Abstract
Background: Breastfeeding may protect against childhood asthma and allergic diseases. Studies have not focused on the mode of feeding human milk and followed children to school age although feeding human milk from a bottle rather than the breast may alter the risk of disease.
Materials and Methods: At 12 months' postpartum, women in the Moms2Moms study (Columbus, OH) completed a survey assessing sociodemographic and infant feeding behaviors. At 6 years' postpartum, they completed a survey and pediatric medical records were abstracted to assess asthma and allergic disease diagnoses. Logistic regression models were used to estimate associations between infant feeding behaviors and asthma or allergic disease.
Results: Of 285 children, 16% had asthma and 44% ever had ≥1 allergy diagnosis. Longer durations of each infant feeding behavior were not clearly associated with increased odds of asthma or allergic disease by age 6. Results suggested that longer durations of breast milk feeding (regardless of the mode of feeding) may be related to a lower risk of food allergy (e.g., odds ratio [OR]1-month, adjusted = 0.96, 95% confidence interval [CI] = 0.87–1.05; OR12-month, adjusted = 0.57, 95% CI = 0.19–1.74), but that the mode of feeding (regardless of the substance fed) may be more meaningful for environmental allergies (e.g., exclusive direct breast milk feeding OR12-month, adjusted = 0.32, 95% CI = 0.06–1.81). However, effect estimates were imprecise and CIs included the null.
Conclusions: Although no clear associations between mode of breast milk feeding (breast versus expressed) and asthma and allergy outcomes were observed, future research with larger samples should further evaluate these associations.
Keywords: breastfeeding, asthma, allergies, food allergy, human milk expression
Introduction
More than 1 in 10 U.S. children have been diagnosed with asthma or an allergic disease, making them two of the most common childhood health conditions.1 Allergic diseases and asthma often require frequent inpatient and outpatient medical care, and can interfere with a child's daily activities including their ability to attend school.2–5 However, identification of early protective factors during infancy that can reduce the risk of disease in early childhood has been challenging.6 Recent advances in the prevention of food allergies have included carefully administered early exposure.7 In addition, numerous observational studies have noted that breastfeeding may protect against the development of asthma or allergic disease.8–10
Possible mechanisms for a protective effect of breastfeeding could be the bioactive components of breast milk (e.g., immunoglobulin A) and their role in supporting the developing immune system.11,12 However, findings supporting this effect on both asthma and allergic disease outcome during early childhood have been inconsistent, possibly because of variation in the operational definitions of infant feeding behaviors, such as a lack of differentiation between mode of feeding (e.g., feeding at the breast versus expressed breast milk).8,13–15
The mode of feeding breast milk may be important because the concentration of macronutrients in breast milk can be negatively altered by common storage and management practices (e.g., freezing and reheating).9,16–18 Therefore, it would be important to distinguish between feeding practices including the mode of feeding breast milk when examining risk of asthma or allergic disease.
Despite how common breast milk expression is among U.S. mothers, very few studies have examined the risk of asthma or allergic diseases in relation to feeding practices including indirect breastfeeding, direct breastfeeding, and formula feeding.8–10,19 Of these, only two studies examined childhood asthma and allergic disease beyond the first year of life.8,10 However, in one study, infant feeding practices were not measured throughout the entire first postnatal year, thereby introducing exposure measurement error that may bias effect estimates.8 The other study relied exclusively on maternal report of the eczema outcome.10 The goal of this prospective study was to examine the association of infant feeding practices during the first year of life and the presence of asthma or allergic disease by 6 years of age determined by both maternal report of medical diagnoses and medical chart abstraction.
