Abstract
Purpose:
Compare the detection rate of seizures on scalp EEG with simultaneous intracranial stereo EEG (SEEG) recordings.
Methods:
Twenty-seven drug-resistant epilepsy patients undergoing SEEG with simultaneous scalp EEG as part of their surgical work-up were included. A total of 172 seizures were captured.
Results:
Of the 172 seizures detected on SEEG, 100 demonstrated scalp ictal patterns. Focal aware and subclinical seizures were less likely to be seen on scalp, with 33% of each observed when compared with focal impaired aware (97%) and focal to bilateral tonic–clonic seizures (100%) (P < 0.001). Of the 72 seizures without ictal scalp correlate, 32 demonstrated an abnormality during the SEEG seizure that was identical to an interictal abnormality. Seizures from patients with MRI lesions were statistically less likely to be seen on scalp than seizures from nonlesional patients (P = 0.0162). Stereo EEG seizures not seen on scalp were shorter in duration (49 seconds) compared with SEEG seizures seen on scalp (108.6 seconds) (P < 0.001).
Conclusions:
Scalp EEG is not a sensitive tool for the detection of focal aware and subclinical seizures but is highly sensitive for the detection of focal impaired aware and focal to bilateral tonic–clonic seizures. Longer duration of seizure and seizures from patients without MRI lesions were more likely to be apparent on scalp. Abnormalities seen interictally may at times represent an underlying seizure. The cognitive, affective, and behavioral long-term effects of ongoing difficult-to-detect seizures are not known.
Keywords: Epilepsy/seizures, EEG, Stereo EEG, Epilepsy monitoring, Intracranial electrodes, Cortical localization
Scalp EEG has long been used in the evaluation of patients with epilepsy, and the literature pertaining to scalp EEG is extensive. However, scalp EEG is a poor spatial localizer, and the pattern of oscillations of electrical potentials seen on scalp EEG could be generated from a near infinite number of potential sources.1 In patients with drug-resistant epilepsy, other noninvasive methods, such as positron emission tomography, single-photon emission computed tomography, magnetoencephalography, source modeling and functional MRI, are often employed but do not directly measure seizure activity and do not accurately distinguish a seizure onset zone from an area of seizure propagation.2–4 Accordingly, in patients with drug-resistant epilepsy who are surgical candidates, invasive monitoring is often necessary to define the seizure-generating network.
There is a dearth of evidence directly comparing intracranial EEG patterns to those seen on scalp. The existing studies either did not collect data simultaneously,5,6 did not use a complete surface electrode montage,7 did not include stereo electrodes,8 investigated temporal lobe seizures only,9,10 or looked exclusively at interictal data.11
A deeper understanding of the scope and limitations of scalp EEG, established through simultaneous comparison, could ultimately lead to improved understanding of epilepsy and increased insight into our patients’ symptoms.12 In this investigation, we analyzed simultaneous scalp and stereo EEG (SEEG) recordings and report their comparative seizure detection sensitivity, the duration of electrographic seizure activity, and the relationship in time between SEEG onset, scalp onset, and clinical signs.
METHODS
Participant Demographics
Patients with drug-resistant epilepsy who underwent simultaneous SEEG with scalp EEG (standard of care at our institution) in the Mount Sinai Epilepsy Program in 2017 were included. As part of standard protocol, these patients underwent extensive presurgical evaluation including video EEG monitoring, MRI (1.5 T and/or 3 T) with/without gadolinium, neuropsychological evaluation, Wada test, and positron emission tomography when appropriate (see Table S1, Supplemental Digital Content 1, http://links.lww.com/JCNP/A113). All patients were presented at multidisciplinary conference, and an intracranial study was deemed necessary for surgical planning. For patients with bilateral seizure onset, a bilateral SEEG was performed. Other reasons for a bilateral study included discordant Wada, nonlesional MRI, bilateral interictal epileptiform discharges, difficult to lateralize scalp onsets, and/or seizure recurrence after prior surgical intervention. The SEEG was then done to see if resection was possible and if not, with the goal of optimizing responsive neurostimulation electrode placements.
If a patient’s scalp EEG electrodes were intact at the start of the study, but then degraded in quality over the recording, the seizures were included only when the scalp electrodes were intact. At least one clinical seizure needed to be captured for inclusion. Both scalp seizures and SEEG seizures had to last for at least 10 seconds and evolve in morphology and location. In total, 27 patients were included (9 men and 18 women) with an average age of 38.2±6.8 years. The planned location of SEEG electrodes varied according to the presurgical work-up (see Table S1, Supplemental Digital Content 1, http://links.lww.com/JCNP/A113). All patients’ antiseizure medications were lowered or discontinued during the monitoring period. As a result of SEEG, patients underwent focal resection (4), unilateral responsive neurostimulation (1), bilateral responsive neurostimulation (7), vagus nerve stimulation (2), laser interstitial thermal therapy (11), or responsive neurostimulation with resection (2).
Standard Protocol Approvals
The study was approved by the Icahn School of Medicine Institutional Review Board.
EEG Acquisition
Electrophysiological data were collected for patients using a Natus XLTEK128 or Natus Quantum amplifier (Natus Medical Incorporated, Pleasanton, CA). In every case, 19 surface electrodes were used to record scalp EEG. No subtemporal chain, sphenoidal, or foramen ovale electrodes were used.
