Abstract
During liver development bipotent progenitor cells differentiate into hepatocytes and biliary epithelial cells (BECs) to ensure a functional liver required to maintain organismal homeostasis. The developmental cues controlling the differentiation of committed progenitors into these cell types, however, are incompletely understood. Here, we discover an essential role for estrogenic regulation in vertebrate liver development to affect hepatobiliary fate decisions. Exposure of zebrafish embryos to 17β-estradiol (E2) during liver development significantly decreased hepatocyte-specific gene expression, liver size, and hepatocyte number. In contrast, pharmacological blockade of estrogen synthesis or nuclear estrogen receptor signaling enhanced liver size and hepatocyte marker expression. Transgenic reporter fish demonstrated nuclear estrogen receptor activity in the developing liver. Chemical inhibition and morpholino knockdown of nuclear estrogen receptor 2b (esr2b) increased hepatocyte gene expression and blocked the effects of E2 exposure. esr2b−/− mutant zebrafish exhibited significantly increased expression of hepatocyte markers with no impact on liver progenitors, other endodermal lineages or vasculature. Significantly, E2-stimulated Esr2b activity promoted biliary epithelial differentiation at the expense of hepatocyte fate, while loss of esr2b impaired biliary lineage commitment. Chemical and genetic epistasis studies identified bone morphogenetic protein (BMP) signaling as a mediator of the estrogen effects. The divergent impact of estrogen on hepatobiliary fate was confirmed in a human hepatoblast cell line, indicating the relevance of this pathway for human liver development.
Conclusion:
Our studies identify E2, esr2b and downstream BMP activity as important regulators of hepatobiliary fate decisions during vertebrate liver development. These results have significant clinical implications for liver development in infants exposed to abnormal estrogen levels or estrogenic compounds during pregnancy.
Introduction
The liver is critical in maintaining metabolic homeostasis of the organism, regulating lipid and carbohydrate metabolism and detoxifying both endogenous and exogenous waste products throughout life. To ensure adequate function at birth, bipotential liver progenitors, hepatoblasts, differentiate into hepatocytes to perform its metabolic tasks, and into biliary epithelial cells (BEC) to transport bile. While many key factors have been identified that regulate particular aspects of liver specification and differentiation, the signaling networks that function to ensure the balanced and timely maturation of hepatoblasts into hepatocyte and biliary lineages are largely unknown.
During pregnancy, temporal and spatial expression of steroid hormones are crucial for fetal development, growth and organogenesis (1,2). Estrogen, in particular, is present throughout all stages of gestation and regulates many intrauterine processes (3). Furthermore, estrogen is necessary for proper embryonic development, as early inhibition of estrogen synthesis leads to defects in embryogenesis (4). Interestingly, excessive exposure to estrogenic compounds, such as diethylstilbestrol (DES), during pregnancy has been shown to alter fetal organogenesis and is associated with later development of cancer (5,6). While these studies indicate the potential effects of estrogenic exposure in the liver later in life, the direct impact of estrogen signaling on liver differentiation and hepatobiliary fate decisions have not been characterized.
17β-estradiol (E2) is the most prevalent and active form of estrogen in vertebrates. Classical E2 signaling involves binding of E2 to the nuclear hormone receptors estrogen receptor alpha (ESR1 or ERα) and estrogen receptor beta (ESR2 or ERβ) in the cytoplasm, which then dimerize, translocate to the nucleus, and activate transcription of target genes via estrogen response elements (ERE). While both ESR1 and ESR2 contain highly conserved DNA-binding and ligand-binding domains and interact with the same co-regulators, they possess an overlapping, yet unique, repertoire of target genes (7,8). Importantly, ESR1 and ESR2 exhibit different tissue distributions, biological functions and pathological phenotypes (9–11). During embryonic development, ESR1 and ESR2 are differentially expressed: in the human mid-gestational fetus (16–23 weeks), ESR1 is abundant in the uterus, while ESR2 is expressed in ovary, testes, adrenal gland, spleen, and liver. ESR2 expression in the embryonic liver is of particular interest (12,13), as it directly contrasts with the adult liver, which predominantly expresses ESR1 (14). These differences in estrogen receptor expression suggest the possibility for a physiological role of ESR2-specific regulation during liver development. While we have recently reported a role for E2 signaling through a G protein-coupled receptor on adult liver growth and cancer formation (15), the role of ESR2 during liver development has not been defined.
Here, we identify an essential novel function for E2 acting through Esr2b to control hepatobiliary differentiation during zebrafish liver development. Enhanced estrogen activity results in decreased liver size and hepatocyte number in a BMP-dependent fashion, while chemical inhibition of E2 synthesis or blockade of receptor signaling leads to expansion of differentiated hepatocytes. Transgenic reporter fish demonstrate activation of estrogen response elements in the liver upon E2 exposure. Chemical inhibition of Esr2, and knockdown or genetic loss of esr2b increased liver size and protected the liver from the inhibitory effects of estrogen exposure. E2/Esr2b regulated the timing and scale of hepatoblast differentiation towards hepatocytes, but had no effect on hepatoblast production or viability, other endodermal organs, or vasculature. Esr2b activity determined hepatobiliary fate decisions, as loss of esr2b enhanced hepatocyte commitment at the expense of biliary differentiation. These findings were confirmed in vitro in human induced hepatoblast culture. Together, these data elucidate a previously uncharacterized function of estrogen in a non-reproductive tissue as a key differentiation determinant during embryonic liver development.
Experimental Procedures
Zebrafish husbandry
Male and female WT Tu zebrafish, Tg(−2.8fabp10a:eGFP)as3 (4,16), abbreviated fabp10a:GFP, Tg(fabp10a:DsRed)gz15 (17) Tg(Tp1bglob:eGFP)um14 abbreviated tp1:GFP (18), Tg(flk:mCherry)is5 (19), Tg(5xERE:GFP)c262 (4,20), and Tg(hsp70l:dnXla.Bmpr1a-GFP) (21) were used.
Chemical exposures
Zebrafish embryos were exposed to chemicals for 48 hours from 24–72 hours post fertilization (hpf) and analyzed at 72hpf (unless otherwise specified). Chemicals used are listed in Supporting Table 1.
Morpholino injection
ATG morpholino oligonucleotides (MO) designed against esr1, esr2a, esr2b (Gene Tools) were previously validated (22). MOs were injected into WT Tu embryos at the one-cell stage. esr1 MO: 5’-AGGAAGGTTCCTCCAGGGCTTCTCT-3’ (10μM), esr2a MO: 5’-ACATGGTGAAGGCGGATGAGTTCAG-3’ (10μM), esr2b MO: 5’-AGCTCATGCTGGAGAACACAAGAGA-3’ (10μM).
Generation of esr2b−/− mutant
TALEs targeting endogenous esr2b were generated according to published protocols (4,23) and obtained from The Broad Institute Genetic Perturbation Platform. mRNAs of TALEN pairs were synthesized by mMESSAGE mMACHINE kit (Ambion) and injected into 1-cell stage WT embryos. Somatic mutation rate was determined from pooled-larvae by sequencing. Primers used for esr2b PCR: Forward 5’-GCCAGGGTCTCTCTTGTGTT-3’, Reverse 5’-TGACAGCTGCCACCTAAAGA-3’. F1 with mutations at TALEN-targeted site were raised and out-crossed for at least 4 generations to avoid possible TALEN-induced off-target effects.
Whole mount in situ hybridization
Embryos were fixed with 4% paraformaldehyde and performed WISH according to standard protocols (24,25) for the following markers: hepatocytes - fabp10a and transferrin, endoderm - foxa3, hepatoblasts - prox1, biliary tree - sox9, endocrine pancreas – insulin, exocrine pancreas - trypsin.
Cell culture and Immunofluorescence
iCell hepatoblasts (Cellular Dynamics International, HBC-100-020-001-PT) were maintained according to manufacturer’s protocol and exposed to DMSO, E2 (1μM, 2μM), or PHTPP (100μM, 250μM) for 48 hrs. Cells were grown in media for additional 3 days and then fixed and stained with antibodies (CK7, albumin) and nuclear staining. The antibodies used are listed in Supporting Table 2.
Expression and fluorescent image analysis
Embryos processed for in situ hybridization, as well as transgenic embryos were imaged on a Zeiss Discovery V8 stereoscope, and Zeiss LSM800 and Nikon/Yokagawa W1 confocal microscopes, respectively. Quantification of gene expression areas and organ volumes was performed using ImageJ and Imaris 9. Analyses shown in each figure represent one of ≥3 technical replicates and ≥3 biological replicates (3 different clutches of embryos).
