Abstract
Analysis of drugs in hair by mass spectrometry imaging (MSI) has great potential as an objective, long-term measure of medication adherence. However, the fidelity of the chemical record in hair may be compromised by any cosmetic hair treatments. Here, we investigate infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) MSI response to multiple antiretrovirals (ARVs) in cosmetically treated hair. Hair strands from patients on different ARV regimens were mechanically treated with dye, bleach, and relaxer. The treatments had little or no effect relative to untreated controls for cobicistat, abacavir, dolutegravir, maraviroc, efavirenz, and darunavir, but all three treatments removed emtricitabine (FTC) to undetectable levels from patient hair strands. We also evaluated hair strands by IR-MALDESI MSI from 8 patients on FTC-based regimens who reported a range of hair treatments at varying recency prior to hair collection. While FTC was undetectable in the treated portion of these hair strands, ARVs coadministered with FTC remained detectable in hair strands after treatment. We conclude that IR-MALDESI MSI can be used when measuring adherence to ARV therapy, provided that ARVs other than FTC are targeted in people using hair treatments.
Introduction
Antiretroviral (ARV) therapy that effectively suppresses HIV replication in people living with HIV can render them noninfectious to their partners.1–5 ARV therapy is generally administered in combinations of three or more drugs, which are often coformulated. In most cases, these drugs are taken as a daily oral dose, and proper adherence to an ARV regimen is essential for efficacy. Nonadherent patients run the risk of viral rebound and drug resistance, while uninfected individuals on pre-exposure prophylaxis with ARVs risk contracting HIV.6,7 Many strategies to monitor ARV adherence have been implemented including patient self-report, pharmacy refill data, electronic monitoring systems, and pharmacologic measures of blood and plasma.8–16 Though these measures seek to give patients and medical providers information to treat and prevent the spread of HIV, their utility is limited by reporting bias, indirect measurement, and short-term measurement of adherence.
Hair testing has been used regularly as a forensic tool for the detection of compounds consumed by drug (ab)users and recently has become of interest for monitoring ARV adherence.17–25 Hair is a particularly useful matrix because it can provide a weeks- to months-long record of drug use. Our group and others have been interested in using hair analysis as an objective measure of patient adherence to ARVs. Accumulation of these drugs in hair has been measured in clinical samples over a wide concentration range of 0.005–40 ng mg−1 hair,26–29 which varies by drug based on its basicity and lipophilicity. Measurements of ARVs in hair have been used to establish exposure thresholds for adherence classification.30
One important consideration in assessing the objective fidelity of targeting drugs in hair as a measure of adherence is the potential effect of different hair treatments on the detection of the target drug(s) of interest. Many groups have investigated the effect of hair treatment on drug detection in forensic applications by LC/MS.31–35 In general, a decrease in concentration was observed for the drug target of interest after cosmetic treatment. Mass spectrometry imaging (MSI) has emerged as an alternative to LC/MS for hair analysis because of its ability to provide high spatial, and therefore high temporal, resolution along the length of hair strand, providing a timeline of drug use. In addition, MSI does not require lengthy sample processing and drug extraction times and can be used to monitor multiple drug targets simultaneously in a single assay, which is critical for rapid screening of hair samples reflecting varied ARV regimens. Few groups have investigated the effect of hair treatment on MSI analysis of hair, but Cuypers and co-workers investigated the effect of bleach (hydrogen peroxide treatment) on the detection of cocaine.36 They found that the use of hydrogen peroxide treatment lowered the detectability of cocaine in hair by MSI, both by generating reaction products and by washing cocaine (and reaction products) out of the hair. This is an important finding, but it only pertains to one hair treatment and one drug. Since the disposition of drug in hair and its affinity for binding to melanin vary based on drug physicochemical properties,37 the influence of hair treatments on MSI response is likely drug-dependent. Given that the basis of ARV regimens for HIV treatment and prevention is a combination of multiple drugs across multiple classes, disposition in hair and effect of treatment for these compounds may differ. We are interested in expanding this work to include multiple hair treatments and their effect on the detection of ARVs in hair by MSI.
Our previous work has shown that MSI, specifically infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI), can be used to detect ARVs in human hair.38 At that time, we had not yet investigated the effect of hair treatments normalized to a marker for melanin. If hair treatments affect ARV concentrations in hair, that must be taken into account when attempting to use MSI as an objective adherence measure. Using samples from patients adherent to ARV regimens, we have examined the effect of mechanical hair treatment with dye, bleach, and relaxer on the detectability by MSI of several ARVs with a range of physicochemical properties. The drugs we investigated, in order of increasing lipophilicity, include: nucleoside analogue reverse transcriptase inhibitors emtricitabine (FTC) and abacavir (ABC), integrase inhibitor dolutegravir (DTG), protease inhibitor darunavir (DRV), the entry inhibitor maraviroc (MVC), the CYP3A inhibitor (or pharmacokinetic “booster”) cobicistat (COBI), and the nonnucleoside reverse transcriptase inhibitor efavirenz (EFV). Except for FTC, which has very low accumulation (0.025–2 ng mg−1 hair) in hair strands,26 all drugs could be monitored in a single assay. Since FTC, a predominant component of HIV prevention and treatment regimens, was found to be most consistently affected by bench-top hair treatment, we then quantified the time since hair treatment needed to detect FTC and attempted to use other ARVs administered in combination with FTC as a proxy for FTC detection.
Experimental
Materials and reagents
Methanol (HPLC grade), acetonitrile (HPLC), water (HPLC), acetic acid (80% w/w), ammonium hydroxide, hydrogen peroxide (30% v/v), and formic acid (Optima) were obtained from Fisher Scientific (Hampton, NH). For positive ion mode acquisition, the electrospray solvent was a 50/50 mixture of methanol/water (v/v) with 0.2% formic acid. For negative mode acquisition, the electrospray solvent was a 50/25/24/1 mixture of acetonitrile/methanol/water/acetic acid (v/v/v/v).
Commercially available cosmetic products for common hair treatments were used to manually manipulate hair strands. Bleaching was performed using a cream developer containing 6% hydrogen peroxide (20 Volume, ColorCharm®) in combination with a persulfate-based booster (Powder Lightener, ColorCharm®) both manufactured by Wella (Coty, Inc., New York, NY). Dyeing was performed using the cream developer with a brown hair dye (ColorCharm® Medium Brown 4N/411, Wella). Straightening was performed using a medium relaxer from Mizani (New York, NY).
Hair collection
The relationship between hair type and ARV IR-MALDESI MSI ion abundance was evaluated in hair samples collected from 36 healthy volunteers (12 for each drug) following 28 days of directly observed daily dosing with FTC, DTG, or MVC during the ENLIGHTEN study (NCT03218592). Hair treatment influence on MSI ion abundance was conducted on hair strands collected from volunteers living with HIV who were prescribed a range of ARV medications and classified as adherent based on plasma concentrations of <50 copies HIV RNA per mL (NCT02768779). Informed consents were obtained from human participants prior to any study activities. Manual hair treatment testing was performed using samples from 4 volunteers living with HIV reporting no hair treatments, with samples from 8 additional volunteers reporting hair treatments evaluated as comparison between in vitro and in vivo treatment effects. In all cases, approximately 20 strands were collected from each individual by cutting strands as close to the scalp as possible. All samples were stored in foil packets at 4 °C prior to analysis.
Sample preparation
Prior to treatment, samples were fixed to a glass microscope slide by taping one end of the hair strand. Bleach powder and dye were mixed in a 1 : 2 ratio with the cream developer in a centrifuge tube prior to application to the hair strands. The compounds were then applied to the hair strands using a pipette or spatula. Treatment contact times with hair followed manufacturer recommendations (Bleach and dye: 30 min; Relaxer: 15 min). The hair strands were then washed thoroughly with water and dried with nitrogen. Finally the hair strands were transferred to double-sided tape (VHB Tape, 3 M, St Paul, MN) on a microscope slide for MSI analysis.