Materials and Methods
Study population and data collection
We used prospective data from the Moms2Moms (M2M) study, a cohort of English-speaking women at least 18 years of age who delivered a singleton, liveborn infant at >24 weeks of gestation at The Ohio State University Wexner Medical Center (Columbus, OH) during 5 months of 2011 (n = 1,244). Exclusions included the following: women whose obstetric medical record indicated their intention to exclusively “bottle feed” (indicating intent to formula feed) their infant (n = 303); no valid contact information for the mother (n = 111); the mother was incarcerated (n = 11); and infant death (n = 6). Twelve months after delivery, a questionnaire was mailed to eligible women to assess their lactation and infant feeding behaviors, and sociodemographic information. Additional data on the mother and infant were abstracted from the obstetric and newborn medical records. The M2M study methods have been previously reported.20,21
In 2017, when children were 6 years of age, the mothers were contacted to participate in a follow-up survey that included sociodemographic and child health-related information. Participants were also invited to sign a pediatric medical record release, and health diagnoses like asthma and allergic disease from the child's primary care provider were requested, accordingly. If a child had more than one provider they saw regularly, a second authorization was obtained. Eligible participants for this analysis were dyads who participated in the original M2M study and who had questionnaire data about asthma and allergy diagnoses or medical records available. This study was reviewed and approved by the Institutional Review Board at Nationwide Children's Hospital.
Study variables
Maternal and child characteristics
The following maternal and child characteristics were reported on the questionnaire at 12 months' postpartum: child's sex (male, female), maternal education (high school/General Educational Development (GED) or less, some college/associate's degree, bachelor's degree, graduate or professional degree), marital status (single/living with partner/divorced versus married), smoking status during pregnancy or infancy (yes, no, based on a question asked at 12 months' postpartum: “At any time during your pregnancy up until now, have you smoked cigarettes?”), annual household income (< $15,000, $15,000–$34,999, $35,000–$54,999, $55,000–$74,999, ≥ $75,000), and daycare attendance (yes, no). The child's race (white, black/African American, other), ethnicity (Hispanic, non-Hispanic), and maternal parity (0, 1, ≥2 pregnancies) were abstracted from the obstetric and newborn medical records.
Infant feeding variables
The questionnaire administered at 12 months' postpartum asked about the age of the infant when they started and stopped feeding directly at the breast, expressed milk feeding, and formula feeding.
Continuous variables for the duration of each feeding practice (in days) were calculated by subtracting the infant's age (in days) when the practice was started from the age (in days) when it stopped. Infants who had not stopped a feeding practice by the time of questionnaire completion were assigned 365 days for the infant's age of when the practice was stopped. Duration variables were created for: (1) breast milk feeding, which included either direct feeding at the breast or expressed milk; (2) direct breast milk feeding (feeding at the breast); (3) expressed breast milk feeding; and (4) formula feeding. Each practice may have occurred simultaneously or consecutively with other practices. The duration of exclusive breast milk feeding and exclusive direct breast milk feeding were calculated as above but only counted days when none of the other feeding practices were used. Exclusive expressed milk feeding was practiced by a small subsample of dyads and typically for short durations; this precluded robust analysis and so was not pursued further.
Asthma and allergic disease
Diagnoses for asthma and allergic diseases were ascertained by a combination of maternal report and medical record abstraction. First, the follow-up survey at age 6 years asked the following yes/no question for each disease outcome, “Has a doctor or other health professional EVER told you that your child has/had: (a). Asthma? (b). Hay fever? (c). Any kind of respiratory allergy? (d). Any kind of food or digestive allergy (e). Eczema or any kind of skin allergy?.” If the mother indicated “yes,” they were then asked to detail the specific allergy type. Second, trained research staff abstracted medical records using 9th/10th revision International Classification of Diseases (ICD) codes for asthma or asthma or allergy-related terms to classify children as having probable or definite asthma or allergic disease.
Children were considered to have a probable or definite diagnosis of asthma if their mother responded “yes” to the question about the child having an asthma diagnosis; if they had an asthma-related phrase or ICD code in their medical record; or if they had a documented history in their medical record of reactive airway disease, shortness of breath, wheezing, or prescriptions for asthma-related medications but no definitive asthma diagnosis (Supplementary Table S1).
