Abstract
Advances in microscale 3D cell culture systems have helped to elucidate cellular physiology, understand mechanisms of stem cell differentiation, produce pathophysiological models, and reveal important cell-cell and cell-matrix interactions. An important consideration for such studies is the choice of material for encapsulating cells and associated extracellular matrix (ECM). This review focuses on the use of alginate hydrogels which are versatile owing to their simple gelation process following an ionic crosslinking mechanism in situ, with no need for procedures that can be potentially toxic to cells such as heating, the use of solvents, and UV exposure. The article aims to give some perspectives, particularly to researchers who typically work more with poly(dimethylsiloxane) (PDMS), on the use of alginate as an alternative material to construct microphysiological cell culture systems. More specifically, this review describes how physicochemical characteristics of alginate hydrogels can be tuned with regards to their biocompatibility, porosity, mechanical strength, ligand presentation, and biodegradability. A number of cell culture applications are also described, and these are subcategorized according to whether the alginate material is used to homogeneously embed cells, to micropattern multiple cellular microenvironments, or to provide an outer shell that creates a space in the core for cells and other ECM components. The article ends with perspectives on future challenges and opportunities for 3D cell culture applications.
Keywords: alginate, three-dimensional, cell culture, microencapsulation, biomaterials
Graphical Abstract
1. Introduction
Microscale three-dimensional (3D) cellular systems allow the study of biological processes that involve the interplay among cells, their surrounding environment, and different external physical and chemical stimuli.1–3 In multicellular organisms, cells in nearly all tissues (and organs) reside in complex 3D networks of macromolecules, known as the extracellular matrix (ECM), that provide structural scaffolding and regulate intercellular communication. The ECM modulates a cell’s dynamic behavior, and is therefore important for morphology, differentiation, proliferation, evolution, and function of the cells and tissues.4,5 In addition to the complexity of ECM components, it is also important to appreciate the diversity among different tissues, as cells of different types require significantly different microenvironments. Although microscale 2D cell culture systems, often comprised of poly(dimethylsiloxane) (PDMS) devices, have considerably improved the understanding of basic cell functions, cell growth and manipulation in 2D can be insufficient to provide physiologically accurate representations of the in vivo microenvironment of many cells and tissues.6–8
3D cell culture systems are often better in vitro models for investigating aspects of the in vivo microenvironment compared to their 2D counterparts.9–11 Table 1 summarizes some representative differences between 2D and 3D cell culture systems. Gene/protein expression is different between 2D and 3D cell cultures and can give rise to differences in morphology, proliferation, cell fate, and other functions. The main goal of 3D cell culture is to provide a realistic microenvironment in which cellular mechanisms are comparable to conditions in vivo.12,13 3D cell cultures have increased our understanding of (1) the ability of cells to sense and respond to signals from the ECM in terms of surface topography;14,15 (2) the effect of mechanical properties (e.g., stiffness, viscosity, and elasticity) on cellular differentiation;16,17 (3) the role of ECM composition in development and morphogenesis;18–20 (4) new cellular signaling pathways;21,22 and (5) the biochemical signaling processes for regulating stem cell differentiation.23–25
Table 1.
Representative differences in cellular properties between 2D and 3D culture systems.
| Cellular properties | 2D | 3D | Refs. |
|---|---|---|---|
| Morphology | Stretched and Sheet-like cells in monolayer | Spheroid and aggregate structure | 225,226 |
| Proliferation | Frequently proliferate at a faster rate than in vivo | Proliferate at a faster/slower rate compared to 2D culture | 227,228 |
| Response to soluble factors | Cell monolayers are equally exposed to nutrients and growth factors | Nutrients and growth factors may not be able to fully penetrate the 3D structure | 229 |
| Gene and protein expression | Gene and protein expression levels often vary from in vivo | Often display gene/protein expressions similar to in vivo | 230,231 |
| Nutrient limited physiology | Cells are not nutrient limited | Some cells are quiescent, hypoxic, or necrotic | 134,232 |
Various methods have been developed for microscale 3D cell culture, such as microwells,26,27 hanging drops,28,29 cellular microarrays,30,31 and microfluidic devices.32,33 First, microwell systems are extensions of the traditional multi-well plate where the size of wells are reduced, the surface rendered non-adhesive, and the well-geometry varied to allow generation of 3D spheroids with controllable sizes and shapes.34–36 Second, in the hanging drop-based method, cells are suspended in media droplets that are freely hanging. Cells aggregate at the bottom of droplets, leading to the formation of 3D spheroids often with tight cell-cell adhesions.37–40 Third, cellular microarrays fabricated using robotic spotting and micropatteming techniques, allow cell-cell and cell-ECM interactions by cell and ECM printing.41–43 Lastly, microfluidic devices can perform controlled microscale 3D culture by introducing fluid flows, enabling precise placement of cells within geometrically confined microchannels and the entrapment of cell suspensions in specific regions of porous membrane.44–46 The above microscale 3D cell culture platforms are all designed to address inherent limitations of 2D culture systems while allowing higher throughput and offering more precise microenvironment control. However, these 3D culture devices can have limitations in providing ECM scaffolds or in controlling the physical and biochemical properties of the culture substrate and environment. Moreover, these methods can be labor-intensive and timeconsuming; often require sophisticated instruments and specially trained users for fabrication and operation of the devices.47 A more comprehensive overview of the commonly used microscale 3D cell culture platforms and their advantage and limitations are provided in Table 2.
Table 2.
Conventional devices and techniques for microscale 3D cell culture.
| 3D cell culture | Advantages | Drawbacks |
|---|---|---|
| Microwell | - Control over spheroid size and shape - Multiple cell types culture - Screening application possible |
- Little work done with ECM molecules - Fabrication of microwells - Requires specialized instrument |
| Hanging drop | - Reproducible procedure - Control over spheroid size - Multiple cell types culture |
- Low stability of droplets - No ECM molecules - Difficult media replacement - Generally short-term culture - Risk of evaporation |
| Cellular mieroarray | - Screening of cell-cell and cell-biomaterials interaction - Biomatenals and ECM molecules can be added |
- Sharing of media - Requires robotic spottmg instrument - Limited substrate materials - Cross talk between samples |
| Microfluidic device | - Offer physical stress by controlling fluids - Chemical gradient generation - Higher permeability to gases - Real tune, on-chip analysis |
- Surface treatment and coating required - Difficult to recover cells from channel - Immunohistochemistry challenging - Can be permeable to water vapor |
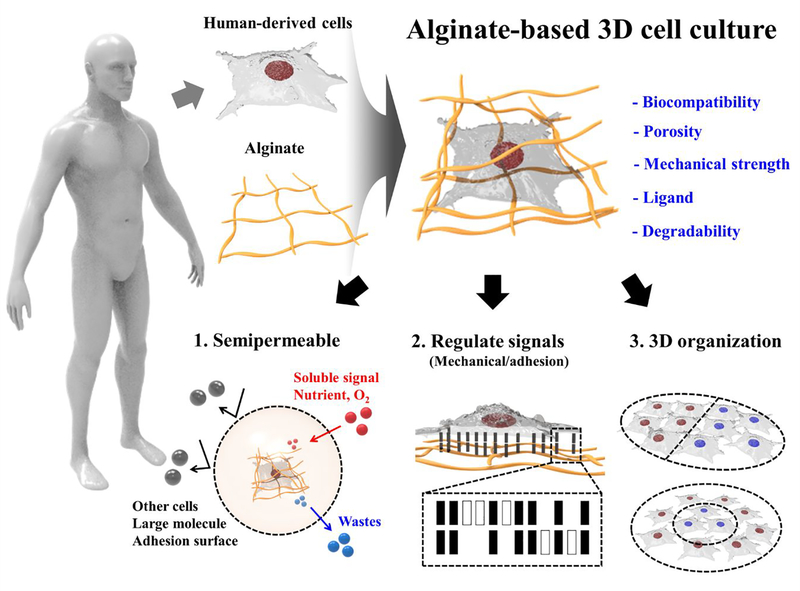
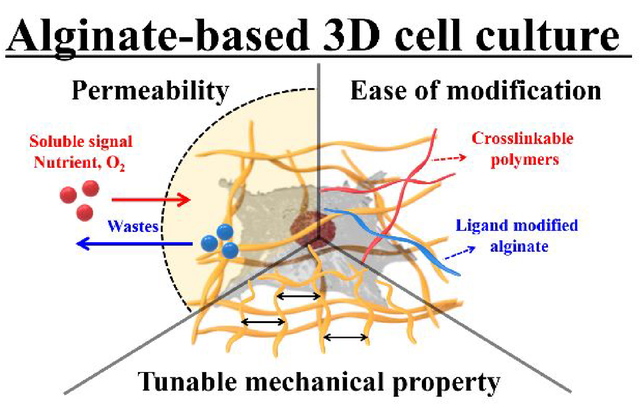
Compared to the above approaches, hydrogel microencapsulation of cells is a promising technique for performing microscale 3D cell culture, as it can provide more physiologicallyaccurate ECM-containing environments.48–50 Hydrogels are porous substrates that can provide footings for cell growth, organization, and differentiation within their network structures. The hydrogels are able to mimic the microenvironments of many tissues due to their high water retention capacity and biocompatibility.51 The versatile characteristics of hydrogels make them excellent scaffolds for 3D cell culture. Thiele et al. published a comprehensive review on desirable hydrogel characteristics for 3D cell culture applications.52 Another advantage of 3D culture using a hydrogel is that the cell culture protocols are robust and simple. However, limitations of hydrogels arise due to their indeterminate and variable composition.53 Nevertheless, studies have performed 3D cell cultures using hydrogels of agarose;54 carrageenan;55 alginate;56 chitosan;57 gellan gum;58 hyaluronic acid;59 collagen;60 gelatin;61 elastin;62 fibrin;63 and silk fibroin.64 Among them, alginate is the most common material for microencapsulation owing to its permeability, biocompatibility, and ability to perform in situ ionic crosslinking of the material.65 In addition, alginate microgel encapsulation of cells can be performed in well-defined processes using isotonic solutions under physiological conditions with regards to temperature and pH.66 Based on these controllable physicochemical properties, alginate offers ease of modulation in terms of biocompatibility, porosity, mechanical strength, ligand presentation, and biodegradability, which are important for modulation of mass transportation, regulation of cellular signaling, and control of 3D cell organization (Figure 1).
Figure 1.
Schematic illustration of alginate-based 3D cell culture system.
An important question is how modulation of alginate affects the fate of encapsulated cells in microscale 3D cell culture. Although many review papers have previously summarized the use of alginate hydrogels for 3D cell culture in more detail,67,68 this article aims to provide a short primer for the use of alginate hydrogels, including methods to modify their properties, and as an alternative to use of PDMS in microphysiological cell culture.69–73 In particular, our focus is to discuss and provide examples of specific features of the alginate hydrogels, including the engineering of mechanical properties, permeability, degradation kinetics, and chemical ligand presentation; features that are complementary to PDMS. This review then describes applications of alginate hydrogels in cell microencapsulation in terms of homogeneous alginate hydrogel encapsulation, patterned alginate hydrogels, and composite alginate hydrogels. Finally, current limitations and future directions for advanced 3D alginate cell culture are discussed.
