Abstract
Early childhood and pregnancy are two sensitive periods of heightened immune plasticity, when exposure to adversity may disproportionately increase health risks. However, we need deeper phenotyping to disentangle the impact of adversity during sensitive periods from that across the total lifespan. This study examined whether retrospective reports of adversity during childhood or pregnancy were associated with inflammatory imbalance, in an ethnically diverse cohort of 53 low-income women seeking family-based trauma treatment following exposure to interpersonal violence. Structured interviews assessed early life adversity (trauma exposure ≤ age 5), pregnancy adversity, and total lifetime adversity. Blood serum was assayed for pro-inflammatory (TNF-a, IL-1ß, IL-6, and CRP) and anti-inflammatory (IL-1RA, IL-4, and IL-10) cytokines. CD14+ monocytes were isolated in a subsample (n = 42) and gene expression assayed by RNA sequencing (Illumina HiSeq 4000; TruSeq cDNA library). The primary outcome was a macrophage-associated M1/M2 gene expression phenotype. To evaluate sensitivity and specificity, we contrasted M1/M2 gene expression with a second, clinically-validated macrophage-associated immunosuppressive phenotype (endotoxin tolerance) and with pro-inflammatory and anti-inflammatory cytokine levels. Adjusting for demographics, socioeconomic status, and psychopathology, higher adversity in early life (ß = .337, p = 0.029) and pregnancy (ß = .332, p = 0.032) were each associated with higher M1/M2 gene expression, whereas higher lifetime adversity (ß = −.341, p = 0.031) was associated with lower immunosuppressive gene expression. Adversity during sensitive periods was uniquely associated with M1/M2 imbalance, among low-income women with interpersonal violence exposure. Given that M1/M2 imbalance is found in sepsis, severe COVID-19 and myriad chronic diseases, these findings implicate novel immune mechanisms underlying the impact of adversity on health.
Subject terms: Predictive markers, Human behaviour, Physiology
Introduction
Exposure to adversity during sensitive periods of immunologic development is associated with heightened risk for poor health outcomes, including cardiovascular [1], metabolic [2], psychiatric [1, 3], and neurodegenerative disorders [4], as well as premature all-cause mortality [5]. Sensitive periods are limited windows of development, during which the central nervous system (CNS) and immune system can both be especially “plastic” or prone to epigenetic changes in response to environmental stimuli. “Biological embedding” [6, 7] of stress through epigenetic changes can lead to heightened health risks later in life [6–8]. While the plasticity of brain, behavior, and immunity is pronounced early in life, it continues across the adult lifespan. We know comparatively little about whether there are specific developmental stages in adulthood that are associated with heightened susceptibility to adversity and sustained phenotypic changes. Pregnancy may be an important “developmental infection point,” during which the maternal immune system undergoes profound and progressive transformations, central to the survival of mother and baby [9]. As not all women exposed to adversity in their lifetime develop poor health, a deeper understanding of the phenotypes and mechanisms associated with adversity exposure, with consideration of potential sensitive periods, is needed. Such evidence would allow development of new diagnostic biomarkers, identification of high-risk individuals, and better disease targeting [10].
Inflammation is one major pathway by which adversity exposure increases later disease risk [11]. The immune system has particular sensitive periods of development, such as early life [12, 13] and pregnancy [14–16], during which it undergoes substantial changes, exhibiting heightened phenotypic plasticity and vulnerability to environmental stressors [17–19]. Although trauma exposure tends to be associated with elevated pro-inflammatory proteins [7, 20], these biomarkers are not highly specific—i.e., they are also elevated in myriad mental and physical conditions [21–23]. Furthermore, because inflammation is multidimensional, employing a single biomarker, like C-Reactive Protein (CRP), tends not to be sensitive enough to optimally identify at-risk individuals [24, 25]. Accordingly, the National Institute of Health recently called for complex, composite biomarkers [26]. Inflammatory cytokines are predominantly produced by myeloid cells of the innate immune system, such as monocyte/macrophages. Hence, this study investigates whether pro-inflammatory monocyte/macrophage phenotypes may elucidate the unique biological fingerprint of adversity experienced during sensitive periods of immunological development.
Early childhood, especially the first 5 years of life, is a sensitive period during which environmental exposures influence the allocation of resources between innate (non-specific) immunity versus adaptive (specific) immunity. Exposure to adversity, especially interpersonal trauma [27], activates the neuroimmune networks that anticipate the threat of wounding and infection [28], thereby mobilizing the pro-inflammatory defenses of the innate immune system. In contrast, adaptive immunity, or targeting of specific pathogens, is more precise, but it takes years to develop [29]. It is increasingly recognized that the innate immune system can form epigenetically mediated immunologic “memory” known as “trained immunity”, which results in heightened reactions to secondary infections or sterile inflammatory triggers (like stress), and which may be intergenerationally transmissible [30–33]. Current available evidence suggests that exposure to adversity during early life drives epigenetic modifications that enhance inflammation, skew T-cell repertoires [34], and may contribute to immunosupression [8, 35, 36]. Hence, these enduring phenotypic alterations facilitate a robust inflammatory response to threats, but over time, can drive chronic disease and accelerate aging of the adaptive immune system.
As in early childhood, pregnancy is a period of an individual’s lifespan characterized by high levels of tissue proliferation, establishment of immune tolerance, and heightened vulnerability to infection—a major cause of maternal and fetal mortality [29, 37]. In apparent contradiction, pregnancy is characterized by both chronic low-grade maternal inflammation and immunosuppression, which helps restrain the inflammatory response [9, 15, 38]. Circulating blood monocytes infiltrate the uterine lining during early pregnancy, becoming “decidual macrophages”. Excess inflammation at the maternal-fetal interface is associated with a pro-inflammatory “M1-like” macrophage phenotype and predicts fetal loss and premature birth [39, 40]. In contrast, macrophages exhibiting greater expression of “M2-like” genes (immunoregulatory and anti-inflammatory) help generate immunosuppressive T-regulatory cells, which prevent rejection of the semi-allogenic (non-self) fetus [14, 15, 41]. These M1-like and M2-like profiles reflect coordinated programs involving hundreds of genes, instantiated in part through epigenetic regulation [42], whereby a relative imbalance favoring M1 versus M2-like expressed genes has been linked with inflammatory disease [43].