For the work where pyrrole-2,3,5-tricarboxylic acid (PTCA) was targeted as a biomarker of melanin, hair strands were fixed to a glass microscope slide with VHB tape and the distal ends were placed in a solution of 1 M ammonium hydroxide in 45/45/10 methanol/water/hydrogen peroxide (v/v/v) for 10 min. The slide was removed from the solution, and the hair strands were rinsed thoroughly with water. The hair strands were then dried with nitrogen before MSI analysis.
IR-MALDESI MSI operation and data processing
Slides containing the samples were placed on a stage controlled by a thermoelectric cooler (TE Technology, Inc., Traverse City, MI) in an enclosed source. Samples were then cooled to −10 °C under nitrogen and given time to stabilize (~12 min). A layer of ice was grown on the sample by opening the source and exposing the sample to ambient humidity. Before MSI analysis, the source was closed, and a steady flow of nitrogen was used to stabilize the relative humidity at approximately 14% using a steady flow of nitrogen.
An IR-MALDESI source was used for all imaging experiments adapted from previous work.25,39,40 A single laser pulse from an IR OPO laser (Opotek, Carlsbad, CA) tuned to 2.94 μm wavelength was used at each spot to ablate the ice-covered samples. The desorbed plume was extracted and ionized by a perpendicular electrospray that carried the sample into the mass spectrometer (ThermoFisher Q Exactive Plus, Bremen, Germany). The stage was translated in 100 μm increments (giving 100 × 100 μm pixel size) to oversample based on the laser spot size (~200 μm). Interrogated regions of interest on hair strands were at least 4 mm in length. For analysis of antiretrovirals, the mass spectrometer was operated in full scan mode with positive polarity (m/z 200 to 800; resolving power: 140 000 at m/z 200; s-lens RF level: 100, all ARVs except FTC) or MS/MS mode (m/z 248.1 ± 4 → m/z 100 to 275; NCE = 10 with charge z set at +2; resolving power: 140 000 at m/z 200; s-lens RF level: 50, for FTC only). MS/MS mode was used to increase sensitivity for detection of FTC. For PTCA analysis, the mass spectrometer was operated in full scan mode with negative polarity (m/z 190–760; resolving power: 140 000 at m/z 200; s-lens RF level: 50). Molecular identities were assigned by accurate mass. Communication and timing between the stage, laser, and mass spectrometer were controlled using a custom MATLAB program (The Mathworks, Inc., Natick, MA) and a microcontroller.
Data were processed using MSiReader41,42 and custom MATLAB software. RAW files were converted to mzML using MSconvert, then converted to imzML before processing by MSiReader. Ion images were generated from m/z values ±2.5 ppm.
Statistical methods
Hair treatment.
Prior to analysis, data were preprocessed using R v.3.5.3 (R Foundation for Statistical Computing, Vienna, Austria.).43 Maximum MSI ion abundances were calculated among radial measurements at each longitudinal sampling position along a hair strand, resulting in approximately 40 values for each strand. The limit of detection (LOD) was considered to be 1000 counts, with zero values given an imputed value of half this limit (500 counts).
Statistical analyses were performed using SAS Software v.9.4 (SAS Software, Cary, NC). A Kruskal–Wallis test within treatments revealed distributional differences across strands, suggesting that it would be inappropriate to pool data within each treatment. To estimate percent change relative to control, and to account for clustering of data within strands,44 a non-linear mixed-effects model for left-censored data was fit to preprocessed data (scaled by the maximum ion abundance measured across all strands being compared). For example, in analyzing differences in ABC signal abundance between bleach treatment (3 strands) and untreated control (3 strands), all values for ion abundance were divided by the maximum across all 6 strands. This scaling factor forces all values to fall between the rescaled LOD and 1. This preserves the original distribution of the variable and is a recommended method for clustered or longitudinal data to help prevent convergence problems.45 P-Values presented in Table 1 are adjusted for multiple comparison testing by controlling for the false discovery rate.
Table 1.
Target ARVs, properties, and effect of cosmetic hair treatments
| Properties | Bleach | Dye | Relaxer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Antiretroviral | Abbreviation | Classa | m/z | pKa | log P | Average changeb | p-Valuec | Average changeb | p-Valuec | Average changeb | p-Valuec |
| A | Abacavir | ABC | NRTI | 287.16149 | 5.01 | 1.22 | +50% | 0.19 | −24% | 0.23 | −76% | 0.024 |
| A | Dolutegravir | DTG | II | 420.13655 | 8.7 | 1.7 | +59% | 0.029 | −28% | 0.26 | −48% | 0.11 |
| B | Maraviroc | MVC | EI | 514.33519 | 7.3 | 3.63 | +69% | 0.023 | −27% | 0.23 | +29% | 0.26 |
| B | Emtricitabine | FTC | NRTI | 248.1 → 130.04100 | 2.65 | −0.9 | −84% | 0.0061 | −85% | 0.0061 | −84% | 0.0061 |
| C | Efavirenz | EFV | NNRTI | 316.03467 | 10.2 | 4.46 | −7% | 0.74 | −28% | 0.23 | −67% | 0.024 |
| D | Darunavir | DRV | PI | 548.24250 | 8.2 | 2.82 | +177% | 0.077 | +130% | 0.23 | −3% | 0.96 |
| D | Cobicistat | COBI | CYP3A I | 776.36169 | 6.69 | 4.36 | +44% | 0.26 | +174% | 0.23 | +235% | 0.024 |
NNRTI: nonnucleoside reverse transcriptase inhibitor, CYP3A I: CYP3A inhibitor, EI: entry inhibitor, PI: protease inhibitor, II: integrase inhibitor, NRTI: nucleoside reverse transcriptase inhibitor.
Average percent change relative to untreated controls.
False discovery rate (FDR)-adjusted p-values.
Hair color.
Association between average ion abundances of ARVs and the melanin biomarker PTCA in hair strands was performed using SAS Software v.9.4 (SAS Software, Cary, NC). In order to account for ties in ordinal rank and avoid distributional assumptions, correlation analyses were carried out using Kendall’s tau-b (τb).
Results and discussion
Manual manipulation of hair treatments
Initial characterization of changes to ARV response in hair following treatments was performed by manually applying cosmetic products to individual strands of hair collected from 4 subjects on combination regimens encompassing 6 different ARVs (EFV, MVC, DRV, DTG, ABC, FTC) and the CYP3A inhibitor COBI. Example ion abundance distribution images following mechanical treatment for hair strands from a subject on an ARV regimen that included MVC and FTC are shown in Fig. 1. A total of 12 strands were analyzed for each drug: 3 strands each were treated with bleach, dye, and relaxer, with 3 strands left untreated. Fig. 1A shows the ion image corresponding to MVC, overlaid with the endogenous ion cholesterol (m/z 369.3516) to indicate the location of the hair strands. For these samples, the bleached strands had slightly higher MVC ion abundance than the control strands (1.5-fold on average), and the dyed strands had slightly lower MVC ion abundance (0.6-fold on average). Fig. 1B shows the ion image (MS/MS) corresponding to FTC overlaid with an unfragmented endogenous ion (m/z 248.2457). FTC was undetectable in full MS mode, so MS/MS mode was used to enhance the sensitivity and selectivity for detection of FTC. FTC ion intensity was completely absent from the ion image in any of the treated strands, while the control strands show detectable FTC ion abundance, indicating that all three treatments remove FTC from the hair strands.
Fig. 1.