2. Properties of alginate beneficial for 3D cell culture
2.1. Tunable mechanical properties
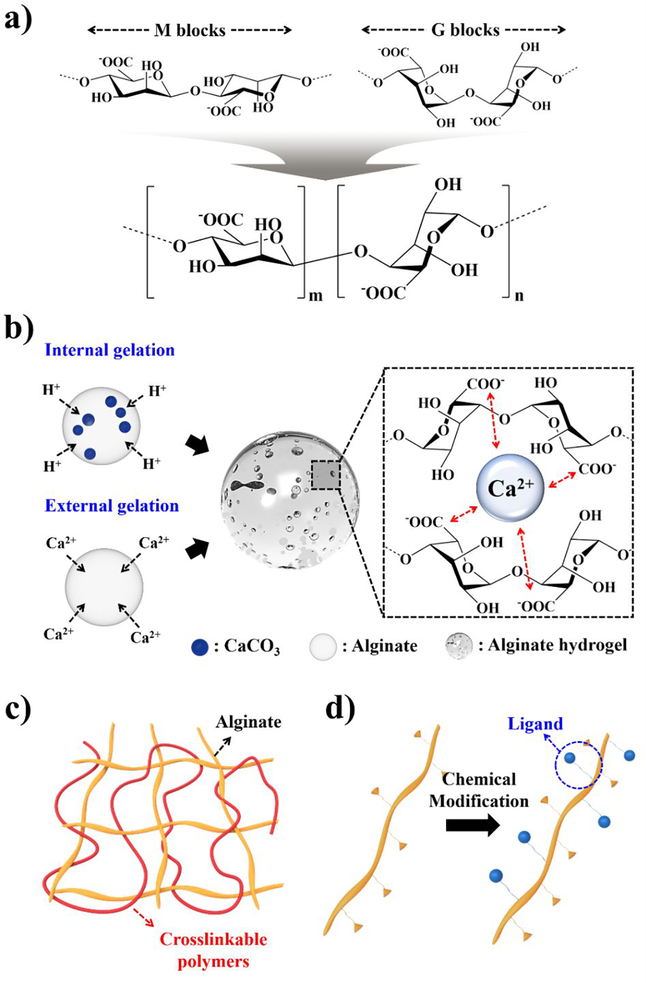
Alginate is a natural hydrophilic polysaccharide derived from the cell walls of brown algae (Phaeophyceceae), and is composed of β-(l-4)-linked d-mannuronic acid repeats (Mblocks) and β-(l-4)-linked 1-guluronic acid repeats (G-blocks) (Figure 2a). These blocks are linked by glycosidic bonds and form either homopolymeric block copolymers (M- or G-blocks), or alternative copolymers (MG-blocks).74,75 The M-blocks possess a linear and flexible configuration, whereas the G-blocks introduce steric hindrance around the carboxyl functional groups. Because of this configuration, the G-blocks form folded and rigid structural feature that governs the overall alginate mechanical property.76 Therefore, M-to-G ratio is a major determinant factor for mechanical properties of resulting hydrogels. For instance, a large proportion of G-blocks (i.e., M/G << 1) would result in production of a strong and rigid hydrogel, whereas alginate with a high M/G ratio (>> 1) produces a soft and elastic hydrogel.77 The distribution of the M- and G-blocks is mainly dependent on the source and type of algae, and its purity depends on extraction processes.
Figure 2.
Characterizations of alginate, a) Chemical structures of D-mannuronic acids (Mblocks), L-guluronic acids (G-blocks), and copolymer repeating unit; b) Two ionic gelation mechanisms (internal and external) of alginate hydrogels and “Egg-box” formation in presence of crosslinking divalent cations such as Ca2+; Schematic illustration of alginate modifications: c) interpenetrating polymer network (IPN), and d) chemical modification for ligand presentation.
Alginate hydrogels can be formed in the presence of a variety of divalent cations such as Ca2+, Ba2+, Sr2+, Zn2+, Cu2+, Cd2+, and Co2+, as these form ionic bonds with carboxyl groups.78 During the crosslinking process, these hydrogels shrink and increase the stiffness of the material relative to alginate in solution. The ionic crosslinking generates a 3D network by coordination between four-carbonyl functional groups of G-blocks and divalent cations, giving an “egg-box” arrangement.79 The chemical composition and distribution of active G-blocks in the alginate chains play a major role in the formation of ionic gels with diverse properties. Furthermore, divalent ions can determine the strength and stability. The crosslinking strength of divalent cations to carboxyl groups increases in the following order of Mn2+ < Zn2+, Ni2+, Co2+< Fe2+ < Ca2+ < Sr2+ < Ba2+ < Cd2+ < Cu2+ < Pb2+.80 Therefore, the mechanical properties of alginate hydrogels can be modulated with different divalent cations. For instance, Ba2+crosslinked gels are stronger than that of their Ca2+ crosslinked counterparts,81 and have been investigated for cell encapsulation.82–84 Furthermore, Brady et al. have demonstrated that additional factors such as alginate molecular weight, chemical composition, and concentration determine the mechanical properties of alginate hydrogels.85 However, it is important to note that many divalent ions show high toxicity, particularly Pb2+, Cu2+, and Cd2+, which should be taken into considerations for the gels to be used as 3D cell culture systems.86
The ionic crosslinking process can be by internal or external gelation (Figure 2b).87 In the internal crosslinking mechanism, gelation occurs in a controlled manner by first reacting insoluble forms of divalent salts with the cations-of-interest with alginate solution, then subsequent acidification induces exchange of ions, hence the release of cations into the solution causing gelation. The main advantage of this method is that it provides better control of gelation kinetics for homogeneous gelation. On the other hand, in the external method, gelation occurs gradually over space and time as cations diffuse throughout the alginate solution. In contrast to the internal gelation method, gelation kinetics are dictated by diffusion of cations, and generally produce inhomogeneous hydrogels with a gradient of the extent of crosslinking; the outer surface is most crosslinked and the interior is least crosslinked.88,89 To summarize, many methods have been developed and implemented to modulate mechanical properties of alginate hydrogels. This mechanical tunability is one key feature that makes alginate an attractive solution to mimics ECM scaffolding in microscale 3D cell culture applications.
2.2. Semipermeable and degradable encapsulation
An important consideration for 3D hydrogel networks used for cell culture is permeability to gases and biological macromolecules. In the in vivo cellular microenvironment, cells obtain essential nutrients and eliminate metabolic wastes via diffusion, convection, secretion, excretion, cellular uptake and filtration. The porosity of hydrogels enables exchange of O2, CO2, nutrients, and metabolic wastes without disrupting the physical structure or mechanical characteristics of the material.
Alginate hydrogels can swell. Swelling is defined by the ratio of hydrogel sizes before and after swelling, and depends on crosslinking density, solvent polarity, and affinity between solvent and polymer.90–92 When a dry hydrogel makes contact with water, the most hydrophilic functional groups interact with the water molecules, forming a primary-bound water layer. Hydrophobic functional groups also interact with water molecules, creating a secondary-bound water layer. Upon saturation of the functional groups, the remaining free or bulk water molecules distribute themselves in the spaces throughout the hydrogel networks, due to osmotic pressure.93 Many researchers have investigated alginate hydrogel swelling and its impact on 3D cell culture.94–97 The capacity of alginate swelling is mainly dependent on the amount and type of divalent cations crosslinking alginate hydrogels.92 For example, Ba2+-incorporated alginate hydrogels show significantly lower swelling ratios compared to Ca2+-alginate hydrogels because the affinity of Ba2+-ions towards the G-blocks in alginate polymer is higher than that of Ca2+ ions, resulting in increased stiffness of hydrogels, as discussed in Section 2.1.
Typically, the average pore size of alginate hydrogels is in the nanometer scale (5–200 nm).98,99 This range of pore size allows molecules less than ~ 650 kDa to be transported in and out through alginate matrices. Examples of such molecules include, oxygen (~16 Da), carbon dioxide (~44 Da), urea (60 Da), glucose (180 Da), insulin (~5700 Da), and other macromolecules.100 Moreover, both the stability and permeability of alginate hydrogels can be modulated with the use of polycations. Examples of such polycations include poly-L-lysine (PLL),101,102 poly-L-ornithine (PLO),103 and poly-methylene co-guanidine.104 The polycations interact electrostatically with the negative charged alginate surface which results in decreased pore sizes.105,106 For example, alginate hydrogels encapsulating insulin-secreting cells such as islets of Langerhans are coated with PLL to decrease the outer pore size, while maintaining a liquid-core.107 Another example is the use of PLL to decrease pore sizes and reduce the diffusion rate of encapsulated hemoglobin.
The crosslinking methods, as described earlier in Section 2.1, have an effect on the pore sizes of the alginate hydrogels produced (Figure 2b). In internally-gelled alginate hydrogels that use insoluble gas-forming divalent cation salts such as carbonates and citrates,108 exposure to acid induces ion exchanges to promote a homogeneous crosslinking profile with large and uniform pore sizes on the hydrogel surface.109 In contrast, inhomogeneous hydrogel structures obtained from external crosslinking has smaller gel networks on the alginate surface with pore sizes of 12–16 nm.110 Note that the pore size also depends on the type of crosslinking cations, alginate M/G ratios, and the concentration of alginate.111–113 For instance, Thu et al. demonstrated that Bovine serum albumin (BSA) diffused out of alginate hydrogels at a higher rate in hydrogels with lower concentration of alginate.114 It is known that the porosity decreases with increasing amounts of G-blocks, which interact with cations to form crosslinks. Kierstan investigated the relationship between the pore size and crosslinking divalent cations such as Ca2+ and Ba2+ ions. As expected, it was demonstrated that Ba2+ produced hydrogels with reduced pore size due to its higher affinity to G-blocks.115 Moreover, the effective pore size can also be altered by introducing other polymers, such as chitosan, which reduced the pore size to 1.74 nm.116 This ability to tune pore sizes makes alginate useful for modulating biomolecule transport into and out of alginate structures. For example, encapsulation of insulin protected it from denaturation in the gastric environment (pH 1.2), while releasing the encapsulated protein with zero-order kinetics under a neutral pH condition.117
The thickness of alginate hydrogels is also a critical consideration for regulating mass transportation.118–120 In general, as the thickness of the alginate hydrogels increases, transport limitations of small molecules including nutrients and wastes will occur from outer shell to center core, causing cellular necrosis under hypoxic and nutrient-limited environments. The concentration of O2 in alginate hydrogels has been evaluated to be relatively constant up to depths of ~100 μm in the hydrogel.121 Based on this O2 limitation, an alginate microcapsule with a diameter of 0.9–1 mm showed βTC3 cells to form a ~0.2 mm thick layer at the periphery, whereas cells located at the center of the microcapsule died.122 This growth pattern implies that nutrient gradients form with a high level at the outer layer and less towards the center of alginate microcapsules.
Certain applications such as tissue replacement and controlled molecule release require gel degradation. Alginate is not metabolized by mammals; however, the crosslinked alginate hydrogels can slowly dissolve through exchange reactions of divalent cations with monovalent cations such as sodium ions in the surrounding milieu.123–125 Physical and chemical modifications can modulate degradation. Degradation can be manipulated by controlling the molecular weight and composition of the alginate polymers.126 A clever approach to create polymers of varying molecular weights and structures uses gamma radiation.127,128 Irradiation doses below 8 Mrad divide MG chains while keeping the G content and G-block length constant. Such gamma-irradiated alginate hydrogels degrade more rapidly, and hence are cleared more quickly in vivo, leading to a significant improvement in bone regeneration from transplanted cells.129,130 Degradation rates can also be controlled by incorporating alginates with different lengths of G-blocks to induce size mismatches among the crosslinking blocks.131 Another chemical method is to perform partial oxidation of alginate with sodium periodate which leads to faster degradation of alginate hydrogels.99,132 This method cleaves cis-diol groups in uronate residues at the carbon-carbon bond and creates a hydrolytically labile bond, enabling degradation of the alginate chains. The rate of degradation is dependent not only on the degree of oxidation, but also on physical conditions of the surrounding environments such as temperature and pH of the media.123,133 This method has been used to produce alginate gels that serve as delivery vehicles for drugs and cells. These findings collectively show that degradation kinetics of alginate hydrogels can be precisely controlled for a wide range of applications in 3D cell culture research. The ability to adjust swelling, porosity, and crosslinking of alginate enables versatile regulation of permeability and degradability of alginate hydrogel microencapsulation for applications in cell transplantation and tissue engineering.
2.3. Ease of modification
The control of soluble factor distribution within 3D hydrogels is important because the response of cells and tissues to biomolecules depends on the bulk concentration in the medium, diffusion-limited gradients within the gel, and rates of change in these parameters.134 Recent advances in targeted delivery systems with 3D hydrogels show promise for drug delivery with enhanced pharmacokinetics and pharmacodynamics.135–137 Soluble biomolecules frequently show enhanced bioactivity when encapsulated and attached to hydrogel networks. Growth factors are proteins that control cell differentiation, proliferation, tissue growth, and vascularization.138–140 Various growth factors have been encapsulated within alginate hydrogels. For example, Mierisch et al. demonstrated the targeted delivery of TGF-β using calcium alginate hydrogels to treat osteochondral defects.141 Additionally, the temporal and spatial availability of growth factors can affect stem cell differentiation. Ansari et al. developed a stem cell delivery system by using alginate-hyaluronic acid (HA) gels loaded with TGF-β1 ligand and encapsulating periodontal ligament stem cells (PDLSCs) for chondrogenic differentiation.142 They demonstrated that sustained release of TGF-β1 can be achieved by regulating the hydrogel composition of alginate/HA, and higher expression of chondrogenesisrelated genes was observed from hydrogels with a 2:1 alginate/HA composition.