It may be “through the eyes” of the macrophage that pregnancy emerges as a sensitive period for immune system development in adulthood, such that the effects of adversity during pregnancy may persist post-natally. Decidual (uterine) macrophages play a pivotal role in a successful pregnancy [44]. In pregnancy, the uterus undergoes an enormous transformation “to a degree unparalleled in most adult tissues” [44]. Whereas many of her tissue macrophage compartments (e.g., the CNS) were populated when the mother herself was in utero, during her pregnancy, the decidual macrophages are largely drawn from bone marrow-derived monocytes. Under chronic stress conditions, these monocytes become biased toward M1-like, pro-inflammatory phenotypes [45–48]. Moreover, decidual macrophage phenotypes are epigenetically modified by the microenvironment [44, 49] of the fetal-maternal interface, and may be skewed toward an M1-like phenotype by stress-associated glucocorticoids [50]. In fact, psychological stress during pregnancy has been associated with leukocyte epigenetic markers of higher inflammation and lower T-regulatory-associated immunosuppression, which persist after birth to predict post-partum depression in a sample of Latinx mothers [51]. Psychosocial stress during pregnancy has also been linked to elevated expression of inflammatory genes (RNA) during pregnancy [52], which can in turn affect fetal tissue development [53] and birth timing and weight [54]. Hence, pregnancy appears to be an epigenetically sensitive window in the life of an adult woman, during which adversity may leave an enduring impact on her immune system and its reactivity to threat, as well as impacting offspring development and outcomes.
The current cross-sectional study sought to understand social adversity predictors of immune phenotypes among ethnically diverse low-income women, with significant exposure to diverse forms of interpersonal trauma. We focus on monocyte/macrophage phenotypes because they (1) exhibit developmental sensitivity during the first several years of life [13], and during pregnancy [9, 14, 15, 38], (2) drive inflammatory disease [33, 43], and (3) help regulate the balance of innate and adaptive immunity, with implications for immunosuppression [41]. Our primary hypothesis was that greater adversity during two sensitive periods of the female lifespan—early life (defined herein as birth to age five) and pregnancy—would be significantly positively associated with alterations in a macrophage-associated M1/M2 RNA phenotype, reflecting a relative upregulation of pro-inflammatory versus anti-inflammatory and immunoregulatory genes.
Furthermore, we reasoned that a novel phenotypic marker of sensitive periods should meet certain criteria for specificity with respect to the timing of exposure and the genomic fingerprint. First, we reasoned that if the M1/M2 phenotype is specific to early life or pregnancy exposures, then it should not be significantly associated with total adversity across the lifespan. Second, if sensitive periods are uniquely associated with M1/M2, then they should not be associated with alternative biomarkers. Thus, to ascertain whether standard protein biomarkers might provide similar insights, we additionally analyzed levels of classic pro-inflammatory and anti-inflammatory cytokines and C-Reactive Protein (CRP). Similarly, we contrasted M1/M2 with an alternative macrophage-associated phenotype, known as endotoxin tolerance (ET), validated across a wide age-range among patients with sepsis [55]. We selected ET based on emerging epigenetic research that describes trained immunity and tolerance as “two opposite functional programs of innate immunity” [30], because, after an initial priming (e.g., by pathogen exposure), the former enhances inflammatory reactivity while the latter suppresses it [42].
Methods
Study population
Data used in the present study came from “The Child Parent Psychotherapy Health study” (CPP-HEALTH), conducted between 2013 and 2015. CPP-HEALTH enrolled 62 women and their children who were seeking treatment for the child’s exposure to interpersonal trauma. The goal was to examine biological correlates of trauma and treatment-related changes in psychological functioning following participation in a previously-validated dyadic intervention, Child-Parent Psychotherapy [56]. Inclusion criteria included biological mothers with children aged 2–6 years who had been exposed to a traumatic event, and were fluent in English or Spanish. Exclusion criteria included homelessness, current family violence, substance abuse, child was a ward of the state, current pregnancy, child developmental disorder, psychosis, and chronic medical conditions in mother or child. Maternal participants provided written consent and were compensated. The research was approved by the Institutional Review Boards at Zuckerberg San Francisco General and the University of California San Francisco.
Participant characteristics
The present study included 53 mother–child dyads who completed the baseline assessment and provided a blood sample for inflammatory cytokine assays (Table 1). Of these, gene expression phenotypes were obtained in a convenience subset of 42. Power analyses demonstrated 80% power to detect a moderate effect size (d = 0.40) at n = 44.
Table 1.
Sociodemographic, adversity exposure, mental health, and medical characteristics of the sample.
| Sample characteristics | Serum cytokine cohort | Gene expression subcohort |
|---|---|---|
| Statistical estimate (n = 53) | Statistical estimate (n = 42) | |
| Mother’s age, yearsa | 32.10 (0.92) | 31.84 (0.91) |
| Non-Hispanic Caucasianb | 7 (13%) | 4 (10%) |
| Hispanic/Latina Caucasianb | 38 (72%) | 32 (76%) |
| African Americanb | 2 (4%) | 2 (5%) |
| Asian Americanb | 6 (11%) | 4 (10%) |
| High school educationb | 34 (64%) | 24 (57%) |
| Family povertyb | 35 (66%) | 29 (69%) |
| US Bornb | 45 (85%) | 37 (86%) |
| Child’s age, monthsa | 50.38 (1.74) | 49.53 (1.93) |
| Early life adversitya | 0.81 (0.15) | 0.81 (0.17) |
| Adversity in pregnancya | 0.91 (0.11) | 0.88 (0.12) |
| Total life adversitya | 11.12 (0.58) | 11.58 (0.64) |
| PTSD severity (PSSI)a | 21.30 (1.55) | 21.81 (1.71) |
| Depressive symptoms (CESD-R)a | 24.98 (1.72) | 24.60 (1.82) |
| Body mass indexa | 27.57 (0.84) | 27.53 (0.96) |
| Current antidepressant useb | 6 (11%) | 4 (10%) |
| NSAID useb | 1 (2%) | 1 (2%) |
n = 53 constitutes the sample with serum cytokine data and n = 42 constitutes the subsample of these 53 women, who had gene expression data. High school education was coded 0 for participants who attended school for less than 12 years, and 1 for 12 or more years of attendance. Poverty was calculated using 2016 Census criteria (see “Methods” section).
PSSI PTSD Symptom Scale Interview, total score, CESD-R Center for Epidemiologic Studies Depression Scale, total score.
aMean (SEM).
bn(%).
Study procedures
At baseline, women provided information on their history of adversity and current psychological functioning. Participants were excluded from study enrollment if they were currently experiencing domestic violence. To ascertain early life adversity (ELA) and total life adversity (TLA) exposure, women completed the 30-item Life Stressor Checklist-Revised (LSC-R) in interview format [57]. Because the current study focused on interpersonal adversity, natural disasters and vehicle accidents were excluded. TLA was computed by summing the number of different types of events experienced throughout the lifespan, and ELA was quantified as the number of different types of events experienced up to age 5. Pregnancy adversity (PA) was assessed using a validated structured interview [58], in which a trained clinician asked mothers to report whether ten different types of violence (e.g., being hit in the head or stomach, choked, pushed, kicked, knifed, threatened) occurred during her pregnancy with the target child (aged 2–6 years). PA was computed as (0) no events, (1) one event, or (2) more than one event. Women also completed the Center for Epidemiologic Studies Depression Scale Revised (CESD-R) [59] and the Posttraumatic Stress Scale Interview (PSSI) [60].
