Abstract
In this study, we aimed at the green synthesis of silver nanoparticles (AgNPs) using Lavandula angustifolia extract and the investigation of the anti-proliferative and apoptotic inducing effects of these nanoparticles in the U87MG glioblastoma cancer cell line. Green synthesized silver nanoparticles were characterized by various analytical techniques such as UV–Visible Spectrophotometer (UV–Vis), scanning electron microscopy (SEM) and Energy Dispersive X-ray (EDX). UV–Vis spectroscopy displayed a specific silver plasmon peak at 430 nm. U87MG cells were treated at increased concentrations with Lavandula angustifolia-AgNPs (La-AgNPs) (0–20 µg/mL) for 72 h and the anti-proliferative effects of green synthesized silver nanoparticles on U87MG cells were evaluated by MTT assay. The La- AgNPs induced a statistically significant dose-dependent decrease in proliferation and increased cytotoxicity in U87MG cells. The IC50 value is 7.536 µg/mL. Furthermore, the expression of apoptosis proteins caspase-3, caspase-8 and caspase-9 was analyzed using ELISA and caspase-3 and p53 using western blotting. The results suggest that La-AgNPs induce cell death in U87MG cells through the p53 mediated intrinsic apoptotic pathway. Together, the present findings suggest that La-AgNPs could be considered as a potential option for the treatment of glioblastoma.
Keywords: Silver nanoparticles, AgNPs, Glioblastoma, U87MG
Introduction
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor accouting for 3–4% of all cancer-related deaths in adults (Batash et al. 2017). GBM, also known as grade IV astrocytoma as classified by the World Health Organization (WHO), is known as the most aggressive and fatal tumors among the brain tumors in adults (Louis et al. 2007). It constitutes 22.6–27% of primary brain tumors, 50–60% of all gliomas, 12–15% of all intracranial tumors, and 50–60% of astrocytic tumors (DeAngelis 2001; Ironside et al. 2002; Levin et al. 2001; Sathornsumetee and Rich 2008). Although GBMs can occur at any age, most of the cases observed are between 45 and 75 years old and rare in childhood (Iacob and Dinca 2009; Newton 2010). The incidence of GBM is higher in men than in women (Ohgaki et al. 2004).
GBM standard care, also known as the Stupp protocol for patients newly diagnosed with glioblastoma, includes the oral consumption of temozolomide (TMZ) administered concurrently with radiotherapy following maximum safe surgical resection (Stupp et al. 2005). Despite the availability of treatments in clinical pratice, such as surgical interventions, radiotherapy, and chemotherapy applications, brain tumors remain an important health problem that needs to be overcome due to their rapid development and poor prognosis. Median survival after diagnosis is generally less than 1 year, and even under the best of circumstances, the majority of patients die within 2 years of diagnosis. However, 5–10% of patients live up to 2 years (Buckner 2003). Due to the limited and less effective treatment methods available for GBM, the development of new techniques and improvement of existing technologies is imperative. In this regard, nanotechnology has shown promise in the treatment of GBM.
Nanotechnology is a technology that covers the production, characterization, and use of nanoparticles ranging from 1 to 100 nm in size. Many technological advances have made it possible to design and characterize nanoparticles using a wide variety of organic and inorganic materials to achieve desired properties (Albrecht et al. 2006). In this context, one of the promising agents in cancer treatment is silver nanoparticles (AgNP). AgNP, which has become popular in recent years, have a wide range of uses, especially in the field of healthcare, due to their physical and chemical properties. These biomedical uses include antimicrobial, antioxidant, anti-diabetic, anti-inflammatory, anticoagulant, anticancer, and thrombolytic applications among others (Badmus et al. 2020; Adebayo et al. 2019; Lateef et al. 2018; Aina et al. 2019; Azeez et al. 2020). Silver nanoparticles can be synthesized using physical, chemical and biological methods. In recent years, green synthesis (biosynthesis) is more preferred than other methods for reasons such as being environmentally friendly, cost-effective, and can be easily synthesized in large quantities (Thakkar et al. 2010). For the biosynthesis of nanoparticles, naturally available sources of plant extracts, bacteria, fungi, algae, yeast, viruses, agrowastes, animal wastes, and metabolites of arthropods can be used (Ahmed et al. 2016; Donmez 2020; Daphne et al. 2018; Raveendran et al. 2003; Adelere and Lateef 2016; Akintayo et al. 2020; Lateef et al. 2016).
Formerly known as Lavandula officinalis, Lavandula angustifolia or Lavender is a genus of the Lamiaceae family and is widely distributed throughout the world. L. angustifolia grows naturally in Turkey. Lavender is a perennial plant with semi-shrubby, lilac or gray-blue flowers, naturally spreading and cultivated in the middle elevations (600–1500 m) of the mountainous regions of the Northern Mediterranean from Spain to Greece, and they can grow 20–60 cm. Fresh flowering branch tips of the plant are used for medicinal purposes, and partially dried flowers and leaves are used in the perfumery and cosmetic industries (Baytop 1999; Ceylan et al. 1988). The Lavender is a major source of polyphenols and pharmacological properties described in the literature. Lavender is comprised of over 100 constituents, including 1,8-cineole, camphor, endo-borneol, α-pinene, camphene, β-pinene, p-cymene, limonene, terpinen-4-ol, cryptone, and flavonoids (Rychlik 2008; Shimizu et al. 1990; Brown 1962). Lavender has been used in traditional medicine for many years, and studies have shown its analgesic (Kane et al. 2004), antioxidant (Hohmann et al. 1999), anticancer (Elegbede et al. 1984), antianxiety (Burnett et al. 2004), antifungal (D'Auria et al. 2005), antibacterial (Gabbrielli et al. 1988) and sedative effects (Goel et al. 2005).