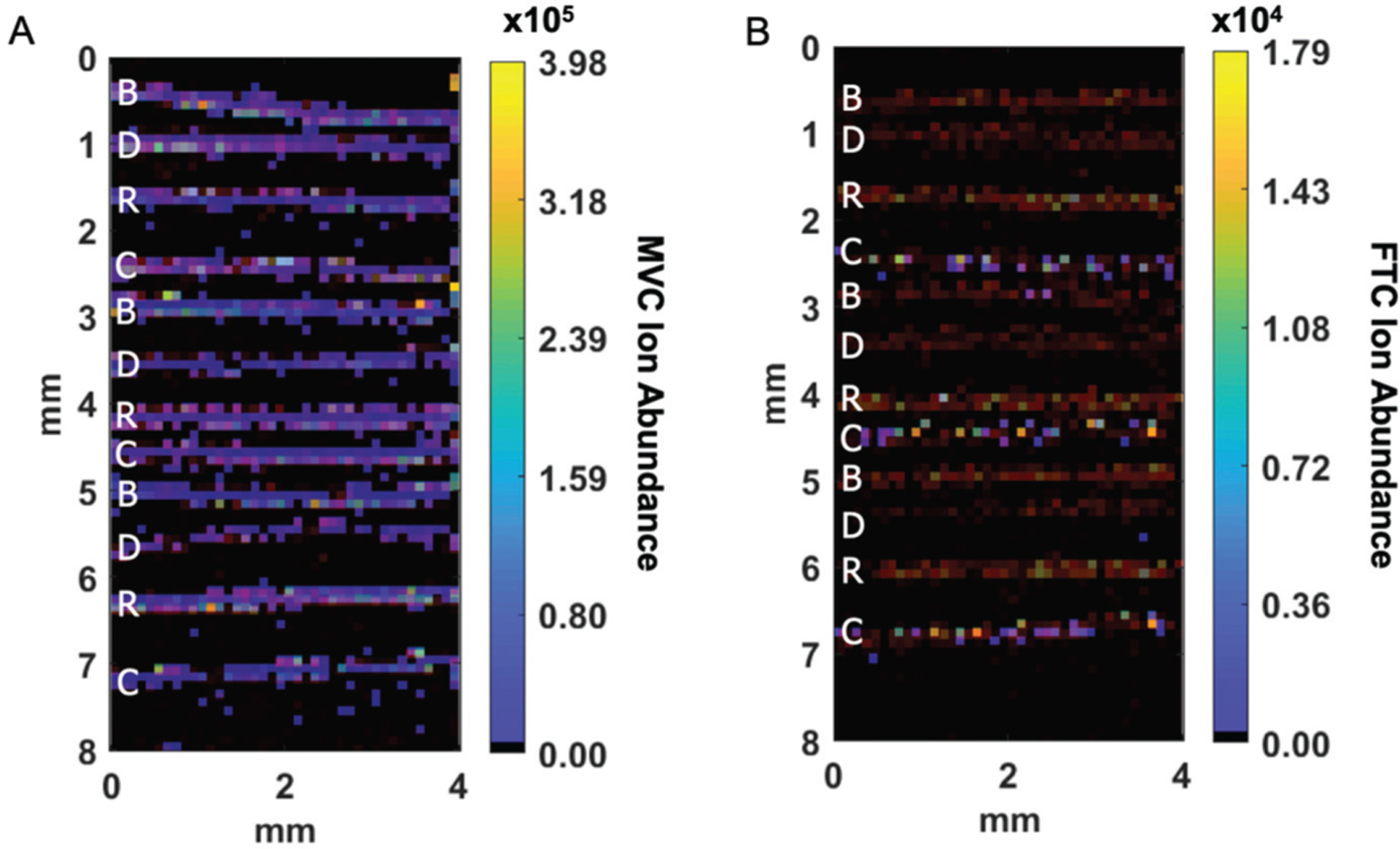
Ion images of (A) MVC (m/z 514.3352) and (B) FTC (m/z 248.1 → 130.041) overlaid with an endogenous ion (red) after manual treatment with bleach (B), dye (D), and relaxer (R) compared to untreated control samples (C). Average ion abundances for each strand are shown with other antiretrovirals in Fig. 2.
Mean ion signal abundances associated with each strand from 4 patients and 7 ARVs are shown for each treatment group in Fig. 2, and summarized in Table 1. Each data point in the figure represents a single hair strand, with all hair strands for a single drug coming from the same patient. In addition, because the ARVs are administered in combination, data for up to two ARVs may have come from the same patient, labeled in Table 1. Bleaching with peroxide (boosted by ammonium persulfate) decreased IR-MALDESI MSI ion abundance for FTC (p = 0.0061), and otherwise yielded the same or increased ion abundance between bleached and control hairs for other ARVs. For MVC and DTG, the increase was statistically significant (p = 0.023 and p = 0.029, respectively). Average ion abundance for DRV increased by 177%, but the change was not statistically significant (p = 0.077). Dyeing also resulted in lower average concentrations compared to untreated controls for 5 of the drugs (EFV, MVC, DTG, ABC, and FTC), but only treatment of FTC resulted in a statistically significant decrease (p = 0.0061). Average ion abundance for COBI and DRV both increased by over 100%, but neither change was statistically significant (p = 0.23 for both). Treatment with the relaxer resulted in significant decreased abundance for EFV, ABC, and FTC, while there was a significant increase observed for COBI. These observations can be interpreted in the context of three distinct mechanisms: (1) incorporation and binding of different drugs into hair based on drug physicochemical properties; (2) chemical and structural alterations to hair strands resulting from hair treatment; and (3) infrared laser desorption mediated by ice.
Fig. 2.
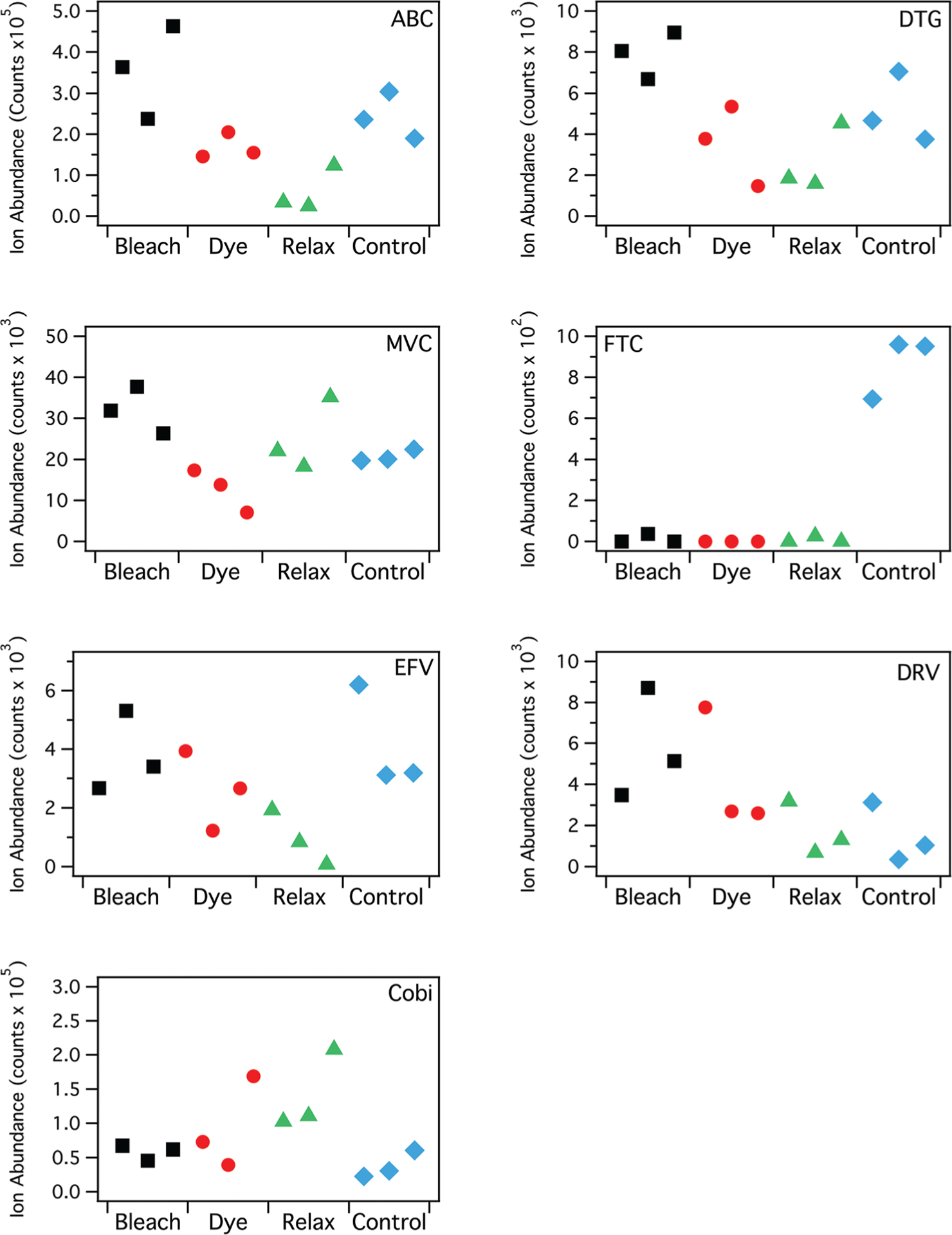
IR-MALDESI MSI ion abundance from individual patient hair strands after manual cosmetic treatment with incorporation of 7 different ARVs, compared to control samples. Each data point represents the average ion abundance from a single hair strand.