Laboratory procedures
Blood draw procedure
Women provided morning fasting blood samples at the hospital laboratory; prior to the draw, mothers were asked to consume nothing other than water or coffee and to reschedule if they felt ill, or had used medications in the prior three days that might affect assays/biomarkers. Serum was stored at −80°.
Immunogenomic phenotypes (M1/M2 ratio)
We selected a set of a priori-determined [61], previously published (44) genes reflecting an M1 and M2-like profile, quantified at baseline, based on previous literature (Supplementary Table 1). This M1/M2 phenotype reflects a relative increase of pro-inflammatory M1-like versus anti-inflammatory and immunoregulatory M2-like expressed genes. Our prior work demonstrated that higher levels of the M1/M2 phenotype prospectively predicted poorer response to behavioral trauma treatment in this same sample [62], indexed as a lesser reduction in depressive and PTSD symptoms. In line with this previous work, we computed an aggregate sum for M1-like genes and another for M2-like genes (focused on M2ab) [61], and then computed a final M1/M2 ratio as an index of relative pro-inflammatory imbalance.
Endotoxin tolerance (ET): an immunosuppresive phenotype
To establish whether associations of adversity during sensitive periods would be specific to M1/M2, or whether there might be a more general pattern of associations with other established inflammatory phenotypes, we included a previously validated, endotoxin tolerance (ET) phenotype for model comparison [55] (Supplementary Table 2). ET was specifically chosen because the profile of macrophage gene expression evoked by social stress overlaps substantially with the macrophage gene expression profile evoked by endotoxins such as lipopolysaccharides (LPS), and ET is specifically elicited in culture models by repeated LPS stimulation, hence, we hypothesized that it might provide insights into total lifetime experiences of social stress or adversity [28]. Repeated in vitro endotoxin stimulation over a 24-h period results in a temporary refractory period, during which cells exhibit sub-normal production of both pro-inflammatory and anti-inflammatory cytokines, described as “immunoparalysis.” In the context of wounding or infection, this immunoparalytic state can result in death [55]. Hence, we reasoned that greater total adversity across the lifespan might be associated with a desensitization of this negative feedback mechanism, resulting in lower ET scores.
Inflammatory protein profiles
The inflammatory cytokines IL-6, IL-1ß, IL-1 receptor antagonist (IL-1RA), IL-4, IL-10, and tumor necrosis factor alpha (TNF-a) were measured in serum in duplicate using a chemiluminescent multiplex assay from Meso Scale Discovery (Rockville, MD). CRP was measured using a high-sensitivity immunoturbidimetric assay from Randox (Kearneysville WV) and a PolyChem clinical chemistry analyzer (PolymedCo, Cortlandt Manor, NY). A pro-inflammatory aggregate score was comprised of the average of the normalized scores for CRP, IL-6, TNF-a, and IL-1ß. An anti-inflammatory aggregate score was constructed by averaging the normalized scores for IL-1RA, IL-4, and IL-10.
Immune RNA phenotyping
These methods are described elsewhere in detail (Supplementary Methods). CD14+ monocytes were isolated from total peripheral blood mononuclear cells using positive selection (anti-CD14 PE by BD Pharmingen), and frozen in RNAprotect Cell Reagent at −80°. Total RNA was isolated, converted to cDNA using with the Illumina TruSeq Stranded enzyme system and sequenced on Illumina HiSeq 4000, reads are aligned to the reference human transcriptome (HISAT2), and gene expression modeled (StringTie [63]).
Statistical analysis
All variables were inspected for normality violations; inflammatory cytokines were blom-transformed and gene counts were log2-normalized. Multivariable regression models inputted each adversity factor as a predictor of the M1/M2 phenotype with and without adjustment for two separate sets of covariates. Model 1 included demographic and medical covariates strongly connected with inflammatory cytokines in the literature [62], and those highly characteristic of this sample: age, BMI, antidepressant use, and Hispanic ethnicity. Four participants indicated they were breastfeeding, however the small number precluded us from including it as a covariate. Model 2 included socioeconomic status factors: family poverty per census definition, being US born, and education [64]. Specificity tests for IP, pro-inflammatory (PRO) and anti-inflammatory (ANTI) cytokines were conducted similarly. Finally, we conducted multivariable regression analyses to better understand whether different adversity factors exhibited associations with immunophenotypes that were independent of one another and of psychiatric symptoms.
Results
Participant characteristics
On average (Table 1), women were 32 years of age (range: 22–51 years), predominantly non-white Hispanic (72%), and living in poverty (66%). The mean number of years since the adversity reported in the pregnancy recorded herein was 4 years (range: 2–6 years). Participants had been exposed to, on average, 11 different types of adverse events across their lifespan (total life adversity; TLA), with 45% reporting early life adversity (ELA), and 66% reported pregnancy adversity (PA), demonstrating high but variable levels of trauma exposure across different developmental periods. ELA and PA were not significantly correlated with one another. On average, participants exhibited elevated depressive and PTSD symptoms, were overweight, and exhibited low-grade chronic inflammation at baseline, with a mean CRP level of 2.50 (SEM: 0.42, range: 0.29–11.60) mg/L.
Associations of adversity with immune biomarkers
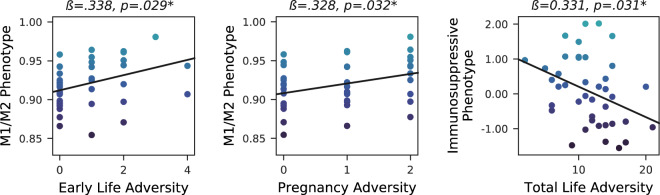
ELA and PA were each significantly associated with a higher M1/M2 RNA score (all p’s < 0.05), whereas TLA was not (Fig. 1). Conversely, higher TLA only (but not ELA or PA) was significantly related to a lower score on the endotoxin intolerance (ET) immunosuppressive phenotype.
Fig. 1. Life adversity is associated with differing immune phenotypes, depending on the timing of the exposure.
Note: *p < 0.05. The scatterplots above depict the unadjusted associations between self-reported interpersonal adversity across various periods of life in relation to normalized immune gene expression signatures, with color mapped to the y-axis biomarker. The M1/M2 phenotype refers to a relative increase in the expression of macrophage-associated pro-inflammatory M1-like genes versus anti-inflammatory and counter-regulatory M2-like genes. The Immunosuppressive phenotype represents a tolerant or desensitized state elicited by repeated cellular stressors (e.g., endotoxin), during which time the typical inflammatory response is deactivated; lower scores imply increased duration of the inflammatory response.