This work is based on the green synthesis approach for the synthesis of silver nanoparticles using lavender extract, which is rich in phytochemicals. These phytochemicals function as reducing agents and stabilizing agents for the bioreduction of ionic silver into nanoparticles. The silver nanoparticles synthesized using lavender extract were characterized by UV–Vis spectrophotometry and scanning electron microscopy (SEM), and their anticancer effects were evaluated using the U87MG glioblastoma cell line. In addition, the mechanism of cell death in cancer cells after treatment with silver nanoparticles, modulation of p53, Caspase-3, -8, and -9 protein expression levels were studied using Western blot and ELISA methods, respectively.
Materials and methods
Chemicals and reagents
The chemical agents silver nitrate (AgNO3) (Sigma Aldrich, St. Louis, Missouri, USA), high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) with L-glutamine, sodium pyruvate and sodium bicarbonate (Biowest, France), penicillin–streptomycin solution (Biowest, France), trypsin- EDTA solution (Biowest, France) and fetal bovine serum (Biowest, France) were used in the synthesis of silver nanoparticles and cell culture. For the evaluation of cell viability, MTT[3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay kit and dimethyl sulfoxide, purchased from Sigma Aldrich (St. Louis, Missouri, USA) were used. Human Caspase-3 (Active) ELISA kit (cat. KHO1091), human caspase-8 flice ELISA kit (cat. BMS2024), human caspase 9 ELISA kit (cat BMS 2025) purchased from ThermoFisher Scientific (Waltham, Massachusetts, USA) were used to evaluate human caspases protein levels. p53 antibody (cat. sc-6243) (Santa Cruz Biotechnology, Dallas, Texas, USA), caspase-3 antibody (cat. bs-2593R) (Bioss Antibodies, Woburn, MA, USA), beta-Actin antibody (cat. A1978) (Sigma Aldrich, St. Louis, Missouri, USA), anti-rabbit secondary antibody (cat. sc2357) (Santa Cruz Biotechnology, Dallas, Texas, USA), anti-mouse secondary antibody (cat. SA00001-1) (Proteintech Group, Inc., Rosemont, USA) were used to determine the p53 and caspase-3 protein level by Western blotting.
Preparation of Lavandula angustifolia aqueous extract
The dried lavender (Lavandula angustifolia) (1 g) was extracted in 100 ml distilled water. The mixture was boiled in a microwave oven for one minute (1200 W, 50 Hz), left to cool, and filtered through Whatman filter paper. The lavender extract obtained was then stored at 4 °C in airtight containers.
Green synthesis and characterization of silver nanoparticles (AgNPs)
In brief, 5 mL of 10 mg/mL aqueous lavender extract was mixed with an aqueous solution of 5 mM AgNO3 solution. The mixture was boiled in a microwave oven for one minute (1200 W, 50 Hz). To study the formation of silver nanoparticles, the UV–Vis spectra of the synthesized silver nanoparticles were monitored using the Shimadzu UV-1801 UV–Vis spectrophotometer. The spectrum of the AgNP solution was recorded in the 300–700 nm wavelength range. Scanning electron microscopy (SEM) was used to determine the morphology of AgNPs. Elemental analysis of AgNPs was confirmed by Energy Dispersive X-ray (EDX).
Cell culture
U87MG human brain tumor cells were obtained from the American Type Culture Collection (ATTC). U87MG human brain tumor cell lines were grown adherently and maintained in DMEM containing L-glutamine, non-essential amino acids, sodium pyruvate, 10% Fetal Bovine serum and 1% Penicillin- Streptomycin as monolayer cultures at 37 °C in 5% CO2 atmosphere.
MTT assay
MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to determine the cytotoxic effects of the AgNPs at various concentrations on cell viability in U87MG cells. The cultured U87MG cells were trypsinized and seeded on a 96 well plate with a seeding density of 5000 cells per well. The plate was then left to incubate for 24 h at 37 °C for attachment of cell lines to the plate. Then, 100 μl of the media having the AgNPs at a concentration of 0–20 μg/mL were added to the wells and incubated. U87MG cells not treated with AgNPs were used as a control group. After 72 h of incubation, the viability of the cells was tested using the MTT assay following the standard protocol. Briefly, 40 μL of MTT (5 mg/mL) was added to each well, and the plate was incubated for a further 4 h at 37 °C. The resulting formazan crystals was dissolved in 100 μL of dimethyl sulfoxide with gentle shaking at 37 °C for 5–10 min. Then, absorbance was measured at 595 nm with an ELISA reader. The results were given as the means of three independent experiments. The cell viability was calculated using the following equation:
Caspase activation assay
The effects of AgNPs on caspase-3, caspase-8, and caspase-9 at the U87MG cell line were measured using human active caspase-3, caspase-8, and caspase-9 ELISA kits against untreated control cells (negative control), according to the manufacturer’s instructions. U87MG cells were cultured onto 96-well plates to obtain a density of 1.2–1.8 × 10,000 cells per well in a 100 µL volume of complete growth medium (DMEM containing 10% FBS). The cells were treated with two different concentrations (5 and 7.5 µg/mL) in 100 μL sample volume per well and incubated for 72 h before the assay. Cells were lysed with the cell extraction buffer and the lysates were diluted by the standard dilution buffer in the range required for the assay, and assessed using human caspase ELISA kits. Optical densities were measured at 450 nm using a Multiskan GO (ThermoFisher Sci., Waltham, Massachusetts, USA) spectrophotometer. The assay was performed in triplicate and the data are expressed as mean ± SD.