Results
Estrogenic regulation is required for normal liver development
We previously performed a chemical genetic screen in zebrafish embryos to identify novel regulators of liver development (4,26) and uncovered several estrogen-related compounds that affected hepatogenesis (Supporting Fig. S1A). Exposure to physiological estrogens E2 and estriol, synthetic estrogens 17α-ethinylestradiol (EE2) and diethylstilbestrol, and the phytoestrogen quercetin from 24–72hpf each decreased liver-specific gene expression, as assessed by whole mount in situ hybridization (WISH) for the hepatocyte-specific gene liver fatty acid binding protein 10a (fabp10a) at 72hpf (Supporting Fig. S1A–C). In contrast, treatment with the aromatase inhibitor chrysin and the estrogen receptor antagonist tamoxifen increased the area of fabp10a expression (Supporting Fig. S1A). Exposure to the environmental estrogenic compound bisphenol A (BPA) decreased fabp10a expression. Any BPA possibly contained in the plastic Petri dishes, however, is not sufficient to affect hepatogenesis (Supporting Fig. S1D–G). In addition, exposure to steroid hormones progesterone and testosterone had no impact on liver formation (Supporting Fig. S2A), indicating the specificity of estrogen-associated signaling or transcriptional activity for this process.
To corroborate these findings and further investigate the impact of E2 on liver development, zebrafish embryos were exposed to physiological levels of E2 (22) from 24–72hpf, a time period during which hepatic specification, progenitor differentiation, and hepatocyte maturation successively take place. Alterations in liver formation were assessed by WISH for fabp10a at 72hpf, with quantification of liver area by ImageJ. Exogenous E2 exposure resulted in a dramatic reduction in liver size compared to DMSO-exposed controls (Fig. 1A,B; p<0.01). Quantitative analysis of hepatocyte number using fluorescence activated cell sorting (FACS) (Supporting Fig. S2B; p<0.001) and liver volume by confocal microscopy (Fig. 1C,D; p<0.0001) in Tg(fabp10a:GFP) reporter embryos at 72hpf confirmed a decrease in total hepatocyte number following E2 exposure. To determine whether the observed decrease in liver area was specific to endogenous estrogen activity, zebrafish were exposed to Anastrozole (ANAS), an inhibitor of the estrogen synthesis enzyme aromatase. ANAS exposure resulted in enlarged livers (Fig. 1A,B; p<0.01, Fig. 1C,D; p<0.0001), indicative of a specific role for endogenous estrogenic modulation in liver development. Taken together, these data indicate that optimal levels of both endogenous and exogenous E2 signaling are important for normal liver formation.
Figure 1. E2 signaling is required for normal embryonic liver formation.
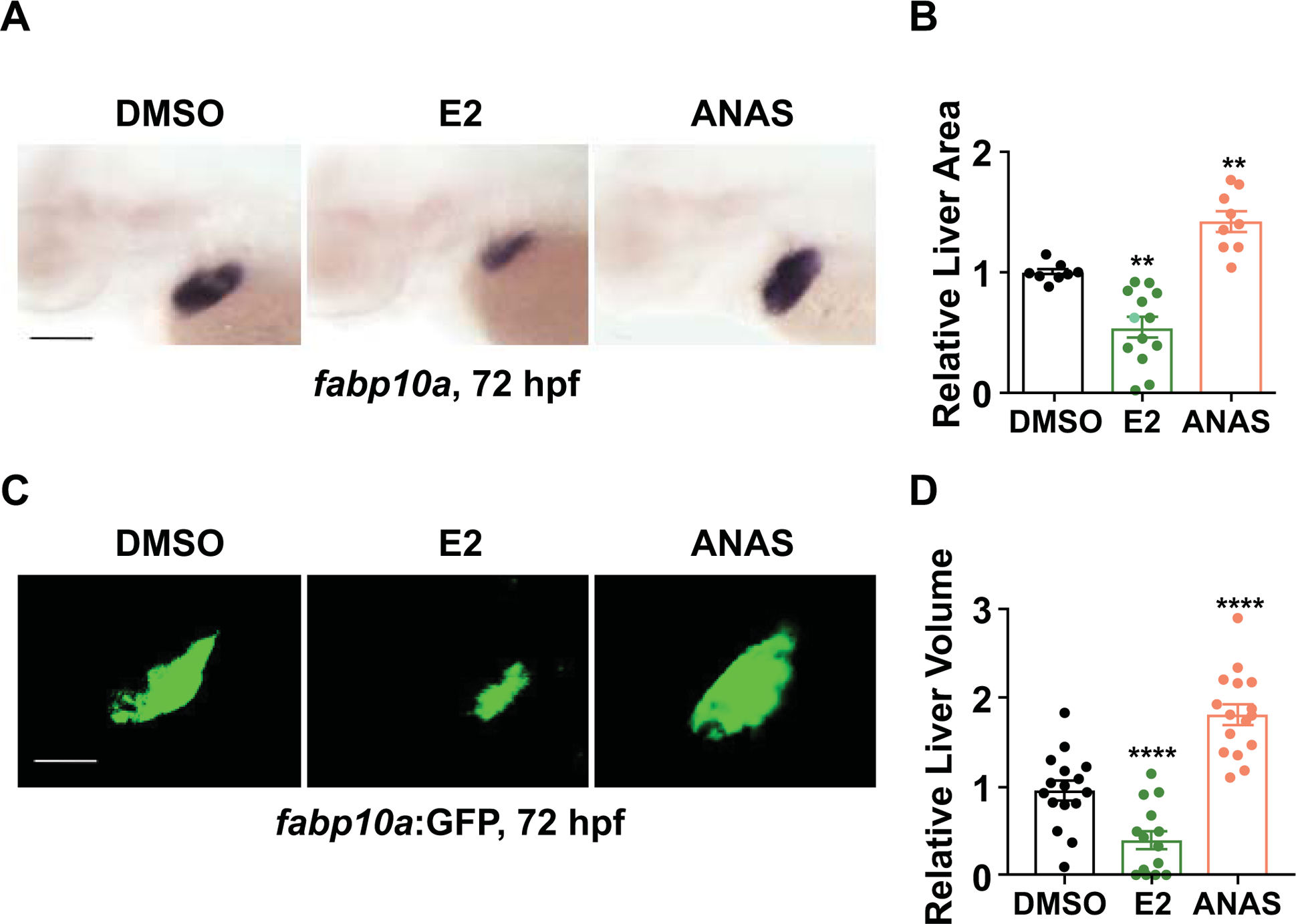
(A) Representative images of zebrafish embryos exposed to DMSO, E2 (10μM), or ANAS (10μM) from 24–72hpf. Liver size assessed by whole mount in situ hybridization (WISH) for liver fatty acid binding protein10a (fabp10a) at 72hpf. (B) Liver marker fabp10a expression area quantified by ImageJ analysis. n ≥ 8, **p<0.01, one-way ANOVA. (C) Representative images of Tg(fabp10a:GFP) embryos exposed to DMSO, E2 (10μM), or ANAS (10μM) from 24–72hpf. (D) Quantification of liver volume in Tg(fabp10a:GFP) embryos exposed to DMSO, E2, or ANAS from 24–72hpf by confocal microscopy analysis at 72 hpf. ****p<0.0001, n ≥ 10, one-way ANOVA. All values represent mean ± SEM, all scale bars, 200μm.
The effect of E2 on liver development is mediated by esr2b
Analysis of previously published microarray expression profiling of endodermal progenitors (27) indicates the presence and dynamic expression of three Esr genes, esr1, esr2a and esr2b, during liver development (Supporting Fig. S2C). To identify the specific estrogen receptor mediating the effects of E2 on early liver development, a chemical inhibitor approach was employed: embryos were exposed to E2 concomitantly with the specific ESR1 antagonist MPP or ESR2 antagonist PHTPP (24–72hpf). Embryos exposed to PHTPP alone had increased fabp10a expression at 72 hpf (p<0.0001), while MPP-exposed embryos were unchanged (Fig. 2A,B). Furthermore, PHTPP, but not MPP, blocked the effect of E2 on liver size, indicating that E2 regulates liver development via ESR2. The results of these chemical modulations were confirmed by morpholino (MO)-mediated knockdown of individual zebrafish estrogen receptors: esr2b morphants exhibited a significant increase in liver size compared to esr1 and esr2a morphants and uninjected controls, as measured by fabp10a expression area (Fig. 2C,D; p<0.0001, Supporting Fig. S2D; p<0.01). Furthermore, while E2 exposure in esr1 and esr2a morphants still reduces liver size, the negative impact of E2 exposure was significantly blunted in esr2b morphants (Fig. 2C,D, Supporting Fig. S2D), further indicating that E2 specifically acts through esr2b to regulate liver development.
Figure 2. Estrogen receptor 2b mediates impact of E2 on embryonic liver development.