ARV incorporation into hair based on color
Using samples collected following directly-observed daily dosing, we evaluated the accumulation of three ARVs (FTC, DTG, and MVC) to understand the extent to which accumulation of a drug in hair is influenced by hair type, characterized by features such as color. Twelve samples were evaluated for each drug, with hair colors ranging from gray to black and representing a broad range of expected melanin content. ARV response to daily dosing was characterized in the proximal 5 mm of the hair strands. The melanin biomarker pyrrole-2,3,5-tricarboxylic acid (PTCA) was evaluated in distal regions of sample strands. Relationships between ARV accumulation and PTCA are shown in Fig. 3 along with Kendall’s τb correlation coefficients, which indicated moderate positive correlation for MVC with PTCA (τb,MVC = 0.55, p = 0.01, asymptotic standard error = 0.19) and no significant correlation between FTC or DTG with PTCA (τb,FTC = −0.18, p = 0.46, asymptotic standard error = 0.19 τb,DTG = 0.03, p = 0.95, asymptotic standard error = 0.18). Drug basicity and hydrophobicity of these ARVs (Table 1) follows a similar trend to the Kendall’s rank correlation coefficients, consistent with evidence from previous research that has shown compounds with basic properties are incorporated more strongly into hair due to binding with melanin.37,46–49 These relationships suggest that the mechanism of incorporation of ARVs differs, with more basic, lipophilic drugs like MVC more tightly bound to melanocytes than less basic, more hydrophilic compounds like FTC.
Fig. 3.
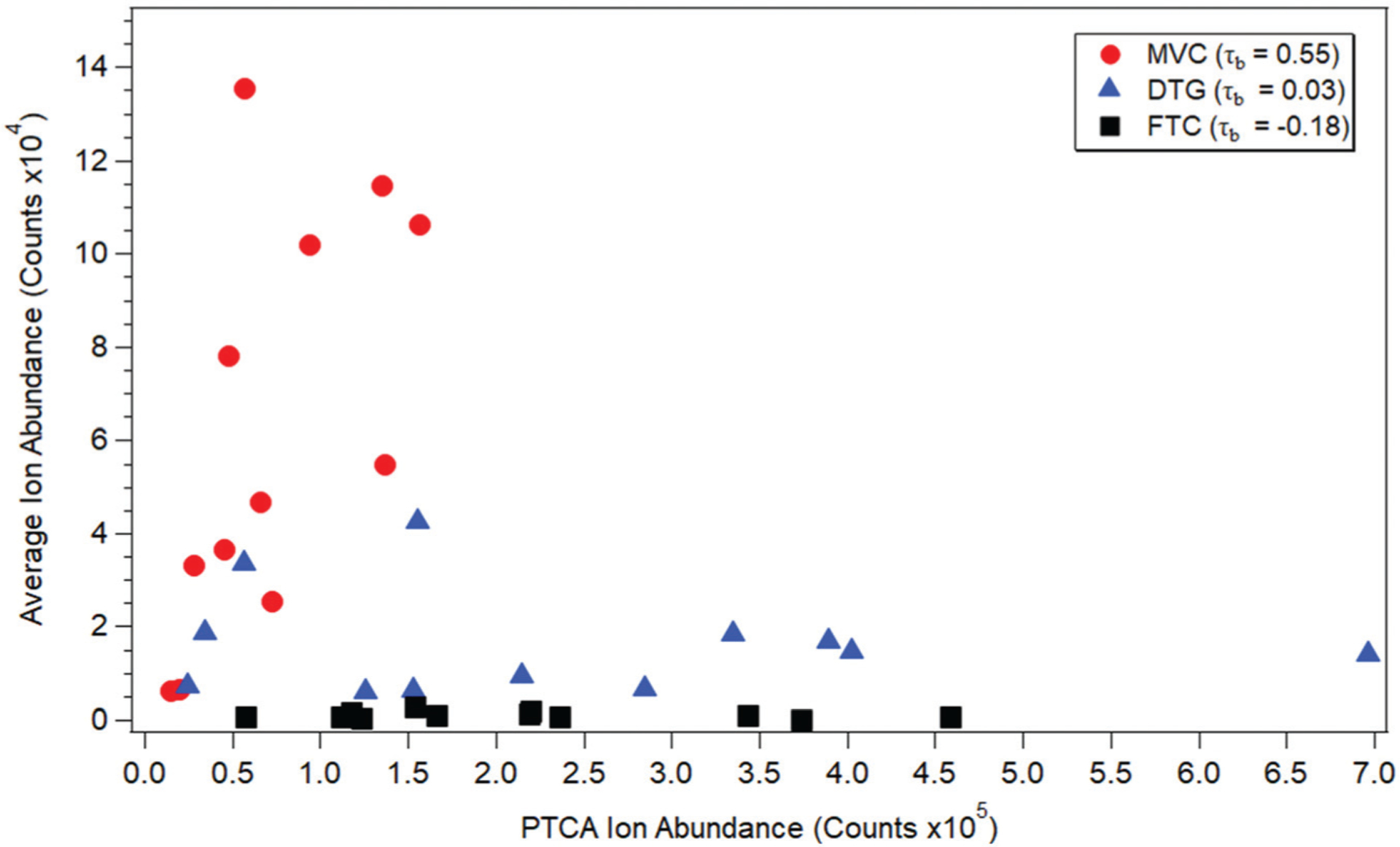
Correlation of PTCA ion abundance with average ion abundance in 12 patient hair samples (3 strands each) for 3 different ARVs after 28 days of directly observed dosing.
Chemical and structural alterations to hair
Ultrastructural effects of hair caused by treatment likely affect ARVs differently depending on how they are incorporated in the hair. The bleaching treatment combines 6% hydrogen peroxide with the alkaline ammonium persulfate to enhance oxidation and promote degradation of the melanin granule. The bleaching agent and contact time used here were designed to lighten the hair shade rather than completely remove all color. Under these conditions, loosely bound compounds like FTC may undergo preferential reactivity with oxidative products relative to other ARVs that are more strongly bound to melanin. The relaxing treatment, like bleaching, is highly alkaline and designed to cleave hair disulfide bonds to yield a straightened hair strand.50 The highly basic conditions causes hair strands to swell and may delocalize more acidic and hydrophilic compounds like FTC. Dyeing had the most broadly deleterious effect on ARV response in hair strands but only resulted in a statistically significant decrease in FTC ion abundance. While this treatment process incorporated 6% hydrogen peroxide, the color dye was suspended in an aqueous/fatty acid mixture that may penetrate keratinous hair layer and pre-ferentially remove hydrophilic compounds. While there was complete removal of FTC due to dyeing, all of the other ARVs remained detectable in hair strands. In addition, because the dye was mixed with a solution containing hydrogen peroxide, there may be reactions of the drugs with the dye solution, therefore reducing the concentration of the parent drug.
However, the bleaching treatment also incorporates hydrogen peroxide, and that treatment resulted in the same or increased ion abundance. Therefore, reactions with hydrogen peroxide seem unlikely to decrease ion abundance of this set of ARVs. Finally, the introduction of dye into the hair could increase the total number of molecules competing for ionization, resulting in ion suppression for the target of interest.