As mentioned previously, alginate hydrogels have many desirable characteristics for 3D cell culture such as controllable mechanical strength, porous structures, permeability to essential substances, and degradation. However, alginate, like most unmodified polysaccharides, lacks binding sites for cell adhesion and attachment, and has low adsorption capacity for proteins. Because of this inherent characteristic, cells encapsulated within native alginate hydrogels do not undergo proliferation, migration or exhibit other cellular responses.143,144 One approach to provide sites for cell adhesion is to form an interpenetrating polymer network (IPN), which is a 3D network structure of at least two polymers consisting of alginate plus one other material such as: collagen;145,146 hyaluronic acid;147,148 gelatin;149,150 and chitosan.151,152 IPN is a combination of more than two polymer chains each in network formation without any covalent bonds between them (Figure 2c). This network structure is classified into two categories, namely, a full IPN and a semi-IPN. The former is formed when two (or more) different polymers are crosslinked independently, whilst the latter refers to networks with only one polymer of the assembly crosslinked, leaving the other in a linear form.153,154 Mahou et al. proposed IPN hydrogels from an alginate-collagen blend and evaluated its property in tissue engineering applications.155 Ionotropic crosslinking of alginate was performed with collagen fibrillogenesis, and the resulting IPN hydrogels exhibited stiff mechanical property with great resistance towards enzymatic degradation. In addition, they showed that the viability of the embedded mesenchymal stromal cells (MSCs) was unaffected, and human umbilical vein endothelial cells (HUVECs) were able to attach themselves and proliferate on the collagen-coated IPN alginate hydrogels. More recently, Vorwald et al. developed fibrin-alginate IPN hydrogels to increase cell adhesion and mechanical strength simultaneously.156 Cellular responses of co-cultures of MSCs and endothelial cells (ECs) was modulated by alginate crosslinking density and fibrinogen concentration. As IPN stiffness increased, total cell density decreased with increases in cell circularity. Challenges for these methods include the need to optimize polymer concentrations and crosslinking ratios to avoid negative effects of unreacted materials.157 Various types of extracellular matrix proteins and synthetic peptides are employed to overcome the need for increasing cell adhesion. Commonly used biomolecules include gelatin, collagen, arginine-glycine-aspartic acid (RGD) peptide derived from fibronectin, Asp-Gly-Glu-Ala (DGEA), and tyrosine-isoleucine-glycine-serinearginine (YIGSR) derived from laminin, that are the functional adhesion ligands in the ECM (Figure 2d). Among them, gelatin has been extensively studied for increasing cell adhesion and differentiation in alginate hydrogels.158,159 For instance, Balakrishnan et al. demonstrated that oxidized alginate having appropriate molecular weight and degree of oxidation rapidly crosslinks with gelatin.160 The gelatin incorporated alginate hydrogels successfully encapsulated hepatocytes and increased their albumin secretion. Inclusion of RGD-peptidefunctionalized co-monomers in alginate hydrogels allows tailored biological recognition to promote cell adhesion, cellular remodeling, and migration.161 The RGD tripeptide motif has been widely used as ligands for cell adhesion, because they are recognized by transmembrane integrin receptor proteins (more specifically, αvβ3 and α5β1 for various cell types).162,163 The conjugation of RGD peptides to alginate chains can be chemically performed by using water soluble carbodiimide chemistry.164 RGD-modified alginates maintain viability and promote (1) proliferation of bone marrow MSCs;165 (2) muscle differentiation of umbilical cord MSCs in alginate-fibrin-RGD hydrogels;166 (3) osteogenic differentiation;167 (4) vascular endothelial growth factor (VEGF) secretion from MSC spheroids.168 Additional incorporation of a metalloproteinase cleavable peptide (proline-valine-glycine-leucine-isoleucine-glycine) further enhances adhesion and provides better elongation of MSCs compared to RGD-alginate hydrogels without the cleavable peptides.169 Different peptide motifs including DGEA170 and YIGSR171 sequences have also been investigated for modification of alginate hydrogels and found to improve the adhesion of various cells.172 The majority of the studies to date, however, has focused on RGD-alginate hydrogels because of its higher solubility in aqueous media, short and easy manufacturing process, and well-defined characteristics.173
3. Alginate encapsulation for microscale 3D cell culture
This section is divided into three sub-sections depending on the structures and compositions of the microscale alginate constructs used. The first sub-section will focus on examples where the material prepared is homogeneous throughout. The second sub-section will focus on use of two different types of alginate material combined in different microstructures and patterns. The third sub-section will describe structures where an alginate shell is used to encapsulate another material in its core.
3.1. Homogeneous alginate hydrogels
Cell microencapsulation is a strategy for 3D cell culture in which cells are isolated by a semipermeable material that permits diffusion of nutrients, gases, and metabolites.65 Alginate solutions can be mixed with a cell suspension, then the mixtures rapidly crosslinked by divalent cations (i.e., Ca2+, Sr2+, Fe2+, and Ba2+) under various physical and chemical conditions.174 This is an advantage for the encapsulation of living cells to enable cell culture in a 3D environment where soluble factors can diffuse into the cell encapsulating gel. When chemically modified alginate is used, adhesive cues can also be presented to cells from all directions.
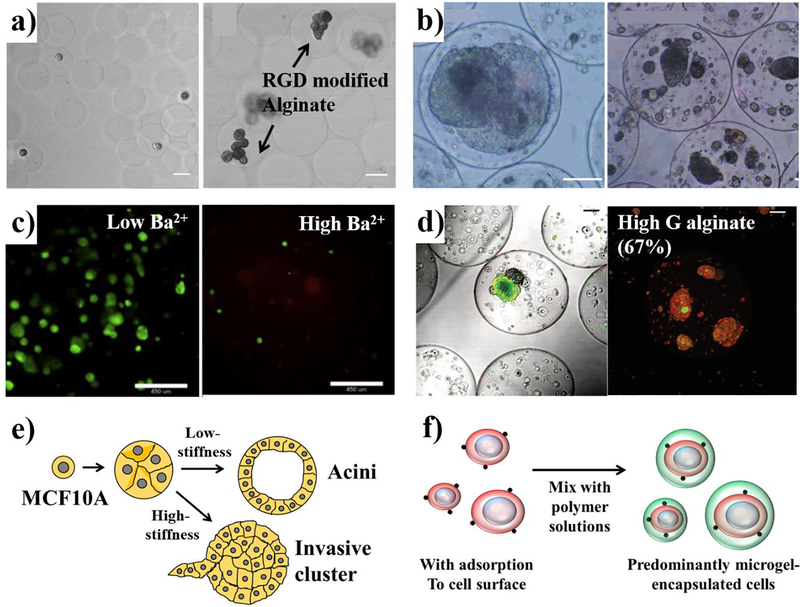
Some examples of homogeneous alginate microencapsulation are shown in Figure 3. Microenvironmental factors influence overall cell fate and is mediated in part through specific cell morphology induced. Utech et al. prepared RGD-peptide-functionalized alginate hydrogels using carbodiimide chemistry and encapsulated MSCs into alginate microgels using PDMS a microfluidic device that produce monodisperse alginate droplets with size adjustability.175 The crosslinking of cell containing alginate-Ca-EDTA mixture takes place by introduction of acetic acid which triggers internal gelation. The encapsulated cells show growth and proliferation in alginate hydrogels with a high viability of 70% after two weeks of seeding (Figure 3a). The mechanical properties of alginates can also alter cellular responses. The growth of the hepatocarcinoma cell line (HepG2) differed significantly in alginate hydrogels with a liquefied core versus a solidified core.176 Alginate-poly-lysine-alginate (APA) hydrogels fabricated with an electrostatic droplet generator are microcapsules (300–400 μm) with high uniformity. APA hydrogels are obtained by external crosslinking and each microcapsule contained 150–180 mouse embryonic stem (ES) cells. As shown in Figure 3b, ES cells aggregate into a clump and proliferate to form a large spheroid in the liquefied core, whereas the cells just proliferate and grow into small multi-spheroids in an alginate hydrogel filled core. This result clearly suggests that the liquefied and soft capsules, rather than rigid gel core beads might provide a more suitable 3D culture environment for the growth of certain types of cells. In another example, Richardson et al. elucidated that the proliferation of human embryonic stem cells (hESCs) is diminished in mechanically strong alginate hydrogels, in which alginate G-blocks were crosslinked with a high affinity cation.177 Homogeneous barium alginates (BAlg) have shown significantly higher proliferation of hESCs compared to inhomogeneous counterparts observed through 10 days post-encapsulation (Figure 3c). In addition, a reduction in proliferation was also observed with alginate hydrogels composed of alginate with high concentration of Ba2+ ions which increase stiffness. The microencapsulation of cancer cells in alginate hydrogels has been used as tumor models.178 In this study, serotonin (5-HT) producing small intestinal neuroendocrine tumor spheroids (KRJ-1) were encapsulated in alginate hydrogels of diverse capsule sizes, M/G ratio, and crosslinking ions. Spheroids encapsulated in alginate with increased G-blocks experienced increased physical stress, which gradually induced cell death (Figure 3d). According to this result, they found that the KRJ-1 cells are sensitive to matrix resistance and require cell-cell signaling for growth. Alginate microencapsulation offers an important gastrointestinal neuroendocrine neoplasms (GI-NEN) models to develop, test and validate new drugs.179 Physical confinement without any basement membrane ligands has been shown to cause malignant transformation.180,181 Chaudhuri et al. demonstrated in vitro that increased ECM stiffness is associated with malignant phenotype transformation of normal human mammary epithelium (MCF10A).182 The growth and proliferation of MCF10A encapsulated within porous IPNs of alginate and Matrigel matrix with stiffness ranging from 30 to 310 Pa was created by modulating the concentration of Ca2+ ions. As shown in Figure 3e, the development into growth-arrested acinar structures or invasive clusters is more apparent with increases in the hydrogel stiffness, independent of ligand density and polymer concentration. Enhanced expression of ESR1, a gene whose function is to initiate an estrogen receptor α (ER α) of breast cancers, was observed within the MCF10A cells in stiff IPNs.182 Moreover, Mao et al. proposed a microfluidic-based single cell encapsulation that increases the proportion of encapsulation efficiencies over 90% (Figure 2f).183 In this study, they created in vitro microenvironments of alginate hydrogel by controlling CaCO3 nanoparticle concentrations, acetic acid concentrations, and alginate molecular weight to achieve higher stiffness (~16 kPa) cell-encapsulating microgels. With their system, murine mesenchymal stem cells (mMSCs) and murine pre-adipocyte cells (OP9s) were exposed to a calcium carbonate (CaCO3) solution, allowing adsorption of CaCO3 to the cell surface. Addition of these nanoparticles to an alginate solution then initiated cross-linking. A cross junction microfluidic device was used to generate the alginate emulsion by applying an acetic acid-dissolved oil phase which induces internal gelation of the alginate hydrogels. Genomic analysis of alkaline phosphatase and osteogenic transcription factor Runs2 show that the stiffer microgels induced an increased osteogenesis of the mMSCs. Furthermore, they demonstrated therapeutic efficacy of MSC therapy in NOD/SCID/IL2γ−/− (NSG) mice by measuring pharmacokinetics and pharmacodynamics of the hydrogel drug. Therefore, recent studies of integration between microfluidics and microencapsulation of cells using alginate hydrogels show several key advancements such as control over mechanical property, narrow size distribution, cell encapsulation with high efficiency, and drug delivery with enhanced efficacy.184 In short, the encapsulation of cells in homogeneous alginate hydrogels is important for regulating cellular behaviors as well as for studying mechanotransduction and malignant phenotypes.
Figure 3.
Homogeneous alginate microencapsulation with various physicochemical properties, a) Micrographs of RGD modified alginate hydrogels encapsulating mesenchymal stem cells (MSCs) at day 0 and day 15.175 Reproduced with permission from ref 175. Copyright 2015 John Wiley and Sons, b) Growth profile difference of mouse embryonic stem (ES) cells enclosed in liquefied and unliquefied alginate microcapsules.176 Reproduced with permission from ref 176. Copyright 2006 John Wiley and Sons, c) Crosslinking density control by using 10 mM Ba2+ and 50 mM Ba2+ alginate hydrogel with human embryonic stem cells (hESCs) at day 10.177 Reproduced with permission from ref 177. Copyright 2016 Elsevier, d) Small intestinal neuroendocrine tumor (KRJ-1) spheroids encapsulating alginate microspheres using high-G alginate.178 Reproduced with permission from ref 178. Copyright 2012 John Wiley and Sons, e) Different stiffness of alginate hydrogels lead to the malignant transformation of MCF10A spheroids.182 f) Schematic illustration showing the single cell encapsulation process.183 Scale bars: a, 25 μm; b, 100 μm; c, 450 μm; d, 100 μm.
3.2. Patterned alginate hydrogels
In vivo microenvironments are composed of anisotropic and hierarchical structures with different types of cells and ECM.185 Different cell types require distinct and particular environments, as cells differ in differentiation profile, proliferation rates, spatial organization, and nutrient metabolism during growth.