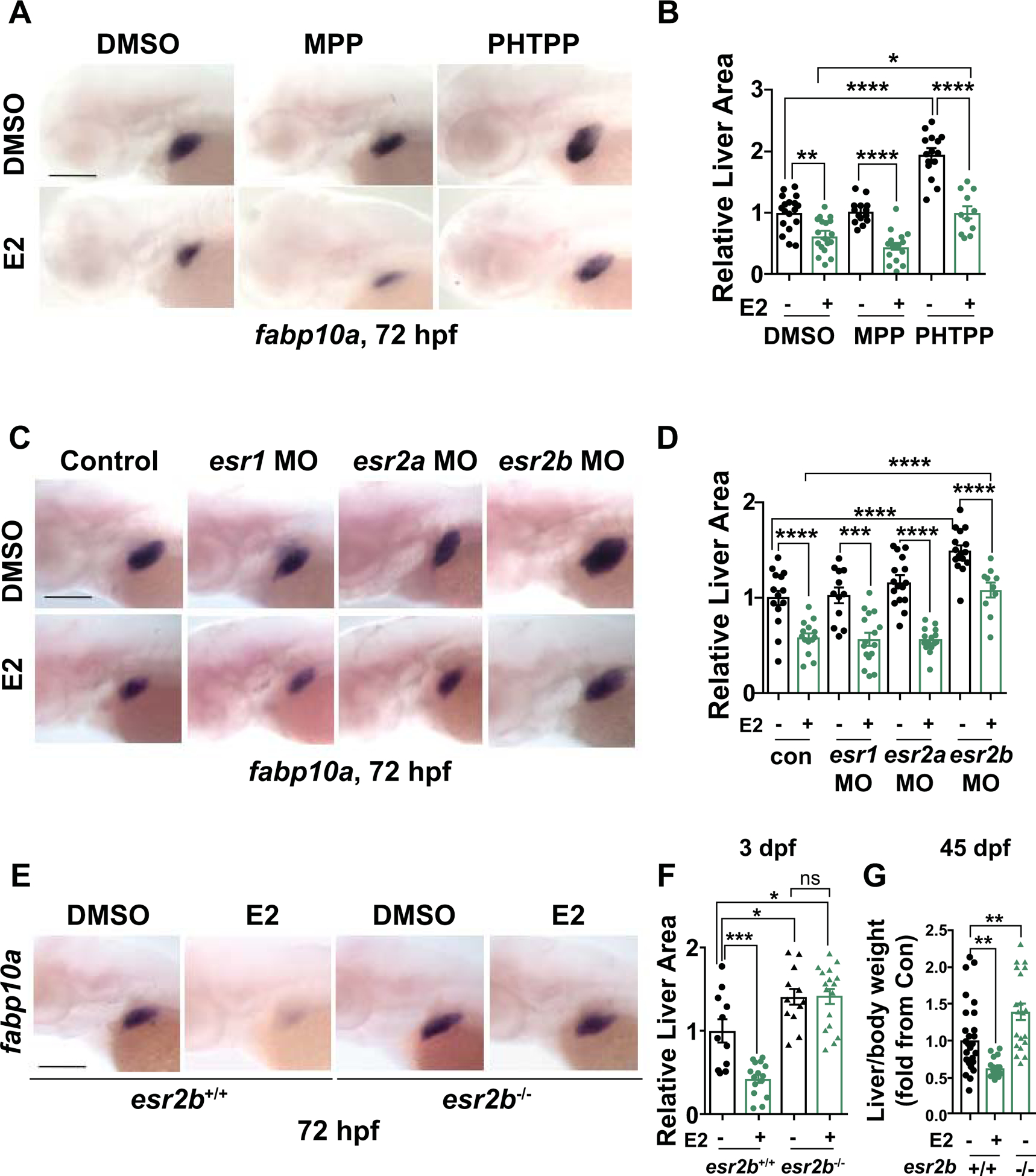
(A) Representative images of WT embryos exposed to DMSO, ESR1 antagonist (MPP), and ESR2 antagonist (PHTPP) alone or together with E2 from 24–72hpf at 72hpf. (B) Quantification of liver size by ImageJ analysis. *p<0.05, **p<0.01, ****p<0.0001, n ≥ 11, one-way ANOVA. (C) Representative images of fabp10a expression at 72hpf in esr1, esr2a, or esr2b morphants exposed to DMSO or E2 from 24–72hpf. (D) Quantification of fabp10a liver area by ImageJ analysis. ***p<0.001, ****p<0.0001, n ≥ 10, one-way ANOVA. (E) Representative images of WISH for fabp10a at 72hpf of esr2b−/− mutants and WT siblings upon exposure to DMSO or E2 from 24–72hpf. (F) Quantification of liver size at 72hpf. ns=not significant, *p<0.05, ***p<0.001, one-way ANOVA. (G) Liver/body weight of 45 dpf esr2b+/+ and esr2b−/− fish exposed to E2 from 24–72hpf, n ≥ 11, **p<0.01, one-way ANOVA. All values represent mean ± SEM, all scale bars = 200 μm.
These chemical and genetic inhibition studies prompted further examination of the developmental role of esr2b by generating esr2b knockout zebrafish using transcription activator-like effector nucleases (TALENs) (4,28). TALEN-generated esr2b mutants contain a 5-base pair deletion in the first exon, predicted to cause a premature stop codon (Supporting Fig. S2E). Indeed, compared to wild-type (WT) siblings, esr2b expression was not detected in esr2b−/− homozygous mutant embryos, indicative of complete loss of esr2b (Fig. S2F). Both esr2b+/− and esr2b−/− embryos exhibited no gross developmental abnormalities compared to their WT siblings (esr2b+/+), survived to adulthood and were fertile as described (4,29). While exhibiting normal liver histology (Supporting Fig. S2G), esr2b−/− embryos, exhibited a ~40% increase in fabp10a expression area compared to WT at 72hpf (Fig. 2E,F); further, esr2b−/− embryos completely lacked a response to E2 in the liver, confirming esr2b as the mediator of estrogenic regulation of liver development. Importantly, the negative impact on hepatocyte growth was maintained into early adulthood, leading to significantly decreased liver:body weight ratios in animals at 6 weeks that were exposed to E2 during development, while esr2b mutants exhibited increased liver:body weight ratios (Fig. 2G).
E2/esr2b signaling functions during a specific temporal window of liver development
To precisely delineate the developmental period influenced by estrogenic activity, WT embryos were exposed to DMSO and E2 at select intervals designed to target hepatic progenitor specification (18–24hpf), hepatoblast budding (24–42hpf), and hepatocyte differentiation (42–72hpf; Fig. 3A). Expression of the endodermal progenitor marker foxa3 and hepatoblast marker hhex were evaluated at 48hpf, while the hepatocyte marker fapb10a was assessed at 72hpf. E2 exposure from 18–24hpf and 24–42hpf had no or minimal effects on both foxa3 or hhex expression (Fig. 3B–F); additionally, no significant effects were observed on hepatocytes during the earliest exposure window, with modest increases in the number of embryos with reduced fabp10a expression after E2 exposure from 24–42hpf (Fig. 3E). In contrast, later developmental exposure to E2 from 42–72hpf, during the window of hepatocyte differentiation, consistently caused the most profound decrease in fapb10a expression (Fig. 3E,F; p<0.01). Importantly, E2 exposure did not alter endodermally derived exocrine or endocrine pancreatic cell populations, as assessed by WISH for trypsin and insulin at 72hpf (Supporting Fig. S3A). Together, these data indicate that E2 signaling specifically impacts the window of hepatoblast differentiation toward mature hepatocytes during liver development.
Figure 3. E2 inhibits hepatocyte differentiation.