Western blot analysis
U87MG cells were treated with AgNPs for 72 h to determine the p53 and caspase-3 proteins. The cells were harvested and lysed, and the total protein concentration was measured using the Bradford protein assay with bovine serum albumin as the standard. Untreated and treated cells with AgNPs were lysed in Triton X-100 buffer containing 50 mM HEPES, pH 7.0, 150 mM NaCl, 10% glycerol, 1.2% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 100 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, 0.15 units/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A. Equal amounts of protein (50 μg) were separated by SDS-PAGE and then transferred to a PVDF membrane. The membranes were blocked in 1% BSA in PBST. The membranes were subsequently incubated with p53 or caspase-3 primary antibodies at 4 °C overnight. After incubation, all membranes were washed with 0.05% Tween 20 Tris-buffered saline (TBST) for 15 min 3 times and then treated with a secondary antibody for one hour at room temperature. After washing the membranes again with TBST three times, immunoreactivity was detected using a WesternBright Sirius kit (cat. K-12043-D20) (Advansta Inc., San Jose, CA, USA). The β-actin protein was used as a loading control. Signal intensity on blots was determined using the Amersham Hyperfilm ECL (GE Healthcare, Chicago, Illinois, USA).
Statistical analysis
Statistical assessment of the data was performed using one-way ANOVA and student’s t test was applied for comparisons as a post-test with the help Graphpad Prism 8 software (GraphPad Software Inc., San Diego, USA), and the results are shown as mean ± SD, and p < 0.05 was calculated as the minimum level of significance.
Results
Green synthesis of AgNPs using L. angustifolia extract
In this study, we used the extract of L. angustifolia for synthesis of AgNPs (La-AgNP). In Fig. 1, silver nitrate, Lavender extract and the extract after reaction with silver ions for 1 h are shown in the tubes. Green synthesis of AgNPs was first confirmed by observing the transformation of the color of the solution from transparent yellow to reddish brown due to the reduction of silver ions and their accumulation as AgNPs (Fig. 1).
Fig. 1.

Schematic representation of the green synthesis of AgNPs using L. angustifolia extract
Characterization of La-AgNPs
The absorption spectrum of the prepared La-AgNPs in the 300–700 nm range was evaluated using UV–Vis (UV–Vis) spectrophotometry. The absorption peak was observed at 430 nm for AgNPs synthesized using lavender extract confirmed their synthesis (Fig. 2A). Scanning electron microscopy (SEM) was used to determine the morphology of AgNPs (Fig. 2B). SEM showed that the AgNPs were smaller than 100 nm and had a spherical morphology. Elemental analysis of La-AgNPs was confirmed by Energy Dispersive X-ray (EDX) as shown in Fig. 2C. EDX revealed an energy line approximately at 3 keV indicating that silver is the basic constituent element. Additionally, the EDX spectrum obtained from the La-AgNPs showed characteristic energy lines corresponding to C (0.2 keV), O (0.5 keV) and N (0.3 keV) arising from the plant extract.
Fig. 2.
Characterization of La-AgNPs. A UV spectrum, B SEM image and C EDX analysis of La-AgNPs
Anti-proliferative activity of La-AgNPs on U87MG cells
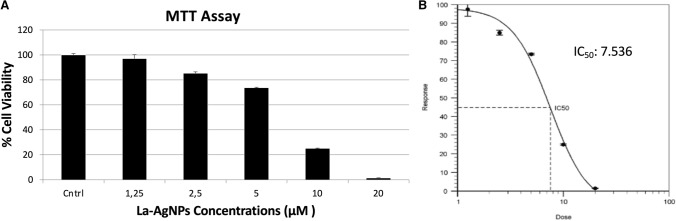
The U87MG cells were treated with 0–20 μg/ml of synthesized silver nanoparticles for 72 h. MTT assay results clearly demonstrated that the growth and proliferation of U87MG cells were significantly inhibited in a dose-dependent manner under La-AgNPs exposure. The results showed that La-AgNPs had the highest inhibitory effect at 20 μg/ml, which was statistically significant compared to the control group (P < 0.001). The results of the MTT assay and induction of cytotoxicity are presented in Fig. 3A. The IC50 value calculated in AAT Bioquest software was 7.536 μg/mL (Fig. 3B).
Fig. 3.