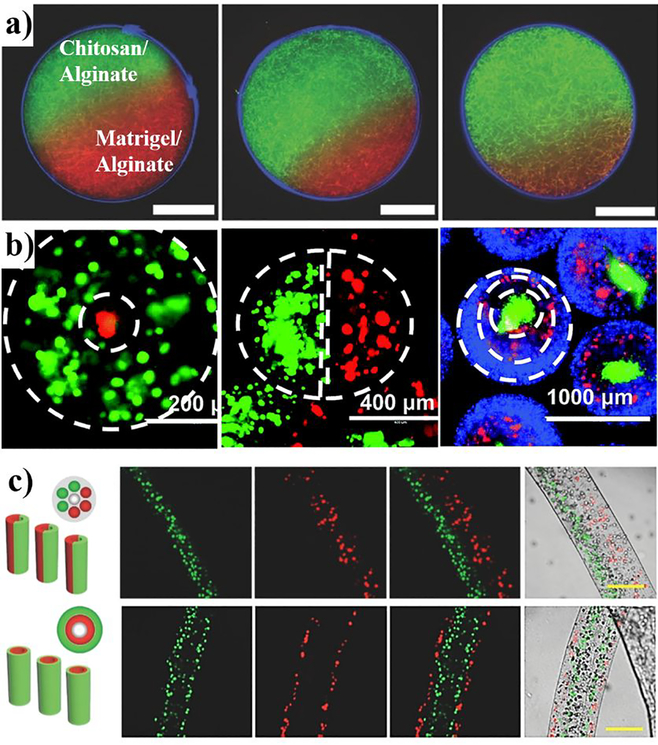
Alginate hydrogels can act as a physical or chemical “barrier” to provide cellular microenvironments for artificial tissue models. Different configurations, such as Janus, coreshell, multicompartment structure, have been achieved by advanced techniques.186–189 These systems mimic physical and chemical characteristics in terms of microenvironment heterogeneity and provide a means of functional regulation of mechanical and biochemical properties. In addition, researchers have demonstrated in vitro systems that mimic anisotropic ECM structures to confirm the adaptation of specific types of cells. For example, Yoshida et al. developed Janus alginate microgels as an anisotropic cellular scaffold for 3D cell culture.190 To include different types of ECM in the alginate microgel hemispheres, they developed a centrifuge based microfluidic device that produces monodispersed alginate microgels as the alginate solution is ejected out of a theta-shaped capillary. As shown in Figure 4a, two different mixtures such as collagen and Matrigel incorporating alginate were separately ejected by centrifugal force (~2000 G) and immersed into a calcium chloride solution for external crosslinking, and the resulting anisotropic gels used to encapsulate mouse embryonic fibroblast cells (NIH/3T3). The viability of the encapsulated cells was more than 79% and the cells proliferated into fibrillar networks. In short, it was found that alginate microgels with different ECM compositions could be utilized for providing spatially anisotropic microenvironments. Cell-cell communication is important for maintaining normal cellular morphology and functionality of tissues.191 Spatially controlled alginate microgels provide useful 3D scaffolds for micropattemed cell co-culture. Figure 4b shows that three different type of alginate microgels (core-shell, side-by-side, and triple-layer) can be fabricated by a multi-fluidic electrostatic spraying technique.192 In this study using model cells including MDA-MB-231, normal human lung fibroblasts (NHLFs), and MCF-10A, the authors demonstrated that different types of cells can be co-encapsulated in different compartments. Recently, Zhang et al. demonstrated a one-step microfluidic method to generate multi-compartment alginate hydrogels, where the fabricated compartments can be independently controlled.193 They used RGD-modified alginate hydrogels for encapsulating MSCs and HUVECs to create complex stem cell niche microenvironments to study single cell-cell interaction. Thus, the alginate microgels offer flexibility in terms of controllable structures and can also reduce spatial distance among co-cultured cells.
Figure 4.
Patterned alginate systems for 3D cell culture, a) Confocal image of heterogeneous material-based alginate hydrogels composed of hemispheres with different volume ratio of collagen and Matrigel.190 Reproduced with permission from ref 190. Copyright 2017 John Wiley and Sons, b) Alginate hydrogel designs for cell encapsulation. Double layer, side-byside, and triple-layer alginate hydrogels encapsulating different types of cells (green cells: MDA-MB-231 expressing GFP; red cells: normal human lung fibroblasts expressing RFP; blue cells: MCF-10A stained with Hoechst).192 Reproduced with permission from ref 192. Copyright 2015 The Royal Society, c) Cell-laden alginate microfibers with Janus-compartment and twoshell layer hollow structure (red cells: NIH 3T3 cells stained with a red fluorescent dye; green cells: HepG2 cells stained with a green fluorescent dye).196 Reproduced with permission from ref 196. Copyright 2014 John Wiley and Sons. Scale bars: a, 50 μm; c, 200 μm.
Fibrous alginates can induce the growth, alignment, and migration of cells and promote cell-cell interactions.194 Cell encapsulating microfibers can be used to generate tubular structures for vascular tissue engineering.195 By using a glass capillary device that modulates alginate crosslinking chemistry, Cheng et al. developed a scalable, one-step, and continuous process for formation of hollow microfibers.196 This procedure produces microfibers by crosslinking multiple, parallel, laminar flows of alginate solutions arranged in different orientations to control overall fiber cross-sectional morphology. Alginate suspensions of HepG2 and NIH/3T3 cells were used to fabricate Janus compartments and layered-shell structures (Figure 4c). Cells in the layered-shell microfibers show higher viability (-85%) than the Janus microfibers (~79%). This result suggests that the layered-shell arrangements enhance cell-cell interactions via a larger interaction area. Other microfluidic approaches for alginate fiber formation include use of a coaxial triple cylinder to form 3D micro-vascularized structures197 and use of microfabricated nozzles198 to produce microfibers for the encapsulation and co-culture of cells.
3.3. Composite alginate hydrogels
Microencapsulation of complementary cell types can recapitulate some in vivo microenvironment to provide more physiologically-relevant in vitro models.199 Multiple cell types have been encapsulated within the same alginate microcapsule to enhance paracrine signaling between cells.200 Alginate microcapsules with hollow cores have been investigated to minimize interactions between the encapsulated cells and the surrounding network. These hollow core-shell structures are comprised of liquid core and/or ECM materials that are surrounded by a semipermeable alginate shell.200–202
One study showed that microencapsulation promotes cellular reorganization within the liquid core to form spheroids. As shown in Figure 5a, Alessandri et al. succeeded in forming spheroids from HeLa cells, which normally have a low propensity to form 3D aggregates.203 They also demonstrated that murine sarcoma S180 cells expressing low levels of E-cadherin can also be induced to form spheroids. The spheroids formed in confined core-shell environments formed stronger cell-cell interactions, and hence were more compact, compared to unconfined multicellular spheroids. In another study, Chen et al. described controlled 3D assembly of liver hepatocellular carcinoma cells (HepG2) in microcapsules.204 Microcapsules with a media core and an alginate shell were generated by using two connected microfluidic flow-focusing PDMS channels. With slight alteration in encapsulating procedure, different cells (HepG2 and NIH/3T3) can be loaded into different regions within microcapsules (Figure 5b). A microstructure with hepatocytes in the core and fibroblasts around an alginate hydrogel shell formed co-culture spheroids with improved heterotopic cell-cell paracrine signaling. In addition, the highly permeable alginate shell allowed long-term culture (> 14 days) of spheroids to develop microtissues in the microcapsules. In some applications, having both a protective shell and an interior space for development is critical. For example, microcapsules with a soft core mimicking the medulla and stiff shell to resemble the cortex have guided ovarian follicle development.205 Early secondary preantral follicles were encapsulated within a soft collagen core inside of a stiff alginate microcapsule, so that they can locate themselves at the boundary between core and shell where they experience mechanical heterogeneity, similar as in the native microenvironment (Figure 5c). A total of ~28% of the preantral follicles in the appropriate biomimetic microcapsule formed antra. In contrast, when embedded in oxidized, soft, alginate material alone, no follicles proceeded to the antral phase because degradation of this alginate occurred rapidly in 5–7 days exposing the follicle prematurely. The authors further demonstrated in vitro ovulation in the microcapsules without addition of any additional growth factors.
Figure 5.
Composite alginate hydrogels, a) Time sequential phase contrast micrographs shows HeLa and murine sarcoma SI80 spheroids encapsulated in media-core and alginate shell structure.203 Reproduced with permission from ref 203. Copyright 2013 National Academy of Sciences, b) Spatial assembly of core-shell structure. HepG2 cells confined in the media core and NIH/3T3 fibroblasts immobilized by the alginate shell forming an artificial liver in a drop.204 Reproduced with permission from ref 204. Copyright 2016 The Royal Society, c) Schematic illustration and micrographs of mouse ovary that consists of two mechanically distinct tissue layers consisting of a rigid cortex and a softer medulla.205 Reproduced with permission from ref 205. Copyright 2014 Elsevier, d) Alginate capsules with a Matrigel inner lining for human neural stem cells (hNSC) differentiation. Inset shows confocal image of the Matrigel forming a basement membrane-mimetic lining (red) on the inner surface of an alginate shell (green) microcapsule.209 Reproduced with permission from ref 209. Copyright 2016 The Royal Society. Scale bar: a, 100 μm; c, 100 μm; d, 10 μm.
Microencapsulation with ECM components such as collagen and Matrigel can modulate cellular differentiation of stem cells and induced pluripotent stem cells (iPSCs).206,208 Human neural stem cells (hNSC) encapsulated in alginate-Matrigel core-shell microcapsules differentiate into neurons with 97.8% viability (Figure 5d).209 Using a 3Dprinted microfluidic device, the authors produced hollow cell-laden spheres where the Matrigel is anchored to the inner surface of the alginate capsule to mimic a basement membrane. Under differentiating conditions the neural stem cells differentiate to form a 3D network of mature neurites that criss-cross each other within a defined size sphere. In another example, Perez at al. demonstrated osteogenic programming of MSCs when encapsulated in collagen or poly(lactic-co-glycolic acid) (PLGA) core-alginate shell microcapsules.210,211 Other studies have shown hepatocyte differentiation212 and differentiation into pancreatic lineages,213 both from encapsulation of human embryonic stem cells. Composite alginate microcapsules with a core-shell structure provide a controlled and protected microenvironment for a variety of core materials, including physiological ECM gels and basement membrane mimetics, to facilitate cell growth, cell-cell interaction, cell transplantation, and stem cell differentiation.
4. Conclusions and Future directions
In the past few decades, alginate-based microscale 3D cell culture platforms have emerged as promising physiological 3D model systems for basic biology, tissue engineering, and stem cell applications. In this review, we summarize the use of alginate hydrogels for microscale 3D cell culture as an alternative to other microscale cell culture systems, including PDMS microdevices. The characteristics of the alginate hydrogels, such as controllable mechanical properties, semipermeable and degradable encapsulation, and ease of modification, are particularly useful for 3D cell culture systems. In particular, advanced techniques with enhanced alginate crosslinking have paved the way for alginate hydrogels to be fabricated with improved control of their structures and functions for applications in 3D cell culture.
Despite these major advances, many challenges remain. The alginate substrates on their own are insufficient to instruct cells to form functional tissue structures. ECM-enriched alginate hydrogels can provide a promising approach to alleviate such problems. It is, however, critically important to carefully consider unknown side effects such as abnormal vascularization,214 diffusion limitations of some amino acid,215 and many other possible complications. In addition, current systems often lack vascularization and therefore fail to fully model adult tissue or organ biology. Nevertheless, maturation of 3D cellular structure has been demonstrated, for example, by using tubular alginate hydrogel networks, fabricated with advanced fabrication techniques such as microfluidics216–218 and bioprinting.219–221 Experimental protocols such as cell seeding, medium change, and analytic methods should all be further assessed to enhance reproducibility. This is critical because variations in, for instance, cell counts and media change cycle disturb systematic comparison of batch-to-batch experimental results. Development of real-time 3D imaging-based readout systems can enable monitoring of cellular behaviors and microenvironmental parameters, which can further accelerate research. Moreover, real-time biochemical readouts may provide quantitative methods tailored for analysis of hydrogel-based 3D cell culture models. For example, optical coherence tomography (OCT)222 can provide a non-destructive and non-invasive way of imaging encapsulated 3D cell structures without cell fixation and staining procedures. This optical system has an advantage over conventional 3D imaging techniques such as confocal laser scanning microscopy (CLSM),223 and multiphoton imaging,224 as OCT-based imaging systems use near-infrared radiation that can enable imaging depth to be in the order of microns up to centimeters in intact biological 3D structures.
Importantly, the ready accessibility and ease of use of alginate hydrogels for microscale 3D cell culture systems provide an attractive materials platform for interdisciplinary collaborations involving bioengineering, materials science, medicine, biology, and chemistry, to better understand and mimic the complicated in vivo microenvironments for tissue engineering, personalized medicine, and high throughput drug screening.