In contrast, neither ELA nor PA were significantly associated with serum pro-inflammatory cytokines (PRO) in any analysis (Table 2). Moreover, TLA was only significantly associated with PRO when adjusting for socioeconomic factors, such as poverty (which also exhibited a significant independent positive association with TLA), being US born, and education (both ns). Higher PA (but not ELA or TLA) was significantly associated with lower anti-inflammatory cytokines (ANTI) in unadjusted analyses; however, it became non-significant when adjusting for age, BMI, antidepressants, and ethnicity.
Table 2.
Association of adversity during sensitive periods with inflammatory biomarkers.
| Timing of adversity exposure | M1/M2-like phenotype | Endotoxin tolerance phenotype | Pro-inflammatory cytokines | Anti-inflammatory cytokines | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t-test (df) | p | β | t-test (df) | p | β | t-test (df) | p | β | t-test (df) | p | |
| Early life | ||||||||||||
| Unadjusted | 0.337 | 2.265 (40) | 0.029* | 0.066 | 0.420 (40) | 0.676 | 0.126 | 0.909 (51) | 0.368 | 0.045 | 0.321 (51) | 0.750 |
| Adjusted model 1 | 0.308 | 2.094 (36) | 0.043* | 0.064 | 0.384 (36) | 0.703 | 0.137 | 1.121 (47) | 0.268 | 0.040 | 0.311 (47) | 0.757 |
| Adjusted model 2 | 0.311 | 2.114 (37) | 0.042* | 0.084 | 0.511 (37) | 0.612 | 0.184 | 1.486 (48) | 0.144 | 0.062 | 0.442 (48) | 0.660 |
| Pregnancy | ||||||||||||
| Unadjusted | 0.332 | 2.226 (40) | 0.032* | 0.150 | 0.962 (40) | 0.342 | −0.185 | −1.344 (51) | 0.185 | −0.333 | −2.519 (51) | 0.015* |
| Adjusted model 1 | 0.404 | 2.764 (36) | 0.009** | 0.180 | 1.061 (36) | 0.296 | −0.069 | −0.529 (47) | 0.599 | −0.230 | −1.745 (47) | 0.087† |
| Adjusted model 2 | 0.324 | 2.049 (37) | 0.048* | 0.203 | 1.187 (37) | 0.243 | −0.123 | −0.933 (48) | 0.355 | −0.294 | −2.081 (48) | 0.043* |
| Total lifespan | ||||||||||||
| Unadjusted | 0.162 | 1.012 (38) | 0.318 | −0.341 | −2.239 (38) | 0.031* | 0.188 | 1.323 (48) | 0.192 | 0.045 | 0.311 (48) | 0.757 |
| Adjusted Model 1 | 0.054 | 0.315 (34) | 0.755 | −0.410 | −2.417 (34) | 0.021* | 0.135 | 1.086 (44) | 0.283 | 0.001 | −0.001 (44) | 0.999 |
| Adjusted Model 2 | 0.051 | 0.301 (35) | 0.765 | −0.415 | −2.544 (35) | 0.016* | 0.314 | 2.398 (45) | 0.021* | 0.101 | 0.661 (45) | 0.512 |
**p ≤ 0.01, *p ≤ 0.05, †p ≤ 0.10. Separate linear regression models were fitted, each specifying one inflammatory marker as the outcome and the specified life adversity factor as the independent variable. The anti-inflammatory and pro-inflammatory aggregates include the average of the blom-standardized scores for IL-1RA, IL-4, IL-10, and IL-1B, IL-6, TNF-a, and CRP respectively. Phenotype definitions are given in the methods. Adjusted model 1 included: age, BMI, and Hispanic ethnicity and current antidepressant use as covariates; adjusted model 2 included: high school education, family poverty, and being US born. Three individuals were missing data for total life adversity.
In multivariate analyses (Table 3), ELA remained significantly associated with the M1/M2 RNA score, independent of PA and PTSD or depressive symptoms (all p’s < 0.05). Of note, the association of PA with M1/M2 showed a non-significant trend in the presence of ELA (p = 0.052). Similarly, the TLA-ET association remained significant when adjusting for potentially confounding effects of ELA and PA (p = 0.031), or symptoms of PTSD or depression (p = 0.026; Table 4). Exploratory post-hoc analyses for hypothesis generation revealed that, of the genes comprising the M1/M2 phenotype, toll-like receptor 2 (TLR2) showed the highest Pearson correlation with both ELA and PA (r’s > 0.20; Supplementary Fig. 1). Due to their exploratory nature, no correction for multiple comparisons was made.
Table 3.
Univariate and multivariate associations of adversity during sensitive periods and mental health symptoms with the M1/M2 phenotype.
| M1/M2 phenotype | |||
|---|---|---|---|
| β | t-test | p-value | |
| Univariate associations (Mental health) | |||
| PTSD symptom severity (PSSI), df = 40 | 0.180 | 1.157 | 0.254 |
| Depressive symptoms (CESD-R), df = 40 | 0.074 | 0.470 | 0.641 |
| Multivariate model 1 (Adversity only, df = 39) | |||
| Early life adversity | 0.297 | 2.049 | 0.047* |
| Adversity in pregnancy | 0.291 | 2.008 | 0.052† |
| Multivariate model 2 (Adversity and mental health, both) | |||
| Early life adversity | 0.330 | 2.171 | 0.036* |
| Adversity in pregnancy | 0.312 | 1.916 | 0.063† |
| PTSD symptom severity (PSSI) | 0.123 | 0.554 | 0.583 |
| Depressive symptoms (CESD-R) | −0.224 | −1.042 | 0.304 |
Univariate associations show that neither mental health factor is significantly associated with the phenotype in regression analyses (equivalent here to Pearson correlations). Multivariate models 1 and 2 demonstrate the relative independent associations of each sensitive period from one another and also from mental health symptoms, taking into account the small sample size may limit statistical power. Correlation of PSSI and CESD-R: r = 0.731, p < 0.001; Adversity in Early Life and Pregnancy are not significantly correlated with one another. The results of Multivariate Model 2 do not substantially change when only including either PSSI or CESD-R rather than both.
PSSI PTSD symptom scale interview, total score, CESD-R Center for epidemiologic studies depression scale, total score.
*p ≤ 0.05, †p ≤ 0.10.
Table 4.