Viability of U87MG cells were determined 72 h after exposure to different concentrations of La-AgNPs with MTT assay. A the results are expressed as the mean ± standard deviation of three independent experiments. B the IC50 value calculated using AAT bioquest software on the basis of MTT assay
The effects of La-AgNPs on U87MG cell apoptosis
The p53 tumor supressor acts as a integrative multiple stress signal within cells. Due to the its ability to activate apoptosis through the intrinsic and the extrinsic apoptotic pathways, we focused on the effect of La-AgNPs on the level of p53 protein in U87MG cells. Our results showed that the level of p53 increased 1.31-fold at 5 uM (p value 0.0018); and 1.62-fold increase at 7.5 uM concentration (p value 0.00076) compared to untreated cells (Fig. 4A, B). These findings indicate that La-AgNPs could induce apoptosis in cancer cells in p53-dependent manner. Since caspase-3 is considered the executioner caspase activation is the optimum goal for any apoptotic pathway, the level of caspase-3 protease was estimated in U87MG La-AgNPs treated and untreated cells. Likewise, when the effect of La-AgNPs on level of caspase-3 expression was evaluated, 4.88-fold increase at 5uM (p value 0.00068); 4.83-fold increase was determined at 7.5uM (p value 0.00051) (Fig. 4A, C). The La-AgNPs at 5 and 7.5 µg/mL concentrations showed signifcant (p < 0.01) expression of all two proteins tested.
Fig. 4.
The La-AgNPs-induced changes in protein levels of active caspase-3 and p53 apoptotic markers in control and treated U87MG cells. A western blot analysis of the levels of caspase-3 and p53 in U87MG cells were treated with 5 and 7.5 μM La-AgNPs for 72 h. Quantitative analysis of p53 (B) and caspase-3 (C) normalized to beta-actin band intensity calculated by ImageJ software. The bars indicate the SD, n = 3, *p < 0.05 compared to control
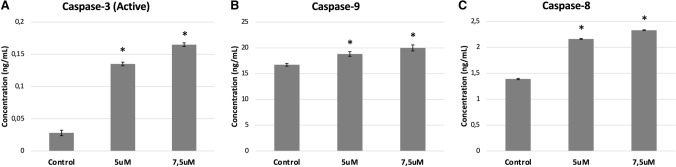
Caspases are a family of conserved cysteine proteases that play an essential role in apoptosis. After treatment with La-AgNPs, expression levels of the caspase-3, -8 and -9 proteins in U87MG cells was evaluated by ELISA to understand the apoptotic mechanism involved in AgNPs treated cancer cells. The levels of caspase expression were compared with the untreated cells arbitrarily set to 1.0. It is known that caspase-8 is associated with the extrinsic apoptotic pathway and caspase-9 is associated with the intrinsic apoptotic pathway. Caspase-3 is a common caspase for both pathways and is indicative of apoptosis. Results showed that the expression of caspase-3 increased by 4.84- and 5.89-fold, respectively, in the 5uM and 7.5uM La-AgNPs treatment groups, compared to the control group. At the same time, the expression of cleaved caspase-9 increased by 1.12 and 1.19-fold whereas the expression of cleaved caspase-8 increased by 1.55 and 1.68-fold in both the treatment groups (5uM and 7.5uM La-AgNP) (Fig. 5).
Fig. 5.
The La-AgNP-associated expression levels of caspase-3 (A), -9 (B), and -8 (C). U87MG cells were treated with 5 and 7.5 μM La-AgNPs for 72 h. The caspase-3, -8 and -9 levels (ng/mL) of cell lysates were determined by ELISA analysis. The bars indicate the SD, n = 3, *p < 0.05 compared to control
Discussion
In this study, aqueous lavender extract was used for the green synthesis of silver nanoparticles. The biomolecules contained in this extract are responsible for the reduction of Ag + ions to Ag0 in a single step. A color change from transparent yellow to reddish brown was observed during the monitoring of the reaction after the addition of lavender extract to the silver nitrate solution and the application of heat. This color change is due to the surface plasmon resonance (SPR), an optical property which is unique to the noble metals, caused by AgNPs (Ghaffari-Moghaddam et al. 2014; Zhang et al. 2016). The presence of alkaloids, phenols, flavonoids, tannins, terpenes, glycosides, and steroids in L. angustifolia extract has been previously reported (Godwin and Egwaikhide, 2020). It has been shown that some of these phytochemicals present in L. angustifolia extract lead to the formation of silver nanoparticles by causing excitation of the SPR of the particles (Marslin et al. 2018; Dada et al. 2019). Thus, the appearance of reddish brown color in the reaction mixture indicated the formation of AgNPs. The result is in line with previously reported studies (Kalimuthu et al. 2008; Rautela et al. 2019).
In this study, AgNPs were successfully synthesized using the aqueous extract of L. angustifolia as a reducing agent. L. angustifolia is a very inexpensive and readily available plant, and no additional substance was needed for synthesis. As recently reported in the literature, many plant extracts have been used in the synthesis of AgNPs, but so far, L. angustifolia extract has not been used for the green synthesis of AgNPs.