The SEEG surgery was performed by two neurosurgeons (F.E.P., S.G.). Trajectories were preplanned using a preoperative postcontrast volumetric fast spoiled gradient echo 3D sequence. Lead numbers were minimized to decrease morbidity.13 After anesthesia induction, bone fiducials were placed in the patient’s skull (Medtronic Inc, Minneapolis, MN). Computed tomography was performed and fused to the preoperative MRI using robotic stereotactic assistance device ROSA software (Rosa; Medtech, Montpellier, France). Each patient had 1.12-mm-diameter Ad-Tech leads (Ad-Tech Medical Instrument Corp, Racine, WI) with between 6 and 14 contacts (dependent upon planned trajectory length) placed using the ROSA robot.
Twenty patients had bilateral stereo electrodes implanted, four had right-sided only stereo electrodes placed, and three had left-sided only stereo electrodes placed. Postoperatively, a CT with fine cuts was obtained and fused with the preoperative MRI for localization and accuracy verification. Scalp electrodes were placed after postoperative imaging was performed using the standard 10–20 system. Occasionally, the scalp electrode placement was adjusted (<1 cm) to avoid the SEEG insertion points. These deviations from the 10–20 system were not documented by the EEG technicians.
EEG Analysis
The official EEG reports, read by the on-service epileptologist (one of a group of nine), were used to obtain onset/offset times for intracranial seizures. EEGs were then reviewed independently by two board-certified epileptologists (L.V.M., M.C.F.), who then confirmed onset/offset times and adjusted them in rare cases. Scalp EEGs were reviewed separately from SEEGs to eliminate bias. Any difference of more than 2 seconds in onset or offset times was reviewed together for a consensus. There were no differing opinions regarding if a seizure was electrographically present on scalp or not. Video, reviewed separately from EEG, was used to characterize seizure semiology, time of first clinical sign, and state of awareness during seizures. All patients had typical clinical seizures during the recording. Clinical seizures included patient pushbuttons and descriptions of focal aware (FA) symptoms as well as more clinically apparent episodes.
Statistical Analysis
Nonparametric statistical analyses were performed. Wilcoxon signed rank tests were used to compute P values unless otherwise specified. A P < 0.05 was considered to imply statistical significance. Reported statistical values were calculated using MATLAB 2018a (Mathworks Inc, Natick, MA). Confidence intervals (CIs) (95% to 5%) were determined by resampling statistics (bootstrap). Sample means were computed for each experimental variable by randomly sampling the set of experimental variables a number of times equal to the size of the set with replacement. This was computed 2,000 times unless otherwise specified. Then, 95% and 5% CIs were calculated by rank ordering the randomly sampled means. Finally, the distribution of sample means was corrected for bias and skewness using the bias-corrected and accelerated bootstrap interval method.14
RESULTS
A total of 172 seizures were included in this study and classified according to International League Against Epilepsy nomenclature.15 Four types of seizures were observed: subclinical, FA, focal impaired awareness, and focal to bilateral tonic clonic.
Characteristics of SEEG and Scalp Seizures
Of the 172 seizures included in this study, 100 (100/172 = 58%) were observed both on scalp EEG and SEEG, whereas 72 (72/172 = 42%) seizures were detected on SEEG only. Figure 1 shows an example of a simultaneous onset (patient A), a nonictal appearing correlation on scalp (patient B), and a delayed onset between SEEG and scalp onset (patient C). Seizures observed on the scalp were more likely to be clinically apparent. Of the 100 seizures observed on the scalp, 72 were clinical (72/100 = 72%) and 28 were subclinical (28/100 = 28%). Of the 72 seizures not observed on the scalp, 15 were clinical (15/72 = 21%) and 57 were subclinical (57/72 = 79%). Of the 87 clinical seizures, a total of 83% (72/87) were seen on the scalp. The 15 clinical seizures not detected on scalp EEG derive from 8 patients (patients 1, 5, 12, 16, 17, 18, 19, and 20). The percentage of clinical:subclinical seizures ranged from 14% to 100% depending on the patient (median: 80%). In two patients (patients 18, 20), scalp EEG failed to register any seizures, including clinical seizures. Patient 18 had a small left middle cranial encephalocele with seizures originating from the left lateral temporal lobe, and patient 20 had increased volume in the right hippocampus and amygdala with right mesial temporal onsets.
FIG. 1.