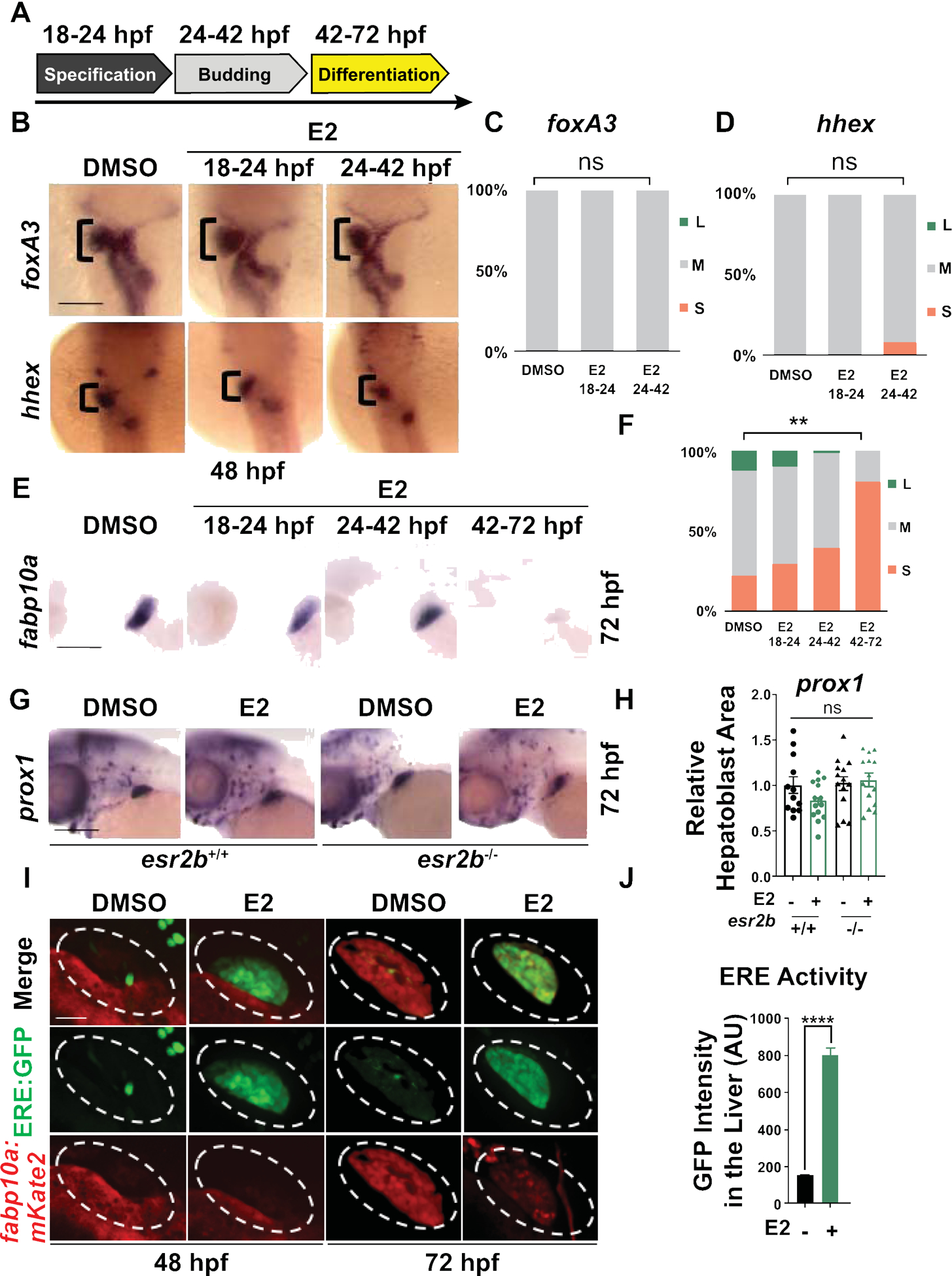
(A) Scheme illustrating E2 exposure time-windows: 18–24hpf targeting hepatic specification, 24–42hpf targeting hepatoblast budding, 42–72hpf targeting hepatoblast differentiation and maturation. (B) Endoderm (foxa3) and hepatoblast (hhex) markers expressions as assayed by WISH at 48hpf remained mostly unaffected by E2 exposures from 18–24hpf or 24–72hpf. (C-D) Distribution graph of liver size showing % of embryos with large (L, blue), medium (M, gray) or small (S, red) liver. n ≥ 20, ns=not significant, two-tailed Student’s t-test. (E) E2 exposure from 42–72hpf exposure time-window has the most significant impact on fabp10a hepatocyte marker expression. (F) Liver size distribution of embryos exposed to E2 as assessed by WISH for fabp10a at 72hpf as % of embryos with large (L, blue), medium (M, gray) or small (S, red) livers. n ≥ 51, **p<0.01, two-tailed Student’s t-test. (G) Expressions of hepatic progenitors (prox1) of esr2b+/+ and esr2b−/− embryos exposed to DMSO or E2 from 24–72hpf at 72hpf. (H) Quantification of prox1 expression at 72hpf by ImageJ. n ≥ 11, ns=not significant, two-way ANOVA. (I) Co-localization of nuclear estrogen receptor activity in hepatocytes in bigenic zebrafish Tg(fabp10a:DsRed; 5xERE:GFP) exposed to DMSO or E2 from 24–72hpf at 48 and 72hpf. Scale bar =70μm (J) Quantification of GFP intensity in the liver area (AU), ****p<0.0001, n ≥ 10, one-way ANOVA. Scale bars =200μm. All values represent mean ± SEM.
To confirm that the impact on hepatocyte differentiation was mediated by Esr2, WT and mutant embryos were exposed to E2 from 24–72hpf, and the relative areas of hepatoblast (prox1) and hepatocyte (transferrin, fabp10a) expression were quantified by WISH and ImageJ analysis at 72hpf. E2 exposure significantly reduced levels of the hepatocyte marker transferrin similarly to fabp10a (Supporting Fig. S3B,C) in WT embryos; in contrast, prox1 expression was unchanged (Figure. 3G,H). While esr2b−/− mutants exhibited increased expression of hepatocyte markers transferrin and fabp10, there was no significant impact on prox1, confirming that hepatoblast formation is not impacted by E2 signaling (Fig. 3G,H, Supporting Fig. S3B,C). These observations indicate that E2/esr2b signaling has a selective and important role in hepatoblast differentiation towards hepatocytes.
We previously demonstrated that E2 is present in the embryo during the window of liver development and significantly increased within the physiological range upon exposure to exogenous E2 (22). To examine whether Esr signaling is active in the region of the developing liver and increases in response to E2 treatment during hepatocyte differentiation, we utilized transgenic reporter zebrafish with estrogen response element (ERE)-driven GFP expression, Tg(5xERE:GFP) (4,20), crossed into a hepatocyte-specific reporter background Tg(fabp10a:DsRed). Baseline levels of ERE activation were observed in the livers of control embryos (Fig. 3I,J). E2 exposure from 24–72hpf increased ERE activity in the hepatoblast region at 48hpf as well as in hepatocytes at 72hpf, and concomitantly reduced hepatocyte-specific fluorescence (Fig. 3I,J). Together, these data demonstrate that precise E2/esr2b signaling is necessary for proper hepatocyte differentiation during liver development.
E2/esr2b signaling controls hepatobiliary fate decisions
By 72hpf, the embryonic liver is comprised of a mixture of bipotent hepatic progenitors, endothelial cells, and differentiating cells of the hepatocyte and biliary lineage. Given that modulation of E2/esr2b activity altered hepatocyte development, we sought to assess whether other hepatic cell types were also affected. We previously showed that early (12–24hpf) vascular development and specification can be influenced by estrogenic activity (22). To determine whether alterations in endothelial population contributed to the effects of E2 on liver development, endothelial-specific reporter fish Tg(flk1:mCherry) were crossed into the Tg(fabp10a:GFP) liver reporter background and exposed to E2 from 24–72hpf. Whereas hepatocyte-specific GFP expression was reduced, no alterations were observed in gross vascular structure by fluorescence imaging (Supporting Fig. S4A–C). Similarly, cloche mutants, which lack all vascular endothelium (30), demonstrated a decrease in hepatocyte-specific gene expression in response to E2 exposure compared to WT embryos (Supporting Fig. S5A,B). Consistent with prior reports indicating that endothelial cells are required for liver growth, but not specification (31,32), these findings demonstrate that the impact of E2/esr2b signaling on hepatic differentiation is independent of vasculature.
During embryonic development, bipotential hepatoblasts are specified from common endodermal precursors, which subsequently differentiate to become hepatocytes or BECs, also known as cholangiocytes. Given the strong impact of E2/esr2b on hepatocytes, we examined the contemporaneous effect on BEC differentiation within the same organism. Bigenic Tg(fabp10a:GFP; tp1blob:mCherry) embryos were examined, marking hepatocytes and BECs. E2 exposure from 24–72hpf decreased liver size (Fig. 4A,B,E; p<0.05) and enhanced BEC formation at 72hpf (Fig. 4A,C–E; p<0.0001). Consistently, esr2b knockdown dramatically reduced area and complexity of notch+ BECs (Fig. 4F,H–J; p<0.0001) despite a significant increase in overall fabp10a+ liver size (Figure. 4F,G,J; p<0.05). Similar impact on hepatobiliary differentiation was seen using sox9b as a biliary marker versus fabp10a-based hepatocyte differentiation after E2 and ESR antagonist Fulvestrant exposure (Supporting Fig. S5C,D) and in esr2b morphants assessing BEC-associated Annexin 4A via 2F11 epitope expression (33) (Supporting Fig. S5E,F; p<0.0001). Time-lapse imaging further detailed the dynamic changes in enhancing BEC growth upon E2 treatment (Fig. 4K,L, Supporting Fig. S6A, Supporting Movies 1,2). Together, these findings demonstrate that E2 controls hepatocyte-versus-biliary fate decisions during development via Esr2b.
