdf [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, & Skinner JS (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise, 41(7), 1510–1530. 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Courneya KS, & McAuley E (2016). Reliability and discriminant validity of subjective norm, social support, and cohesion in an exercise setting. Journal of Sport & Exercise Psychology, 17(3), 325–337. 10.1123/jsep.17.3.325 [DOI] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, & Zamboni M (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and Ageing, 39(4), 412–423. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Medeiros Guerra LM, Galvao DE Souza HA, Mesquita Soares TC, Gomes DA Silva J, DA Rocha Morgan DA, Monteiro Melo FC, Jefferson DE Medeiros H, Tenorio DA Cunha AJ, & Knackfuss MI (2016). Resisted exercise, morphological and functional standards, and quality of life of people living with HIV/AIDS. The Journal of Sports Medicine and Physical Fitness, 56(4), 470–475. [PubMed] [Google Scholar]
- Deeks SG (2010). HIV infection, inflammation, immunosenescence, and aging. Annual Review of Medicine, 62, 141–155. 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, & Havlir DV (2013). The end of AIDS:HIV infection as a chronic disease. The Lancet, 382(9903), 1525–1533. 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feito Y, Heinrich K, Butcher S, & Poston W (2018). High-intensity functional training (HIFT): Definition and research implications for improved fitness. Sports, 6(3), 76. 10.3390/sports6030076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van. K., Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime GF, Onder G, Papanicolaou D, & Zamboni M (2011). Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International Working Group on Sarcopenia. Journal of the American Medical Directors Association, 12(4), 249–256. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, & Lakatta EG (2005). Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation, 112(5), 674–682. 10.1161/CIRCULATIONAHA.105.545459 [DOI] [PubMed] [Google Scholar]
- Frederix I, Vanhees L, Dendale P, & Goetschalckx K (2015). A review of telerehabilitation for cardiac patients. Journal of Telemedicine and Telecare, 21(1), 45–53. 10.1177/1357633X14562732 [DOI] [PubMed] [Google Scholar]
- Gine-Garriga M, Guerra M, Pages E, Manini TM, Jimenez R, & Unnithan VB (2010). The effect of functional circuit training on physical frailty in frail older adults: A randomized controlled trial. Journal of Aging and Physical Activity, 18(4), 401–424. 10.1123/japa.18.4.401 [DOI] [PubMed] [Google Scholar]
- Hawkins KL, Zhang L, Ng DK, Althoff KN, Palella FJJ, Kingsley LA, Jacobson LP, Margolick JB, Lake JE, Brown TT, & Erlandson KM (2018). Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS, 32(10), 1257–1266. 10.1097/QAD.0000000000001829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John MD, Greene M, Hessol NA, Zepf R, Parrott AH, Foreman C, Bourgeois J, Gandhi M, & Hare CB (2016). Geriatric assessments and association with VACS index among HIV-infected older adults in San Francisco. Journal of Acquired Immune Deficiency Syndromes, 72(5), 534–541. 10.1097/QAI.0000000000001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, & Beam WC (1999). A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research Quarterly for Exercise and Sport, 70(2), 113–119. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, & Bryant K, (2012). Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clinical Infectious Diseases, 54(7),984–994. 10.1093/cid/cir989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, & Tate JP (2019). Strengths and limitations of the Veterans Aging Cohort Study Index as a measure of physiologic frailty. AIDS Research and Human Retroviruses, 35(11–12), 1023–1033. 10.1089/AID.2019.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Marquez DX, Elavsky S, & Blissmer B (2003). Exercise self-efficacy in older adults: Social, affective, and behavioral influences. Annals of Behavioral Medicine, 25(1), 1–7. 10.1207/S15324796ABM2501_01 [DOI] [PubMed] [Google Scholar]
- Montoya JL, Wing D, Knight A, Moore DJ, & Henry BL (2015). Development of an mHealth intervention (iSTEP) to promote physical activity among people living with HIV. Journal of the International Association of Providers of AIDS Care, 14(6), 471–475. 10.1177/2325957415601505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey MC, Lee CC, Castle S, Valencia WM, Katzel L, Giffuni J, Kopp T, Cammarata H, McDonald M, Oursler KA, Wamsley T, Jain C, Bettger JP, Pearson M, Manning KM, Intrator O, Veazie P, Sloane R, Li J, & Parker DC (2018). Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. Journal of the American Geriatrics Society, 66(5), 1009–1016. 10.1093/cid/cir989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes TD (1988). Implications of exercise testing for prediction of athletic performance: A contemporary perspective. Medicine and Science in Sports and Exercise, 20(4), 319–330. 10.1249/00005768-198808000-00001 [DOI] [PubMed] [Google Scholar]
- Oursler KA, & Sorkin JD (2016). HIV and aging. International Journal of Infectious Diseases, 53, 59–60. 10.1016/j.ijid.2016.11.414 [DOI] [PubMed] [Google Scholar]