Acknowledgement
We would like to thank Dr. Cameron Yamanishi for his valuable feedback and for proofreading the manuscript. We thank the NIH (R01 CA196018, R01 HL136141, U19 AI16482, R21 AG061687) and NSF (CBET0939511) for funding.
Footnotes
The authors declare no competing financial interest.
References
- 1.Discher DE; Janmey P; Wang Y.-l., Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310 (5751), 1139–1143. [DOI] [PubMed] [Google Scholar]
- 2.Beningo KA; Dembo M; Wang Y.-l., Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc. Natl. Acad. Sci. U. S. A. 2004,101 (52), 18024–18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht DR; Underhill GH; Wassermann TB; Sah RL; Bhatia SN, Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods 2006, 3 (5), 369–375. [DOI] [PubMed] [Google Scholar]
- 4.Chen W; Villa-Diaz LG; Sun Y; Weng S; Kim JK; Lam RH; Han L; Fan R; Krebsbach PH; Fu J, Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 2012, 6 (5), 4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbi ME; Ahn EH; Downey J; Afzal J; Kim D-H; Rey S; Chang C; Kundu A; Semenza GL; Abraham RM, Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Sci. Sig. 2012, 5 (227), ra41–ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmondson R; Broglie JJ; Adcock AF; Yang L, Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014,12(4), 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridky TW; Chow JM; Wong DJ; Khavari PA, Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 2010,16 (12), 1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabata Y; Lutolf MP, Multiscale microenvironmental perturbation of pluripotent stem cell fate and self-organization. Sci. Rep. 2017, 7, 44711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong EL; Wan X; Yang J; Morgado M; Mikos AG; Harrington DA; Navone NM; Farach-Carson MC, A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions. Biomaterials 2016, 77, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centeno EG; Cimarosti H; Bithell A, 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018,13 (1), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaukonen R; Jacquemet G; Hamidi H; Ivaska J, Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc 2017, 12(11), 2376–2390. [DOI] [PubMed] [Google Scholar]
- 12.Yamada KM; Cukierman E, Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130 (4), 601–610. [DOI] [PubMed] [Google Scholar]
- 13.Caliari SR; Burdick JA, A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13(5), 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abadi PP; Garbem JC; Behzadi S; Hill MJ; Tresback JS; Heydari T; Ejtehadi MR; Ahmed N; Copley E; Aghaverdi H, Engineering of mature human induced pluripotent stem cell-derived cardiomyocytes using substrates with multiscale topography. Adv. Func. Mater 2018, 28 (19) 1707378. [Google Scholar]
- 15.Zhang W; Chen J; Backman LJ; Malm AD; Danielson P, Surface topography and mechanical strain promote keratocyte phenotype and extracellular matrix formation in a biomimetic 3D comeal model. Adv. Healthc. Mater 2017, 6 (5), 1601238. [DOI] [PubMed] [Google Scholar]
- 16.Haugh MG; Vaughan TJ; Madl CM; Raftery RM; McNamara LM; O'Brien FJ; Heilshom SC, Investigating the interplay between substrate stiffness and ligand chemistry in directing mesenchymal stem cell differentiation within 3D macro-porous substrates. Biomaterials 2018,171, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charrier EE; Pogoda K; Wells RG; Janmey PA, Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commnn. 2018, 9 (1), 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strachan L; Condic M, Neural crest motility and integrin regulation are distinct in cranial and trunk populations. Dev. Biol. 2003, 259 (2), 288–302. [DOI] [PubMed] [Google Scholar]
- 19.Simão D; Silva MM; Terrasso AP; Arez F; Sousa MF; Mehijardi NZ; Šarić T; Gomes-Alves P; Raimundo N; Alves PM, Recapitulation of human neural microenvironment signatures in iPSC-derived NPC 3D differentiation. Stem Cell Rep. 2018,11 (2), 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho Y; Moon WK; Kim HS; Na K; Yang JH; Huh YH; Kim JA; Chung S; Lee SH, Construction of a 3D mammary duct based on spatial localization of the extracellular matrix. NPG Asia Mater. 2018,10 (10), 970–981. [Google Scholar]
- 21.Wang H; Feng Z,; Xu B, Instructed Assembly as Context-Dependent Signaling for the Death and Morphogenesis of Cells. Angew. Chem. Int. Ed. 2019, 58 (17), 5567–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang R; Li H; Zhang S; Zhang Y; Wang N; Zhou H; He H; Hu G; Zhang T-C; Ma W, RXRa provokes tumor suppression through p53/p21/p16 and PI3K-AKT signaling pathways during stem cell differentiation and in cancer cells. Cell Death Dis. 2018, 9 (5), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A; Kanzaki LF; Galloway JL; Schilling TF, Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. Elife 2018, 7, e38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumbal J; Koledova Z, FGF signaling in mammary gland fibroblasts regulates multiple fibroblast functions and mammary epithelial morphogenesis. Development 2019, 146 (23) devl85306. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury F: Na S; Li D; Poh, YC: Tanaka TS; Wang F; Wang N, Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9(1), 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khademhosseini A; Langer R; Borenstein J; Vacanti JP, Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. U. S. A. 2006,103 (8), 2480–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napolitano AP; Chai P; Dean DM; Morgan JR, Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007,13 (8), 2087–2094. [DOI] [PubMed] [Google Scholar]
- 28.Tung Y-C; Hsiao AY; Allen SG; Torisawa Y. s.; Ho M; Takayama S, Highthroughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136 (3), 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao AY; Tung Y-C; Kuo C-H; Mosadegh B; Bedenis R; Pienta KJ; Takayama S, Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed. Microdevices 2012,14 (2), 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacBeath G; Schreiber SL, Printing proteins as microarrays for high-throughput function determination. Science 2000, 289 (5485), 1760–1763. [DOI] [PubMed] [Google Scholar]
- 31.Quist AP; Oscarsson S, Micropattemed surfaces: techniques and applications in cell biology. Expert Opin. Drug Discov. 2010, 5 (6), 569–581. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B; Korolj A; Lai BFL; Radisic M, Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3 (8), 257–278. [Google Scholar]
- 33.Viola H; Chang J; Grunwell JR; Hecker L; Tirouvanziam R; Grotberg JB; Takayama S, Microphysiological systems modeling acute respiratory distress syndrome that capture mechanical force-induced injury-inflammation-repair. APL Bioeng. 2019, 3 (4), 041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howes AL; Richardson RD; Finlay D; Vuori K, 3-Dimensional culture systems for anti-cancer compound profding and high-throughput screening reveal increases in EGFR inhibitormediated cytotoxicity compared to monolayer culture systems. PLoS One 2014, 9 (9), el08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong SF; Choi YY; Kim DS; Chung BG; Lee S-H, Concave microwell based sizecontrollable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011, 32 (32), 8087–8096. [DOI] [PubMed] [Google Scholar]
- 36.Lee GH; Lee JS; Lee G-H; Joung WY; Kim SH; Lee SH; Park JY; Kim DH, Networked concave microwell arrays for constructing 3D cell spheroids. Biofabrication 2017,10 (1), 015001. [DOI] [PubMed] [Google Scholar]
- 37.Harrison RG, Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1906,4(1), 140–143. [Google Scholar]
- 38.Hsiao AY; Tung YC; Qu X; Patel LR; Pienta KJ; Takayama S, 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol. Bioeng 2012,109 (5), 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung BM; Lesher-Perez SC; Matsuoka T; Moraes C; Takayama S, Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater. Sci. 2015, 3 (2), 336–344. [DOI] [PubMed] [Google Scholar]
- 40.Cavnar SP; Salomonsson E; Luker KE; Luker GD; Takayama S, Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J. Lab. Autom. 2014,19(2), 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbulovic-Nad I; Lucente M; Sun Y; Zhang M; Wheeler AR; Bussmann M, Biomicroarray fabrication techniques—a review. Crit. Rev. Biotechnol. 2006, 26 (4), 237–259. [DOI] [PubMed] [Google Scholar]
- 42.Lee M-Y; Kumar RA; Sukumaran SM; Hogg MG; Clark DS; Dordick JS, Three-dimensional cellular microarray for high-throughput toxicology assays. Proc. Natl. Acad. Sci. U. S.A. 2008,105 (1), 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge X; Gebe JA; Bollyky PL; James EA; Yang J; Stem LJ; Kwok WW, PeptideMHC cellular microarray with innovative data analysis system for simultaneously detecting multiple CD4 T-cell responses. PLoS One 2010, 5 (6), el 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huh D; Leslie DC; Matthews BD; Fraser JP; Jurek S; Hamilton GA; Thomeloe KS; McAlexander MA; Ingber DE, A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4 (159), 159ral47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song JW; Cavnar SP; Walker AC; Luker KE; Gupta M; Tung Y-C; Luker GD; Takayama S, Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One 2009, 4 (6), e5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heo YS; Cabrera LM; Song JW; Futai N; Tung Y-C; Smith GD; Takayama S, Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly (dimethylsiloxane) devices. Anal. Chem. 2007, 79 (3), 1126–1134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta G; Hsiao AY; Ingram M; Luker GD; Takayama S, Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164 (2), 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin YM; Kim TG; Park J-S; Gwon H-J; Jeong S1,1 Shin H; Kim K-S; Kim D; Yoon M-H; Lim Y-M, Engineered ECM-like microenvironment with fibrous particles for guiding 3D-encapsulated hMSC behaviours. J. Mater. Chem. B 2015, 3 (13), 2732–2741. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y; Wang J, Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014,139 (10), 2449–2458. [DOI] [PubMed] [Google Scholar]
- 50.Gothard D; Smith EL; Kanczler JM; Black CR; Wells JA; Roberts CA; White LJ; Qutachi O; Peto H; Rashidi H, In vivo assessment of bone regeneration in alginate/bone ECM hydrogels with incorporated skeletal stem cells and single growth factors. PLoS One 2015, 10 (12), e0145080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sani ES; Kheirkhah A; Rana D; Sun Z; Foulsham W; Sheikhi A; Khademhosseini A; Dana R; Annabi N, Sutureless repair of comeal injuries using naturally derived bioadhesive hydrogels. Sci. Adv 2019, 5 (3), eaavl281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiele J; Ma Y; Bruekers SM; Ma S; Huck WT, 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv. Mater. 2014, 26 (1), 125–148. [DOI] [PubMed] [Google Scholar]
- 53.Cushing MC; Anseth KS, Hydrogel cell cultures. Science 2007, 316 (5828), 1133–1134. [DOI] [PubMed] [Google Scholar]
- 54.Luan NM; Iwata H, Xenotransplantation of islets enclosed in agarose microcapsule carrying soluble complement receptor 1. Biomaterials 2012, 33 (32), 8075–8081. [DOI] [PubMed] [Google Scholar]
- 55.Popa E; Reis R: Gomes M, Chondrogenic phenotype of different cells encapsulated in K-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol. Appl. Biochem. 2012, 59 (2), 132–141. [DOI] [PubMed] [Google Scholar]
- 56.de Vos P; Faas MM; Strand B; Calafiore R, Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27 (32), 5603–5617. [DOI] [PubMed] [Google Scholar]
- 57.Ramesh S; Rajagopal K; Vaikkath D; Nair PD; Madhuri V, Enhanced encapsulation of chondrocytes within a chitosan/hyaluronic acid hydrogel: a new technique. Biotechnol. Lett. 2014, 36(5), 1107–1111. [DOI] [PubMed] [Google Scholar]
- 58.Silva-Correia J; Zavan B; Vindigni V; Silva TEL; Oliveira JM; Abatangelo G; Reis RL, Biocompatibility evaluation of ionic-and photo-crosslinked methacrylated gellan gum hydrogels: in vitro and in vivo study. Adv. Healthc. Mater. 2013, 2 (4), 568–575. [DOI] [PubMed] [Google Scholar]