Figure 4. E2/esr2b activity controls hepatobiliary fate decisions.
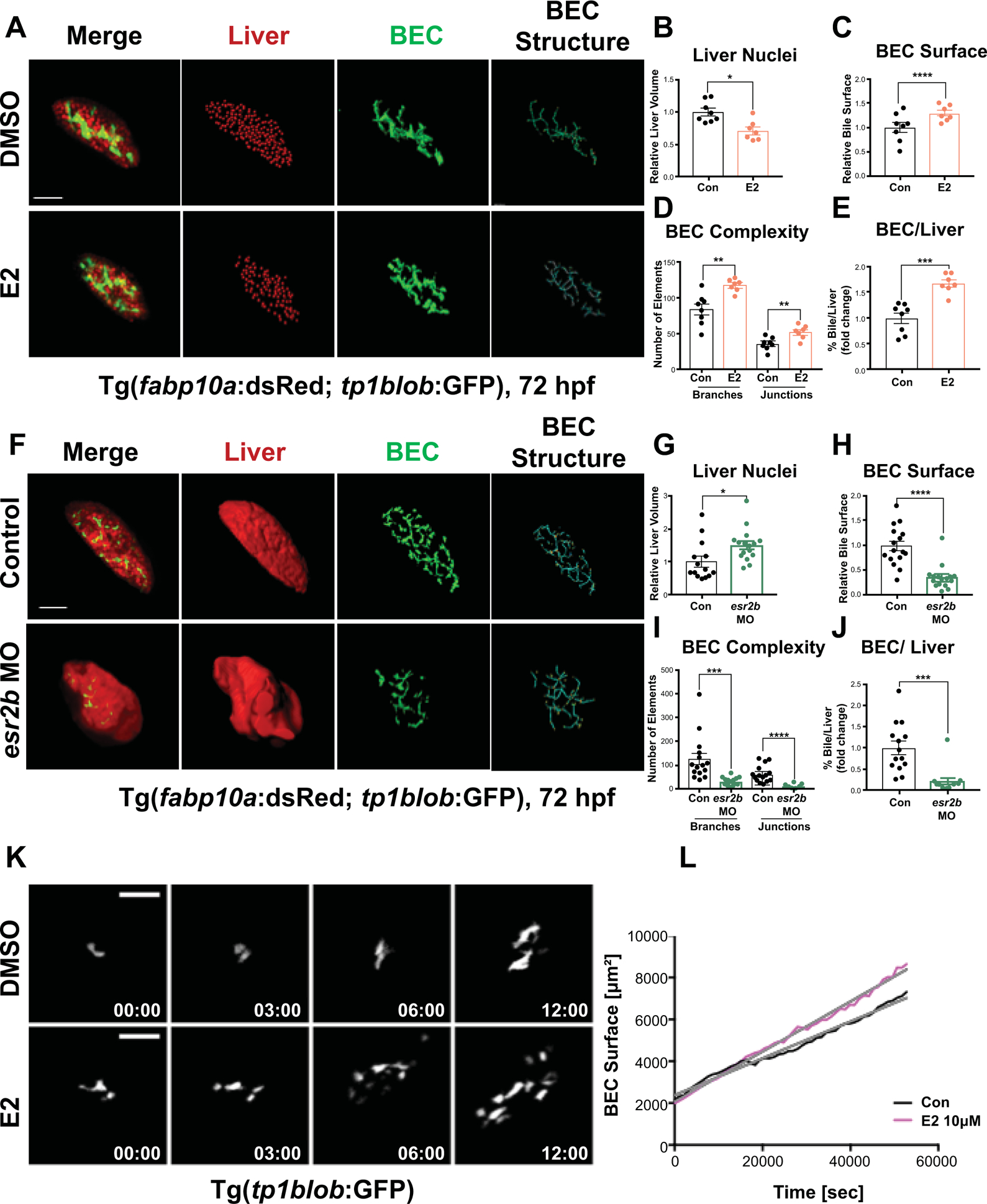
(A) Representative images of bigenic Tg(fabp10a:dsRed; tp1blob:GFP) embryos at 72hpf exposed to DMSO or E2 from 24–72hpf. Quantification of number of liver nuclei (B), Biliary surface area (C), Biliary tree complexity (D), and biliary surface area/number of liver nuclei ratio for individual embryos (E). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, one-way ANOVA. (F) Representative images of esr2b MO injected or non-injected control bigenic Tg(fabp10a:dsRed; tp1blob:GFP) embryos at 72 hpf. Quantification of number of liver nuclei (G), Biliary surface area (H), Biliary tree complexity (I), and biliary surface area/number of liver nuclei ratio for individual embryos (J). *p<0.05, ***p<0.001, ****p<0.0001, one-way ANOVA. (K) Time-lapse images showing dynamic growth of BEC in Tg(tp1blob:GFP) embryos for 16 hours from 55–72 hpf. These embryos were exposed to DMSO or E2 starting at 30 hpf. (L) Quantification of BEC surface areas (μm2) of Tg(tp1blob:GFP) embryos exposed to DMSO or E2 from 30 hpf. All images were quantified by Imaris 9 software, all values represent mean ± SEM, scale bars = 70μm.
E2/ESR2 signaling affects hepatobiliary differentiation in human hepatoblast
To directly test the impact of E2/Esr2b signaling on hepatoblast differentiation and to determine the conservation of the roles of E2 signaling in hepatobiliary development across species, we utilized bipotent hepatoblasts derived from human induced pluripotent stem (iPS) cells. These progenitor cells are capable of differentiating into hepatocytes and BEC/cholangiocytes as assayed by Albumin and cytokeratin 7 (CK7) expression (34), respectively (Fig. 5A). Upon E2 exposure for 48 hours, CK7+ BECs increased, while Albumin+ hepatocytes significantly decreased (Fig. 5B,C,D; p<0.0001). In contrast, the ESR2 antagonist PHTPP decreased CK7+ BECs, while enhancing Albumin+ hepatocytes (Fig. 5B,C,D; p<0.0001). These changes in hepatocyte and BEC populations upon E2 and PHTPP exposures occurred in a dose-dependent manner suggesting the specific impact of E2/ESR2 modulation on hepatocyte and BEC differentiation. Importantly, cell number by nuclear staining was not changed compared to DMSO-treated controls (Fig. 5B,E), demonstrating that the impact of E2 and PHTPP are specific to hepatoblast differentiation rather than overall cellular proliferation. These results reveal that E2/ESR2 regulation of hepatobiliary fate decisions of hepatoblasts is conserved across vertebrate species.
Figure 5. E2/ESR2 signaling affects hepatobiliary differentiation in human hepatoblasts.