IR laser desorption
Structural changes resulting from treatment may also influence desorption conditions. Laser desorption by IR-MALDESI MSI is accomplished with an infrared laser (λ = 2.94 μm) with emission tuned to absorption features of water. The conditions promoting uptake of water under basic conditions and following bleaching may also increase the amount of absorbed ice during sample preparation, resulting in enhanced coupling of laser energy into the sample and improved desorption and enhanced detection.
FTC detection after hair treatment
Since manual treatment of individual hair strands under laboratory conditions may not reflect typical hair treatment exposure on a full head of hair, we next selected incurred samples from subjects on an FTC-based regimen reporting a variety of treatments and times since most recent treatment relative to sample collection. Data for 8 patients with recent (<8 weeks prior) hair treatment including hair color, type of treatment, and time since the treatment (reported by the patient) are shown in Table 2. There were 4 different hair colors and 5 different treatments evaluated. Images from the proximal (closest to scalp) 7 mm of hair strands are shown in Fig. 4, where intensity from FTC (shown in cyan) is overlaid with an endogenous ion to indicate the location of the hair strands (shown in red/yellow). The proximal ends are on the left side of the images. The percentages in the figure represent the ratio of voxels with both FTC and endogenous detected to all voxels with endogenous detected. In general, there is a trend of increasing frequency of detection of FTC with increasing time since treatment. The patients P-1 and P-2 had different treatments the day immediately before sampling, but some FTC signal is observed in each hair strand. In the case of P-2, only the proximal end of the hair strands contain FTC, which suggests that the hair treatment (dye) may not have reached the base of the scalp. Observations for P-3, 4, and 5 highlight biological and treatment variability. For P-3, FTC was observed along the length of the middle strand, was intermittent in the bottom strand, and was undetectable in the top strand. In the case of the undetectable strand, this is likely due to this particular hair being in the dormant growth phase. For P-4, whose hair was similar in color, had similar treatment, and had hair treatment on a similar timeline, FTC was observed consistently throughout all three hair strands. The response to FTC for P-5 was similar to P-3 but was the only patient who reported using a relaxer. FTC detection was intermittent throughout the hair strand but concentrated more toward the proximal end. This suggests that relaxer and bleach both removed some FTC, but the same effect is not observed with P-4. These differences are the subject of future investigation. Hair from patients P-6, P-7, and P-8 showed consistent detection of FTC throughout each of the strands.
Table 2.
Hair treatment, color, and timeline for hair strands from all patients
| Patient ID | Targeted ARVs | Hair color | Treatment | Treatment timeline |
|---|---|---|---|---|
| P-1 | FTC, DTG | Brown | Perm | Previous day |
| P-2 | FTC, DRV | Grey | Dye (brown) | Previous day |
| P-3 | FTC, EFV | Brown | Dye | A couple of weeks ago |
| P-4 | FTC, COBI | Brown | Bleach and dye | 3 weeks ago |
| P-5 | FTC | Black | Relaxer | 4 weeks ago |
| P-6 | FTC | Black | Brazilian blowout | 6.5 weeks ago |
| P-7 | FTC | Blonde | Dye (red) | 6 weeks |
| P-8 | FTC | Blonde | Dye | 8 weeks |
Fig. 4.
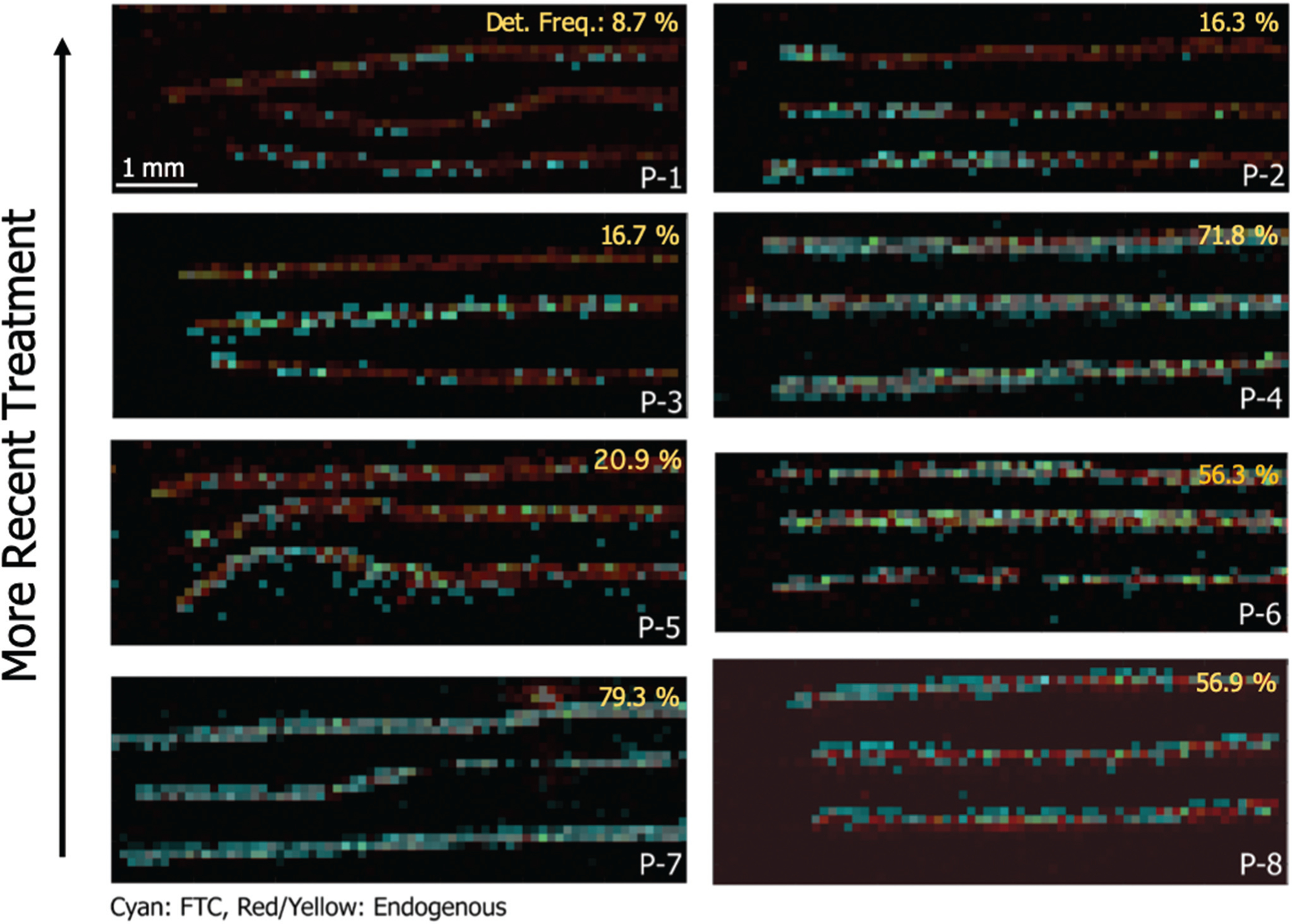
MS/MS images of FTC (m/z 248.1 ± 4 → m/z 130.041) from 8 patients with different cosmetic hair treatments and varying times after treatment. Patient IDs and corresponding treatments and timeline are detailed in Table 2. The percentages on the images represent the frequency of detection of FTC as a ratio of number of voxels where FTC was colocalized with the endogenous ion to the number of voxels where only the endogenous ion was detected.
Based on an average growth rate of ~1 cm per month,51 7 mm of hair corresponds to approximately 3 weeks (21 days) of hair growth. Thus, we would expect to see FTC signal regardless of treatment for hair that was treated more than 3 weeks prior. However, because our method of acquiring the hair was cutting with scissors rather than plucking, approximately 3 to 5 mm of hair may remain below the scalp, and some hairs may not be cut at exactly the same point. This relative imprecision of this cutting method may account for the inter-strand variability in FTC response seen in P-3 and P-5. Nevertheless, if hair was treated very close to sample collection and FTC signal was observed, then that indicated that the hair treatment did not remove all of the drug from the hair strands, as with P-1 and P-2.