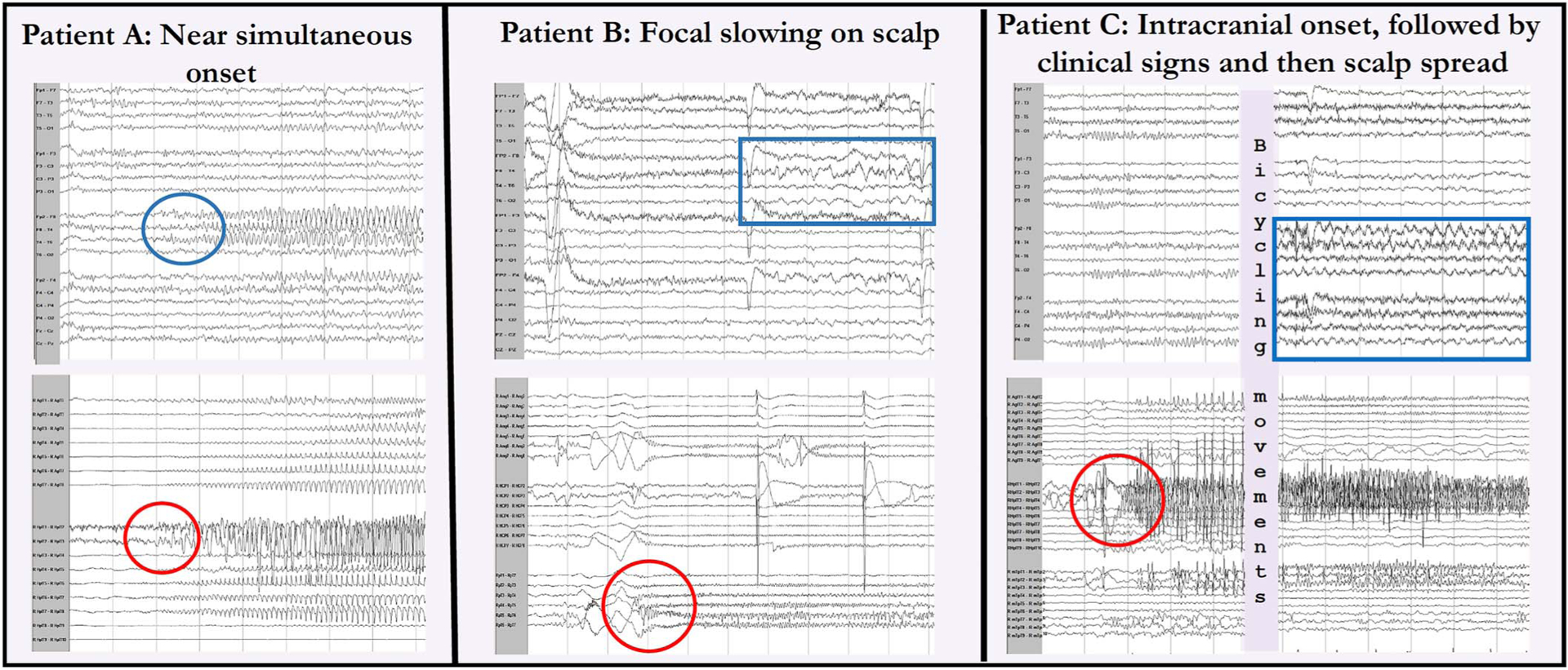
Comparison of select simultaneous scalp and intracranial EEGs. In patient A, the intracranial onset (bottom circle) in the right hippocampus was seen near simultaneously on scalp (top circle) in the right temporal chain. For patient B, an intracranial right lateral temporal onset (circle) was followed by 5 seconds of polymorphic right temporal slowing (box), which was not sustained and not different from the interictal slowing. In patient C, there was an intracranial right hippocampal onset (circle) with no scalp EEG finding followed by clinical signs and then rhythmic diffuse right hemispheric theta slowing seen on scalp (box).
Scalp EEG detected all focal to bilateral tonic–clonic seizures (33/33 = 100%) and most focal impaired awareness seizures (32/33 = 97%) but was poor at detecting subclinical and FA seizures (28/85 = 33%; 7/21 = 33%). There was a significant relationship between seizure type and the proportion of seizures seen on scalp EEG (P < 0.001). Specifically, FA and subclinical seizures were less likely than other seizure types to be detected on scalp EEG.
All of the brain regions from which seizures originated, along with the percentage of seizures observed on scalp EEG in each location, are listed in Table 1. No statistically significant differences were found between brain regions.
TABLE 1.
Seizure Types, Locations, and Percentage of Seizures Seen on Scalp EEG
| Types of Seizures | No. of Seizures | No. of Seizures Seen on Scalp EEG (Percent) |
|---|---|---|
| Subclinical | 85 | 28 (33) |
| Focal aware | 21 | 7 (33) |
| Focal impaired awareness | 33 | 32 (97) |
| Focal to bilateral tonic clonic | 33 | 33 (100) |
| Location | No. of Seizures | No. of Seizures Seen on Scalp EEG (Percent) | ||||
|---|---|---|---|---|---|---|
| Mesial temporal | R (n = 84) | L (n = 27) | B/L (n = 0) | R (n = 49) (58) | L (n = 21) (78) | B/L (n = 0) |
| Subclinical | 45 | 9 | — | 20 (44) | 5 (56) | — |
| Focal aware | 16 | 2 | — | 6 (44) | 1 (50) | — |
| Focal impaired awareness | 18 | 4 | — | 18 (100) | 3 (75) | — |
| Focal to bilateral tonic-clonic | 5 | 12 | — | 5 (100) | 12 (100) | — |
| Frontal | R (n = 0) | L (n = 4) | B/L (n = 5) | R (n = 0) | L (n = 3) (75) | B/L (n = 5) (100) |
| Subclinical | — | 2 | 0 | — | 1 (50) | n/a |
| Focal aware | — | 0 | 0 | — | n/a | n/a |
| Focal impaired awareness | — | 0 | 4 | — | n/a | 4 (100) |
| Focal to bilateral tonic-clonic | — | 2 | 1 | — | 2 (100) | 1 (100) |
| Insula/lateral temporal | R (n = 24) | L (n = 3) | B/L (n = 2) | R (n = 1) (4) | L (n = 3) (100) | B/L (n = 2) (100) |
| Subclinical | 23 | 2 | 0 | 0 (0) | 2 (100) | n/a |
| Focal aware | 0 | 0 | 0 | n/a | n/a | n/a |
| Focal impaired awareness | 1 | 0 | 0 | n/a | n/a | n/a |
| Focal to bilateral tonic-clonic | 0 | 1 | 2 | 1 (100) | 1 (100) | 2 (100) |
| Lateral temporal | R (n = 1) | L (n = 8) | B/L (n = 0) | R (n = 1) (100) | L (n = 2) (25) | B/L (n = 0) |
| Subclinical | 0 | 3 | — | n/a | 0 (0) | — |
| Focal aware | 0 | 3 | — | n/a | 0 (0) | — |
| Focal impaired awareness | 0 | 0 | — | n/a | n/a | — |
| Focal to bilateral tonic-clonic | 1 | 2 | — | 1 (100) | 2 (100) | — |
| Occipital | R (n = 0) | L (n = 3) | B/L (n = 0) | R (n = 0) | L (n = 3) (100) | B/L (n = 0) |
| Subclinical | — | 0 | — | — | n/a | — |
| Focal aware | — | 0 | — | — | n/a | — |
| Focal impaired awareness | — | 1 | — | — | 1 (100) | — |
| Focal to bilateral tonic-clonic | — | 2 | — | — | 2 (100) | — |
| Bilateral diffuse onset | R (n/a) | L (n/a) | B/L (n = 11) | R (n/a) | L (n/a) | B/L (n = 10) (91) |
| Subclinical | — | — | 1 | — | — | 0 (0) |
| Focal aware | — | — | 0 | — | — | n/a |
| Focal impaired awareness | — | — | 4 | — | — | 4 (100) |
| Focal to bilateral tonic-clonic | — | — | 6 | — | — | 6 (100) |
Note (n/a) is marked under scalp EEG when no seizure of that type was observed in the patient either clinically or on stereo EEG (SEEG).