Univariate and multivariate associations of total life adversity, adversity during sensitive periods, and mental health with the endotoxin tolerance phenotype.
| Endotoxin tolerance immunosuppressive phenotype | |||
|---|---|---|---|
| β | t-test | p-value | |
| Univariate associations (Mental health) | |||
| PTSD symptom severity (PSSI), df = 40 | 0.115 | 0.730 | 0.470 |
| Depressive symptoms (CESD-R), df = 40 | 0.189 | 1.216 | 0.231 |
| Multivariate model 1 (df = 36) | |||
| Total life adversity | −0.442 | −2.607 | 0.031* |
| Early life adversity | 0.118 | 0.741 | 0.464 |
| Adversity in pregnancy | 0.213 | 1.375 | 0.178 |
| Multivariate model 2 (df = 36) | |||
| Total life adversity | −0.374 | −2.321 | 0.026* |
| PTSD symptom severity (PSSI) | 0.034 | 0.143 | 0.887 |
| Depressive symptoms (CESD-R) | 1.182 | 0.805 | 0.426 |
Univariate associations show that neither mental health factor is significantly associated with the phenotype in regression analyses (equivalent here to Pearson correlations). Multivariate models 1 and 2 demonstrate the relative independent association of total life adversity from adversity during sensitive periods and from mental health symptoms.
PSSI PTSD symptom scale interview, total score, CESD-R Center for Epidemiologic Studies depression scale, total score.
*p ≤ 0.05, †p ≤ 0.10.
Conclusions
Exposure to psychosocial adversity during sensitive periods of child and adult development exerts a particularly potent impact on health across the lifespan [1, 65–68]; however, the mechanisms by which adversity becomes “biologically embedded” remain elusive, and not all exposed individuals are equally at-risk. For Precision Medicine care, we need better screening tools that combine an assessment of adversity, through a developmental lens, with actionable biomarker panels [25]. Inflammation is a major mechanism that links adversity, during developmental inflection points, with increased health risks across the lifespan [8, 16–18]. Nonetheless, little research has validated diagnostic immune biomarkers that are specific to adversity during two crucial sensitive periods of a woman’s lifespan [69]—her early life and her own pregnancy—during which her immune system undergoes significant transformation. This study leverages a unique, cross-sectional clinical sample of low-income, ethnically diverse women with a history of exposure to interpersonal violence, to identify associations between adversity during early life and pregnancy, in comparison to total lifetime exposure, with current immune phenotypes.
This study identifies the macrophage-associated M1/M2 phenotype as a specific biomarker of exposure to adversity during early life (ELA) and during pregnancy (PA). Specifically, we found that greater ELA and greater PA were associated with a greater relative expression of M1 versus M2-like genes in CD14+ circulating monocytes, indicating a pro-inflammatory imbalance, even when adjusting for sociodemographic, medical, and psychological factors. Adding confidence to the specificity of M1/M2 as a biomarker of adversity during these two sensitive periods, the M1/M2 phenotype was not significantly associated with total lifetime adversity (TLA) exposure.
Macrophages are the primary cellular mediators of inflammation, a complex response to actual and anticipatory threats [28], involving coordinated transcriptional programs across hundreds of expressed genes [70]. Hence, inflammation cannot be meaningfully understood by one or two serum protein biomarkers. During the arc of an inflammatory response, marophage phenotypes are highly dynamic, transitioning from a pro-inflammatory M1 (classically activated) phenotype across a spectrum of anti-inflammatory/immunoregulatory M2-like (alternatively activated) phenotypes [61, 70]. Prior evidence demonstrates that a pro-inflammatory M1/M2 polarization skew is associated with heightened risk for myriad adverse clinical outcomes, including atherosclerosis, diabetes, neurodegeneration, pregnancy complications, and mental health treatment non-response [14, 43, 61, 62, 71–73]. As such, these findings demonstrating potential sensitive windows of development in the etiology of a pro-inflammatory M1/M2 phenotype have significant potential for advancing understanding of the origins of myriad disease risks.
In contrast, adversity during sensitive periods did not significantly correlate with a pro-inflammatory aggregate score, composed of CRP, IL-6, TNF-a, and IL-1ß. Despite other studies linking early life adversity and CRP [74], in the present study, only total lifetime adversity correlated with the pro-inflammatory protein profile, and only when adjusting for socioeconomic factors. Notably, the average level of the pro-inflammatory protein CRP in this sample was 2.5 mg/L (range: 0.29–11.60), which indicates prevalent low-grade inflammation associated with heightened cardiovascular risk [75]. As such, while the small sample size may have limited statistical power, nonetheless, there was good statistical distribution in the pro-inflammatory outcome, and the effect sizes were small (β < 0.20), suggesting that the M1/M2 phenotype may be a more sensitive biomarker than serum proteins.
The present findings paint pregnancy as a sensitive period in a woman’s life span for bioembedding of experience within her own immune system, rather than solely as a “vessel” for the fetus. It is established that balance of maternal M1 versus M2 phenotypes of decidual macrophages in the uterine lining plays a crucial role in a successful pregnancy, from implantation, to fetal tolerance, to parturition [14]. Other studies have reported that stress during pregnancy is cross-sectionally associated with a pro-inflammatory milieu and markers of impaired adaptive immunity [17]. In this study, women who retrospectively reported greater adversity during pregnancy not only had an increased M1/M2 skew, but also had lower levels of anti-inflammatory cytokines, at least two, and as many as 6 years after the pregnancy ended. Furthermore, this correlation was not better explained by total life adversity, which included assessment of recent stressful events. At least one prior study has reported that adversity during pregnancy was associated with epigenetic alterations consistent with an M1/M2 imbalance that persisted post-natally [51]. A second study also linked psychosocial stress during pregnancy (but not prior to pregnancy) with elevated inflammatory gene expression profiles consistent with M1/M2 imbalance [52]. Moreover, these stress-related material immunologic alterations appear to impact fetal tissue development and immune regulation [53, 54] in ways that can potentially shape neonatal development. Altogether, these findings align with recent research highlighting women’s neurobiological plasticity during pregnancy and the potential for biological embedding of stress during this time, for the mother herself, as well as for her baby [76].
These findings revealed an intriguing contrast between two macrophage-associated phenotypes with potential implications for the discovery of novel diagnostic biomarkers, experimental paradigms, and treatment approaches [77]. First, adversity during sensitive periods (but not total life adversity) was associated with an increased pro-inflammatory M1/M2 phenotype skew. Second, total adversity across the life span was associated with reduced expression of an immunosuppressive, endotoxin tolerance phenotype, validated among adults and children with sepsis [55]. Futhermore, this association remained significant, even when adjusting for sensitive period exposure.