- 59.Shen Y-I; Abaci HE; Krupski Y; Weng L-C; Burdick JA; Gerecht S, Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater. Sci. 2014,2(5), 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee W; Debasitis JC; Lee VK; Lee J-H; Fischer K; Edminster K; Park J-K; Yoo S-S, Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30 (8), 1587–1595. [DOI] [PubMed] [Google Scholar]
- 61.Ma S; Natoli M; Liu X; Neubauer MP; Watt FM; Fery A; Huck WT, Monodisperse collagen-gelatin beads as potential platforms for 3D cell culturing. J. Mater. Chem. B 2013,1 (38), 5128–5136. [DOI] [PubMed] [Google Scholar]
- 62.Betre H; Ong SR; Guilak F; Chilkoti A; Fermor B; Setton LA, Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 2006, 27(1), 91–99. [DOI] [PubMed] [Google Scholar]
- 63.Liu J; Xu HH; Zhou H; Weir MD; Chen Q,; Trotman CA, Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013, 9 (1), 4688–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis NE; Beenken-Rothkopf LN; Mirsoian A; Kojic N; Kaplan DL; Barron AE; Fontaine MJ, Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials 2012, 33 (28), 6691–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passemard S; Szabo L; Noverraz F; Montanari E; Gonelle-Gispert C; Biihler L. o. H.; Wandrey C; Gerber-Lemaire S, Synthesis strategies to extend the variety of alginate-based hybrid hydrogels for cell microencapsulation. Biomacromolecules 2017,18 (9), 2747–2755. [DOI] [PubMed] [Google Scholar]
- 66.Villani S; Marazzi M; Bucco M; Faustini M; Klinger M; Gaetani P; Crovato F; Vigo D; Caviggioli F; Torre M, Statistical approach in alginate membrane formulation for cell encapsulation in a GMP-based cell factory. Acta Biomater. 2008, 4 (4), 943–949. [DOI] [PubMed] [Google Scholar]
- 67.Lee KY; Mooney DJ, Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012, 37(1), 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Augst AD; Kong HJ; Mooney DJ, Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6(8), 623–633. [DOI] [PubMed] [Google Scholar]
- 69.Huh D; Matthews BD; Mammoto A; Montoya-Zavala M; Hsin HY; Ingber DE, Reconstituting organ-level lung functions on a chip. Science 2010, 328 (5986), 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jang KJ; Suh KY, A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010,10 (1), 36–42. [DOI] [PubMed] [Google Scholar]
- 71.Marsano A; Conficconi C; Lemme M; Occhetta P; Gaudiello E; Votta E; Cerino G; Redaelli A; Rasponi M Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016,16 (3), 599–610. [DOI] [PubMed] [Google Scholar]
- 72.Kim HJ; Huh D; Hamilton G; Ingber DE, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012,12 (12), 2165–2174.. [DOI] [PubMed] [Google Scholar]
- 73.Kane BJ; Zinner MJ; Yarmush ML; Toner M, Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal. Chem. 2006, 78 (13), 4291–4298. [DOI] [PubMed] [Google Scholar]
- 74.Morris ER; Rees DA; Thom D, Characterisation of alginate composition and blockstructure by circular dichroism. Carbohydr. Res. 1980, 81 (2), 305–314. [Google Scholar]
- 75.Sperger DM; Fu S; Block LH; Munson EJ, Analysis of composition, molecular weight, and water content variations in sodium alginate using solid-state NMR spectroscopy. J. Pharm. Sci. 2011,100 (8), 3441–3452. [DOI] [PubMed] [Google Scholar]
- 76.Chui C-Y; Bonilla-Brunner A; Seifert J; Contera S; Ye H, Atomic force microscopyindentation demonstrates that alginate beads are mechanically stable under cell culture conditions. J. Mech. Behav. Biomed. Mater. 2019, 93, 61–69. [DOI] [PubMed] [Google Scholar]
- 77.Costa MJ; Marques AM; Pastrana LM; Teixeira JA; Sillankorva SM; Cerqueira MA, Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoil. 2018, 81, 442–448. [Google Scholar]
- 78.Remuñán-López C; Bodmeier R, Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Control. Release 1997, 44 (2–3), 215–225. [Google Scholar]
- 79.Grant GT; Morris ER; Rees DA; Smith PJ; Thom D, Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 1973, 32 (1), 195–198. [Google Scholar]
- 80.Haug A; Smidsrød O, The effect of divalent metals on the properties of alginate solutions. Acta Chem. Scand. 1965,19 (2), 341–351. [Google Scholar]
- 81.Mørch ÝA; Donati I; Strand BL; Skjåk-Bræk G, Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7 (5), 1471–1480. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J; Ke J; Zhu Y; Song J; Yang J; Wen C; Zhang L, Influence of divalent cations on the biofouling behaviors of alginate hydrogels. Biomed. Mater. 2019,15 (1), 015003. [DOI] [PubMed] [Google Scholar]
- 83.Muthyala S; Safley S; Gordan K; Barber G; Weber C; Sambanis A, The effect of hypoxia on free and encapsulated adult porcine islets—an in vitro study. Xenotransplantation 2017, 24 (1), el2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saxena A; Bhattacharya A; Kumar S; Epstein IR; Sahney R, Biopolymer matrix for nano-encapsulation of urease-A model protein and its application in urea detection. J. Colloid Interface Sci. 2017, 490, 452–461. [DOI] [PubMed] [Google Scholar]
- 85.Brady S; Fox E; Lally C; Clarkin O, Optimisation of a novel glass-alginate hydrogel for the treatment of intracranial aneurysms. Carbohydr. Polym. 2017,176, 227–235. [DOI] [PubMed] [Google Scholar]
- 86.Smidsrød O; Skja G, Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- 87.Chan LW; Lee HY; Heng PW, Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63 (2), 176–187. [Google Scholar]
- 88.Strobel SA; Scher HB; Nitin N; Jeoh T, Control of physicochemical and cargo release properties of cross-linked alginate microcapsules formed by spray-drying. J. Drug Deliv. Sci. Technol. 2019, 49, 440–447. [Google Scholar]
- 89.Lee P; Rogers M, Effect of calcium source and exposure-time on basic caviar spherification using sodium alginate. Int. J. Gastron. Food Sci. 2012,1 (2), 96–100. [Google Scholar]
- 90.Lee KY; Rowley JA; Eiselt P; Moy EM; Bouhadir KH; Mooney DJ, Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density.Macromolecules 2000, 33 (11), 4291–4294. [Google Scholar]
- 91.Bajpai S; Sharma S, Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Fnnct. Polym. 2004, 59 (2), 129–140. [Google Scholar]
- 92.Darrabie MD; Kendall WF; Opara EC, Effect of alginate composition and gelling cation on micro-bead swelling. J. Microencapsul. 2006, 23 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- 93.Kopecek J, Swell gels. Nature 2002, 417 (6887), 389–391. [DOI] [PubMed] [Google Scholar]
- 94.Schiitz K; Placht AM; Paul B; Briiggemeier S; Gelinsky M; Lode A, Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2017,11 (5), 1574–1587. [DOI] [PubMed] [Google Scholar]
- 95.Bozza A; Coates EE; Incitti T; Ferlin KM; Messina A; Menna E; Bozzi Y; Fisher JP; Casarosa S, Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials 2014, 35 (16), 4636–4645. [DOI] [PubMed] [Google Scholar]
- 96.Nam S; Stowers R; Lou J; Xia Y; Chaudhuri O, Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019, 200, 15–24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun J; Wei D; Yang K; Yang Y; Liu X; Fan H; Zhang X, The development of cellinitiated degradable hydrogel based on methacrylated alginate applicable to multiple microfabrication technologies. J. Mater. Chem. B 2017, 5 (40), 8060–8069. [DOI] [PubMed] [Google Scholar]
- 98.Andresen I-L; Skipnes O; Smidsrod O; Ostgaard K; Hemmer PC„ Some biological functions of matrix components in benthic algae in relation to their chemistry and the composition of seawater. 3rd edn. ACS Svmp. Ser. 1977, 48, 361–381. [Google Scholar]
- 99.Boontheekul T; Kong H-J; Mooney DJ, Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26 (15), 2455–2465.. [DOI] [PubMed] [Google Scholar]
- 100.Lanza RP; Kiihtreiber WM; Ecker D; Staruk JE; Chick WL, Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres. Transplantation 1995, 59 (10), 1377–1384. [DOI] [PubMed] [Google Scholar]
- 101.Muzzio NE; Pasquale MA; Rios X; Azzaroni O; Llop J; Moya SE, Adsorption and exchangeability of fibronectin and serum albumin protein corona on annealed polyelectrolyte multilayers and their consequences on cell adhesion. Adv. Mater. Interfaces 2019, 6 (8), 1900008. [Google Scholar]
- 102.Canibano-Hemandez A; Saenz del Burgo L; Espona-Noguera A; Orive G; Hernandez RM; Ciriza J. s.; Pedraz JL, Alginate microcapsules incorporating hyaluronic acid recreate closer in vivo environment for mesenchymal stem cells. Mol. Pharm. 2017,14 (7), 2390–2399. [DOI] [PubMed] [Google Scholar]
- 103.Yang Y; Opara EC; Liu Y; Atala A; Zhao W, Microencapsulation of porcine thyroid cell organoids within a polymer microcapsule construct. Exp. Biol. Med. 2017, 242 (3), 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rokstad AM; Brekke O-L; Steinkjer B; Ryan L; Kollarikova G; Strand BL; SkjakBrsek G; Lacik I; Espevik T; Mollnes TE, Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011, 7 (6), 2566–2578. [DOI] [PubMed] [Google Scholar]
- 105.Hajifathaliha F; Mahboubi A; Mohit E; Bolourchian N; Khalaj V; Nematollahi L, Comparison of Linear Poly Ethylene Imine (LPEI) and Poly L-Lysine (PLL) in Fabrication of CHOK1 Cell-Loaded Multilayer Alginate Microcapsules. Adv. Pharm. Bull. 2020,10 (2), 290–296.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loh QL; Wong YY; Choong C, Combinatorial effect of different alginate compositions, polycations, and gelling ions on microcapsule properties. Colloid Polym. Sci. 2012, 290 (7), 619–629. [Google Scholar]
- 107.Sakai S; Ono T; Ijima H; Kawakami K, In vitro and in vivo evaluation of alginate/sol-gel synthesized aminopropyl-silicate/alginate membrane for bioartificial pancreas. Biomaterials 2002, 23 (21), 4177–4183. [DOI] [PubMed] [Google Scholar]
- 108.Reis CP; Neufeld RJ; Vilela S; Ribeiro AJ; Veiga F, Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006, 23 (3), 245–257. [DOI] [PubMed] [Google Scholar]
- 109.Zhuang Y; Yu F; Ma J; Chen J, Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel. J. Colloid Interface Sci. 2017, 507, 250–259. [DOI] [PubMed] [Google Scholar]
- 110.Thu B; Smidsrod O; Skjåk-Bræk G, Alginate gels—some structure-function correlations relevant to their use as immobilization matrix for cells. Progr. Biotechnol. 1996,11, 19–30. [Google Scholar]
- 111.Zhou Q; Kang H; Bielec M; Wu X; Cheng Q; Wei W; Dai H, Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018,197, 292–304. [DOI] [PubMed] [Google Scholar]
- 112.Zeeb B; Saberi AH; Weiss J; McClements DJ, Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015, 50, 27–36. [Google Scholar]
- 113.Pravilovic RN; Balanč BD; Djordjević VB; Boškovic-Vragolović NM; Bugarski BM; Pjanović RV, Diffusion of polyphenols from alginate, alginate/chitosan, and alginate/inulin particles. J. Food Process Eng. 2019, 42 (4), el3043. [Google Scholar]
- 114.Thu B; Bruheim P; Espevik T; Smidsrød O; Soon-Shiong P; Skjåk-Bræk G, Alginate polycation microcapsules: II. Some functional properties. Biomaterials 1996, 17 (11), 1069–1079.. [DOI] [PubMed] [Google Scholar]
- 115.Kierstan M; Darcy G; Reilly J, Studies on the characteristics of alginate gels in relation to their use in separation and immobilized applications. Biotechnol. Bioeng. 1982, 24 (7), 1507–1517.. [DOI] [PubMed] [Google Scholar]
- 116.Asthana A; Ho Lee K; Kim K-O; Kim D-M; Kim D-P, Rapid and cost-effective fabrication of selectively permeable calcium-alginate microfluidic device using "modified'’ embedded template method. Biomicrofluidics 2012, 6 (1), 012821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan AW; Neufeld RJ, Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials 2010, 31 (34), 9040–9047.. [DOI] [PubMed] [Google Scholar]
- 118.Li L; Chen Y; Wang Y; Shi L; Nie Y; Liu T; Song K, Effects of concentration variation on the physical properties of alginate-based substrates and cell behavior in culture. Int. J. Biol. Macromol. 2019,128, 184–195. [DOI] [PubMed] [Google Scholar]
- 119.Gomaa M; Hifiney AL; Lawzy MA; Abdel-Gawad KM, Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar]