We chose to image further along the length of hair strands from one patient with consistent FTC response (P-4) to observe the time point of treatment. Fig. 5 shows the MS image of FTC overlaid with an endogenous ion, sampled on the proximal 15 mm of the hair strand. In the top strand, there was a clear transition from hair containing FTC to hair without any FTC at about 8 mm on the image, or about 7 mm from the proximal end of the hair strand. As stated above, 7 mm would correspond to about 3 weeks of hair growth, which would agree with the self-reported time since treatment. The transition was less stark in the other two strands, but there was difference in FTC detection around 12 mm in the image, or about 11 mm from the proximal end of the strands. For the strands sampled here, the transition from detection of FTC to no detectable FTC shows that treatment removed FTC from hair strands, consistent with the results on the benchtop.
Fig. 5.
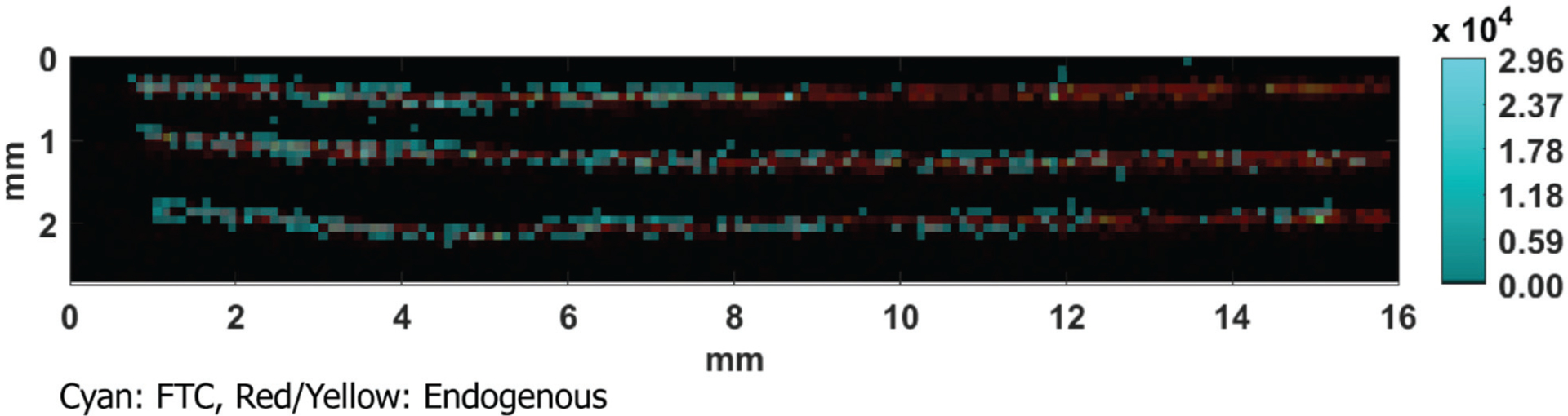
MS/MS image of FTC (m/z 248.1 ± 4 → m/z 130.041) and endogenous (m/z 248.2457) from P-4 sampled on the proximal 15 mm of hair.
While FTC was removed by both manual treatment after removal from the scalp and typical treatment, our trends underscore that other ARVs behave differently, and FTC was the only drug that became undetectable in the hair strands. Because FTC is most often administered in a fixed dose combination with other ARVs, it may be possible to determine patient adherence to an FTC-based regimen by targeting the other drugs in the regimen. To illustrate this point, we analyzed the hair strands from the four most recent treatments shown in Fig. 4 (P-1, P-2, P-3, and P-4), this time targeting the other ARVs administered in combination with FTC. Fig. 6 shows ion images for hair strands from these four patients, targeting DTG, DRV, EFV, and COBI along the proximal 7 mm of the strands. In each of these images, the drug administered in combination with FTC was detectable along the length of at least 1 of the 3 hair strands analyzed. In Fig. 6A, DTG was detected in 1 of the 3 strands, indicating that 2 of the strands were likely in the dormant growth phase. For Fig. 6B, DRV was detected along the length of all 3 strands. For Fig. 6C and D, EFV and COBI were both detected along the length of all 3 strands. These data demonstrate that adherence to these regimens can be determined by analyzing another drug if FTC is undetectable.
Fig. 6.
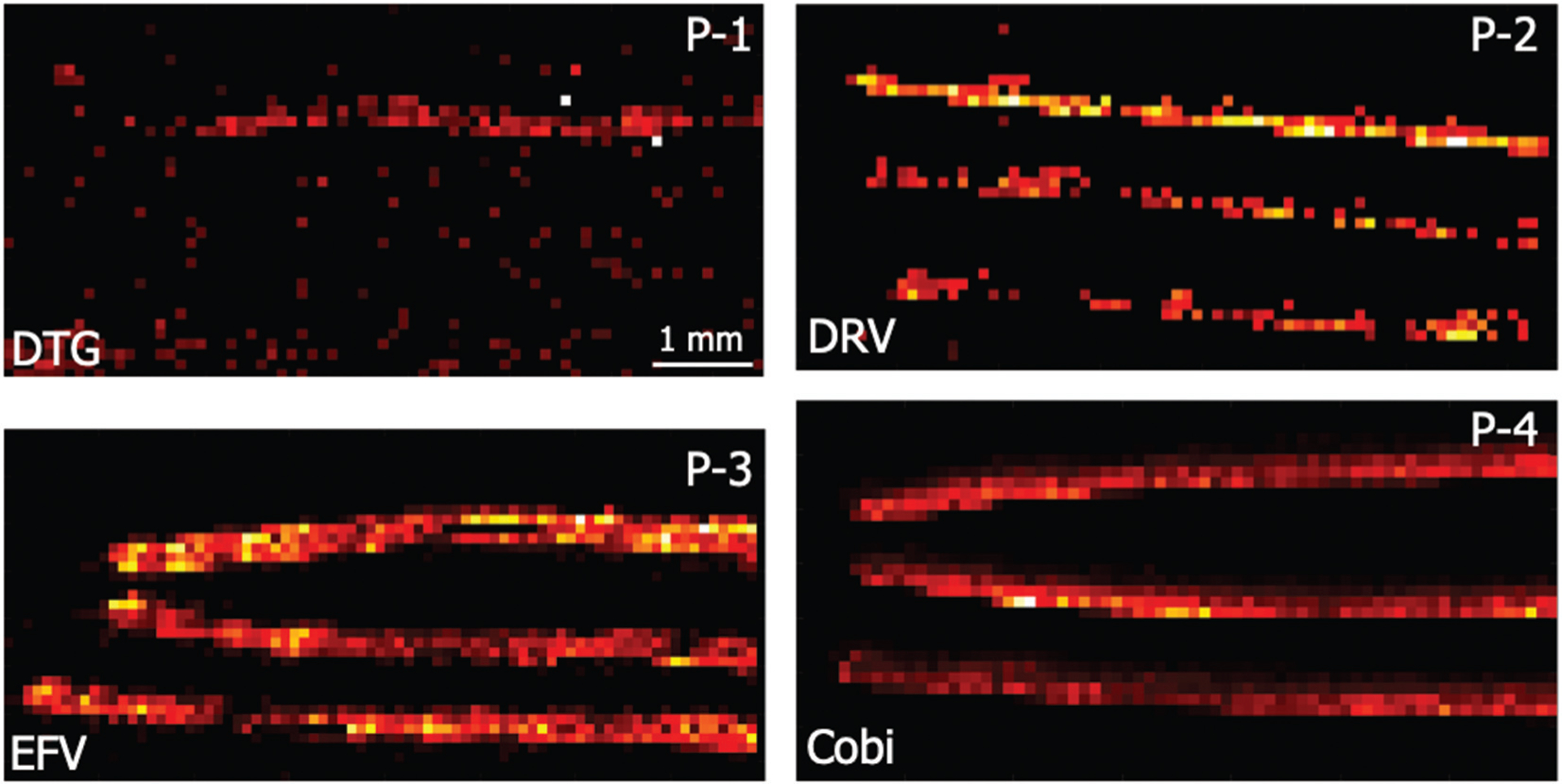
ARV ion abundance images for drugs co-administered with FTC from the first 4 patients shown in Fig. 4.