To provide context for the significance of these findings, the body’s defense against infection balances “host resistance” with “disease tolerance”. Resistance kills or clears pathogens (e.g., via inflammatory responses), whereas, tolerance helps limit tissue damage (e.g., via prolonged inflammation) [78], and is a response to repeated exposure [79, 80]. Experimental models using a single endotoxin exposure have provided significant insights into inflammation as an important pathway by which adversity leads to mental and physical health risks [81, 82]. However, to our knowledge, this is the first time a human study has reported an association of any psychosocial factor with an endotoxin tolerance phenotype, a novel translational model for repeated adversity exposures. These findings extend prior evidence in mice reporting that chronic unpredictable stress suppressed the development of LPS-induced endotoxin intolerance, and induced heightened pro-inflammatory markers in the hippocampus [83]. Our findings may reflect a “weathering effect” [84], such that a lifetime of recurrent adversity may blunt tolerance mechanisms of cellular resilience and enable more reactive or prolonged inflammatory responses, thereby potentially accelerating disease risk.
We speculate that the association between adversity exposure during sensitive periods and the M1/M2 phenotype might be an adaptive manifestation of “trained immunity” [30, 78], a long-lasting, epigenetically-mediated form of innate immunologic memory. The M1/M2 phenotype may reflect a bias toward greater M1-mediated resistance and lower M2-mediated tolerance, although future studies would ideally validate the functional phenotype. This study’s specific M2-like signature closely follows the so-called M2a and M2b subtypes [61]. Hence, this M1/M2 signature reflects not only increased inflammatory mediators, but reduced expression of genes that code for several critical functions: (1) “self-recognition” complexes and T-helper cell signaling (Human Leukocyte Antigen-DR isotype (HLA-DR), CD80 and CD86), (2) critical activators of immunosuppressive T-regulatory cells (transforming growth factor-beta (TGF-β) and IL-10), and (3) cytokines/receptors that inhibit IL-1β-mediated inflammation (IL-1RA, IL-1R2, and IL-10) [15, 85, 86]. Reduced expression of these M2-like genes suggests that cross-talk with adaptive immune cells may be reduced, particularly immunsuppressive T-regulatory cells and T-helper cells that orchestrate the adaptive immune repertoire [87].
Crucially, although trained immunity is typically studied in vaccination and infection models, it relies on cellular stress and damage pathways that can be activated by psychological stress [28]. Exciting new evidence suggests that high catecholamine can also induce trained immunity in human monocytes both in vitro and in vivo [88], resulting in epigenetic markers (H3K4me3), as seen in vaccination models [30]. In that study, in vivo norepinephrine induction did not explicitly utilize an in vivo experimental stress model, providing an important direction for future research. Another cardinal characteristic of trained immunity is a heightened pro-inflammatory response to challenge, after an initial priming exposure [30]. Among humans and animals in vivo, experimental psychological stress tests acutely increase secretion of M1-associated pro-inflammatory and pro-atherogenic cytokines like IL-1β [82, 89] via sympathetic arousal, and enhance the binding of nuclear factor kappa-B (NF-kB) in peripheral leukocytes [28], a transcriptional regulator that induces a pro-inflammatory M1-like phenotype. Conversely, relative decreases in M2-associated IL-10, as suggested by this M1/M2 phenotype, may reduce tolerance and delay stress recovery. Consistent with the functional phenotype expected if adversity during sensitive periods results in trained immunity and long-lasting innate immunologic memory, depressed individuals with early life adversity exhibited greater inflammatory reactivity to a standardized acute stress task than similarly depressed individuals without early adversity [90]. Hence, we posit that adversity during sensitive periods “educates” the macrophage to mount a heightened inflammatory response, whereas repeated life adversity “wears down” or desensitizes the mechanisms that constrain the inflammatory response. Through a developmental perspective, these findings might suggest that an M1/M2 skew associated with adversity during sensitive periods may reflect an anticipatory adaption (intelligent resource allocation), whereas reduced ET associated with total lifetime adversity may reflect a reactionary adaption (limiting damage induced by weathering).
Strengths and limitations
These data come from a sample of diverse, low-income women with high exposure to violence and other adversities, and findings may not generalize to men or different populations of women. As a limitation, women were interviewed about adversity experienced during one particular pregnancy due to clinic-based recruitment; hence, possible additional adversity during other pregnancies was not assessed. Although other sensitive periods of development may be of interest, available research suggests monocyte/macrophages are likely to be most developmentally sensitive during early life [13] and during pregnancy [9, 14, 51]. We acknowledge that in vivo macrophage phenotypes are dynamic, multidimensional, and tissue-specific [70, 91] and may differ from over-simplified in vitro frameworks of the M1/M2 spectrum. Future studies should triangulate gene expression phenotypes with cell surface and functional phenotyping. Single cell RNA seq would be needed to resolve whether M1 and M2 expression is co-expressed on single cells or represents a shift in underlying myeloid lineage cell subsets.
We now understand that adversity exposure is as veritable a risk factor for chronic disease as poor diet or exercise; yet, we understand little about the immunological mechanisms that help explain this link. This study identifies the M1/M2 RNA phenotype as a novel and specific adulthood biomarker of adversity exposure during two sensitive developmental periods in the female lifespan and points to paths for advancing translational research targeting these mechanisms. Such discoveries may further Precision Medicine through developmentally-oriented adversity screening paired with sophisticated biomarker panels to support individuals and prevent adversity-associated morbidity.
Supplementary information
Acknowledgements
The authors would like to thank W. Thomas Boyce (UCSF), Robert Hendren (UCSF) and Elizabeth Sinclair (UCSF) for their intellectual support on this project, James Graham (UC Davis) for his expertise and support in completing the protein assays, and the Child Trauma Research Program clinicians and participating families. The research was supported by awards from the SAMHSA Grant Number 5U79SM080030 (PI Alicia Lieberman) and from the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004 to (sub PI Nicole Bush). Additional support was provided by the JPB Research Network on Toxic Stress funded by the JPB Foundation of New York (PIs W. Thomas Boyce and Nicole Bush), the Institute for Integrative Health (TIIH; PI Kirstin Aschbacher), and the Robert Wood Johnson Foundation Health Disparity Seed Grant (M Hagan). Dr. Bush is the Lisa and John Pritzker Distinguished Professor of Developmental and Behavioral Health and receives support from the John and Lisa Pritzker Family Foundation. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript; its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Author contributions
K.A. conceived of the presented idea, took the lead in writing the manuscript, conducted the statistical analyses, and programmed visualizations and data science components. N.B. initiated and carried out the study in collaboration with A.L. and K.A.; N.B, A.L., and M.H. obtained funding for the work. N.B. contributed to the integrative theory and the writing of the manuscript. S.C. supervised the RNA-seq assays and provided intellectual guidance in analyses. A.B. prepared the RNA-seq transcriptomic data for analyses and wrote the related methods. A.L. developed and led the clinical research program, and collaborated on the study design. M.H. and L.R. assisted with the clinical research program, data collection and management, provided intellectual guidance on measurement of adversity, and helped write the associated methods. A.L., M.H., E.E., I.S., and L.R. provided critical feedback and assistance with manuscript preparation.
