Abstract
Small renal masses are commonly diagnosed with modern medical imaging. Renal tumour volume has been explored as a prognostic tool to help decide when intervention is needed and appears to provide additional prognostic information for smaller tumours compared with tumour diameter. However, the current method of calculating tumour volume in clinical practice uses the ellipsoid equation (π/6 × length × width × height) which is an oversimplified approach. Some research groups trace the contour of the tumour in every image slice which is impractical for clinical use. In this study, we demonstrate a method of using 3D segmentation software and the 3D interpolation method to rapidly calculate renal tumour volume in under a minute. Using this method in 27 patients that underwent radical or partial nephrectomy, we found a 10.07% mean absolute difference compared with the traditional ellipsoid method. Our segmentation volume was closer to the calculated histopathological tumour volume than the traditional method (p = 0.03) with higher Lin’s concordance correlation coefficient (0.79 vs 0.72). 3D segmentation has many uses related to 3D printing and modelling and is becoming increasingly common. Calculation of tumour volume is one additional benefit it provides. Further studies on the association between segmented tumour volume and prognosis are needed.
Keywords: Renal cancer, Prognosis, Diagnosis, Organ volume
Background
With the increased use of modern imaging techniques, diagnosis of small renal masses (SRM) has become increasingly commonplace. About 14.4% of adults were found to incidentally have SRMs > 1 cm in one study [1]. As a result, less invasive nephron-sparing methods are increasingly being used to preserve renal function [2, 3]. Although treatment methods for renal cancer have evolved dramatically, diagnosis and monitoring of them remain unchanged. As the diagnosis of SRMs continues to rise, there is a need to explore other diagnostic and prognostic methods to aid in the decision of when to intervene and treat the pathology.
Renal tumours have traditionally been graded and classified based on maximal diameter [4]. This method incorrectly assumes that the tumour is spherical and growing uniformly in all directions. Therefore, there has been interest in using renal tumour volume rather than diameter as a predictor for prognosis as it more accurately captures the size and shape of the tumour and has shown promising results [5, 6]. However, these studies use an ellipsoid formula (π/6 × length × width × height) to calculate tumour volume based on the radiologist’s measurements in three views (coronal, sagittal, axial) which does not account for irregular shapes. If tumour volume were to be used for grading, there is a need for a more accurate and rapid method that can be integrated into clinical practice.
3D segmentation is a process commonly used in 3D modelling and 3D printing to create digital models of target anatomy from medical imaging which is increasingly being explored in urology [7–9]. Additionally, we have also developed a method using segmentation software to rapidly calculate renal tumour volume, taking advantage of features such as 3D interpolation that are easily accessible and intuitive for clinicians to employ. In this study, we aim to describe a new method of calculating radiological segmentation volume (RSV), examine if it differs significantly from the radiological ellipsoid volume (REV) method and examine if one method is closer to histopathological ellipsoid volume (PEV).
Methods
Segmentation Method
A video summarising our method is available in the electronic supplementary material. The software used in this study was Mimics v21.0 (Materialise, Leuven, Belgium) which is an FDA-approved medical segmentation software. A patient’s computed tomography (CT) or magnetic resonance imaging (MRI) scan is loaded into the software which displays it in axial, coronal and sagittal views. This requires access to the patient’s scan which is uploaded in the DICOM format to the software.
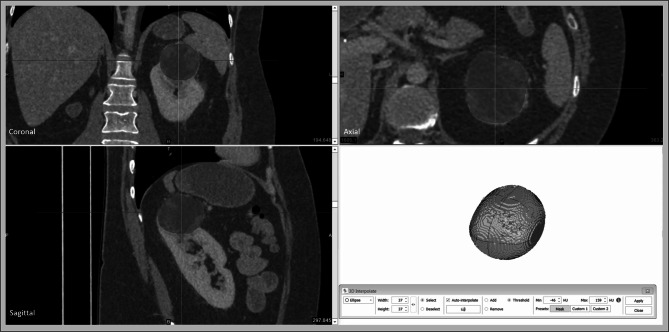
A density threshold is set for the desired tissue using Hounsfeld units (HU) on CT scans and gray value (GV) on MRI scans which aims to include all of the tumours while minimising the inclusion of other areas (Fig. 1).