Children were considered to have an allergy diagnosis if their mother responded “yes” to the question about the child having any type of allergy diagnosis or if they had an allergy-related phrase in their medical record (Supplementary Table S2). For analysis purposes, allergies were categorized as food allergy, environmental allergy (hay fever or respiratory allergy), and eczema/skin allergy.
Statistical analysis
Univariate statistics were used to characterize the sample. Logistic regression models were used to estimate associations between each infant feeding practice and asthma (probable or definite combined), food allergy, environmental allergy, and eczema/skin allergy. Models were adjusted for established asthma and allergy risk factors selected a priori (sex, child's race, child's ethnicity, maternal smoking status, maternal education, and daycare attendance during infancy). Unadjusted and adjusted odds ratios were calculated at 1, 3, 6, 9, and 12 months for each infant feeding practice, with 0 months' duration of that feeding practice as the reference group. However, because unadjusted and adjusted estimates were similar, only adjusted estimates were reported. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Of the 305 dyads who participated in the follow-up study, 285 had complete data on asthma and allergies and were included in analyses (Supplementary Fig. S1). Overall, children were primarily white (80%) with similar proportions of males (53%) and females (47%). Most mothers were married and over a third of them obtained a bachelor's degree (Table 1). The median duration of expressed milk feeding was 149 days (IQR = 220 days) and of direct breast milk feeding was 180 days (IQR = 323). The median duration of formula feeding was 319 days (IQR = 300).
Table 1.
Characteristics of Moms2Moms Follow-Up Study Dyads
| Characteristics | Dyads that participated in M2M follow-up with asthma and allergy data (N = 285) |
|
|---|---|---|
| n | % | |
| Child sex | ||
| Male | 150 | 53 |
| Female | 135 | 47 |
| Child race | ||
| White | 227 | 80 |
| Other | 21 | 7 |
| Black/African American | 20 | 7 |
| Missing | 17 | 6 |
| Child ethnicity | ||
| Non-Hispanic | 257 | 90 |
| Missing | 17 | 6 |
| Hispanic | 11 | 4 |
| Maternal education | ||
| High school graduate/GED or less | 31 | 11 |
| Some college/associate's degree | 37 | 13 |
| Bachelor's degree | 113 | 40 |
| Graduate or professional degree | 104 | 36 |
| Marital status | ||
| Married | 239 | 84 |
| Single/living with partner/divorced | 46 | 16 |
| Household income | ||
| < $15,000 | 27 | 9 |
| $15,000–$34,999 | 45 | 16 |
| $35,000–$54,999 | 33 | 12 |
| $55,000–$74,999 | 46 | 16 |
| $75,000+ | 133 | 47 |
| Missing | 1 | 0 |
| Maternal smoking during pregnancy | ||
| No | 268 | 94 |
| Yes | 17 | 6 |
| Maternal parity | ||
| 0 | 157 | 55 |
| 1 | 77 | 27 |
| ≥2 | 51 | 18 |
| Ever attended child care during infancy | ||
| No | 145 | 51 |
| Yes | 139 | 49 |
| Missing | 1 | 0 |
GED, General Educational Development; M2M, Moms2Moms.