Data and materials availability
FASTQ files are available from the NCBI Sequence Read Archive (SRA) under BioProject PRJNA626346.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01498-1.
References
- 1.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–66. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 2.Jakubowski KP, Cundiff JM, Matthews KA. Cumulative childhood adversity and adult cardiometabolic disease: a meta-analysis. Health Psychol. 2018;37:701–15. doi: 10.1037/hea0000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective-and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–71. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desplats P, Gutierrez AM, Antonelli MC, Frasch MG. Microglial memory of early life stress and inflammation: susceptibility to neurodegeneration in adulthood. Neurosci Biobehav Rev. 2019. 10.1016/j.neubiorev.2019.10.013. [DOI] [PMC free article] [PubMed]
- 5.Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. 2013;28:721–34. doi: 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–9. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 7.Danese A, Lewis J. Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma. Neuropsychopharmacology. 2017;42:99–114. doi: 10.1038/npp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berens AE, Jensen SKG, Nelson CA. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017. 10.1186/s12916-017-0895-4 [DOI] [PMC free article] [PubMed]
- 9.Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol. 2020;11:2627. doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa R, Newsom G. Proposition 56: trauma Screenings. Sacramento, 2019. www.dhcs.ca.gov. Accessed 4 Apr 2020.
- 11.Danese A, van Harmelen A-L. The hidden wounds of childhood trauma. Eur J Psychotraumatol. 2017;8:1375840. doi: 10.1080/20008198.2017.1375840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Lacey RE. Adverse childhood experiences and adult inflammation: findings from the 1958 British birth cohort. Brain Behav Immun. 2018;69:582–90. doi: 10.1016/j.bbi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Georgountzou A, Papadopoulos NG. Postnatal innate immune development: from birth to adulthood. Front Immunol. 2017;8:957. doi: 10.3389/fimmu.2017.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120. doi: 10.3389/fimmu.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson-Arvelund J, Ernerudh J. The role of macrophages in promoting and maintaining homeostasis at the fetal-maternal interface. Am J Reprod Immunol. 2015;74:100–9. doi: 10.1111/aji.12357. [DOI] [PubMed] [Google Scholar]
- 16.Davis EP, Narayan AJ. Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Dev Psychopathol. 2020;32:1625–39. doi: 10.1017/S0954579420001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veru F, Dancause K, Laplante DP, King S, Luheshi G. Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: Project Ice Storm. Physiol Behav. 2015;144:137–45. doi: 10.1016/j.physbeh.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Barak B, Feldman N, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front Neurosci. 2014;8:272. doi: 10.3389/fnins.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, et al. Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry. 2014;4:e413.. doi: 10.1038/tp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–70. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–12. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 23.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–44. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen LJH, Moffitt TE, Arseneault L, Danese A, Eugen-Olsen J, Fisher HL, et al. Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. 2020;174:38–47. doi: 10.1001/jamapediatrics.2019.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush NR, Aschbacher K. Immune biomarkers of early-life adversity and exposure to stress and violence—searching outside the streetlight. JAMA Pediatr. 2019. 10.1001/jamapediatrics.2019.3882. [DOI] [PubMed]
- 26.Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243:213–21. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, et al. PTSD blood transcriptome mega-analysis: shared inflammatory pathways across biological sex and modes of trauma. J Neuropsychopharmacol. 2017;43:469.. doi: 10.1038/npp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2016;42:36. doi: 10.1038/npp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berendsen MLT, Øland CB, Bles P, Jensen AKG, Kofoed PE, Whittle H, et al. Maternal priming: Bacillus Calmette-Guérin (BCG) vaccine scarring in mothers enhances the survival of their child with a BCG vaccine scar. J Pediatr Infect Dis Soc. 2020;9:166–72. doi: 10.1093/jpids/piy142. [DOI] [PubMed] [Google Scholar]
- 32.Moore RS, Kaletsky R, Murphy CT. Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell. 2019;177:1827–41. doi: 10.1016/j.cell.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Yang J, Wei Y, Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol. 2020;17:36–49. doi: 10.1038/s41423-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid BM, Coe CL, Doyle CM, Sheerar D, Slukvina A, Donzella B, et al. Persistent skewing of the T-cell profile in adolescents adopted internationally from institutional care. Brain Behav Immun. 2019;77:168–77. doi: 10.1016/j.bbi.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Cao X. Epigenetic regulation of the innate immune response to infection. Nat Rev Immunol. 2019. 10.1038/s41577-019-0151-6. [DOI] [PubMed]
- 36.Lisciandro JG, Van Den Biggelaar AHJ. Neonatal immune function and inflammatory illnesses in later life: lessons to be learnt from the developing world? Clin Exp Allergy. 2010;40:1719–31. doi: 10.1111/j.1365-2222.2010.03629.x. [DOI] [PubMed] [Google Scholar]
- 37.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:323.. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 38.Tang MX, Hu XH, Liu ZZ, Kwak-Kim J, Liao AH. What are the roles of macrophages and monocytes in human pregnancy? J Reprod Immunol. 2015;112:73–80. doi: 10.1016/j.jri.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev. 2012;36:350–61. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schjenken JE, Moldenhauer LM, Zhang B, Care AS, Groome HM, Chan HY, et al. MicroRNA miR-155 is required for expansion of regulatory T cells to mediate robust pregnancy tolerance in mice. Mucosal Immunol. 2020;13:1–7. doi: 10.1038/s41385-020-0255-0. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt A, Zhang XM, Joshi RN, Iqbal S, Wahlund C, Gabrielsson S, et al. Human macrophages induce CD4 + Foxp3 + regulatory T cells via binding andre-release of TGF-β. Immunol Cell Biol. 2016;94:747–62. doi: 10.1038/icb.2016.34. [DOI] [PubMed] [Google Scholar]
- 42.Álvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol. 2015;15:7–17. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 43.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. J Cell Mol Life Sci. 2015;72:4111–26. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumacher A, Sharkey DJ, Robertson SA, Zenclussen AC. Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. J Immunol. 2018;201:325–34. doi: 10.4049/jimmunol.1800058. [DOI] [PubMed] [Google Scholar]
- 45.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. nature.com. 2014. https://www.nature.com/nm/journal/v20/n7/abs/nm.3589.html. Accessed 22 Aug 2019. [DOI] [PMC free article] [PubMed]
- 46.Aschbacher K, Milush JM, Gilbert A, Almeida C, Sinclair E, Epling L, et al. Chronic stress is associated with reduced circulating hematopoietic progenitor cell number: a maternal caregiving model. Brain Behav Immun. 2017;59:245–52. doi: 10.1016/j.bbi.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF. Social stress mobilizes hematopoietic stem cells to establish persistent splenic myelopoiesis. Cell Rep. 2018;25:2552–62. doi: 10.1016/j.celrep.2018.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574–9. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 50.Solano ME, Arck PC. Steroids, pregnancy and fetal development. Front Immunol. 2020;10:3017. doi: 10.3389/fimmu.2019.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sluiter F, Incollingo Rodriguez AC, Nephew BC, Cali R, Murgatroyd C, Santos HP. Pregnancy associated epigenetic markers of inflammation predict depression and anxiety symptoms in response to discrimination. Neurobiol Stress. 2020;13:100273. doi: 10.1016/j.ynstr.2020.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross KM, Cole SW, Carroll JE, Dunkel, Schetter C. Elevated pro-inflammatory gene expression in the third trimester of pregnancy in mothers who experienced stressful life events. Brain Behav Immun. 2019;76:97–103. doi: 10.1016/j.bbi.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller GE, Borders AE, Crockett AH, Ross KM, Qadir S, Keenan-Devlin L, et al. Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav Immun. 2017;64:276–84. doi: 10.1016/j.bbi.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross KM, Carroll JE, Dunkel Schetter C, Hobel C, Cole SW. Pro-inflammatory immune cell gene expression during the third trimester of pregnancy is associated with shorter gestational length and lower birthweight. Am J Reprod Immunol. 2019;82:e13190. doi: 10.1111/aji.13190. [DOI] [PubMed] [Google Scholar]
- 55.Pena OM, Hancock DG, Lyle NH, Linder A, Russell JA, Xia J, et al. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine. 2014;1:64–71. doi: 10.1016/j.ebiom.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieberman AF, Ippen CG, Van Horn P. “Don’t Hit My Mommy!”: a manual for child-parent psychotherapy with young children exposed to violence and other trauma. Zero Three. Washington, DC, 2015.