Fig. 1.
a CT scan of superior pole left renal tumour with threshold density values adjusted to between − 46 and 159 Hounsfeld units (HU); b MRI scan of left midpole renal tumour with threshold density values adjusted to between 182 and 571 Gy value (GV). These values can be adjusted as needed with visual feedback. The green and red crosshairs are centred on the renal tumour in each image
Using the 3D interpolation tool, the tumour is highlighted manually once in each of the three views. The software combines this information with the density threshold to create a 3D model (Fig. 2). The user is able to scroll through the scan to make any further adjustments to the segmentation as needed.
Fig. 2.
Highlighting the tumour in axial, coronal and sagittal planes (red) with the 3D interpolation tool creates a 3D digital model in combination with the threshold density information creates a digital 3D model of the tumour (green) from the CT scan
The software is then able to calculate the volume based on this 3D segmentation which gives the RSV. In our experience, this process can be performed in under 1 min by a skilled user as shown in our supplementary video. In this study, the segmentation was performed by a urology trainee with 3-month experience with the software. The software also states the maximal diameter in axial, coronal and sagittal planes. These three dimensions can then be inputted into the ellipsoid equation used in conventional volume calculation (π/6 × length × width × height) which gives the REV.
Histopathology
The study was approved by the hospital’s human research ethics committee (study reference: LNR/2019/QRBW/51927). Patients were identified that had radical or partial nephrectomy performed. As this was a retrospective study and we did not have access to the pathological specimens, we used the ellipsoid equation to calculate the histopathological volume of the tumours (π/6 × length × width × height) based on the dimensions recorded by the pathologist in their report to give us the PEV.
Statistical Analysis
To determine the difference between RSV and REV, the absolute difference in volume between the two measurement methods was converted to a percentage of the RSV. To determine which method was closer to PEV, the mean absolute difference percentage to PEV in each method was compared and analysed using the Wilcoxon signed-rank test. To graph the correlation of each method, Bland-Altman plots were created and Lin’s concordance correlation coefficient was calculated. Statistical analysis and graphing were performed using Stata/SE version 15.1 (StataCorp LP, College Station, TX, USA).
Results
Twenty-seven patients were included in the study: 12 of which underwent radical nephrectomy and 15 underwent partial nephrectomy. The mean maximal radiological diameter was 33.29 mm. Twenty-one patients were grade T1a (< 40 mm), and six were grade T1b (> 40 mm) based on imaging. Five patients had segmentation from MRI scans and 22 from CT scans.
There was variation in the time interval between the pre-operative scan and the date of the operation. The mean time interval was 89.66 days (SD 88.25 days) with a range of 5–387 days.
The mean RSV was 24.90 cm3 (SD 38.91 cm3), the mean REV was 27.76 cm3 (SD 45.84 cm3) and the mean PEV was 15.74 cm3 (SD 21.28 cm3).
There was a 10.07% mean absolute difference (95% CI 7.12–13.0) between RSV and REV. RSV showed less absolute difference compared with PEV than REV (58.6% vs 64.2%) suggesting that this segmentation method is more accurate (p = 0.03). Both methods showed a considerable difference in volume compared with PEV (Table 1).
Table 1.