This is to avoid confusion with the instances in which seizures of a particular type are observed clinically/with SEEG, but then are all missed by scalp EEG.
For the 72 SEEG seizures without scalp EEG correlate, any scalp patterns occurring at the time of the SEEG seizure were noted (Table 2). These findings were not clearly different from the patients’ interictal patterns.
TABLE 2.
Scalp EEG Findings During the 72 Stereo EEG Seizures With No Scalp Ictal Pattern
| Scalp EEG Pattern During Stereo EEG Seizure | No. of Seizures (Percent) |
|---|---|
| No scalp changes | 40 (56) |
| Diffuse scalp slowing | 25 (35) |
| Focal polymorphic slowing | 3 (4) |
| Focal slowing with mixed epileptiform potentials | 3 (4) |
| Generalized spikes | 1 (1) |
Results From the Bilateral SEEG Studies
For the 100 seizures observed on both stereo and scalp EEG, the overwhelming majority (93/100 = 93%) were from the 20 patients with bilateral SEEG implants. Of these 93 seizures, 62 (62/93 = 67%) had focal SEEG onset with ipsilateral onset on scalp, 13 (13/93 = 14%) had focal SEEG onset with diffuse onset on scalp, and 1 with focal SEEG onset had contralateral onset on scalp (1/93 = 1%). This seizure with contralateral focal scalp onset originated in the insula/lateral temporal lobe (patient 6). Sixteen seizures had bilateral SEEG onset with bilateral onset on scalp (16/93 = 17%) and 1 seizure with bilateral SEEG onset had unilateral onset on scalp (1/93 = 1%) (Table 3).
TABLE 3.
Seizure Propagation Patterns Seen on Bilateral Stereo EEG Studies From Intracranial (I) to Scalp (S)
| Total Seizures (93) | (I) Focal to (S) Focal, Ipsilateral | (I) Focal to (S) Diffuse | (I) Focal to (S) Focal, Contralateral | (I) Diffuse to (S) Diffuse | (I) Diffuse to (S) Focal |
|---|---|---|---|---|---|
| Subclinical | 25 | 2 | 0 | 0 | 0 |
| Focal aware | 5 | 0 | 0 | 0 | 0 |
| Focal impaired awareness | 13 | 9 | 0 | 8 | 0 |
| FTBTC | 19 | 2 | 1 | 8 | 1 |
| Total | 62 (67%) | 13 (14%) | 1 (1%) | 16 (17%) | 1 (1%) |
FTBTC, focal to bilateral tonic clonic.
Results From the Unilateral SEEG Studies
Seven of the 100 seizures observed on both stereo and scalp EEG were obtained from unilateral SEEG implants in a total of seven patients (3 patients with left-sided studies, 4 patients with right-sided studies). Of these seizures, 6 were seen to propagate ipsilaterally from stereo to scalp EEG (6/7 = 86%), and 1 (1/7 = 14%) seizure was diffuse on scalp.
Lesional Versus Nonlesional SEEG Cases
Of the 27 patients studied, 17 patients had epileptogenic lesions on MRI, whereas the remaining 10 patients were nonlesional. In total, these 17 lesional patients accounted for 124 seizures (124/172 = 72%). Seizures in lesional cases were less likely (65/124 = 52%) than seizures in nonlesional cases to be seen on scalp (35/48 = 73%; P = 0.0162). Surprisingly, the duration of seizures in lesional cases was significantly longer than the nonlesional cases (97.6 vs. 70 seconds, P = 0.0088). Of the 124 lesional seizures, 106 (106/124 = 85%) had an onset concordant with the MRI lesion intracranially, whereas 18 seizures (18/124 = 15%) were discordant. There was no significant difference in seizure types between the groups.
Seizure Duration on Scalp Versus SEEG
We calculated the average electrographic seizure duration on both EEG modalities. The mean duration of seizures on scalp EEG was 89.1 seconds (95% CI: 78.7–105.5 seconds), and the average SEEG seizure duration was 83.7 seconds (95% CI: 75.3–94.7 seconds). Ninety-eight of 100 seizures observed on scalp EEG were included in this calculation, with one seizure excluded as a result of premature electrode disconnection (patient 11) and another as a result of obscuring artifact (patient 13). On SEEG, 171 of the 172 seizures were included. One seizure was excluded (patient 11) because of electrode disconnection prior to seizure conclusion.