Asthma
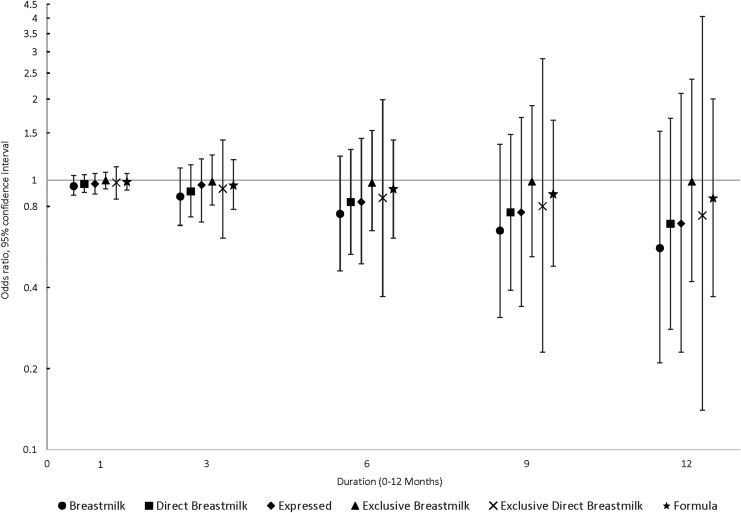
Forty-seven children (16%) were classified into the asthma group (8 probable, 39 definite). Duration of each feeding practice during the first postnatal year was not clearly associated with probable or definite asthma by 6 years of age, after adjustment for confounders (Fig. 1). However, effect estimates moved progressively lower and away from the null as the duration of breast milk feeding increased (e.g., direct breast milk feeding odds ratio [OR]1-month, adjusted = 0.97, 95% [confidence interval] CI = 0.90–1.05; OR12-month, adjusted = 0.69, 95% CI = 0.28–1.70). In addition, CIs were wide and included the null and results for formula feeding were similar.
FIG. 1.
Associations between infant feeding practices at 3, 6, 9, and 12 months and the odds of asthma by 6 years of age (N = 285).
Allergies
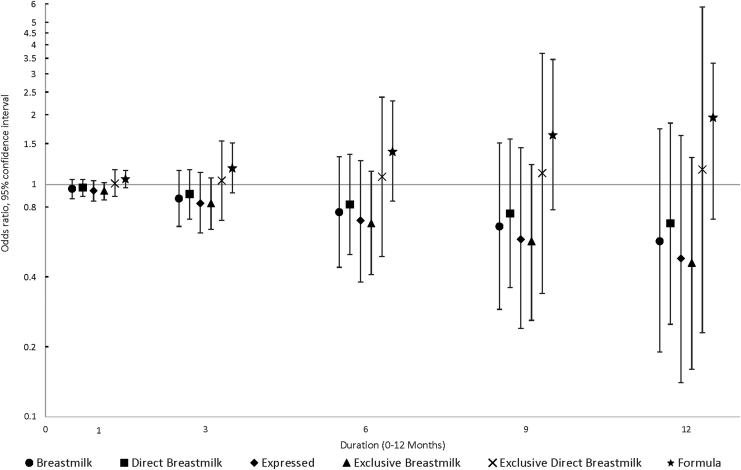
Thirty-five (12%) children were diagnosed with a food allergy, 66 (23%) with an environmental allergy, and 89 (31%) with eczema/skin allergy. Forty-six (16%) children were diagnosed with more than one type of allergy. Duration of each infant feeding practice during the first postnatal year was not clearly associated with any type of allergic disease by 6 years of age, after adjustment for confounders. However, as breast milk feeding duration by any mode increased, the odds of a food allergy appeared to decrease, although effect estimates were imprecise and CIs included the null (Fig. 2). For instance, as breast milk (combined direct and expressed) duration increased, effect estimates for food allergy (OR1-month, adjusted = 0.96, 95% CI = 0.87–1.05; OR12-month, adjusted = 0.57, 95% CI = 0.19–1.74) moved progressively lower and away from the null. Correspondingly, effect estimates for formula feeding increased with longer duration.
FIG. 2.
Associations between infant feeding practices at 3, 6, 9, and 12 months and the odds of food allergy by 6 years of age (N = 285).