Results of segmentation volume compared with ellipsoid equation volume using histopathological volume as a gold standard
| Segmentation (RSV) | Ellipsoid (REV) | Histopathology (PEV) | |
|---|---|---|---|
| Mean volume (cm3) (SD) | 24.90 (38.91) | 27.76 (45.84) | 15.74 (21.28) |
| Mean absolute difference to PEV (%) | 58.6 | 64.2 | |
| Lin’s concordance correlation coefficient | 0.79 | 0.72 |
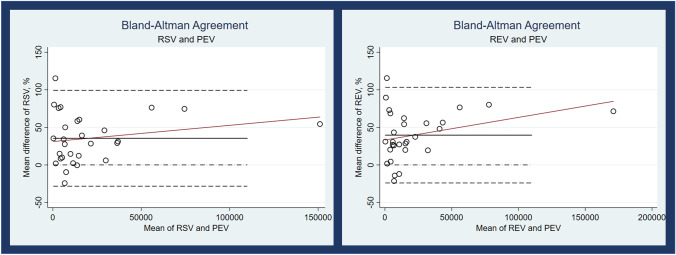
Bland-Altman plots for RSV and REV show that as the size of the tumour increases, the discrepancy with PEV increases for both methods but this bias is more pronounced with REV (Fig. 3). Lin’s concordance correlation coefficient is lower with RSV compared with REV (0.79 vs 0.72).
Fig. 3.
Bland-Altman plots for RSV and REV when compared to PEV
Discussion
We have presented a method of calculating renal tumour volume using 3D segmentation software which can be performed in under a minute. This method adds minimal workload for a radiologist in clinical practice to evaluate a tumour, and we have demonstrated that it differs significantly from the traditional ellipsoid volume calculation while better correlating with histopathological volume. 3D segmentation is a relatively new process but is something we expect to become more common in clinical practice for surgical planning and education. There is increasing research in using 3D segmentation in planning surgery for renal tumours [10–13], and we have highlighted an additional use for segmentation in providing additional prognostic information which may aid in decision-making on treatment options.
Using volume rather than diameter is a promising and intuitive method of evaluating the prognosis of renal tumours. Jorns et al. [5] found that tumour volume could provide more accurate prognostic information for pT1a renal tumours in a sample of 955 patients with clear cell renal cell carcinoma compared with maximal diameter. In another study, these same authors also showed that calculating volume using the ellipsoid equation and only using the maximal tumour volume was not accurate for volume calculation which highlights the need for accurate volume measurement methods [14]. A meta-analysis found that volumetric growth was predictive of progression to metastases and the authors recommended calculating volume when possible [15]. In contrast, Seçil et al. [16] found that renal tumour volume was not independently predictive of prognosis compared with traditional TNM staging in 46 patients.
The limitation of these studies in the literature is the use of the ellipsoid equation to calculate volume. Our results show that 3D segmentation differs significantly to ellipsoid calculation. Breau et al. [17] found a similar mean difference of 8.2% between segmented tumour volume and ellipsoid equation volume in their study evaluating much larger tumours with a mean volume of 170 cm3.
Zhang et al. [18] manually traced the contour of renal tumours on every image slice and multiplied the total surface area by the section thickness to improve accuracy of volume calculation. The disadvantage of this method is the time taken which would make it impractical to implement in clinical practice. A 5-cm tumour on a CT scan with 1-mm slices would therefore have 50 slices to manually segment which we estimate would take approximately 20 min. As we have shown, our method can be performed in under one minute.
The difference between radiological and pathological renal tumour volume is vast, and drawing significant conclusions is difficult. It has been established in literature that radiological volume of renal tumours is larger than histological tumour volume [6]. Other studies have found that radiological maximal diameter is greater than the histopathological maximal diameter [19–21]. This is thought to be related to tumour shrinkage after surgery as well as necrosis in larger tumours [6]. In our cohort, both RSV and REV were vastly different to PEV; however, RSV appears to correlate slightly better than REV.
In this study, there were five patients that had segmented performed on MRI scans rather than CT. The literature suggests that renal tumour size measurements do not differ significantly between MRI and CT [22]; however, a separate analysis in a larger cohort would be of value.
To confirm if this method should be used in clinical practice, further studies to examine the association between segmented renal tumour volume and prognosis are needed.