- 120.Zhang W; Zhao S; He X, Proliferation and differentiation of mesenchymal stem cells encapsulated in miniaturized 3D liquid core of alginate-chitosan-alginate (ACA) microcapsules. Arch. Stem Cell Res. 2015, 2 (1), 1004. [Google Scholar]
- 121.Velasco D; Tumarkin E; Kumacheva E, Microfluidic encapsulation of cells in polymer microgels. Small 2012, 8 (11), 1633–1642. [DOI] [PubMed] [Google Scholar]
- 122.Stabler C; Wilks K; Sambanis A; Constantinidis I, The effects of alginate composition on encapsulated βTC3 cells. Biomaterials 2001, 22 (11), 1301–1310. [DOI] [PubMed] [Google Scholar]
- 123.Lansdown A; Payne M, An evaluation of the local reaction and biodegradation of calcium sodium alginate (Kaltostat) following subcutaneous implantation in the rat. J. R. Coll. Surg. Edinb. 1994, 39 (5), 284–288. [PubMed] [Google Scholar]
- 124.Peirone M; Delaney K; Kwiecin J; Fletch A; Chang P, Delivery of recombinant gene product to canines with nonautologous microencapsulated cells. Hum. Gene Ther. 1998, 9 (2), 195–206. [DOI] [PubMed] [Google Scholar]
- 125.De Vos P; Van Straaten J; Nieuwenhuizen AG; de Groot M; Ploeg RJ; De Haan BJ; Van Schilfgaarde R, Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes 1999, 48 (7), 1381–1388. [DOI] [PubMed] [Google Scholar]
- 126.Kong HJ; Kaigler D; Kim K; Mooney DJ, Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 2004, 5 (5), 1720–1727.. [DOI] [PubMed] [Google Scholar]
- 127.Elbarbary AM; El-Rehim HAA; El-Sawy NM; Hegazy E-SA; Soliman E-SA, Radiation induced crosslinking of polyacrylamide incorporated low molecular weights natural polymers for possible use in the agricultural applications. Carbohydr. Polym. 2017, 176. 19–28. [DOI] [PubMed] [Google Scholar]
- 128.Williams PA; Campbell KT; Silva EA, Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis. J. Biomed. Mater. Res. A 2018,106 (1), 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alsberg E; Kong H; Hirano Y; Smith M; Albeiruti A; Mooney D, Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003, 82 (11), 903–908. [DOI] [PubMed] [Google Scholar]
- 130.Simmons CA; Alsberg E; Hsiong S; Kim WJ; Mooney DJ, Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 2004, 35 (2), 562–569. [DOI] [PubMed] [Google Scholar]
- 131.Bendtsen ST; Quinnell SP; Wei M, Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. A 2017,105 (5), 1457–1468. [DOI] [PubMed] [Google Scholar]
- 132.Wright B; De Bank PA; Luetchford KA; Acosta FR; Connon CJ, Oxidized alginate hydrogels as niche environments for comeal epithelial cells. J. Biomed. Mater. Res. Part A 2014, 102 (10), 3393–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bouhadir KH; Lee KY; Alsberg E; Damm KL; Anderson KW; Mooney DJ, Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001,17 (5), 945–950. [DOI] [PubMed] [Google Scholar]
- 134.Tibbitt MW; Anseth KS, Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009,103 (4), 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brudno Y; Silva EA; Kearney CJ; Lewin SA; Miller A; Martinick KD; Aizenberg M; Mooney DJ, Refdling drug delivery depots through the blood. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (35), 12722–12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joseph JJ; Sangeetha D; Shivashankar M, In vitro Release and Cytotoxic Studies of Novel Alginate Nanocarrier for the Antitumor Drug: Sunitinib. Regen. Eng. Transl. Med. 2019, 5 (2), 220–227. [Google Scholar]
- 137.Schmidt J; Lee MK; Ko E; Jeong JH; DiPietro LA; Kong H, Alginate sulfates mitigate binding kinetics of proangiogenic growth factors with receptors toward revascularization. Mol. Pharm. 2016,13 (7), 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yi H; Forsythe S; He Y; Liu Q; Xiong G; Wei S; Li G; Atala A; Skardal A; Zhang Y, Tissue-specific extracellular matrix promotes myogenic differentiation of human muscle progenitor cells on gelatin and heparin conjugated alginate hydrogels. Acta Biomater. 2017, 62, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quinlan E; López-Noriega A; Thompson EM; Hibbitts A; Cryan SA; O'Brien FJ, Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J. Tissue Eng. Regen. Med. 2017,11 (4), 1097–1109. [DOI] [PubMed] [Google Scholar]
- 140.O'Neill HS; O'Sullivan J; Porteous N; Ruiz-Hemandez E; Kelly HM; O'Brien FJ; Duffy GP, A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J. Tissue Eng. Regen. Med. 2018, 12 (1), e384–e394.. [DOI] [PubMed] [Google Scholar]
- 141.Mierisch CM; Cohen SB; Jordan LC; Robertson PG; Balian G; Diduch DR, Transforming growth factor-β in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy 2002,18 (8), 892–900. [DOI] [PubMed] [Google Scholar]
- 142.Ansari S; Diniz IM; Chen C; Aghaloo T; Wu BM; Shi S; Moshaverinia A, Alginate/hyaluronic acid hydrogel delivery system characteristics regulate the differentiation of periodontal ligament stem cells toward chondrogenic lineage. J. Mater. Sci. Mater. Med. 2017, 28 (10), 162. [DOI] [PubMed] [Google Scholar]
- 143.Wang L; Shansky J; Borselli C; Mooney D; Vandenburgh H, Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng. Part A 2012,18 (19–20), 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rowley JA; Madlambayan G; Mooney DJ, Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20 (1), 45–53. [DOI] [PubMed] [Google Scholar]
- 145.Wong FSY; Tsang KK; Chu AMW; Chan BP; Yao KM; Fo ACY, Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials 2019, 201, 53–67. [DOI] [PubMed] [Google Scholar]
- 146.Studer D; Cavalli E; Formica FA; Kuhn GA: Salzmann G; Mumme M; Steinwachs MR; Faurent-Applegate FA; Maniura-Weber K; Zenobi-Wong M, Human chondroprogenitors in alginate-collagen hybrid scaffolds produce stable cartilage in vivo. J. Tissue Eng. Regen. Med. 2017,11 (11), 3014–3026. [DOI] [PubMed] [Google Scholar]
- 147.Yang S; Zhu B; Yin P; Zhao F; Wang Y; Fu Z; Dang R; Xu J; Zhang J; Wen N, Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6 (3), 1590–1602. [DOI] [PubMed] [Google Scholar]
- 148.Canibano-Hemandez A; Saenz del Burgo F; Espona-Noguera A; Orive G; Hernandez RM; Ciriza J. s.; Pedraz JF, Alginate microcapsules incorporating hyaluronic acid recreate closer in vivo environment for mesenchymal stem cells. Mol. Pharm. 2017,14 (7), 2390–2399. [DOI] [PubMed] [Google Scholar]
- 149.Sarker B; Zehnder T; Rath SN; Horch RE; Kneser U; Detsch R; Boccaccini AR, Oxidized alginate-gelatin hydrogel: A favorable matrix for growth and osteogenic differentiation of adipose-derived stem cells in 3d. ACS Biomater. Sci. Eng 2017, 3 (8), 1730–1737.. [DOI] [PubMed] [Google Scholar]
- 150.Hsieh C-T; Hsu S.-h., Double-Network Polyurethane-Gelatin Hydrogel with Tunable Modulus for High-Resolution 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11 (36), 32746–32757. [DOI] [PubMed] [Google Scholar]
- 151.Yan Y; Li M; Yang D; Wang Q; Liang F; Qu X; Qiu D; Yang Z, Construction of injectable double-network hydrogels for cell delivery. Biomacromolecules 2017, 18 (7), 2128–2138.. [DOI] [PubMed] [Google Scholar]
- 152.Radhakrishnan A; Jose GM; Kurup M, PEG-penetrated chitosan-alginate copolysaccharide-based partially and fully cross-linked hydrogels as ECM mimic for tissue engineering applications. Prog. Biomater. 2015, 4 (2–4), 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gangopadhyay R; De A, Conducting semi-IPN based on polyaniline and crosslinked poly (vinyl alcohol). Synth. Met. 2002,132 (1), 21–28. [Google Scholar]
- 154.Rokhade AP; Patil SA; Aminabhavi TM, Synthesis and characterization of semiinterpenetrating polymer network microspheres of acrylamide grafted dextran and chitosan for controlled release of acyclovir. Carbohydr. Polym. 2007, 67 (4), 605–613. [Google Scholar]
- 155.Mahou R; Vlahos AE; Shulman A; Sefton MV, Interpenetrating alginate-collagen polymer network microspheres for modular tissue engineering. ACS Biomater. Sci. Eng. 2017, 4 (11), 3704–3712. [DOI] [PubMed] [Google Scholar]
- 156.Vorwald CE; Gonzalez-Femandez T; Joshee S; Sikorski P; Leach JK, Tunable FibrinAlginate Interpenetrating Network Hydrogels to Support Cell Spreading and Network Formation. Acta Biomater. 2020, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brigham MD; Bick A; Lo E; Bendali A; Burdick JA; Khademhosseini A, Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semiinterpenetrating networks. Tissue Eng. Part A 2009,15 (7), 1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Soltan N; Ning L; Mohabatpour F; Papagerakis P; Chen X, Printability and cell viability in bioprinting alginate dialdehyde-gelatin scaffolds. ACS Biomater. Sci. Eng. 2019, 5 (6), 2976–2987.. [DOI] [PubMed] [Google Scholar]
- 159.Li Z; Huang S; Liu Y; Yao B; Hu T; Shi H; Xie J; Fu X, Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 2018, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Balakrishnan B; Jayakrishnan A, Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26 (18), 3941–3951. [DOI] [PubMed] [Google Scholar]
- 161.Dumbleton J; Agarwal P; Huang H; Hogrebe N; Han R; Gooch KJ; He X, The effect of RGD peptide on 2D and miniaturized 3D culture of HEPM cells, MSCs, and ADSCs with alginate hydrogel. Cell. Mol. Bioeng. 2016, 9 (2), 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lehenkari P; Horton M, Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem. Biophys. Res. Commun. 1999, 259 (3), 645–650. [DOI] [PubMed] [Google Scholar]
- 163.Koo LY; Irvine DJ; Mayes AM; Lauffenburger DA; Griffith LG, Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J. Cell Sci. 2002, 115 (7), 1423–1433. [DOI] [PubMed] [Google Scholar]
- 164.Lee KY; Kong HJ; Mooney DJ, Quantifying Interactions between Cell Receptors and Adhesion Ligand-Modified Polymers in Solution. Macromol. Biosci. 2008, 8 (2), 140–145. [DOI] [PubMed] [Google Scholar]
- 165.Jeon O; Alsberg E, Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng. Part A 2013, 19 (11–12), 1424–1432.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu J; Zhou EL; Weir MD; Xu HEL; Chen Q; Trotman CA, Fast-degradable microbeads encapsulating human umbilical cord stem cells in alginate for muscle tissue engineering. Tissue Eng. Part A 2012,18 (21–22), 2303–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maia FR; Lourenco AH; Granja PL; Goncalves RM; Barrias CC, Effect of cell density on mesenchymal stem cells aggregation in RGD-alginate 3D matrices under osteoinductive conditions. Macromol. Biosci 2014,14 (6), 759–771. [DOI] [PubMed] [Google Scholar]
- 168.Ho SS; Murphy KC; Binder BY; Vissers CB; Leach JK, Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl. Med. 2016, 5 (6), 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fonseca KB; Bidarra SJ; Oliveira MJ; Granja PL; Barrias CC, Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater. 2011, 7 (4), 1674–1682. [DOI] [PubMed] [Google Scholar]
- 170.Mehta M; Madl CM; Lee S; Duda GN; Mooney DJ, The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. J. Biomed. Mater. Res. Part A 2015,103 (11), 3516–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee JW; Lee KY, Dual peptide-presenting hydrogels for controlling the phenotype of PC 12 cells. Colloids Surf. B Biointerfaces 2017,152, 36–41. [DOI] [PubMed] [Google Scholar]
- 172.Luo Z; Zhang S; Pan J; Shi R; Liu H; Lyu Y; Han X; Li Y; Yang Y; Xu Z, Timeresponsive osteogenic niche of stem cells: a sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials 2018,163, 25–42.. [DOI] [PubMed] [Google Scholar]
- 173.Huettner N; Dargaville TR; Forget A, Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 2018, 36 (4), 372–383. [DOI] [PubMed] [Google Scholar]
- 174.Machida-Sano I; Hirakawa M; Matsumoto H; Kamada M; Ogawa S; Satoh N; Namiki H, Surface characteristics determining the cell compatibility of ionically cross-linked alginate gels. Biomed. Mater. 2014, 9 (2), 025007. [DOI] [PubMed] [Google Scholar]
- 175.Utech S; Prodanovic R; Mao AS; Ostafe R; Mooney DJ; Weitz DA, Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater. 2015, 4 (11), 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang X; Wang W; Ma J; Guo X; Yu X; Ma X, Proliferation and differentiation of mouse embryonic stem cells in APA microcapsule: A model for studying the interaction between stem cells and their niche. Biotechnol. Prog. 2006, 22 (3), 791–800. [DOI] [PubMed] [Google Scholar]