For the 100 seizures detected on both stereo and scalp EEG, the mean SEEG duration was 108.6 seconds (95% CI: 97.8–125.7 seconds). This was statistically different from the mean scalp EEG seizure duration (89.1 seconds; P = 0.001). Similarly, for the 72 seizures that were only seen on SEEG, the average seizure duration was 49.0 seconds (95% CI: 39.9–62.3 seconds). This value was statistically significant from the average seizure duration of all scalp EEG seizures (P < 0.001). The mean seizure duration for SEEG seizures also observed on scalp EEG (108.6 seconds) was also significantly different from the mean duration of SEEG seizures not observed on scalp EEG (49.0 seconds) (P < 0.001).
Lag Between Stereo and Scalp EEG Seizure Recordings
Time lag between onset of seizure activity on SEEG and scalp EEG was calculated for the 100 seizures observed on both modalities (Fig. 2). A mean delay of 17.3 seconds (95% CI: −14.2 to −22.5 seconds) was observed between onset of seizures on SEEG and scalp EEG, with SEEG seizure onset beginning prior to scalp seizure onset in 84 seizures. An example is shown in Figure 1B. In 15 seizures, seizure onset was simultaneous between scalp and SEEG. An example is shown in Figure 1C. In one seizure, scalp onset preceded stereo onset. The time difference was significantly different from zero (P < 0.001).
FIG. 2.
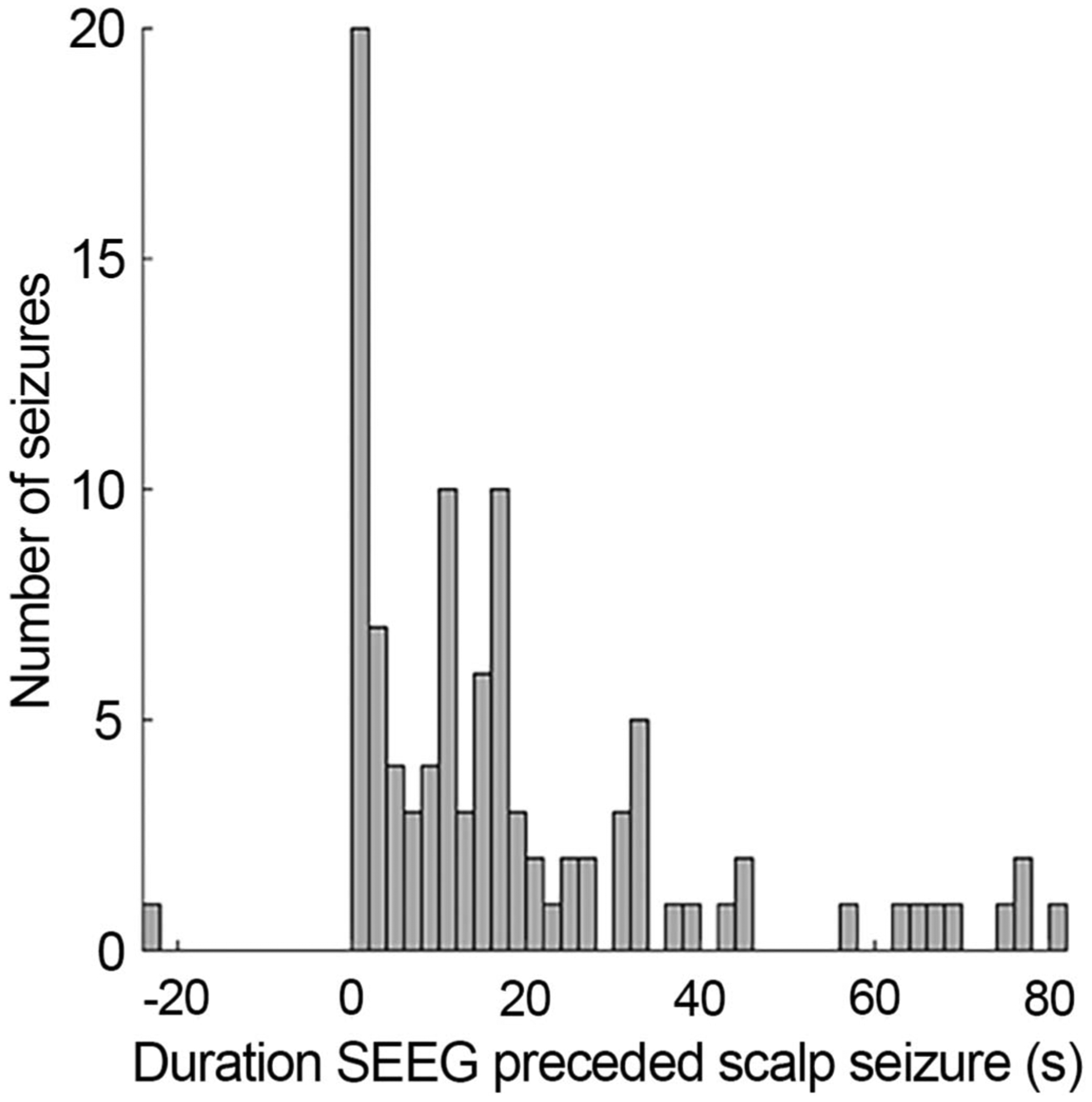
Time lag between SEEG onset and scalp onset. A single seizure had a scalp onset before SEEG onset. Note that only seizures with both stereo and scalp components were included here.
Delay Between Electrographic Activity and Clinical Signs
Delay between electrographic seizure onset and the onset of clinical symptoms was determined (Fig. 3). Eighty-seven of 172 seizures had clinical signs. The mean delay to clinical signs on SEEG was 29.7 seconds (95% CI: 23.6–37.5 seconds). Regarding scalp EEG, 72 of 172 seizures were included. The excluded 100 seizures either had no scalp EEG component, were subclinical, or both. The mean scalp EEG delay to clinical signs was 14.0 seconds (95% CI: 8.6–22.8 seconds). The difference between the delay on SEEG compared with scalp EEG was significantly different (P < 0.001). Of note, 12 seizures had SEEG onset, followed by clinical signs, and then scalp EEG onsets.