UV-visible spectroscopy is an important and valuable technique for the characterization of AgNPs (Sastry et al. 1997). A single absorption peak was observed at 430 nm in the UV-Vis spectrum, indicating that the biosynthesized AgNPs were pure (Fig. 2). It is well known that a strong yet large surface plasmon peak is observed in the presence of various metal nanoparticles of between 2 and 100 nm in size (Sastry et al. 1997, 1998). SEM images of the La-AgNPs showed mostly spherical particles of a size below 100 nm as shown in Fig. 2B. The image was blurry as our current SEM could not take images of particles below 100 nm. The elemental mapping results showed the presence of the silver element, suggested that silver was the dominant element in the respective product (Fig. 2C). The EDX spectrum obtained from the La-AgNPs shows characteristic energy lines corresponding to C (0.2 keV), O (0.5 keV) and N (0.3 keV) arising from the plant extract, while distinctive energy line at 3 keV corresponding to Ag, confirming the successful synthesis of Ag nanoparticles. (Salehi et al. 2016; Gowramma et al. 2015; Badmus et al. 2020; Khan et al. 2014; Nadagouda et al. 2011; Karim et al. 2018).
In this study, the in vitro cytotoxic effect of La-AgNPs prepared by biosynthesis was evaluated with the MTT assay. The results showed a significant decrease in the viability of U87MG cells at 72 h due to the increasing dose of biosynthesized La-AgNPs. It was noted that La-AgNPs caused a 100% reduction in cell viability at a high concentration of 20 µg/mL. Based on the MTT results, the IC50 value (half of the concentration that provides inhibition) of biosynthesized La-AgNPs was calculated as 7.536 µg/mL. The results showed that the proliferation rate decreased with increasing doses of AgNPs. Many studies have evaluated the cytotoxic effects of green synthesized AgNPs on various types of cancer cells. Similarly, AgNPs have exhibited anti-proliferative effects in a variety of cancer cells, including lung cancer cells (Han et al. 2014), breast cancer cells (Azizi et al. 2017), colon cancer cells (Dehghanizade et al. 2018), cervical cancer cells (Murugesan et al. 2019) and neuroblastoma cells (Yuan et al. 2017). The number of studies on the effects of green synthesized AgNPs on glioblastoma is very limited. Eugenio et al. (2018) evaluated the effects of Ag/AgCl-NP, which they synthesized biogenically using yeast culture, on GBM02 glioblastoma and human astraocytes alone and combined with TMZ. Eugenio et al. reported that at high concentrations, Ag/AgCl-NPs inhibited GBM02 proliferation more effectively than TMZ (up to 82 and 62% inhibition, respectively), while the opposite occurred at low concentrations (up to 23 and 53% inhibition, respectively). The combined treatment (Ag/AgCl-NPs and TMZ) inhibited GBM02 proliferation by 54–83%. Liang et al. (2017) synthesized AgNPs with a sodium citrate system and investigated the effects of their combined use with TMZ on human glioma U251 cells. In this study, it was reported that AgNPs created cytotoxicity in U251 cells, depending on the dose, and they increased the sensitivity of the TMZ drugs. In another study in which colloidal AgNPs were used, the effects of AgNPs were evaluated on GBM cells cultured in an in ovo model. The study showed that AgNPs inhibited proliferation in GBM cells and exhibited proapoptotic properties with an increase in caspase -3 and caspase-9 protein levels (Urbańska et al. 2015).
Previous studies concluded that AgNPs can enter cells in different ways, such as by phagocytosis, pinocytosis, or endocytosis, and interact with genetic material. It has also been shown that AgNPs can trigger the production of toxic oxygen radicals, which can lead to DNA fragmentation and apoptosis induction (Chan et al. 2015; Wang et al. 2013). p53 and caspase proteins are involved in the sequence of events in the execution of apoptosis. The apoptotic activities of p53 could occur through transcription-dependent and -independent mechanisms. p53 leads the way of cytochrome c release from mitochondria and activation of capsases and also transactivates the Apaf-1 gene, which is a coactivator of caspase-9 (Fridman et al. 2003). Activated caspase-9 could further progress caspase-8 activation. Caspase-8 is the well-known upstream caspase of extrinsic death receptor signals and is also involved in detachment-induced apoptosis (anoikis). Like other caspases, inactive caspase-8 resides in the cytosol until is activated by proteolytic cleavage process. Followed by the activation of caspase-8, like caspase-9 could cleaves to and activates caspase-3 (Kruidering and Evan 2000). Caspase-3 is one of the best understood crucial mediators of apoptosis. Caspase-3 catalyzes the autoproteolytic cleavage of cellular proteins and could activate death proteases for destruction of cellular structures such as DNA and cytoskeletal proteins (Porter and Jänicke 1999).
The apoptotic caspases are subdivided into initiator and executioner caspases. Together, they coordinate the apoptotic cell death program. One of the initiator caspases is caspase-8 which contains two death effector domains (DEDs). These domains are necessary to the death-inducing signaling complex (DISC) and its activation. In this way, activation of caspase-8 can cleave its well-known apoptosis substrates: caspase-3 and Bid. The enzymatic cleaving of Bid (Asp60) into tBid (truncated form) leads the conformational changes of protein via the exposition of hydrophobic residues. Thus, tBid can insert itself into the mitochondrial outer membrane and binding to Bcl-2 family members by its BH3 domain. Following tBid’s translocation and accumulation, it can induce mitochondrial outer membrane permeabilization (MOMP), which leads to the release of cytochrome c from the mitochondria to the cytosol. Bid activation and MOMP induction lead to mitochondrial apoptotic pathway following death receptor induction. By this means, caspase-8-cleaved tBid links up death receptor stimulation and mitochondria-dependent apoptosis (Kantari and Walczak 2011).