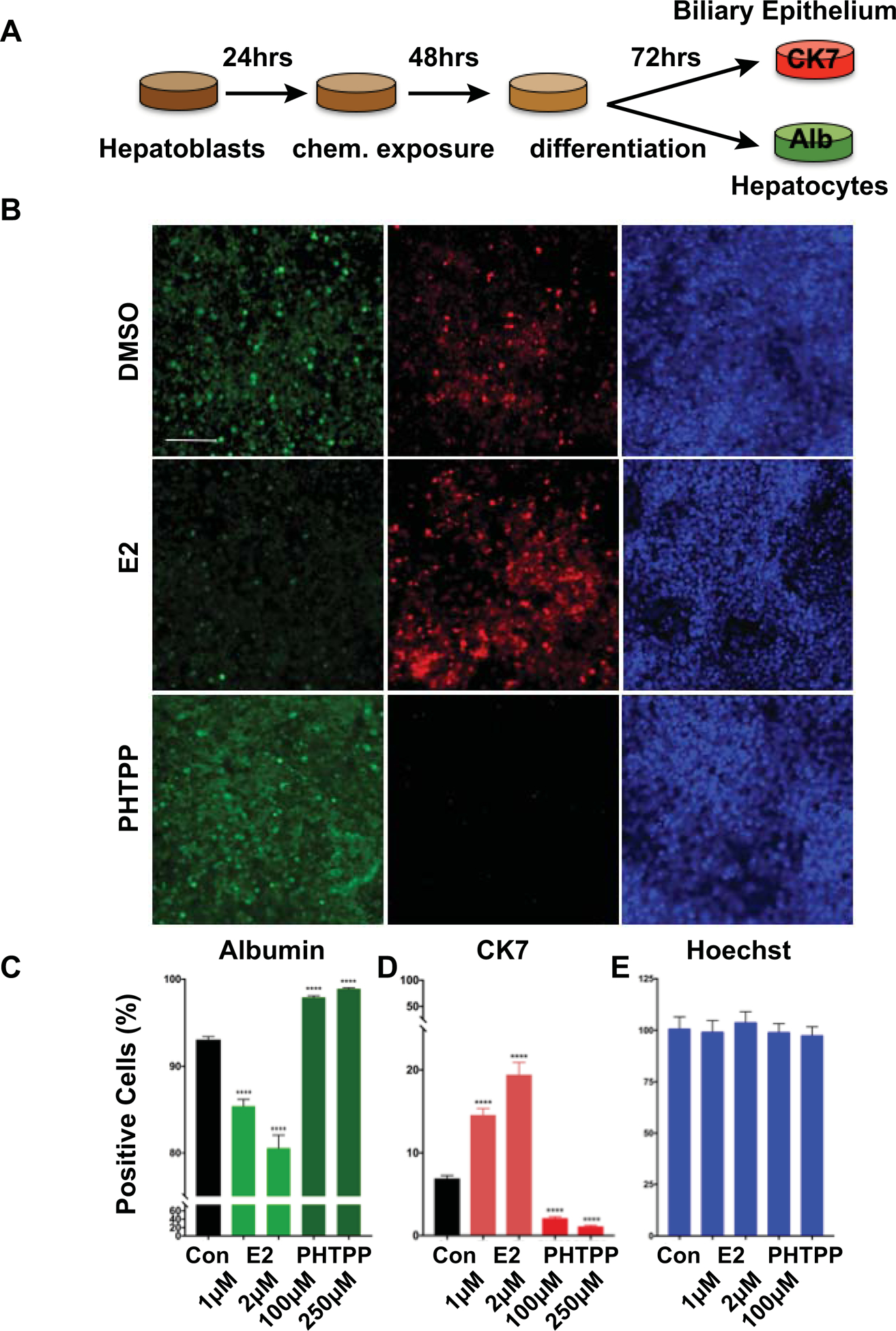
(A) Schematic illustration of the human hepatoblast differentiation experiment. Human hepatoblasts were cultured for 24 hours prior to exposure to E2 (1μM, 2 μM) or PHTPP (100nM, 250nM) for 48 hours. The culture was allowed to grow for another 72 hours before immunofluorescent analysis labeling CK7 (BEC) and Albumin (hepatocyte). (B) Representative images of cells after immunofluorescence marking hepatocytes (Albumin), BEC (CK7), and DNA (Hoechst). Quantification of Albumin+ (C), CK7+ (D), and Hoechst+ (E) cells in E2 or PHTPP treated hepatoblasts relative to DMSO treated controls. ****p<0.0001, one-way ANOVA. All values represent mean ± SEM, scale bar = 100 μm.
E2 signals through BMP pathway to impact hepatobiliary development
To delineate the downstream signaling pathways mediating E2 effects on hepatobiliary differentiation, we utilized a targeted approach: Bone morphogenetic proteins (BMPs) signaling has been shown to be activated downstream of ESR in osteoblasts and mesenchymal stem cells (35,36). We exposed zebrafish to selective BMP inhibitors, Dorsomophin and K02288, to determine the involvement of BMP signaling in E2-mediated impact on hepatobiliary differentiation. Inhibition of BMP during hepatobiliary differentiation had minimal impact on liver and BEC formation compared to DMSO-treated embryos (Fig. 6A–C,E–F). Co-exposure of E2 with Dorsomorphin or K02288 from 42–72hpf, however, significantly blocked the E2-mediated reduction in liver size (Fig. 6A–C; p<0.001, 6E–F; p<0.0001, Supporting Fig. S7A; p<0.01) and normalized the E2-mediated increase in BECs (Fig. 6B,C; p<0.001). Western blot for p-Smad confirmed specific activation of BMP signaling in E2-exposed embryos (Supporting Fig. S7B). Increased p-Smad levels were normalized when E2 was co-exposed with Dorsomorphin. To confirm these results, we employed Tg(hsp70l:dnBmpr-GFP) (dnBmpr) zebrafish that express a dominant-negative form of the BMP receptor upon heat-shock activation (21). dnBmpr was induced at 28hpf, prior to the onset of hepatoblast differentiation, and subsequently at 44hpf and 56hpf to achieve sustained expression throughout liver differentiation. Heat-shocked embryos displayed normal liver formation at 72hpf and significantly mitigated the E2 impact on liver size (Fig. 6G,H; p<0.0001). These data indicate that E2 specifically activates BMP signaling pathway to promote BEC differentiation at the expense of hepatocytes.
Figure 6. E2 signals through BMP pathway to impact hepatobiliary development.
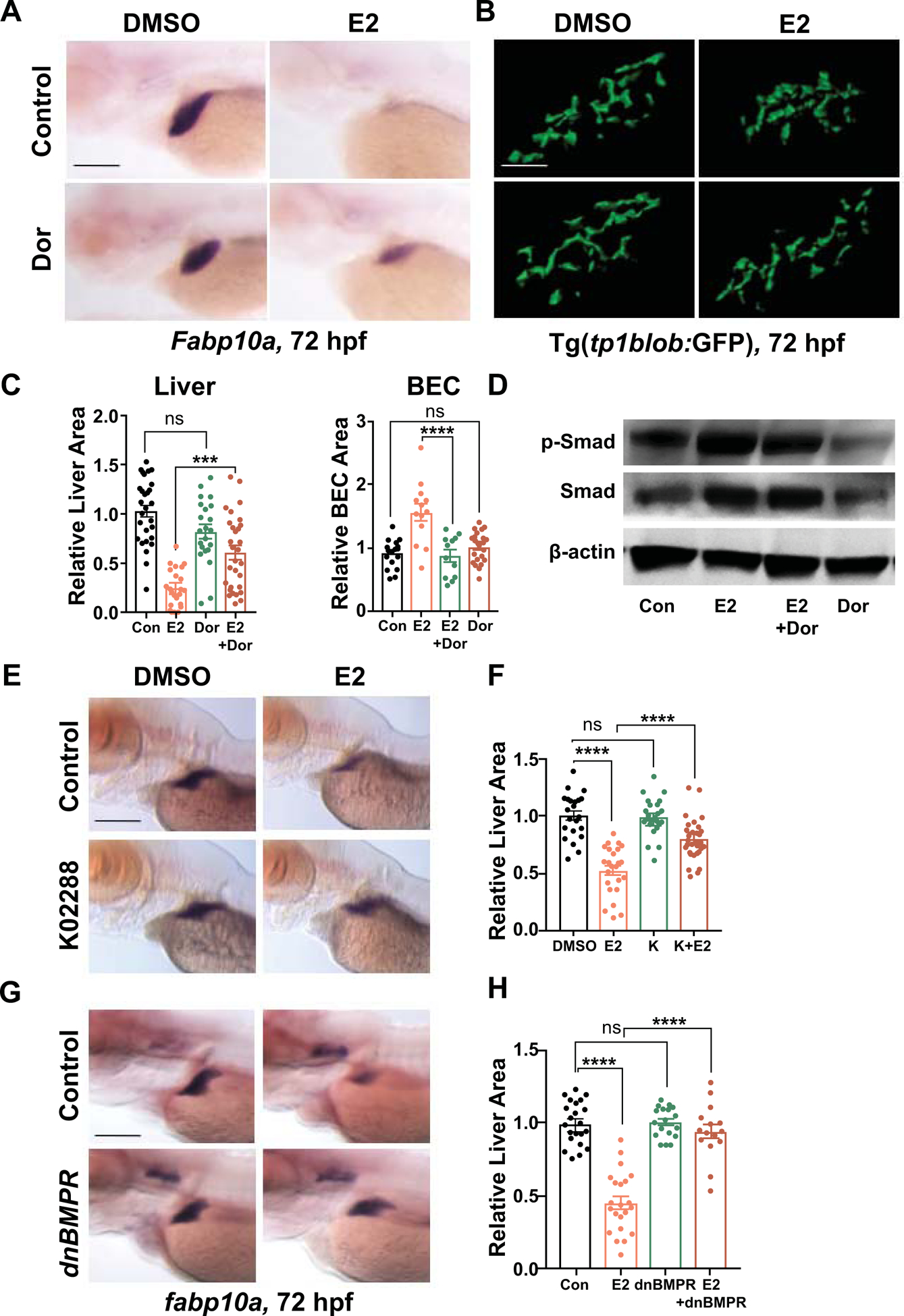
(A) Representative images of zebrafish embryos exposed to DMSO, E2 (10 μM), Dorsomorphin (10μM), or E2+Dorsomophin from 24–72hpf at 72hpf. Liver size assessed by WISH for fabp10a at 72 hpf. (B) Confocal images of BEC surface area of Tg(tp1blob:GFP) embryos exposed to DMSO, E2 (10μM), Dorsomorphin (10μM), or E2+Dorsomophin from 24–72hpf at 72hpf. Scale bar = 40μm. (C) Liver area quantified by ImageJ analysis and BEC surface area quantified by Imaris 9 imaging software. n ≥ 12, ns=not significant, ***p<0.001, ****p<0.0001, one-way ANOVA. (D) Immunoblot of p-Smad, total Smad, and β-actin of embryos treated with DMSO, E2 (10μM), Dorsomorphin (10μM), or E2+Dorsomophin from 24–72 hpf at 72 hpf. (E) Representative images of embryos exposed to DMSO, E2 (10μM), K02288 (5μM), or E2+K02288 from 24–72hpf at 72hpf. (F) Quantification of liver area at 72hpf. ns=not significant, ****p<0.0001, n ≥ 22, one-way ANOVA. (G) Representative images of dnBMPR embryos exposed to DMSO or E2 (10μM), from 24–72hpf at 72hpf. (H) Quantification of liver area at 72hpf. ns=not significant, ****p<0.0001, n ≥ 15, one-way ANOVA. All values represent mean ± SEM, scale bars = 200μm.