This method could also be applied to other types of tumours or anatomy. Tumour volume segmentation has been explored in head and neck tumours as well as oesophageal tumours using machine learning [23, 24]. Whilst in the future artificial intelligence (AI) is likely to automate medical imaging segmentation, barriers around regulation and safety suggest that manual segmentation is still likely to play a role in foreseeable future. Thus, methods that can be rapidly performed as shown in our study may provide an impetus for radiologist segmentation to be integrated into clinical practice.
Conclusion
3D segmentation of renal tumours allows for calculation of volume and the 3D interpolation method makes this process rapid and accessible for clinicians as it can be performed in under a minute. Volume calculation could provide additional prognostic information to help guide management as diagnosis of renal masses becomes increasingly commonplace. Segmented volume differs significantly from traditional ellipsoid equation calculations and correlates more closely with histopathological volume. This method could also be employed for other types of tumours and anatomy. 3D segmentation is likely to become more widespread in clinical practice, and volume calculation is an additional benefit it provides. Studies correlating segmented tumour volume and prognosis are needed to further validate this method.
Supplementary Information
Below is the link to the electronic supplementary material.
Compliance with Ethical Standard
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Informed Consent
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the human research ethics committee of Royal Brisbane and Women’s Hospital who determined that our study did not need ethical approval. An official waiver of ethical approval was granted from the Royal Brisbane and Women’s Hospital human research ethics committee (HREC) study reference LNR/2019/QRBW/51,927. Written consent was not required for this retrospective study with no identifying information.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10278-020-00416-z.
References
- 1.O'Connor SD, Pickhardt PJ, Kim DH, Oliva MR, Silverman SG. Incidental finding of renal masses at unenhanced CT: Prevalence and analysis of features for guiding management. AJR Am J Roentgenol. 2011;197(1):139–145. doi: 10.2214/AJR.10.5920. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Ahn T, Papa N, Perera M, Teloken P, Coughlin G, et al; Changing trends in surgical management of renal tumours from 2000 to 2016: a nationwide study of Medicare claims data. ANZ J Surg, 2019 [DOI] [PubMed]
- 3.Wah TM, Irving HC, Gregory W, Cartledge J, Joyce AD, Selby PJ. Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int. 2014;113(3):416–428. doi: 10.1111/bju.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol. 2018;36(12):1913–1926. doi: 10.1007/s00345-018-2447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorns J, Thiel DD, Lohse CM, Williams A, Arnold ML, Cheville JC, et al. Three-dimensional tumour volume and cancer-specific survival for patients undergoing nephrectomy to treat pT1 clear-cell renal cell carcinoma. BJU Int. 2012;110(7):956–960. doi: 10.1111/j.1464-410X.2012.10937.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi SM, Choi DK, Kim TH, Jeong BC, Seo SI, Jeon SS, et al; A comparison of radiologic tumor volume and pathologic tumor volume in renal cell carcinoma (RCC). PloS one. 10(3):e0122019-e, 2015 [DOI] [PMC free article] [PubMed]
- 7.Chen MY, Skewes J, Desselle M, Wong C, Woodruff MA, Dasgupta P, et al. Current applications of three-dimensional printing in urology. BJU Int. 2020;125(1):17–27. doi: 10.1111/bju.14928. [DOI] [PubMed] [Google Scholar]
- 8.Chen MY, Skewes J, Woodruff MA, Rukin NJ. Using bespoke 3D-printed models to improve patient understanding of an encrusted ureteric stent. J Clin Urol. 2051415819876514, 2019