- 57.Wolfe J, Kimerling R, Brown PJ, Chestman K, Levin K. Psychometric review of the Life Stressors Checklist-Revised. In: Stamm B, editor. Measurement of stress, trauma and adaptation. Sidrian Press: Lutherville, MD; 1996. p. 198–201.
- 58.Narayan AJ, Hagan MJ, Cohodes E, Rivera LM, Lieberman AF. Early childhood victimization and physical intimate partner violence during pregnancy: a developmental and person-oriented approach. J Interpers Violence. 2019;34:3–26. doi: 10.1177/0886260516639261. [DOI] [PubMed] [Google Scholar]
- 59.Eaton W, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). 2004. https://psycnet.apa.org/record/2004-14941-011. Accessed 4 Jun 2019.
- 60.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–73. doi: 10.1002/jts.2490060405. [DOI] [Google Scholar]
- 61.Labonte AC, Tosello-Trampont A-C, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37:275–85. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aschbacher K, Cole S, Hagan M, Rivera L, Baccarella A, Wolkowitz OM, et al. An immunogenomic phenotype predicting behavioral treatment response: toward precision psychiatry for trauma exposure. Brain Behav Immun. 2021; under review. [DOI] [PubMed]
- 63.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–67. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry. 2018;25:1–11. doi: 10.1038/s41380-018-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37:389–96. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 66.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–20. doi: 10.1017/S003329170300134X. [DOI] [PubMed] [Google Scholar]
- 67.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25:141–8. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244–6. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heim CM, Entringer S, Buss C. Translating basic research knowledge on the biological embedding of early-life stress into novel approaches for the developmental programming of lifelong health. Psychoneuroendocrinology. 2019;105:123–37. doi: 10.1016/j.psyneuen.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams H, Cassorla G, Pertsoulis N, Patel V, Vicaretti M, Marmash N, et al. Human classical monocytes display unbalanced M1/M2 phenotype with increased atherosclerotic risk and presence of disease. Int Angiol. 2017;36:145–55. doi: 10.23736/S0392-9590.16.03661-0. [DOI] [PubMed] [Google Scholar]
- 72.Funk N, Wieghofer P, Grimm S, Schaefer R, Bühring H-J, Gasser T, et al. Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov Disord. 2013;28:392–5. doi: 10.1002/mds.25300. [DOI] [PubMed] [Google Scholar]
- 73.Wang WJ, Hao CF, De Lin Q. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol. 2011;92:97–102. doi: 10.1016/j.jri.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry. 2016;21:642–9. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halcox JPJ, Roy C, Tubach F, Banegas JR, Dallongeville J, De Backer G, et al. C-reactive protein levels in patients at cardiovascular risk: EURIKA study. BMC Cardiovasc Disord. 2014;14:1–9. doi: 10.1186/1471-2261-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim P. How stress can influence brain adaptations to motherhood. Front Neuroendocrinol. 2021;60:100875. doi: 10.1016/j.yfrne.2020.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeljeli M, Riccio LGC, Doridot L, Chêne C, Nicco C, Chouzenoux S, et al. Trained immunity modulates inflammation-induced fibrosis. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-019-13636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17:83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 79.Divangahi M, Khan N, Kaufmann E. Beyond killing Mycobacterium tuberculosis: disease tolerance. Front Immunol. 2018;9:2976. doi: 10.3389/fimmu.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock REW. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–54. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 81.Miller A, Raison C. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2015;16:22. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabhar FS. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav Immun. 2012;26:346–52. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan J, Bai J, Gao C, Liang Y, Zhao B, Bian Y. Chronic unpredictable stress abrogates the endotoxin tolerance induced by repeated peripheral LPS challenge via the TLR4 signaling pathway. Neurosci Lett. 2017;645:7–13. doi: 10.1016/j.neulet.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 84.Geronimus AT, James SA, Destin M, Graham LF, Hatzenbuehler ML, Murphy MC, et al. Jedi public health: co-creating an identity-safe culture to promote health equity. SSM Popul Health. 2016;2:105–16. doi: 10.1016/j.ssmph.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taneja V, David CS. HLA transgenic mice as humanized mouse models of disease and immunity. J Clin Invest. 1998;101:921–6. doi: 10.1172/JCI2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petro TM, Chen SSA, Panther RB. Effect of CD80 and CD86 on t cell cytokine production. Immunol Invest. 1995;24:965–76. doi: 10.3109/08820139509060721. [DOI] [PubMed] [Google Scholar]
- 87.Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007;250:1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Van Der Heijden CDCC, Groh L, Keating ST, Kaffa C, Noz MP, Kersten S, et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ Res. 2020;127:269–83. doi: 10.1161/CIRCRESAHA.119.315800. [DOI] [PubMed] [Google Scholar]
- 89.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 91.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2015;6:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
FASTQ files are available from the NCBI Sequence Read Archive (SRA) under BioProject PRJNA626346.