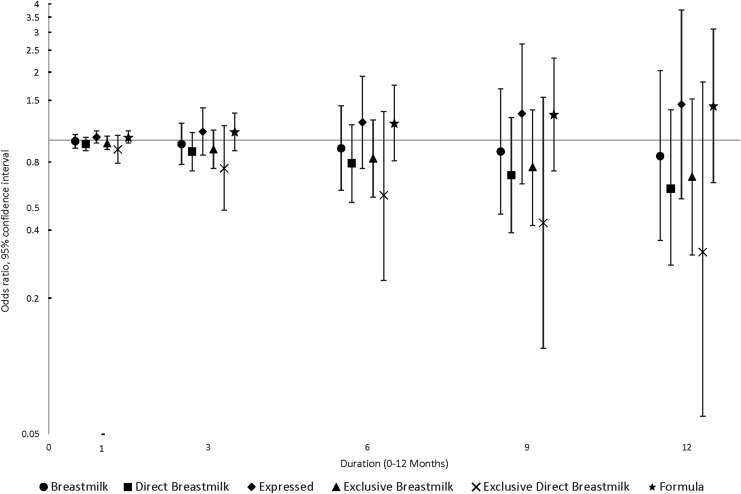
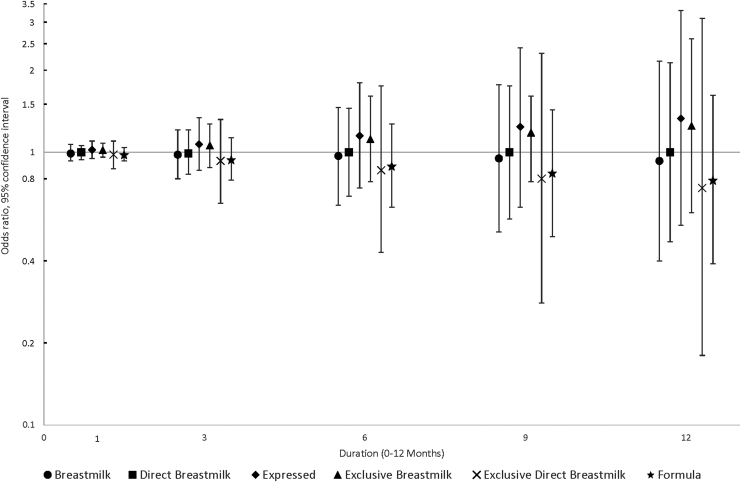
The results for environmental allergy suggested that the mode of feeding may be important, regardless of whether it is breast milk or formula, in that effect estimates for expressed milk feeding and formula feeding were both above the null, whereas effect estimates for direct breast milk feeding, especially exclusive direct breast milk feeding, were progressively below the null with longer duration (OR12-month, adjusted = 0.32, 95% CI = 0.06–1.81) (Fig. 3). Effect estimates for eczema/skin allergy were more consistent and relatively close to the null even as duration increased, for all feeding practices (Fig. 4).
FIG. 3.
Associations between infant feeding practices at 3, 6, 9, and 12 months and the odds of environmental allergy by 6 years of age (N = 285).
FIG. 4.
Associations between infant feeding practices at 3, 6, 9, and 12 months and the odds of eczema/skin allergy by 6 years of age (N = 285).
Discussion
In this prospective cohort of mothers and their children followed from infancy to age 6 years, infant feeding practices during the first postnatal year were not clearly associated with allergic disease or asthma. Visual inspection of the effect estimates suggested that longer durations of breast milk feeding (regardless of the mode of feeding) may be related to a lower risk of food allergy, but that the mode of feeding (regardless of the substance fed) may be more meaningful for environmental allergy risk. However, these results were imprecise. All associations controlled for potential confounding by sociodemographic variables and environmental factors like maternal smoking and childcare attendance.