Discussion
In this study, we employed a chemical genetic screening approach to discover a novel role for estrogen during liver development. Optimal levels of E2 and Esr2b activity are essential for normal liver development, specifically during the time window of hepatobiliary differentiation. Importantly, elevated E2 activity results in enhanced BMP signaling to impair hepatocyte differentiation and enhance biliary lineage development, while inhibition of E2 or loss of esr2b directs hepatoblast differentiation towards hepatocytes at the expense of biliary lineage.
Estrogen regulates hepatobiliary fate decisions
In the liver, hepatocytes and BECs arise from the same hepatic progenitor pool (37). While several pathways have been described that are important for the differentiation of either hepatocytes or BECs, only very few signals have been found to affect hepatobiliary fate decisions in embryonic hepatoblasts. The transcription factor Sall4 promoted biliary over hepatocyte fate in isolated murine hepatoblasts by enhancing BEC proliferation, while suppressing hepatic differentiation (4,38). Conversely, loss of Tbx3 in murine embryos resulted in impaired hepatocyte differentiation with increased BEC differentiation (4,39). These studies, highlighting transcription factors acting in opposite directions, together with our own findings, indicate the critical need for a precisely balanced and tightly regulated hepatobiliary differentiation process. Furthermore, prior studies suggest the relevance of E2 signaling for BEC proliferation: adult vertebrate animal studies have shown differential expressions of Esr subtypes in distinct adult liver cell populations with Esr2 being absent in hepatocytes, but highly expressed in BECs (14). Functionally, Esr2 has been shown to promote proliferation of biliary cells, and its high expression levels have been implicated in biliary cirrhosis and cholangiocarcinoma (4,40). BMPs are part of the Transforming Growth Factor-β superfamily of proteins and play crucial roles during embryogenesis, including gastrulation, hepatic specification (41), and liver regeneration (42). Shin et al. describe a required role for BMP signaling and alk8 activity during hepatoblast formation (41) and in biliary-driven liver regeneration (42). While our data demonstrate BMP activation in response to E2 exposure, there was no effect of E2 signaling on hepatoblasts, demonstrating the importance of precise timing of developmental signals for cell-specific effects, and further demonstrate the complexity of BMP signaling in various contexts.
Estrogen signaling modulates hepatocyte differentiation in vitro
Currently, the only clinically viable approach to acute and acute-on-chronic liver failure is liver transplantation. While theoretically attractive (43), fully functioning human hepatocytes cannot be generated in vitro at scale for both therapeutic and investigational purposes. While several protocols have been generated to produce hepatocyte-like cells from various progenitor populations, these cells do not capture the complexity of metabolic and synthetic functions of mature hepatocytes (44–46). Our previous work has highlighted the impact of chemical screens to improve in vitro differentiation and proliferation of hepatocytes (47). Our current data demonstrate increased hepatocyte differentiation from human bipotent hepatoblasts, generated from iPSCs, with chemical blockade of ESR2, arguing further for the importance of developmental signals for in vitro differentiation of functional hepatocytes.
Embryonic estrogen signaling and adult diseases
While alteration of E2 signaling during gestation can directly affect embryonic organogenesis, it may also lead to developmental reprogramming, resulting in long-lasting impact on adult homeostasis. Multiple studies have shown that maternal exposure to high levels of environmental estrogenic compounds led to fetal epigenetic reprogramming that predisposed these offspring to diseases in adulthood. Prior studies have noted the plasticity of the ER regulatory region epigenomes as they could be altered upon prenatal exposure to estrogenic compounds such as BPA (48). Interestingly, our results revealed the long-term impact of E2 exposure during liver development in adult liver size. Given that epigenetic modifications take place throughout organ differentiation and that epigenetic reprogramming during organogenesis can affect disease susceptibility in adulthood, it is tempting to speculate that the observed effect of E2 signaling on hepatocyte differentiation could imprint a long-term influence on adult liver functions and homeostasis.
In summary, our work reveals a novel and unexpected role for estrogen in regulating hepatobiliary fate during liver development. Our findings are of immediate relevance for human physiology, and in particular for improving protocols for in vitro differentiation of hepatoblasts for research and clinical purposes and for anticipating the increasing impact of environmental exposure to estrogenic pollutants. Further studies will be needed to prospectively determine the long-term impact of embryonic estrogenic exposure on liver function, disease, and regenerative capacity.
Supplementary Material
Acknowledgements:
We thank staff of the Beth Israel Deaconess Medical Center zebrafish facility for zebrafish husbandry, and Arnaud Menuet (esr2b) and John Postlethwait (sox9b) for in situ probes.
Financial Support
This work was supported by NIH R01DK090311, R01DK105198, R24OD017870 (W.G.), the Harvard Digestive Diseases Center (P30 DK034854 W.G.) and the Harvard Stem Cell Institute (M.K.G., W.G.). W.G. is a Pew Scholar in the Biomedical Sciences. There are no conflicts of interest to report.