FIG. 3.
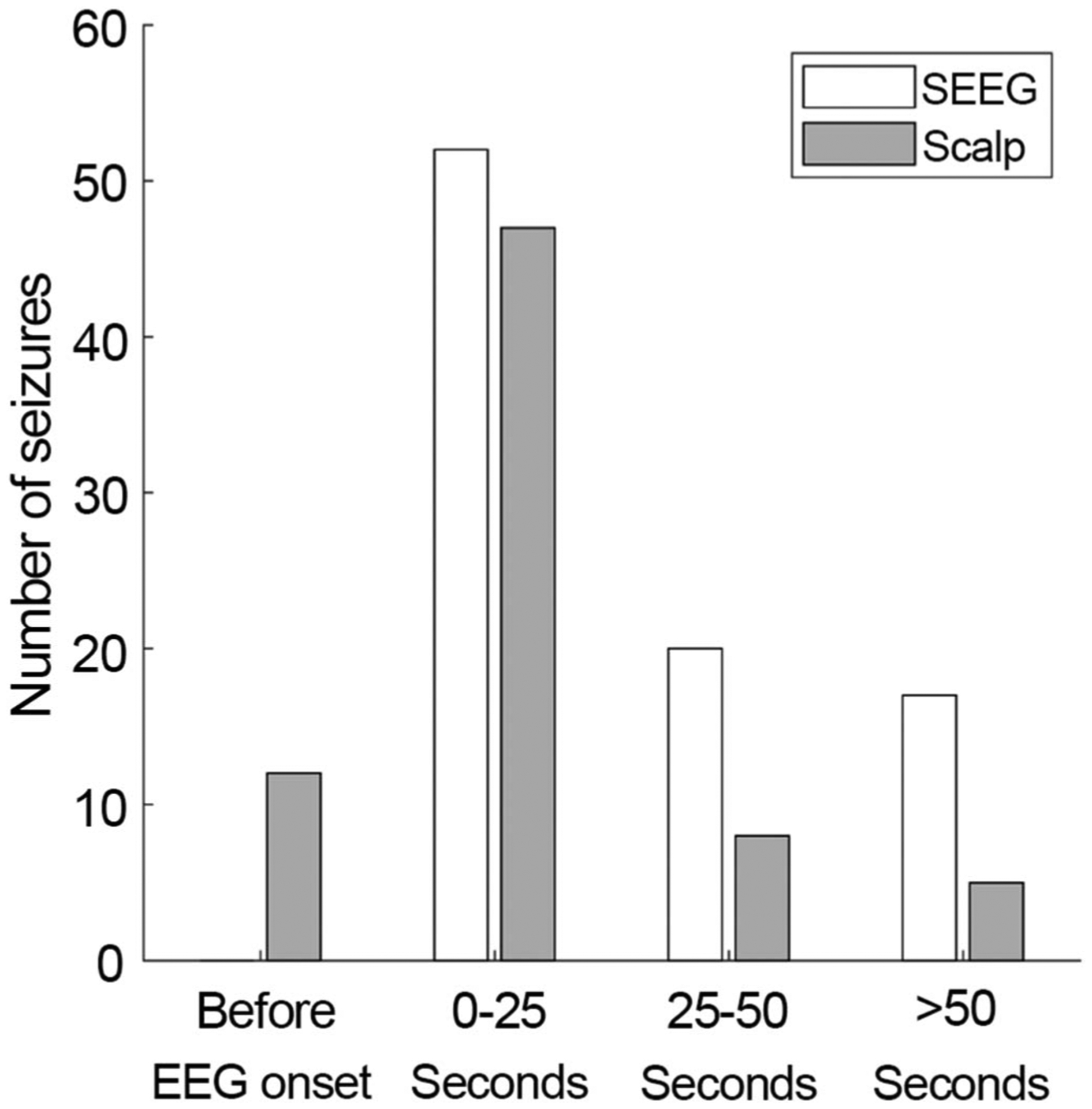
Time difference between clinical signs and EEG ictal patterns on SEEG. In all cases, the time between SEEG onset and clinical onset was longer than the time between scalp onset and clinical onset. Twelve seizures had clinical onset after the SEEG onset and before scalp EEG onset.
DISCUSSION
Few studies have simultaneously compared intracranial and scalp EEG seizures, although none have looked at broad comparisons of ictal data across multiple brain regions specifically using stereo electrodes.5–11 Ours is an attempt to build on and better establish the relationships between these EEG modalities and improve our overall understanding of their individual qualities and limitations.
Similar to previous work, this study showed that FA and subclinical seizures were less likely to be seen on scalp EEG than other seizure types. Prior studies reported FA detection rates of between 10.7% and 21% on scalp EEG, compared with 33% in our study on scalp and 100% on SEEG.5,16 Furthermore, the reason FA, and indeed subclinical seizures, were often not seen on scalp is because these seizures involve smaller regions of cortical activation. To produce a scalp EEG pattern, the seizures must involve at least 6 cm2 of cortical matter, and in most cases, more than 10 cm2.10,17
To the best of our knowledge, no studies other than Lieb et al.,7 which used incomplete surface electrode montages, have reported on scalp EEG subclinical seizure detection rate when compared with simultaneous SEEG. The 33% detection rate of subclinical seizures in our study, as well as that of Lieb et al. 10%, suggests that some patients are having seizures apparent neither clinically nor on scalp EEG.