Our results for asthma do not align with evidence from two prior studies that showed that compared with direct breastfeeding, any other mode of feeding was associated with an increased risk of asthma or coughing/wheezing episodes.8,9 However, prior meta-analysis research has similarly shown inconsistent evidence that breastfeeding (presumably all at the breast) decreases risk of asthma or wheezing diagnosis in children older than 5 years as compared with formula feeding, particularly when infants were breast and formula (mixed) fed.22 A follow-up study from the large randomized trial, PROBIT, did not report a significant reduction in the odds of asthma diagnosis at 6 years of age.14,23 However, PROBIT did not differentiate between mode of breastfeeding (e.g., feeding at the breast versus expressed breast milk).14
Numerous prior studies have reported a reduced risk of eczema with longer duration breastfeeding, including the PROBIT trial (OR = 0.54, 95% CI = 0.31–0.95).23 This study observed no associations between any feeding practice and eczema/skin allergy risk. This result is in line with that of Soto-Ramirez et al. who reported no association between the duration of feeding breast milk (including pumped milk) and eczema/skin allergy in the Infant Feedings Practices Study II.10 However, that study also reported that children who started receiving a combination of direct breastfeeding, pumped breast milk, and formula had a 46% higher risk of eczema/skin allergy based on maternal report by 6 years of age compared with children who were directly breastfed for at least the first 3 months.10 Our sample was too small to examine longitudinal feeding patterns like in the Soto-Ramirez study, to provide direct comparison.
Although prior studies have reported possible protective benefits of breastfeeding for asthma and eczema/skin allergies, this protective benefit has been less conclusive for food allergies.11,22–25 This lack of clarity has been often attributed to the heterogeneity of allergy diagnostic tools, ranging from parent report to skin prick test.11 A large longitudinal study in Australia reported that children who were exclusively breastfed for the first 3 months of life had a reduced risk of food allergy at age 7 years, but this risk reversed later in life, and they had an increased risk of food allergy at age 44 years.26
Another study conducted in Germany examining children at high-risk of atopy found that infants who had been exclusively breastfed for at least 5 months had an increased risk of egg white sensitization at age 1 year, although this association did not exist at age 2 years.27 Future research looking at breastfeeding and the odds of food allergies is warranted to determine what benefits may exist.
Our results for environmental allergies are aligned with a meta-analysis of the association between breastfeeding and allergic rhinitis, which found that breastfeeding did not have a significant protective effect on the development of allergic rhinitis.28 However, this meta-analysis did not examine the mode of feeding, which our results suggested may be most meaningful for environmental allergies.28 In addition, none of the included studies completed follow-up with children past age 4 years, whereas our results examined outcomes at age 6 years.28 Future research is needed to further probe the possible associations between breastfeeding, mode of feeding, and environmental allergies.
Prior research has suggested differences in the nutrient concentration of human milk in direct versus expressed breastfeeding may be one mechanism to explain differences in outcomes.12,14–17 These differences are often attributed to pumping, storing, and reheating practices because the concentration of macronutrients in breast milk may be negatively altered by common storage and management practices.9,18 In addition, varying levels of immune mediators found in human milk fed by different modes may be particularly relevant for allergic outcomes.29
Strengths and limitations
Our study had several limitations, including a limited sample size that made some effect estimates imprecise and limited our ability to draw definite conclusions. Although we adjusted for several maternal and child characteristics thought to confound the relationship between infant feeding and asthma and allergic disease, it is possible that our results will be influenced by residual confounding. For instance, we did not have data on exposure to environmental tobacco smoke. In addition, our study did not have data about family history of asthma or allergic diseases, so we were unable to examine that high-risk group of children separately, or to control for a family history of these diseases. Although medical chart abstraction was used, a thorough, conclusive examination confirming asthma or allergic disease diagnosis was not performed.
It should also be noted that our sample showed a higher prevalence of allergies, particularly of eczema/skin allergies, than U.S. estimates, although the prevalence of definite asthma in our sample (14%) was consistent with national asthma estimates for U.S. children.1 This overestimate in the prevalence of skin allergies could be attributed to the difficulty of definitively diagnosing eczema/skin allergies and mothers potentially reporting more mild and intermittent conditions as eczema/skin allergy. However, asthma and allergic diseases are well-known to be difficult to diagnose in early childhood, and we were inclined to err on the side of being inclusive in our classification.30,31
Also, participants were asked to recall infant feeding practices at 12 months' postpartum for the whole year since delivery, and this may have introduced measurement error. If this error was differential by outcome, it may have introduced some systematic bias in the effect estimates. However, it was not possible with the available data to assess this. Finally, we did not have data on the exact timing of the onset of the outcomes, and decisions to continue or cease particular feeding methods may have been influenced by the emergence of allergic symptoms, a form of reverse causation.