- 9.Checcucci E, de Cillis S, Porpiglia F. 3D-printed models and virtual reality as new tools for image-guided robot-assisted nephronsparing surgery. Curr Opin Urol. 2020;30(1):55–64. doi: 10.1097/MOU.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 10.Hyde ER, Berger LU, Ramachandran N, Hughes-Hallett A, Pavithran NP, Tran MGB, et al. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume-rendered images. Int J Comput Assist Radiol Surg. 2019;14(4):723–732. doi: 10.1007/s11548-019-01913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamai T, Furuya N, Kambara T, Abe H, Honda M, Shioyama Y, et al. Single minimum incision endoscopic radical nephrectomy for renal tumors with preoperative virtual navigation using 3D-CT volume-rendering. BMC Urol. 2010;10:7. doi: 10.1186/1471-2490-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porpiglia F, Amparore D, Checcucci E, Manfredi M, Stura I, Migliaretti G, et al. Three-dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores. BJU Int. 2019;124(6):945–954. doi: 10.1111/bju.14894. [DOI] [PubMed] [Google Scholar]
- 13.Shirk JD, Thiel DD, Wallen EM, Linehan JM, White WM, Badani KK, et al; Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial. JAMA Netw Open. 2(9):e1911598-e, 2019 [DOI] [PMC free article] [PubMed]
- 14.Thiel DD, Jorns J, Lohse CM, Cheville JC, Thompson RH, Parker AS. Maximum tumor diameter is not an accurate predictor of renal cell carcinoma tumor volume. Scandinavian journal of urology. 2013;47(6):472–475. doi: 10.3109/21681805.2013.814071. [DOI] [PubMed] [Google Scholar]
- 15.Smaldone MC, Kutikov A, Egleston BL, Canter DJ, Viterbo R, Chen DYT, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118(4):997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Secil M, Cullu N, Aslan G, Mungan U, Uysal F, Tuna B, et al. The effect of tumor volume on survival in patients with renal cell carcinoma. Diagn Interv Radiol (Ankara, Turkey) 2012;18(5):480–487. doi: 10.4261/1305-3825.DIR.5346-11.1. [DOI] [PubMed] [Google Scholar]
- 17.Breau RH, Clark E, Bruner B, Cervini P, Atwell T, Knoll G, et al. A simple method to estimate renal volume from computed tomography. Can Urol Assoc J. 2013;7(5–6):189–192. doi: 10.5489/cuaj.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Kang SK, Wang L, Touijer A, Hricak H. Distribution of renal tumor growth rates determined by using serial volumetric CT measurements. Radiology. 2009;250(1):137–144. doi: 10.1148/radiol.2501071712. [DOI] [PubMed] [Google Scholar]
- 19.Herr HW, Lee CT, Sharma S, Hilton S. Radiographic versus pathologic size of renal tumors: Implications for partial nephrectomy. Urology. 2001;58(2):157–160. doi: 10.1016/S0090-4295(01)01173-6. [DOI] [PubMed] [Google Scholar]
- 20.Kurta JM, Thompson RH, Kundu S, Kaag M, Manion MT, Herr HW, et al. Contemporary imaging of patients with a renal mass: does size on computed tomography equal pathological size? BJU Int. 2009;103(1):24–27. doi: 10.1111/j.1464-410X.2008.07941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Wu Y, Wang J, Xu J, Na R, Wang X. The effect of discrepancy between radiologic size and pathologic tumor size in renal cell cancer. SpringerPlus. 2016;5(1):899. doi: 10.1186/s40064-016-2645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan I, Beksac AT, Paulucci DJ, Abaza R, Eun DD, Bhandari A, et al. Differences in renal tumor size measurements for computed tomography versus magnetic resonance imaging: implications for patients on active surveillance. J Laparoendosc Adv Surg Tech. 2017;27(12):1275–1278. doi: 10.1089/lap.2017.0234. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Guo N, Gong K, Zhong S, Li Q. Gross tumor volume segmentation for head and neck cancer radiotherapy using deep dense multi-modality network. Phys Med Biol. 2019;64(20):205015. doi: 10.1088/1361-6560/ab440d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousefi S, Sokooti H, Elmahdy MS, Peters FP, Shalmani MTM, Zinkstok RT, et al; editors. Esophageal Gross Tumor Volume Segmentation Using a 3D Convolutional Neural Network. Medical Image Computing and Computer Assisted Intervention – MICCAI. 2018 2018//; Cham: Springer International Publishing, 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.