Conclusion
The effect of a range of hair treatments on the detectability of several antiretrovirals was assessed by IR-MALDESI MSI. Our results indicated that a commercial relaxer, dye, and bleach had varying impact on MSI response to ARVs across multiple drug classes, with instances of both enhanced and diminished ARV detection observed. Any perturbation to the fidelity of hair concentrations could lead to unintended effects if used as the basis for assessment of medication adherence, leading to either false-positive or false-negative tests. Since the detection of FTC was most significantly affected by treatment and the only drug that became undetectable, treated hair samples from patients on FTC-based regimens were assessed to quantify the time between hair treatment and detection of FTC. The transition from the treated portion of the hair to the untreated portion confirmed our benchtop results that hair treatment removes FTC. However, although FTC was removed by hair treatment, other ARVs administered in combination with FTC were detectable, indicating that those ARVs could be used as a proxy for FTC adherence. Two limitations of this investigation were the small sample size and the reliability of self-reported hair treatments. As MSI of hair strands is developed as an objective measure of patient adherence, these results indicate that hair treatment effects must be quantified and taken into consideration to avoid false classifications of adherence to therapy. In the case of ARVs, either a gap between treatment and sample collection should be used to ensure drug detection or an ARV other than FTC should be used to assess adherence.
Acknowledgements
The authors would like to thank Dr Monica Gandhi for supplying the hair sample for a patient on a MVC and FTC regimen. We would like to thank Dr David Muddiman for the development of and assistance with IR-MALDESI MSI. We would also like to thank Michael Hudgens for his statistical support provided through the University of North Carolina at Chapel Hill Center for AIDS Research, an NIH funded program P30 AI050410. We gratefully acknowledge the National Institutes of Health (grants R01 AI122319, P30 AI050410) and the UNC Clinical and Translational Research Center for financially supporting this work.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
References
- 1.Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV http://aidsinfo.nih.gov/content-files/lvguidelines/AdultandAdolescentGL.pdf (accessed May 3, 2019).
- 2.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, Sax PE, Smith DM, Thompson MA and Buchbinder SP, et al. , Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults, JAMA, 2018, 320(4), 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR and Katabira E, et al. , Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women, N. Engl. J. Med, 2012, 367(5), 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV and Ramirez-Cardich ME, et al. , Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men, N. Engl. J. Med, 2010, 363(27), 2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eshleman SH, Hudelson SE, Redd AD, Swanstrom R, Ou S-S, Zhang XC, Ping L-H, Piwowar-Manning E, Porcella SF and Sievers MF, et al. , Treatment as Prevention: Characterization of Partner Infections in the HIV Prevention Trials Network 052 Trial, J. Acquired Immune Defic. Syndr, 2017, 74(1), 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parienti J-J, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R and Bangsberg DR, Not All Missed Doses Are the Same: Sustained NNRTI Treatment Interruptions Predict HIV Rebound at Low-to-Moderate Adherence Levels, PLoS One, 2008, 3(7), e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross R, Yip B, Re III VL, Wood E, Alexander CS, Harrigan PR, Bangsberg DR, Montaner JSG and Hogg RS, A Simple, Dynamic Measure of Antiretroviral Therapy Adherence Predicts Failure to Maintain HIV-1 Suppression, J. Infect. Dis, 2006, 194(8), 1108–1114. [DOI] [PubMed] [Google Scholar]
- 8.Alcaide ML, Ramlagan S, Rodriguez VJ, Cook R, Peltzer K, Weiss SM, Sifunda S and Jones DL, Self-Report and Dry Blood Spot Measurement of Antiretroviral Medications as Markers of Adherence in Pregnant Women in Rural South Africa, AIDS Behav., 2017, 21(7), 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agot K, Taylor D, Corneli AL, Wang M, Ambia J, Kashuba ADM, Parker C, Lemons A, Malahleha M, Lombaard J, et al. , Accuracy of Self-Report and Pill-Count Measures of Adherence in the FEM-PrEP Clinical Trial: Implications for Future HIV-Prevention Trials, AIDS Behav, 2015, 19(5), 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon JH, Jordan MR, Kelley K, Bertagnolio S, Hong SY, Wanke CA, Lewin SR, Elliott JH and Elliott JH, Pharmacy Adherence Measures to Assess Adherence to Antiretroviral Therapy: Review of the Literature and Implications for Treatment Monitoring, Clin. Infect. Dis, 2011, 52(4), 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mekuria LA, Prins JM, Yalew AW, Sprangers MAG and Nieuwkerk PT, Which Adherence Measure - Self-Report, Clinician Recorded or Pharmacy Refill - Is Best Able to Predict Detectable Viral Load in a Public ART Programme without Routine Plasma Viral Load Monitoring?, Trop. Med. Int. Health, 2016, 21(7), 856–869. [DOI] [PubMed] [Google Scholar]
- 12.El Alili M, Vrijens B, Demonceau J, Evers SM and Hiligsmann M, A Scoping Review of Studies Comparing the Medication Event Monitoring System (MEMS) with Alternative Methods for Measuring Medication Adherence, Br. J. Clin. Pharmacol, 2016, 82(1), 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, Haberer JE, Rooney J, Hendrix CW, Anderson PL, et al. , Comparing the Novel Method of Assessing PrEP Adherence/Exposure Using Hair Samples to Other Pharmacologic and Traditional Measures, J. Acquired Immune Defic. Syndr, 2015, 68(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louissaint NA, Cao Y-J, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, Anderson JR, Everts S, Bakshi R, Fuchs EJ, et al. , Single Dose Pharmacokinetics of Oral Tenofovir in Plasma, Peripheral Blood Mononuclear Cells, Colonic Tissue, and Vaginal Tissue, AIDS Res. Hum. Retroviruses, 2013, 29(11), 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wertheimer B, Freedberg K, Walensky R, Yazdan Y and Losina E, Therapeutic Drug Monitoring in HIV Treatment: A Literature Review, HIV Clin. Trials, 2006, 7(2), 59–69. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapía M, Montoya-Herrera O, et al. , Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men, Sci. Transl. Med, 2012, 4(151), 151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxi SM, Vittinghoff E, Bacchetti P, Huang Y, Chillag K, Wiegand R, Anderson PL, Grant R, Greenblatt RM and Buchbinder S, et al. , Comparing Pharmacologic Measures of Tenofovir Exposure in a U.S. Pre-Exposure Prophylaxis Randomized Trial, PLoS One, 2018, 13(1), e0190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE, Horng H, Louie A, Kuncze K, Koss CA, et al. , Hair Levels of Preexposure Prophylaxis Drugs Measure Adherence and Are Associated with Renal Decline among Men/Transwomen, AIDS, 2017, 31(16), 2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, Benet LZ, Kuncze K, Louie A and Saberi P, et al. , Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States, Clin. Infect. Dis, 2018, 66(2), 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, Mirembe BG, Gomez Feliciano K, Horn S and Liu AY, et al. , Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, IPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations, AIDS Res. Hum. Retroviruses, 