Seizure activity has been shown to contribute to memory and cognitive impairment as well as psychiatric instability.18–20 In populations known to have increased seizure risks such as Alzheimer disease and autism, seizures can be adversely affecting their disease severity.21,22 For instance, two patients with Alzheimer disease were found to have abundant, mesial, temporal, subclinical seizures identified with foramen ovale electrodes, despite prior negative scalp EEGs.23 Cognitive improvement followed subsequent treatment of these subclinical seizures. Furthermore, in patients with epilepsy and autism, epilepsy surgery has been shown to both reduce seizure frequency and improve behavioral symptoms previously associated with autism.24 It is possible that some of the cognitive and psychiatric morbidities of epilepsy are secondary to ongoing subclinical seizure activity that scalp EEG cannot quantify.
Typical interictal findings on scalp EEG, such as diffuse or focal slowing, have traditionally been associated with encephalopathies, diffuse brain dysfunction, and/or focal lesions.25–32 However, we demonstrated that intracranial seizures may cause such scalp patterns. In our study, a nonevolving, nonictal abnormality was present in 44% of the SEEG seizures without a scalp EEG seizure. Similarly, Pacia et al. reported diffuse slowing on scalp EEG with simultaneous seizure activity on SEEG, whereas Koessler et al. demonstrated weak, nonictal electrical contributions from deep, mesial, temporal, ictal sources to scalp EEG patterns.10,33 In the absence of intracranial electrodes, there is no way to distinguish seconds of an EEG abnormality that may correlate with a seizure.
In this study, 17 of 27 patients had MRI lesions thought responsible for their epilepsy. Two features emerge from this data. First, most seizures (85%) were concordant with MRI lesions. Second, lesional cases were less likely to have scalp EEG correlates, 52% versus 73%, respectively. This was not related to the length of the seizure; as surprisingly, the seizures in lesional cases were significantly longer. One possibility is that lesions and the surrounding cortex involved in the epileptogenic region are somewhat more confined than the more widely distributed “network” involved with nonlesional cases. Further research is needed to confirm this hypothesis and to fully analyze the possibility of other confounders, such as seizure onset zone location or differences in SEEG sampling because of electrode placement.
The average seizure duration was shorter for SEEG compared with scalp (83.7 vs. 89.1 seconds). Similar differences between intracranial and scalp data were seen in previous studies on FA seizures (47–48 seconds with depth/subdural electrodes vs. 70 seconds with scalp electrodes).5 The shorter overall duration of SEEG may seem counterintuitive because of how intracranial electrodes detect nearly all seizures either before or simultaneously with, scalp electrodes; a theory corroborated by our data. However, we were able to demonstrate a mean duration of 108.6 seconds for SEEG seizures also seen on scalp and a mean duration of 49.0 seconds for SEEG seizures not seen on scalp. This suggests that seizures with focal onsets take time to recruit ample cortex large enough for scalp detection.
The SEEG onset preceded scalp onset most of the time (87%) with an average lead time of 17.3 seconds. Similar data were found for time to clinical symptom onset when comparing SEEG and scalp (29.7 seconds for SEEG and 14.0 seconds for scalp). This is likely because of the proximity of SEEG electrodes to the source generator. In 1% of clinical seizures, scalp onset preceded SEEG onset. Here, the stereo electrodes may not have been optimally placed over the seizure onset zone such that seizure activity propagated to the cortical surface before the intracranial electrodes.
Concurrent acquisition of intracranial and scalp EEG enables direct comparisons between the two signal sources. The number of undetectable scalp seizures subclinically and clinically can only accurately be assessed with simultaneous intracranial and scalp recordings. Standardizing the simultaneous recording of both scalp and intracranial EEG in appropriate patients will increase our understanding of the complexity of different seizure types, seizure networks, and clinical outcomes. During SEEG, performing cognitive and neuropsychiatric assessments after these subclinical seizures may help us understand their impact in our patients. Currently, we believe that this work should add to our humility, as we do not have objective tools to determine when patients are truly seizure free.
Regarding study limitations, the number of patients was small, although seizure onset zones were diverse. Although our study did not find differences in detection by brain region, 65% of seizures demonstrated mesial temporal onset while other onset locations were diverse with small seizure numbers in each category, in part because of a greater number of electrodes, and therefore sampling capabilities, in temporal regions. Accordingly, comparisons between brain regions are limited, and differences may exist between brain regions, seizure etiology, epilepsy type, and MRI lesion that this study was too small to detect. Further research with larger numbers may be useful.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Ava Ferdinand, MD, Nisali Gunawardane MD, and the staff of the Mount Sinai Epilepsy Program for making this study possible.
F. E. Panov has received lecture fees from Zimmer Biomet and Neuropace, outside the related work. S. Ghatan has received personal fees from Neuropace, outside the submitted work. J. Y. Yoo has received personal compensation for serving on the advisory board of Zimmer Biomet. The other authors have no funding or conflicts of interest to disclose.
Footnotes
Presented in part at the 144th Annual Meeting of the American Neurological Association, St Louis, MO, October 13–15, 2019.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.clinicalneurophys.com).