- Oursler KK, Iranmanesh A, Jain C, Birkett KL, Briggs BC, Garner DC, Sorkin JD, & Ryan AS (2019). Low muscle mass is associated with osteoporosis in older adults living with HIV. AIDS Research and Human Retroviruses, 36(4), 300–302. 10.1089/AID.2019.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KK, Sorkin JD, Ryan AS, & Katzel LI (2018). A pilot randomized aerobic exercise trial in older HIV-infected men: Insights into strategies for successful aging with HIV. PLoS One, 13(6), e0198855. 10.1371/journal.pone.0198855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler KK, Sorkin JD, Smith BA, & Katzel LI (2006). Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Research and Human Retroviruses, 22(11), 1113–1121. 10.1089/aid.2006.22.1113 [DOI] [PubMed] [Google Scholar]
- O’Connell KA, & Skevington SM (2012). An international quality of life instrument to assess wellbeing in adults who are HIV-positive: A short form of the WHOQOL-HIV (31 items). AIDS and Behavior, 16(2), 452–460. 10.1007/s10461-010-9863-0 [DOI] [PubMed] [Google Scholar]
- Paoli A, Pacelli F, Bargossi AM, Marcolin G, Guzzinati S, Neri M, Bianco A, & Palma A (2010). Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. The Journal of Sports Medicine and Physical Fitness, 50(1), 43–51. 10.1186/1476-511X-12-131 [DOI] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(7), 833–842. 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DM, Fritz SL, & Krotish DE (2013). Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. Journal of Geriatric Physical Therapy (2001), 36(1), 24–30. 10.1519/JPT.0b013e318248e20d [DOI] [PubMed] [Google Scholar]
- Piggott DA, Erlandson KM, & Yarasheski KE (2016). Frailty in HIV: Epidemiology, biology, measurement, interventions, and research needs. Current HIV/AIDS Reports, 13(6), 340–348. 10.1007/s11904-016-0334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraux E, Reychler G, Forget P, Yombi J-C, & Caty G (2019). Feasibility and preliminary effects of a telerehabilitation program for people living with HIV: A pilot randomized study. The Journal of the Association of Nurses in AIDS Care: JANAC, 30(2), 176–185. 10.1097/JNC.0000000000000005 [DOI] [PubMed] [Google Scholar]
- Rawstorn JC, Gant N, Direito A, Beckmann C, & Maddison R (2016). Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart, 102(15), 1183–1192. 10.1136/heartjnl-2015-308966 [DOI] [PubMed] [Google Scholar]
- Rikli RE, & Jones CJ (1997). Development and validation of a functional fitness test for community-residing older adults. Journal of Aging and Physical Activity, 7, 129–161. 10.1123/japa.7.2.129 [DOI] [Google Scholar]
- Rikli RE, & Jones CJ (2013). Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. The Gerontologist, 53(2), 255–267. 10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- Romero-Arenas S, Martinez-Pascual M, & Alcaraz PE (2013). Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging and Disease, 4(5), 256–263. 10.14336/AD.2013.0400256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, … Gange SJ (2013). Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 8(12), e81355. 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, & Semba RD (2005). Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. Journal of the American Geriatrics Society, 53(5), 747–754. 10.1111/j.1532-5415.2005.53250.x [DOI] [PubMed] [Google Scholar]
- Schrack JA, Jacobson LP, Althoff KN, Erlandson KM, Jamieson BD, Koletar SL, Phair J, Brown TT, & Margolick JB (2016). Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS, 30(17), 2645–2652. 10.1097/QAD.0000000000001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, & Okada A (2004). Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. European Journal of Applied Physiology, 93(1–2), 173–182. 10.1007/s00421-004-1193-3 [DOI] [PubMed] [Google Scholar]
- Tate JP, Sterne JAC, & Justice AC (2019). Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS, 33(5), 903–912. 10.1097/QAD.0000000000002140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, Boesecke C, Patterson S, Grabar S, Cazanave C, Cavassini M, Shepherd L, d’Arminio Monforte A, van Sighem A, Saag M, Lampe F, Hernando V, Montero M, Zangerle R, … Sterne JAC (2017). Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. The Lancet HIV, 4(8),e349–e356. 10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Mugisha J, Rosenbaum S, Firth J, De Hert M, Probst M, & Stubbs B (2016). Cardiorespiratory fitness levels and moderators in people with HIV: A systematic review and meta-analysis. Preventive Medicine, 93, 106–114. 10.1016/j.ypmed.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, & Fried LP (2006). Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. Journal of the American Geriatrics Society, 54(6), 991–1001. 10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- Wennergren J, Munshi I, Fajardo AD, & George VV (2014). Implementation of clinical video telemedicine (CVT) within a VA medical center is cost effective and well received by Veterans. International Journal of Clinical Medicine, 5, 711–716. 10.4236/ijcm.2014.512097 [DOI] [Google Scholar]
- Whitehurst MA, Johnson BL, Parker CM, Brown LE, & Ford AM (2005). The benefits of a functional exercise circuit for older adults. Journal of Strength and Conditioning Research, 19(3), 647–651. [DOI] [PubMed] [Google Scholar]
- Yahiaoui A, McGough EL, & Voss JG (2012). Development of evidence-based exercise recommendations for older HIV-infected patients. The Journal of the Association of Nurses in AIDS Care, 23(3), 204–219. 10.1016/j.jana.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Scherzer R, Kotler DP, Dobs AS, Tien PC, Lewis CE, Kronmal RA, Heymsfield SB, Bacchetti P, & Grunfeld C (2011). Age-related skeletal muscle decline is similar in HIV-infected and uninfected individuals. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 66A(3), 332–340. 10.1093/gerona/glq228 [DOI] [PMC free article] [PubMed] [Google Scholar]