2017, 33(8), 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K, Horng H, Zheng J-H, Bushman LR and Kiser JJ, et al. , Brief Report: Adherence Biomarker Measurements in Older and Younger HIV-Infected Adults Receiving Tenofovir-Based Therapy, JAIDS, J. Acquired Immune Defic. Syndr, 2018, 77(3), 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phung N, Kuncze K, Okochi H, Louie A, Benet LZ, Ofokotun I, Haas DW, Currier JS, Chawana TD and Sheth AN, et al. , Development and Validation of an Assay to Analyze Atazanavir in Human Hair via Liquid Chromatography/Tandem Mass Spectrometry, Rapid Commun. Mass Spectrom, 2018, 32(5), 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi M, Bacchetti P, Ofokotun I, Jin C, Ribaudo HJ, Haas DW, Sheth AN, Horng H, Phung N and Kuncze K, et al. , Antiretroviral Concentrations in Hair Strongly Predict Virologic Response in a Large HIV Treatment-Naive Clinical Trial, Clin. Infect. Dis, 2018, 68(6), 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabb ZJ, Mmbaga BT, Gandhi M, Louie A, Kuncze K, Okochi H, Shayo AM, Turner EL, Cunningham CK and Dow DE, Antiretroviral Drug Concentrations in Hair Are Associated with Virologic Outcomes among Young People Living with HIV in Tanzania, AIDS, 2018, 32(9), 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen EP, Thompson CG, Bokhart MT, Prince HMA, Sykes C, Muddiman DC and Kashuba ADM, Analysis of Antiretrovirals in Single Hair Strands for Evaluation of Drug Adherence with Infrared-Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging, Anal. Chem, 2016, 88(2), 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, Guanira JV, Grinsztejn B, Chariyalertsak S and Bekker L-G, et al. , Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the IPrEx Open Label Extension: Implications for Pre-Exposure Prophylaxis Adherence Monitoring, J. Infect. Dis, 2015, 212(9), 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah SAB, Mullin R, Jones G, Shah I, Barker J, Petroczi A and Naughton DP, Simultaneous Analysis of Antiretroviral Drugs Abacavir and Tenofovir in Human Hair by Liquid Chromatography–Tandem Mass Spectrometry, J. Pharm. Biomed. Anal, 2013, 74, 308–313. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, Young M, Milam J, Cohen MH and Sharp GB, et al. , Atazanavir Concentration in Hair Is the Strongest Predictor of Outcomes on Antiretroviral Therapy, Clin. Infect. Dis, 2011, 52(10), 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, Horng H, Clark TD, Plenty A and Ruel TD, et al. , Hair Concentrations of Antiretrovirals Predict Viral Suppression in HIV-Infected Pregnant and Breastfeeding Ugandan Women, AIDS, 2015, 29(7), 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, Goggin K, Stojanovski K, Grant R and Buchbinder SP, et al. , Strong Relationship between Oral Dose and Tenofovir Hair Levels in a Randomized Trial: Hair as a Potential Adherence Measure for Pre-Exposure Prophylaxis (PrEP), PLoS One, 2014, 9(1), e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka S, Iio R, Chinaka S, Takayama N and Hayakawa K, Analysis of Reaction Products of Cocaine and Hydrogen Peroxide by High-Performance Liquid Chromatography/Mass Spectrometry, Biomed. Chromatogr, 2002, 16(6), 390–394. [DOI] [PubMed] [Google Scholar]
- 32.Pötsch L and Skopp G, Stability of Opiates in Hair Fibers after Exposure to Cosmetic Treatment, Forensic Sci. Int, 1996, 81(2–3), 95–102. [DOI] [PubMed] [Google Scholar]
- 33.Kerekes I and Yegles M, Coloring, Bleaching, and Perming, Ther. Drug Monit, 2013, 35(4), 527–529. [DOI] [PubMed] [Google Scholar]
- 34.Jurado C, Kintz P, Menéndez M and Repetto M, Influence of the Cosmetic Treatment of Hair on Drug Testing, Int. J. Legal Med, 1997, 110(3), 159–163. [DOI] [PubMed] [Google Scholar]
- 35.Yegles M, Marson Y and Wennig R, Influence of Bleaching on Stability of Benzodiazepines in Hair, Forensic Sci. Int, 2000, 107(1–3), 87–92. [DOI] [PubMed] [Google Scholar]
- 36.Cuypers E, Flinders B, Bosman IJ, Lusthof KJ, Van Asten AC, Tytgat J and Heeren RMA, Hydrogen Peroxide Reactions on Cocaine in Hair Using Imaging Mass Spectrometry, Forensic Sci. Int, 2014, 242, 103–110. [DOI] [PubMed] [Google Scholar]
- 37.Pragst F and Balikova MA, State of the Art in Hair Analysis for Detection of Drug and Alcohol Abuse, Clin. Chim. Acta, 2006, 370(1–2), 17–49. [DOI] [PubMed] [Google Scholar]
- 38.Gilliland WM, Prince HMA, Poliseno A, Kashuba ADM and Rosen EP, Infrared Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging of Human Hair to Characterize Longitudinal Profiles of the Antiretroviral Maraviroc for Adherence Monitoring, Anal. Chem, 2019, 91(16), 10816–10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robichaud G, Barry JA and Muddiman DC, IR-MALDESI, Mass Spectrometry Imaging of Biological Tissue Sections Using Ice as a Matrix, J. Am. Soc. Mass Spectrom, 2014, 25(3), 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson CG, Bokhart MT, Sykes C, Adamson L, Fedoriw Y, Luciw PA, Muddiman DC, Kashuba ADM and Rosen EP, Mass Spectrometry Imaging Reveals Heterogeneous Efavirenz Distribution within Putative HIV Reservoirs, Antimicrob. Agents Chemother, 2015, 59(5), 2944–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robichaud G, Garrard KP, Barry JA and Muddiman DC, MSiReader: An Open-Source Interface to View and Analyze High Resolving Power MS Imaging Files on Matlab Platform, J. Am. Soc. Mass Spectrom, 2013, 24(5), 718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bokhart MT, Nazari M, Garrard KP and Muddiman DC, MSiReader v1.0: Evolving Open-Source Mass Spectrometry Imaging Software for Targeted and Untargeted Analyses, J. Am. Soc. Mass Spectrom, 2018, 29(1), 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team, R: A Language and Environment for Statiscal Computing, R Foundation for Statistical Computing, Vienna, Austria, 2019. [Google Scholar]
- 44.Gelman A and Hill J, Data Analysis Using Regression and Multilevel/Hierarchical Models, Cambridge University Press, 2007. [Google Scholar]
- 45.Little TD, Methodology in the Social Sciences, Longitudinal Structural Equation Modeling, Guilford Press, 2013. [Google Scholar]
- 46.Nakahara Y, Kikura R and Takahashi K, Hair Analysis for Drugs of Abuse XX. Incorporation and Behaviors of Seven Methamphetamine Homologs in the Rat Hair Root, Life Sci, 1998, 63(10), 883–893. [DOI] [PubMed] [Google Scholar]
- 47.Nakahara Y and Kikura R, Hair Analysis for Drugs of Abuse XIII. Effect of Structural Factors on Incorporation of Drugs into Hair: The Incorporation Rates of Amphetamine Analogs, Arch. Toxicol, 1996, 70(12), 841–849. [DOI] [PubMed] [Google Scholar]
- 48.Scheidweiler KB, Cone EJ, Moolchan ET and Huestis MA, Dose-Related Distribution of Codeine, Cocaine, and Metabolites into Human Hair Following Controlled Oral Codeine and Subcutaneous Cocaine Administration, J. Pharmacol. Exp. Ther, 2005, 313(2), 909–915. [DOI] [PubMed] [Google Scholar]
- 49.Shima N, Nitta A, Kamata T, Sasaki K, Matsuta S, Ishikawa A, Asai R, Wada M, Kakehashi H, Nakano S, et al. , Incorporation of Zolpidem and Methoxyphenamine into White Hair Strands after Single Administrations: Influence of Hair Pigmentation on Drug Incorporation, Forensic Sci. Int, 2019, 301, 67–75. [DOI] [PubMed] [Google Scholar]
- 50.Pritchett JS and Phinney KW, Influence of Chemical Straightening on the Stability of Drugs of Abuse in Hair, J. Anal. Toxicol, 2015, 39(1), 13–16. [DOI] [PubMed] [Google Scholar]
- 51.Pragst F, Rothe M, Spiegel K and Sporkert F, Illegal and Therapeutic Drug Concentrations in Hair Segments - A Timetable of Drug Exposure?, Forensic Sci. Rev, 1998, 10(2), 81–111. [PubMed] [Google Scholar]