This study offers several strengths, including being one of few prospective studies that has been able to separately examine expressed milk feeding and direct breast milk feeding in relation to the odds of asthma and allergic disease. In addition, because this study measured each feeding practice to the precision of days, we were able to determine the duration of each feeding practice precisely. Unlike some prior studies, this study assessed infant feeding throughout the whole first year of life, and, to reduce poor maternal recall and increase sensitivity, we assessed asthma and allergic disease outcomes through both maternal report and medical record(s).8,10 Using medical record abstraction also decreased the likelihood of misclassifying another type of skin allergy as eczema.10
Conclusions
Our results provide no clear indication that breast milk feeding, whether at the breast or expressed, is associated with a reduced risk of asthma or allergic diseases by age 6 years. Although our results were imprecise, some suggestive patterns were noted for the mode of feeding (bottle versus breast) being influential on the risk of environmental allergies and the substance being fed (breast milk by any mode) influential on food allergies. This provides support for future studies to distinguish between different infant feeding practices in these ways when assessing the protective role breast milk may have on these outcomes.
Supplementary Material
Acknowledgments
The authors thank the women for their participation in the Moms2Moms studies. The authors also thank The Ohio State University Center for Clinical and Translational Science; and Kelly Boone, Thalia Cronin, Kendra Heck, Taniqua Ingol, Rachel Klenzman, Rachel Ronau, Hanna Schlaack, Erin Shafer, and Katie Smith of the Center for Biobehavioral Health of Nationwide Children's Hospital for their support in data collection, analysis, and/or administrative support. The authors have no consultantships, honoraria, stock ownership, equity interests, arrangements regarding patents, or other vested interests related to the content of this article.
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by the Centers for Disease Control and Prevention (Grant No. 1R03SH000048), and the National Center for Advancing Translational Sciences (Grant No. UL1TR001070). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention, the National Center for Advancing Translational Sciences, the National Institutes of Health, or the Department of Health and Human Services.
Supplementary Material
References
- 1. Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital Health Stat 10 2013:1–81 [PubMed] [Google Scholar]
- 2. Jackson KD, Howie LD, Akinbami LJ. Trends in Allergic Conditions Among Children: United States, 1997–2011. National Center for Health Statistics (NCHS Data Brief, No 121), 2013 [PubMed] [Google Scholar]
- 3. Zahran HS, Bailey CM, Damon SA, et al. Vital signs: Asthma in children—United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018;67:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akinbami LJ, Moorman JE, Garbe PL, et al. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009;123(Suppl 3):S131–S145 [DOI] [PubMed] [Google Scholar]
- 5. Pawankar R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ J 2014;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nieto A, Wahn U, Bufe A, et al. Allergy and asthma prevention 2014. Pediatr Allergy Immunol 2014;25:516–533 [DOI] [PubMed] [Google Scholar]
- 7. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klopp A, Vehling L, Becker AB, et al. Modes of infant feeding and the risk of childhood asthma: A prospective birth cohort study. J Pediatr 2017;190:192–199 [DOI] [PubMed] [Google Scholar]
- 9. Soto-Ramírez N, Karmaus W, Zhang H, et al. Modes of infant feeding and the occurrence of coughing/wheezing in the first year of life. J Hum Lact 2013;29:71–80 [DOI] [PubMed] [Google Scholar]