REFERENCES
- 1.Grech R, Cassar T, Muscat J, et al. Review on solving the inverse problem in EEG source analysis. J Neuroeng Rehabil 2008;7:5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centeno M, Tierney TM, Perani S, et al. Combined electroencephalography-functional magnetic resonance imaging and electrical source imaging improves localization of pediatric focal epilepsy. Ann Neurol 2017;82:278–287. [DOI] [PubMed] [Google Scholar]
- 3.Megevand P, Seeck M. Electroencephalography, magnetoencephalography and source localization: their value in epilepsy. Curr Opin Neurol 2018;31:176–183. [DOI] [PubMed] [Google Scholar]
- 4.Ebersole JS. Noninvasive localization of epileptogenic foci by EEG source modeling. Epilepsia 2000;41:S24–S33. [DOI] [PubMed] [Google Scholar]
- 5.Devinsky O, Sato S, Kufta CV, et al. Electroencephalographic studies of simple partial seizures with subdural electrode recordings. Neurology 1989;39:527–533. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Khoo HM, Dubeau F, Gotman J. Association between scalp and intracerebral electroencephalographic seizure-onset patterns: a study in different lesional pathological substrates. Epilepsia 2018;59:420–430. [DOI] [PubMed] [Google Scholar]
- 7.Lieb JP, Walsh GO, Babb TL, Walter RD, Crandall PH. A comparison of EEG seizure patterns recorded with surface and depth electrodes in patients with temporal lobe epilepsy. Epilepsia 1976;17:137–160. [DOI] [PubMed] [Google Scholar]
- 8.Ramantani G, Dumpelmann M, Koessler L, et al. Simultaneous subdural and scalp EEG correlates of frontal lobe epileptic sources. Epilepsia 2014;55:278–288. [DOI] [PubMed] [Google Scholar]
- 9.Ebersole JS, Pacia SV. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia 1996;37:386–399. [DOI] [PubMed] [Google Scholar]
- 10.Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia 1997;38:642–654. [DOI] [PubMed] [Google Scholar]
- 11.Ramantani G, Maillard L, Koessler L. Correlation of invasive EEG and scalp EEG. Seizure 2016;41:196–200. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard WD, Cross JH, Duncan JS, Stefan H, Theodore WH. Epilepsy imaging study guideline criteria: commentary on diagnostic testing study guidelines and practice parameters. Epilepsia 2011;52:1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardinale F, Casaceli G, Raneri F, Miller J, Lo Russo G. Implantation of stereoelectroencephalography electrodes: a systematic review. J Clin Neurophysiol 2016;33:490–502. [DOI] [PubMed] [Google Scholar]
- 14.Efron B Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171–185. [Google Scholar]
- 15.Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017;58:531–542. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O, Kelley K, Porter RJ, Theodore WH. Clinical and electroencephalographic features of simple partial seizures. Neurology 1988;38:1347–1352. [DOI] [PubMed] [Google Scholar]
- 17.Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 2005;46:669–676. [DOI] [PubMed] [Google Scholar]
- 18.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol 2003;2:725–730. [DOI] [PubMed] [Google Scholar]
- 19.Tatum WOt, Ross J, Cole AJ. Epileptic pseudodementia. Neurology 1998;50:1472–1475. [DOI] [PubMed] [Google Scholar]
- 20.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013;81:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCue LM, Flick LH, Twyman KA, Xian H, Conturo TE. Prevalence of non-febrile seizures in children with idiopathic autism spectrum disorder and their unaffected siblings: a retrospective cohort study. BMC Neurol 2016;16:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 2013;54:700–707. [DOI] [PubMed] [Google Scholar]
- 23.Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med 2017;23:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokoszka MA, McGoldrick PE, La Vega-Talbott M, et al. Epilepsy surgery in patients with autism. J Neurosurg Pediatr 2017;19:196–207. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya-Matsuoka C, Tummala S. Electrographic patterns in patients with posterior reversible encephalopathy syndrome and seizures. J Neurol Sci 2017;375:294–298. [DOI] [PubMed] [Google Scholar]
- 26.Praveen-kumar S, Sinha S, Taly AB, et al. Electroencephalographic and imaging profile in a subacute sclerosing panencephalitis (SSPE) cohort: a correlative study. Clin Neurophysiol 2007;118:1947–1954. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet J, Mulleners W, Meulstee J. EEG leading to the diagnosis of limbic encephalitis. Clin EEG Neurosci 2012;43:161–164. [DOI] [PubMed] [Google Scholar]
- 28.Albert DV, Pluto CP, Weber A, et al. Utility of neurodiagnostic studies in the diagnosis of autoimmune encephalitis in children. Pediatr Neurol 2016;55:37–45. [DOI] [PubMed] [Google Scholar]
- 29.Wasay M, Channa R, Jumani M, Shabbir G, Azeemuddin M, Zafar A. Encephalitis and myelitis associated with dengue viral infection clinical and neuroimaging features. Clin Neurol Neurosurg 2008;110:635–640. [DOI] [PubMed] [Google Scholar]
- 30.Riva D, Franceschetti S, Erbetta A, Baranello G, Esposito S, Bulgheroni S. Congenital brain damage: cognitive development correlates with lesion and electroencephalographic features. J Child Neurol 2013;28:446–454. [DOI] [PubMed] [Google Scholar]
- 31.Chayasirisobhon S, Menoni R, Chayasirisobhon W, Locke GE. Correlation of electroencephalography and the active and inactive forms of neurocysticercosis. Clin Electroencephalogr 1999;30:9–11. [DOI] [PubMed] [Google Scholar]
- 32.Labate A, Briellmann RS, Harvey AS, et al. Temporal lobe dysembryo-plastic neuroepithelial tumour: significance of discordant interictal spikes. Epileptic Disord 2004;6:107–114. [PubMed] [Google Scholar]
- 33.Koessler L, Cecchin T, Colnat-Coulbois S, et al. Catching the invisible: mesial temporal source contribution to simultaneous EEG and SEEG recordings. Brain Topogr 2015;28:5–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