Accordingly, in our study, p53 along with and caspase-3, -8 and -9 proteins were examined to explain the apoptotic mechanism that AgNPs use to exert anticancer effects. The expression levels of caspase-3, -8 and -9, and p53 proteins were increased in La-AgNPs treated with U87MG cells compared with untreated cells. These findings demonstrate that La-AgNPs inhibit cell proliferation by inducing the p53-mediated intrinsic apoptotic pathway.
Conclusion
Our study has shown that La-AgNPs have a cytotoxicity against cancer cells at low doses. These results implicated the potential use of La-AgNPs as a therapeutic agent for GMB therapy. In addition, the use of lavender extract in the synthesis of silver nanoparticles can be used as a suitable methodology because it can provide large amounts of nanoparticle synthesis in a short time, environmentally friendly, cheap compared to traditional methods.
Acknowledgements
This research was supported by the Burdur Mehmet Akif Ersoy University Scientific Research Rrojects Unit [Project number: 0617-YL-19].
Author contributions
AS and CAA designed the study, performed the synthesis and characterization of nanoparticles, cell culture and MTT assay, drafted the paper, and contributed equally to this work. SP participated in ELISA and Western blot analyses. AS and SP involved in statistical analysis. All authors have read and approved the final manuscript.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Adebayo EA, Ibikunle JB, Oke AM, Lateef A, Azeez MA, Oluwatoyin AO, AyanfeOluwa AV, Blessing OT, Comfort OO, Adekunle OO, Badmus JA, Asafa TB, Beukes LS, Gueguim-Kan EB, Hakeem AS. Antimicrobial and antioxidant activity of silver, gold and silver-gold alloy nanoparticles phytosynthesized using extract of Opuntia ficus-indica. Rev Adv Mater Sci. 2019;58(1):313–326. doi: 10.1515/rams-2019-0039. [DOI] [Google Scholar]
- Adelere IA, Lateef A. A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes and pigments. Nanotechnol Rev. 2016;5(6):567–587. doi: 10.1515/ntrev-2016-0024. [DOI] [Google Scholar]
- Ahmed S, Annu S, Ikram S, Yudha S. Biosynthesis of gold nanoparticles: a green approach. J Photochem Photobiol B Biol. 2016;161:141–153. doi: 10.1016/j.jphotobiol.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Aina DA, Owolo O, Lateef A, Aina FO, Hakeem AS, Adeoye-Isijola M, Okon V, Asafa TB, Elegbede JA, Olukanni OD, Adediji I. Biomedical applications of Chasmanthera dependens stem extract mediated silver nanoparticles as antimicrobial, antioxidant, anticoagulant, thrombolytic, and larvicidal agents. Karbala Int J Mod Sci. 2019;5(2):71–80. doi: 10.33640/2405-609X.1018. [DOI] [Google Scholar]
- Akintayo GO, Lateef A, Azeez MA, Asafa TB, Oladipo IC, Badmus JA, Ojo SA, Elegbede JA, Gueguim-Kana EB, Beukes LS. Synthesis, bioactivities and cytogenotoxicity of animal fur-mediated silver nanoparticles. IOP Conf Ser: Mat Sci Eng. 2020;805:012041. doi: 10.1088/1757-899X/805/1/012041. [DOI] [Google Scholar]
- Albrecht MA, Evan CW, Raston CR. Green chemistry and the health implications of nanoparticles. J Green Chem. 2006;8:417–432. doi: 10.1039/B517131H. [DOI] [Google Scholar]
- Azeez MA, Durodola FA, Lateef A, Yekeen TA, Adubi AO, Oladipo IC, Adebayo EA, Badmus JA, Abawulem AO. Green synthesized novel silver nanoparticles and their application as anticoagulant and thrombolytic agents: a perspective. IOP Conf Ser: Mat Sci Eng. 2020;805:012043. doi: 10.1088/1757-899X/805/1/012043. [DOI] [Google Scholar]
- Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci Rep. 2017;7:5178. doi: 10.1038/s41598-017-05461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badmus JA, Oyemomi SA, Adedosu OT, Yekeen TA, Azeez MA, Adebayo EA, Lateef A, Badeggi UM, Botha S, Hussein AA, Marnewick JL. Photo-assisted bio-fabrication of silver nanoparticles using Annona muricata leaf extract: exploring the antioxidant, anti-diabetic, antimicrobial, and cytotoxic activities. Heliyon. 2020;6(11):e05413. doi: 10.1016/j.heliyon.2020.e05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment recent literature review. Curr Med Chem. 2017;24(27):3002–3009. doi: 10.2174/0929867324666170516123206. [DOI] [PubMed] [Google Scholar]
- Baytop T. Therapy with plants in Turkey (Past and Present) (in Turkish) Istanbul: Nobel Tip Press; 1999. [Google Scholar]
- Brown SA. Biosynthesis of coumarin and herniarin in lavender. Science. 1962;137:977–978. doi: 10.1126/science.137.3534.977. [DOI] [PubMed] [Google Scholar]
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(6 Suppl 19):10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Burnett KM, Solterbeck LA, Strapp CM. Scent and mood state following an anxiety-provoking task. Psychol Rep. 2004;95(2):707–722. doi: 10.2466/pr0.95.2.707-722. [DOI] [PubMed] [Google Scholar]