List of Abbreviations
- ANAS
anastrozole
- BEC
biliary epithelial cell
- BMP
Bone morphogenetic protein
- BPA
bisphenol A
- DES
diethylstilbestrol
- dlc
delta C
- DMSO
dimethylsulfoxide
- E2
17β estradiol
- EE2
ethinyl estradiol
- ESR
estrogen receptor
- ERE
estrogen response element
- fabp10a
fatty acid binding protein 10a
- FACS
fluorescence activated cell sorting
- flk
fetal liver kinase
- foxa3
forkhead box A3
- FUL
fulvestrant
- hhex
hematopoietically-expressed homeobox
- iPSC
induced pluripotent stem cell
- MO
morpholino
- prox1
prospero homeobox 1
- SALL4
Spalt Like Transcription Factor 4
- Sox9
SRY Box9
- TALEN
transcription activator-like effector nuclease
- Tbx3
T-Box3
- tfa
transferrin A
- WISH
whole mount in situ hybridization
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/HEP.31184
References:
- 1.Mesiano S, and Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. United States; 1997;18(3):378–403. [DOI] [PubMed] [Google Scholar]
- 2.Thompson A, Han VKM, and Yang K. Spatial and temporal patterns of expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 messenger RNA and glucocorticoid receptor protein in the murine placenta and uterus during late pregnancy. Biol Reprod. United States; 2002;67(6):1708–18. [DOI] [PubMed] [Google Scholar]
- 3.Wu JT, and Doong RL. Effect of antiestrogen CI-628 on the morphology and 17 beta-hydroxysteroid dehydrogenase activity of mouse blastocysts in culture. Contraception. United States; 1984;30(3):271–8. [DOI] [PubMed] [Google Scholar]
- 4.Wu JT, and Doong RL. Effect of antiestrogen CI-628 on the morphology and 17 beta-hydroxysteroid dehydrogenase activity of mouse blastocysts in culture. Contraception. United States; 1984;30(3):271–8. [DOI] [PubMed] [Google Scholar]
- 5.Bibbo M, Al-Naqeeb M, Baccarini I, Gill W, Newton M, Sleeper KM, Sonek RN, and Wied GL. Follow-up study of male and female offspring of DES-treated mothers a preliminary report. J Reprod Med. United States; 1975;15(1):29–32. [PubMed] [Google Scholar]
- 6.Ostrander PL, Mills KT, and Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst. United States; 1985;74(1):121–35. [PubMed] [Google Scholar]
- 7.Liu Y, Gao H, Marstrand TT, Ström A, Valen E, Sandelin A, Gustafsson J-A, and Dahlman-Wright K. The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci U S A. United States; 2008;105(7):2604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JM, Couse JF, and Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. United States; 2001;276(40):36869–72. [DOI] [PubMed] [Google Scholar]
- 9.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, and Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. England; 2000;127(19):4277–91. [DOI] [PubMed] [Google Scholar]
- 10.Couse JF, and Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. United States; 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 11.Liu M-M, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, and Kushner PJ. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. United States; 2002;277(27):24353–60. [DOI] [PubMed] [Google Scholar]
- 12.Brandenberger AW, Tee MK, Lee JY, Chao V, and Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. United States; 1997;82(10):3509–12. [DOI] [PubMed] [Google Scholar]
- 13.Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, and Sasano H. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. J Clin Endocrinol Metab. United States; 2001;86(5):2258–62. [DOI] [PubMed] [Google Scholar]
- 14.Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F, and Gaudio E. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. United States; 2000;119(6):1681–91. [DOI] [PubMed] [Google Scholar]
- 15.Chaturantabut S, Shwartz A, Evason KJ, Cox AG, Labella K, Schepers AG, Yang S, Acuña M, Houvras Y, Mancio-Silva L, Romano S, Gorelick DA, Cohen DE, Zon LI, Bhatia SN, North TE, and Goessling W. Estrogen Activation of G-Protein-Coupled Estrogen Receptor 1 Regulates Phosphoinositide 3-Kinase and mTOR Signaling to Promote Liver Growth in Zebrafish and Proliferation of Human Hepatocytes. Gastroenterology. United States; 2019;156(6):1788–1804.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Her GM, Chiang C-C, Chen W-Y, and Wu J-L. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett. England; 2003;538(1–3):125–33. [DOI] [PubMed] [Google Scholar]
- 17.Farooq M, Sulochana KN, Pan X, To J, Sheng D, Gong Z, and Ge R. Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Dev Biol. United States; 2008;317(1):336–53. [DOI] [PubMed] [Google Scholar]
- 18.Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, and Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. Ireland; 2009;126(10):898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M, and Essner JJ. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. England; 2010;137(18):3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelick DA, and Halpern ME. Visualization of estrogen receptor transcriptional activation in zebrafish. Endocrinology. United States; 2011;152(7):2690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, and Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. England; 2006;133(11):2275–84. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KJ, Esain V, Garnaas MK, Cortes M, Dovey MC, Nissim S, Frechette GM, Liu SY, Kwan W, Cutting CC, Harris JM, Gorelick DA, Halpern ME, Lawson ND, Goessling W, and North TE. Estrogen defines the dorsal-ventral limit of VEGF regulation to specify the location of the hemogenic endothelial niche. Dev Cell. United States; 2014;29(4):437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, and Zhang F. A transcription activator-like effector toolbox for genome engineering. Nat Protoc. England; 2012;7(1):171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thisse C, and Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. England; 2008;3(1):59–69. [DOI] [PubMed] [Google Scholar]
- 25.Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis A-P, Puder M, Clevers H, Moon RT, and Zon LI. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. United States; 2008;320(1):161–74. [DOI] [PubMed] [Google Scholar]
- 26.Garnaas MK, Cutting CC, Meyers A, Kelsey PB, Harris JM, North TE, and Goessling W. Rargb regulates organ laterality in a zebrafish model of right atrial isomerism. Dev Biol. United States; 2012;372(2):178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuckenholz C, Lu L, Thakur P, Kaminski N, and Bahary N. FACS-assisted microarray profiling implicates novel genes and pathways in zebrafish gastrointestinal tract development. Gastroenterology. United States; 2009;137(4):1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, and Yeh J-RJ. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature biotechnology. NIH Public Access; 2011;29(8):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H, Cui Y, Jiang L, and Ge W. Functional Analysis of Nuclear Estrogen Receptors in Zebrafish Reproduction by Genome Editing Approach. Endocrinology. United States; 2017;158(7):2292–2308. [DOI] [PubMed] [Google Scholar]
- 30.Reischauer S, Stone OA, Villasenor A, Chi N, Jin S-W, Martin M, Lee MT, Fukuda N, Marass M, Witty A, Fiddes I, Kuo T, Chung W-S, Salek S, Lerrigo R, Alsiö J, Luo S, Tworus D, Augustine SM, Mucenieks S, Nystedt B, Giraldez AJ, Schroth GP, Andersson O, and Stainier DYR. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature. England; 2016;535(7611):294–8. [DOI] [PubMed] [Google Scholar]
- 31.Korzh S, Pan X, Garcia-Lecea M, Winata CL, Pan X, Wohland T, Korzh V, and Gong Z. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. England; 2008;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi TF, Sadler KC, Crosnier C, and Stainier DYR. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol. England; 2008;18(20):1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorent K, Moore JC, Siekmann AF, Lawson N, and Pack M. Reiterative use of the notch signal during zebrafish intrahepatic biliary development. Dev Dyn. United States; 2010;239(3):855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saborowski A, Wolff K, Spielberg S, Beer B, Hartleben B, Erlangga Z, Becker D, Dow LE, Marhenke S, Woller N, Unger K, Schirmacher P, Manns MP, Marquardt JU, Vogel A, and Saborowski M. Murine Liver Organoids as a Genetically Flexible System to Study Liver Cancer InVivoand In Vitro. Hepatol Commun. United States; 2019;3(3):423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee W, Ko KR, Kim H-K, Lim S, and Kim S. Dehydrodiconiferyl alcohol promotes BMP-2-induced osteoblastogenesis through its agonistic effects on estrogen receptor. Biochem Biophys Res Commun. United States; 2018;495(3):2242–2248. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Turgeman G, Harris SE, Leitman DC, Komm BS, Bodine PVN, and Gazit D. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Mol Endocrinol. United States; 2003;17(1):56–66. [DOI] [PubMed] [Google Scholar]
- 37.Zong Y, and Stanger BZ. Molecular mechanisms of liver and bile duct development. Wiley Interdiscip Rev Dev Biol. United States; 2012;1(5):643–55. [DOI] [PubMed] [Google Scholar]
- 38.Oikawa T, Kamiya A, Kakinuma S, Zeniya M, Nishinakamura R, Tajiri H, and Nakauchi H. Sall4 regulates cell fate decision in fetal hepatic stem/progenitor cells. Gastroenterology. United States; 2009;136(3):1000–11. [DOI] [PubMed] [Google Scholar]
- 39.Lüdtke TH-W, Christoffels VM, Petry M, and Kispert A. Tbx3 promotes liver bud expansion during mouse development by suppression of cholangiocyte differentiation. Hepatology. United States; 2009;49(3):969–78. [DOI] [PubMed] [Google Scholar]
- 40.Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Folli F, Attili AF, and Gaudio E. Alfa and beta estrogen receptors and the biliary tree. Mol Cell Endocrinol. Ireland; 2002;193(1–2):105–8. [DOI] [PubMed] [Google Scholar]
- 41.Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, and Stainier DYR. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. England; 2007;134(11):2041–50. [DOI] [PubMed] [Google Scholar]
- 42.Choi T-Y, Khaliq M, Tsurusaki S, Ninov N, Stainier DYR, Tanaka M, and Shin D. Bone morphogenetic protein signaling governs biliary-driven liver regeneration in zebrafish through tbx2b and id2a. Hepatology. United States; 2017;66(5):1616–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squires JE, Soltys KA, McKiernan P, Squires RH, Strom SC, Fox IJ, and Soto-Gutierrez A. Clinical Hepatocyte Transplantation: What Is Next? Curr Transplant Rep. Switzerland; 2017;4(4):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Si-Tayeb K, Lemaigre FP, and Duncan SA. Organogenesis and development of the liver. Dev Cell. United States; 2010;18(2):175–89. [DOI] [PubMed] [Google Scholar]
- 45.Baxter M, Withey S, Harrison S, Segeritz C-P, Zhang F, Atkinson-Dell R, Rowe C, Gerrard DT, Sison-Young R, Jenkins R, Henry J, Berry AA, Mohamet L, Best M, Fenwick SW, Malik H, Kitteringham NR, Goldring CE, Piper Hanley K, Vallier L, and Hanley NA. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. Netherlands; 2015;62(3):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jozefczuk J, Prigione A, Chavez L, and Adjaye J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev. United States; 2011;20(7):1259–75. [DOI] [PubMed] [Google Scholar]
- 47.Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA, North TE, Goessling W, Carpenter AE, and Bhatia SN. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. United States; 2013;9(8):514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos JG, Varayoud J, Kass L, Rodríguez H, Costabel L, Muñoz-De-Toro M, and Luque EH. Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. United States; 2003;144(7):3206–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