- 10. Soto-Ramirez N, Kar S, Zhang H, et al. Infant feeding patterns and eczema in children in the first 6 years of life. Clin Exp Allergy 2017;47:1285–1298 [DOI] [PubMed] [Google Scholar]
- 11. Munblit D, Peroni DG, Boix-Amoros A, et al. Human milk and allergic diseases: An unsolved puzzle. Nutrients 2017;9:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr 2005;135:1–4 [DOI] [PubMed] [Google Scholar]
- 13. Azad MB, Vehling L, Lu Z, et al. Breastfeeding, maternal asthma and wheezing in the first year of life: A longitudinal birth cohort study. Eur Respir J 2017;49:1602019. [DOI] [PubMed] [Google Scholar]
- 14. Kramer MS, Matush L, Vanilovich I, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: Cluster randomised trial. BMJ 2007;335:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greer FR, Sicherer SH, Burks AW, et al. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019;143:e20190281. [DOI] [PubMed] [Google Scholar]
- 16. García-Lara NR, Escuder-Vieco D, García-Algar O, et al. Effect of freezing time on macronutrients and energy content of breastmilk. Breastfeed Med 2012;7:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tacken KJ, Vogelsang A, van Lingen RA, et al. Loss of triglycerides and carotenoids in human milk after processing. Arch Dis Child Fetal Neonatal Ed 2009;94:F447–F450 [DOI] [PubMed] [Google Scholar]
- 18. Lawrence RA. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr 1999;88:14–18 [DOI] [PubMed] [Google Scholar]
- 19. Labiner-Wolfe J, Fein SB, Shealy KR, et al. Prevalence of breast milk expression and associated factors. Pediatrics 2008;122(Suppl 2):S63–S68 [DOI] [PubMed] [Google Scholar]
- 20. Keim SA, McNamara KA, Dillon CE, et al. Breastmilk sharing: Awareness and participation among women in the Moms2Moms study. Breastfeed Med 2014;9:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boone K, Geraghty S, Keim S. Feeding at the breast and expressed milk feeding: Associations with otitis media and diarrhea in infants. J Pediatr 2016;174:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lodge C, Tan DJ, Lau M, et al. Breastfeeding and asthma and allergies: A systematic review andmeta-analysis. Acta Paediatr 2015;104:38–53 [DOI] [PubMed] [Google Scholar]
- 23. Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001;285:413–420 [DOI] [PubMed] [Google Scholar]
- 24. Kull I, Wickman M, Lilja G, et al. Breast feeding and allergic diseases in infants-a prospective birth cohort study. Arch Dis Child 2002;87:478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matheson MC, Allen KJ, Tang ML. Understanding the evidence for and against the role of breastfeeding in allergy prevention. Clin Exp Allergy 2012;42:827–851 [DOI] [PubMed] [Google Scholar]
- 26. Matheson MC, Erbas B, Balasuriya A, et al. Breast-feeding and atopic disease: A cohort study from childhood to middle age. J Allergy Clin Immunol 2007;120:1051–1057 [DOI] [PubMed] [Google Scholar]
- 27. Wetzig H, Schulz R, Diez U, et al. Associations between duration of breast-feeding, sensitization to hens' eggs and eczema infantum in one and two year old children at high risk of atopy. Int J Hyg Environ Health 2000;203:17–21 [DOI] [PubMed] [Google Scholar]
- 28. Mimouni Bloch A, Mimouni D, Mimouni M, et al. Does breastfeeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatr 2002;91:275–279 [DOI] [PubMed] [Google Scholar]
- 29. Munblit D, Boyle RJ, Warner JO. Factors affecting breast milk composition and potential consequences for development of the allergic phenotype. Clin Exp Allergy 2015;45:583–601 [DOI] [PubMed] [Google Scholar]
- 30. Papadopoulos NG, Arakawa H, Carlsen KH, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67:976–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lockett GA, Patil VK, Soto-Ramirez N, et al. Epigenomics and allergic disease. Epigenomics 2013;5:685–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.