- Ceylan AA, Vomel N, Kaya N, Celik E, Nigdeli E. A investigation on effects to yield and qulity of plant space in lavender (in Turkish) Ege Univ J Agric Fac. 1988;25(2):135–145. [Google Scholar]
- Chan EL, Zhang C, Cheung GS. Cytotoxicity of a novel nano-silver particle endodontic irrigant. Cosmet Investig Dent. 2015;7:65–74. doi: 10.2147/CCIDE.S68874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada AO, Adekola FA, Dada FE, Adelani-Akande AT, Bello MO, Okonkwo CR, Inyinbor AA, Oluyori AP, Olayanju A, Ajanaku KO, Adetunji CO. Silver nanoparticle synthesis by Acalypha wilkesiana extract: phytochemical screening, characterization, influence of operational parameters, and preliminary antibacterial testing. Heliyon. 2019;5(10):e02517. doi: 10.1016/j.heliyon.2019.e02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daphne J, Francis A, Mohanty R, Ojha N, Das N. Green synthesis of antibacterial silver nanoparticles using yeast isolates and its characterization. J Phar Tech Res. 2018;11(1):83–92. doi: 10.5958/0974-360X.2018.00016.1. [DOI] [Google Scholar]
- D'Auria FD, Tecca M, Strippoli V, Salvatore G, Battinelli L, Mazzanti G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med Mycol. 2005;43(5):391–396. doi: 10.1080/13693780400004810. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol. 2018;46(1):160–168. doi: 10.1080/21691401.2017.1304402. [DOI] [PubMed] [Google Scholar]
- Donmez S. Green synthesis of zinc oxide nanoparticles using Zingiber officinale root extract and their applications in glucose biosensor. El-Cezerî J Sci Eng. 2020;7(3):1191–1200. doi: 10.31202/ecjse.729462. [DOI] [Google Scholar]
- Elegbede JA, Elson CE, Qureshi A, Tanner MA, Gould MN. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis. 1984;5(5):661–664. doi: 10.1093/carcin/5.5.661. [DOI] [PubMed] [Google Scholar]
- Eugenio M, Campanati L, Müller N, Romão LF, de Souza J, Alves-Leon S, de Souza W, Sant'Anna C. Silver/silver chloride nanoparticles inhibit the proliferation of human glioblastoma cells. Cytotechnology. 2018;70(6):1607–1618. doi: 10.1007/s10616-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22(56):9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Gabbrielli G, Loggini F, Cioni PL, Giannaccini B, Mancuso E. Activity of lavandino essential oil against non-tubercular opportunistic rapid grown mycobacteria. Pharmacol Res Commun. 1988;20(Suppl 5):37–40. doi: 10.1016/s0031-6989(88)80836-1. [DOI] [PubMed] [Google Scholar]
- Ghaffari-Moghaddam M, Hadi-Dabanlou R, Khajeh M, Rakhshanipour M, Shameli K. Green synthesis of silver nanoparticles using plant extracts. Korean J Chem Eng. 2014;31:548–557. doi: 10.1007/s11814-014-0014-6. [DOI] [Google Scholar]
- Godwin OE, Egwaikhide PA. Phytochemical and antimicrobial properties of Lavandula angustifolia. Int J Modern Chem. 2020;12(1):1–12. [Google Scholar]
- Goel N, Kim H, Lao RP. An olfactory stimulus modifies nighttime sleep in young men and women. Chronobiol Int. 2005;22(5):889–904. doi: 10.1080/07420520500263276. [DOI] [PubMed] [Google Scholar]
- Gowramma B, Keerthi U, Rafi M, Muralidhara Rao D. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech. 2015;5(2):195–201. doi: 10.1007/s13205-014-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, Kim JH. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett. 2014;9:459. doi: 10.1186/1556-276x-9-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann J, Zupkó I, Rédei D, Csányi M, Falkay G, Máthé I, Janicsák G. Protective effects of the aerial parts of Salvia officinalis, Melissa officinalis and Lavandula angustifolia and their constituents against enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med. 1999;65(6):576–578. doi: 10.1055/s-2006-960830. [DOI] [PubMed] [Google Scholar]
- Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2(4):386–393. [PMC free article] [PubMed] [Google Scholar]
- Ironside CV, Moss TH, Louis DN. Astrocytic tumours. In diagnostic pathology of nervous system tumours. 1. London: Churchill-Livingstone; 2002. [Google Scholar]
- Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65(1):150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kane FM, Brodie EE, Coull A, Coyne L, Howd A, Milne A, Niven CC, Robbins R. The analgesic effect of odour and music upon dressing change. Br J Nurs. 2004;13(19):4–12. doi: 10.12968/bjon.2004.13.Sup4.16343. [DOI] [PubMed] [Google Scholar]