- 177.Richardson T; Bamer S; Candiello J; Kumta PN; Baneijee I, Capsule stiffness regulates the efficiency of pancreatic differentiation of human embryonic stem cells. Acta Biomater. 2016, 35, 153–165. [DOI] [PubMed] [Google Scholar]
- 178.Rokstad AM; Gustafsson BI; Espevik T; Bakke I; Pfragner R; Svejda B; Modlin IM; Kidd M, Microencapsulation of small intestinal neuroendocrine neoplasm cells for tumor model studies. Cancer Sci. 2012,103 (7), 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kidd M; Modlin I; Shapiro M; Camp R; Mane S; Usinger W; Murren J, CTGF, intestinal stellate cells and carcinoid fibrogenesis. World J. Gastroenterol. 2007,13 (39), 5208–5216.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y-C; Chu T; Hall MS; Fu D-J; Shi Q; Chiu A; An D; Wang F-H; Pardo Y; Southard T, Physical confinement induces malignant transformation in mammary epithelial cells. Biomaterials 2019, 217, 119307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wisdom KM; Adebowale K; Chang J; Fee JY; Nam S; Desai R; Rossen NS; Rafat M; West RB; Hodgson F, Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018, 9 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chaudhuri O; Koshy ST; Da Cunha CB; Shin J-W; Verbeke CS; Allison KH; Mooney DJ, Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014,13 (10), 970–978. [DOI] [PubMed] [Google Scholar]
- 183.Mao AS: Shin JW; Utech S; Wang H; Uzun O; Fi W; Cooper M.; Hu Y; Zhang F; Weitz DA; Mooney DJ, Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat. Mater 2017,16 (2), 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mao AS; Ozkale B; Shah NJ; Vining KH; Descombes T; Zhang F; Tringides CM; Wong SW; Shin JW; Scadden DT; Weitz DA, Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl. Acad. Sci. U.S.A. 2019,116(31), 15392–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fratzl P; Weinkamer R, Nature's hierarchical materials. Prog. Mater. Sci. 2007, 52 (8), 1263–1334.. [Google Scholar]
- 186.Wu Q; Yang C; Fiu G; Xu W; Zhu Z; Si T; Xu RX, Multiplex coaxial flow focusing for producing multicompartment Janus microcapsules with tunable material compositions and structural characteristics. Lab Chip 2017, 17 (18), 3168–3175. [DOI] [PubMed] [Google Scholar]
- 187.Kang S-M; Fee G-W; Huh YS, Centrifugal Force-Driven Modular Micronozzle System: Generation of Engineered Alginate Microspheres. Sci. Rep 2019, 9 (1), 12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao H; Chen Y; Shao F; Xie M; Nie J; Qiu J; Zhao P; Ramezani H; Fu J; Ouyang H, Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle. Small 2018,14 (39), 1802630. [DOI] [PubMed] [Google Scholar]
- 189.Huang H; Choi JK; Rao W; Zhao S; Agarwal P; Zhao G; He X, Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Adv. Fnnct. Mater. 2015, 25 (44), 6839–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yoshida S; Takinoue M; Onoe H, Compartmentalized spherical collagen microparticles for anisotropic cell culture microenvironments. Adv. Hecdthc. Mater. 2017, 6 (8), 1601463. [DOI] [PubMed] [Google Scholar]
- 191.Huang H; Yu Y; Hu Y; He X; Usta OB; Yarmush MF, Generation and manipulation of hydrogel microcapsules by droplet-based microfluidics for mammalian cell culture. Lab Chip 2017,77(11), 1913–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lu Y-C; Song W; An D; Kim BJ; Schwartz R; Wu M; Ma M, Designing compartmentalized hydrogel microparticles for cell encapsulation and scalable 3D cell culture. J. Mater. Chem. B 2015, 3 (3), 353–360. [DOI] [PubMed] [Google Scholar]
- 193.Zhang L; Chen K; Zhang H; Pang B; Choi CH; Mao AS; Liao H; Utech S; Mooney DJ,; Wang H; Weitz DA, Microfluidic templated multicompartment microgels for 3D encapsulation and pairing of single cells. Small 2018,14 (9), 1702955. [DOI] [PubMed] [Google Scholar]
- 194.Lee BR; Lee KH; Kang E; Kim D-S; Lee S-H, Microfluidic wet spinning of chitosan-alginate microfibers and encapsulation of HepG2 cells in fibers. Biomicrofluidics 2011, 5 (2), 022208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yu Yj Shang L; Guo J; Wang J; Zhao Y, Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc 2018,13 (11), 2557–2579. [DOI] [PubMed] [Google Scholar]
- 196.Cheng Y; Zheng F; Lu J; Shang L; Xie Z; Zhao Y; Chen Y; Gu Z, Bioinspired multicompartmental microfibers from microfluidics. Adv. Mater. 2014, 26 (30), 5184–5190. [DOI] [PubMed] [Google Scholar]
- 197.Takei T; Kishihara N; Sakai S; Kawakami K, Novel technique to control inner and outer diameter of calcium-alginate hydrogel hollow microfibers, and immobilization of mammalian cells. Biochem. Eng. J. 2010, 49 (1), 143–147. [Google Scholar]
- 198.Sugiura S; Oda T; Aoyagi Y; Satake M; Ohkohchi N; Nakajima M, Tubular gel fabrication and cell encapsulation in laminar flow stream formed by microfabricated nozzle array. Lab Chip 2008, 8 (8), 1255–1257. [DOI] [PubMed] [Google Scholar]
- 199.Caldwell AS; Aguado BA; Anseth KS, Designing Microgels for Cell Culture and Controlled Assembly of Tissue Microenvironments. Adv. Funct. Mater. 2019, 1907670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chan HF; Zhang Y; Leong KW, Efficient one-step production of microencapsulated hepatocyte spheroids with enhanced functions. Small 2016,12 (20), 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sun Q; Tan SH; Chen Q; Ran R; Hui Y; Chen D; Zhao C-X, Microfluidic formation of coculture tumor spheroids with stromal cells as a novel 3D tumor model for drug testing. ACS Biomater. Sci. Eng. 2018, 4 (12), 4425–4433. [DOI] [PubMed] [Google Scholar]
- 202.Lin Z; Wu J; Qiao W; Zhao Y; Wong KH; Chu PK; Bian L; Wu S; Zheng Y; Cheung KM, Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials 2018,174, 1–16. [DOI] [PubMed] [Google Scholar]
- 203.Alessandri K; Sarangi BR; Gurchenkov VV; Sinha B; KicBling TR; Fetler L; Rico F; Scheming S; Lamaze C; Simon A, Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. U. S. A. 2013,110 (37), 14843–14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen Q; Utech S; Chen D; Prodanovic R; Lin J-M; Weitz DA, Controlled assembly of heterotypic cells in a core-shell scaffold: organ in a droplet. Lab Chip 2016,16 (8), 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Choi JK,; Agarwal P; Huang H; Zhao S; He X, The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014, 35 (19), 5122–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Agarwal P; Zhao S; Bielecki P; Rao W; Choi JK; Zhao Y; Yu J; Zhang W; He X, One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip 2013,13 (23), 4525–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hidalgo San Jose L; Stephens P: Song B; Barrow D, Microfluidic encapsulation supports stem cell viability, proliferation, and neuronal differentiation. Tissue Eng. Part C Methods 2018, 24 (3), 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guerzoni LP; Tsukamoto Y; Gehlen DB; Rommel D; Haraszti T. s.; Akashi M; De Laporte L, A layer-by-layer single-cell coating technique to produce injectable beating mini heart tissues via microfluidics. Biomacromolecules 2019, 20 (10), 3746–3754. [DOI] [PubMed] [Google Scholar]
- 209.Alessandri K: Feyeux M; Gurchenkov B; Delgado C; Trushko A; Krause K-H; Vignjevic D; Nassoy P; Roux A, A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 2016,16(9), 1593–1604. [DOI] [PubMed] [Google Scholar]
- 210.Perez RA; Kim M; Kim T-H; Kim J-H; Lee JH; Park J-H; Knowles JC; Kim HW, Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A 2014, 20 (1–2), 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Choi DH; Park CH; Kim IH; Chun HJ; Park K; Han DK, Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release 2010,147(2), 193–201. [DOI] [PubMed] [Google Scholar]
- 212.Maguire T; Novik E; Schloss R; Yarmush M, Alginate-PLL microencapsulation: Effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol. Bioeng. 2006, 93 (3), 581–591. [DOI] [PubMed] [Google Scholar]
- 213.Richardson T; Kumta PN; Baneijee I, Alginate encapsulation of human embryonic stem cells to enhance directed differentiation to pancreatic islet-like cells. Tissue Eng. Part A 2014, 20 (23–24), 3198–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jain RK; Au P; Tam J; Duda DG; Fukumura D, Engineering vascularized tissue. Nat. Biotechnol. 2005, 23 (7), 821–823. [DOI] [PubMed] [Google Scholar]
- 215.Smith AM; Hunt NC; Shelton RM; Birdi G; Grover LM, Alginate hydrogel has a negative impact on in vitro collagen 1 deposition by fibroblasts. Biomacromolecules 2012, 13 (12), 4032–4038. [DOI] [PubMed] [Google Scholar]
- 216.Kim D; Jo A; Imani KBC; Kim D; Chung JW; Yoon J, Microfluidic fabrication of multistimuli-responsive tubular hydrogels for cellular scaffolds. Langmuir 2018, 34 (14), 4351–4359.. [DOI] [PubMed] [Google Scholar]
- 217.Onoe H; Kato-Negishi M; Itou A; Takeuchi S, Differentiation induction of mouse neural stem cells in hydrogel tubular microenvironments with controlled tube dimensions. Adv. Healthc. Mater. 2016, 5 (9), 1104–1111. [DOI] [PubMed] [Google Scholar]
- 218.Kinoshita K; Iwase M; Yamada M; Yajima Y; Seki M, Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J 2016, 11 (11), 1415–1423. [DOI] [PubMed] [Google Scholar]
- 219.Jia W; Gungor-Ozkerim PS; Zhang YS; Yue K; Zhu K; Liu W; Pi Q; Byambaa B; Dokmeci MR; Shin SR; Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016,106, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mistry P; Aied A; Alexander M: Shakesheff K; Bennett A; Yang J, Bioprinting using mechanically robust core-shell cell-laden hydrogel strands. Macromol. Biosci. 2017, 17 (6), 1600472. [DOI] [PubMed] [Google Scholar]
- 221.Akkineni AR; Ahlfeld T; Lode A; Gelinsky M, A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs. Biofabrication 2016, 8 (4), 045001. [DOI] [PubMed] [Google Scholar]
- 222.Yang L; Yu X; Fuller AM; Troester MA; Oldenburg AL, Characterizing optical coherence tomography speckle fluctuation spectra of mammary organoids during suppression of intracellular motility. Quant. Imaging Med. Surg 2020,10 (1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rothmund G; Sattler EC; Kaestle R; Fischer C; Haas CJ; Starz H; Welzel J, Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosiscomparison of six diagnostic methods. Mycoses 2013, 56 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- 224.Mansour AAj Gonsalves JI; Bloyd CW; Li H; Fernandes S; Quang D; Johnston S; Parylak SL; Jin X| Gage FH, An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36 (5), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grover AC; Tangrea MA; Woodson KG; Wallis BS; Hanson JC; Chuaqui RF; Gillespie JW; Erickson HS; Bonner RF; Pohida TJ, Tumor-associated endothelial cells display GSTP1 and RAR|32 promoter methylation in human prostate cancer. J. Transl. Med. 2006,4(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huang H; Ding Y; Sun XS; Nguyen TA, Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS One 2013, 8 (3), e59482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Xu X; Gurski LA; Zhang C; Harrington DA; Farach-Carson MC; Jia X, Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012, 33 (35), 9049–9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gurski LA; Petrelli NJ; Jia X; Farach-Carson MC, 3D matrices for anti-cancer drug testing and development. Oncol. Issues 2010, 25 (1), 20–25. [Google Scholar]
- 229.Yip D; Cho CH, A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commnn. 2013, 433 (3), 327–332. [DOI] [PubMed] [Google Scholar]
- 230.Price KJ; Tsykin A; Giles KM; Sladic RT; Epis MR; Ganss R; Goodall GJ; Leedman PJ, Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem. Biophys. Res. Commnn. 2012, 427 (2), 343–348. [DOI] [PubMed] [Google Scholar]
- 231.Szot CS; Buchanan CF; Freeman JW; Rylander MN, 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32 (31), 7905–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tredan O; Galmarini CM; Patel K; Tannock IF, Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99 (19), 1441–1454. [DOI] [PubMed] [Google Scholar]