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochem Biophys Acta. 2011;1813(2011):558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Karim MN, Anderson SR, Singh S, Ramanathan R, Bansal V. Nanostructured silver fabric as a free-standing nanozyme for colorimetric detection of glucose in urine. Biosens Bioelectron. 2018;110:8–15. doi: 10.1016/j.bios.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Khan M, Khan ST, Khan M, Adil SF, Musarrat J, Al-Khedhairy AA, Al-Warthan A, Siddiqui MR, Alkhathlan HZ. Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant. Int J Nanomedicine. 2014;9:3551–3565. doi: 10.2147/IJN.S61983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50(2):85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- Lateef A, Ojo SA, Elegbede JA. The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol Rev. 2016;5(6):601–622. doi: 10.1515/ntrev-2016-0049. [DOI] [Google Scholar]
- Lateef A, Folarin BI, Oladejo SM, Akinola PO, Beukes LS, Gueguim-Kana EB. Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep Biochem Biotechnol. 2018;48(7):646–652. doi: 10.1080/10826068.2018.1479864. [DOI] [PubMed] [Google Scholar]
- Levin VA, Leibel SA, Gutin PH, Hellman S, Rosenberg SA, DeVita VT. Cancer: principles and practice of oncology. 6. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Liang P, Shi H, Zhu W, Gui Q, Xu Y, Meng J, Guo X, Gong Z, Chen H. Silver nanoparticles enhance the sensitivity of temozolomide on human glioma cells. Oncotarget. 2017;8(5):7533–7539. doi: 10.18632/oncotarget.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, Kachlicki P, Franklin G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials (basel) 2018;11(6):940. doi: 10.3390/ma11060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K, Koroth J, Srinivasan PP, Singh A, Mukundan S, Karki SS, Choudhary B, Gupta CM. Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. Int J Nanomedicine. 2019;14:5257–5270. doi: 10.2147/IJN.S202404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nadagouda MN, Speth TF, Varma RS. Microwave-assisted green synthesis of silver nanostructures. Acc Chem Res. 2011;44(7):469–478. doi: 10.1021/ar1001457. [DOI] [PubMed] [Google Scholar]
- Newton HB. Overview of the molecular genetics and molecular chemotherapy of GBM. In: Ray SK, editor. Molecular mechanisms of pathogenesis and current therapeutic strategies. London: Springer; 2010. [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nıshıkawa T, Dı Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maıorka PC, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Rautela A, Rani J, Debnath M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J Anal Sci Technol. 2019;10:5. doi: 10.1186/s40543-018-0163-z. [DOI] [Google Scholar]
- Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- Rychlik M. Quantification of free coumarin and its liberation from glucosylated precursors by stable isotope dilution assays based on liquid chromatography-tandem mass spectrometric detection. J Agric Food Chem. 2008;56(3):796–801. doi: 10.1021/jf0728348. [DOI] [PubMed] [Google Scholar]
- Salehi S, Shandiz SA, Ghanbar F, Darvish MR, Ardestani MS, Mirzaie A, Jafari M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomedicine. 2016;11:1835–1846. doi: 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry M, Mayyaa KS, Bandyopadhyay K. pH dependent changes in the optical properties of carboxylic acid derivatized silver colloid particles. Colloids Surf A Physicochem Eng Asp. 1997;127:221–228. doi: 10.1016/S0927-7757(97)00087-3. [DOI] [Google Scholar]
- Sastry M, Patil V, Sainkar SR. Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J Phys Chem. 1998;102(8):1404–1410. doi: 10.1039/A704355D. [DOI] [Google Scholar]
- Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008;1142:108–132. doi: 10.1196/annals.1444.009. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Shogawa H, Matsuzawa T, Yonezawa S, Hayashi T, Arisawa M, Suzuki S, Yoshizaki M, Morita N, Ferro E, et al. Anti-inflammatory constituents of topically applied crude drugs IV. Constituents and anti-inflammatory effect of Paraguayan crude drug “alhucema” (Lavandula latifolia Vill.) Chem Pharm Bull (tokyo) 1990;38(8):2283–2284. doi: 10.1248/cpb.38.2283. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Urbańska K, Pająk B, Orzechowski A, Sokołowska J, Grodzik M, Sawosz E, Szmidt M, Sysa P. The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells. Nanoscale Res Lett. 2015;10:98. doi: 10.1186/s11671-015-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu S, Ma J, Qu G, Wang X, Yu S, He J, Liu J, Xia T, Jiang GB. Silver nanoparticles induced RNA polymerase-silver binding and RNA transcription inhibition in erythroid progenitor cells. ACS Nano. 2013;7(5):4171–4186. doi: 10.1021/nn400594s. [DOI] [PubMed] [Google Scholar]
- Yuan YG, Wang YH, Xing HH, Gurunathan S. Quercetin-mediated synthesis of graphene oxide–silver nanoparticle nanocomposites: a suitable alternative nanotherapy for neuroblastoma. Int J Nanomed. 2017;12:5819–5839. doi: 10.2147/ijn.s140605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.