Key Points
Question
Have neurodevelopmental outcomes to 2 years’ corrected age for infants born extremely preterm improved with advances in perinatal and neonatal intensive care?
Findings
In this cohort study of extremely preterm infants at 2 years, survival free of major neurodevelopmental disability increased steadily over time, from 42% in 1991-1992, 51% in 1997, 53% in 2005, to 62% in 2016-2017. Annual numbers of survivors with major neurodevelopmental disability per year were 22 (1991-1992), 39 (1997), 24 (2005), and 26 (2016-2017).
Meaning
In this study, advances in care over time have been associated with increased survival free of major disability at 2 years of age, with no increase in absolute numbers with major neurodevelopmental disability.
Abstract
Importance
Survival of infants born extremely preterm (EP) (<28 weeks’ gestation) has increased since the early 1990s. It is necessary to know whether increased survival is accompanied by increased neurodevelopmental disability.
Objective
To examine changes in major (ie, moderate or severe) neurodevelopmental disability and survival free of major neurodevelopmental disability at 2 years in infants born EP.
Design, Setting, and Participants
Four prospective longitudinal cohort studies comprising all EP live births at 22 to 27 weeks’ gestation from April 1, 2016, to March 31, 2017, and earlier eras (1991-1992, 1997, and 2005), and contemporaneous term-born controls in the state of Victoria, Australia. Among 1208 live births during the periods studied, data were available for analysis of 2-year outcomes in 1152 children: 422 (1991-1992), 215 (1997), 263 (2005), and 252 (2016-2017). Data analysis was performed from September 17, 2020, to April 15, 2021.
Exposures
Extreme preterm live birth.
Main Outcomes and Measures
Survival, blindness, deafness, cerebral palsy, developmental delay, and neurodevelopmental disability at 2 years’ corrected age. Developmental delay comprised a developmental quotient less than −1 SD relative to the control group means on the Bayley Scales for each era. Major neurodevelopmental disability comprised blindness, deafness, moderate to severe cerebral palsy, or a developmental quotient less than −2 SDs. Individual neurodevelopmental outcomes in each era were contrasted relative to the 2016-2017 cohort using logistic regression adjusted for gestational age, sex, birth weight z score, and sociodemographic variables. Changes in survival free of major neurodevelopmental disability over time were also assessed using logistic regression.
Results
Survival to 2 years was highest in 2016-2017 (73% [215 of 293]) compared with earlier eras (1991-1992: 53% [225 of 428]; 1997: 70% [151 of 217]; 2005: 63% [170 of 270]). Blindness and deafness were uncommon (<3%). Cerebral palsy was less common in 2016-2017 (6%) than in earlier eras (1991-1992: 11%; 1997: 12%; 2005: 10%). There were no obvious changes in the rates of developmental quotient less than −2 SDs across eras (1991-1992: 18%; 1997: 22%; 2005: 7%; 2016-2017: 15%) or in rates of major neurodevelopmental disability (1991-1992: 20%; 1997: 26%; 2005: 15%; 2016-2017: 15%). Rates of survival free of major neurodevelopmental disability increased steadily over time: 42% (1991-1992), 51% (1997), 53% (2005), and 62% (2016-2017) (odds ratio, 1.30; 95% CI, 1.15-1.48 per decade; P < .001).
Conclusions and Relevance
These findings suggest that survival free of major disability at age 2 years in children born EP has increased by an absolute 20% since the early 1990s. Increased survival has not been associated with increased neurodevelopmental disability.
This cohort study compares changes in neurodevelopmental outcomes at 2 years in extremely preterm children born between 1991 and 2017.
Introduction
Advances in perinatal and neonatal care were accompanied by a substantial increase in the survival of extremely preterm (EP) (<28 weeks’ gestation) newborns, from 25% in the late 1970s to more than 50% by the early 1990s, and with a steady increase since then.1,2,3,4 A concern with innovations in care is that improved survival may be accompanied by an increase in neurodevelopmental disability, as observed in an early trial of intensive care in the late 1960s.5 Furthermore, improvements in neurodevelopment have not been consistently reported; in fact, neurodevelopment at school age in EP children may be worsening.6,7,8 Increased survival without concurrent improvements in neurodevelopment will result in more EP children with disabilities who need more care and intervention.
Substantial improvements in perinatal/neonatal intensive care in the past 3 decades include increased exposure to antenatal corticosteroids for women at risk of preterm birth, antenatal magnesium sulfate for fetal neuroprotection, exogenous surfactant to treat newborn respiratory distress syndrome, and gentler methods of respiratory support.9,10,11,12 Although some randomized clinical trials of new treatments have reported long-term neurodevelopmental outcomes,10,11,12 the full measure of the integration of new therapies into clinical care can be determined only by repeated assessments of complete populations.
In Victoria, Australia, 4 discrete geographic cohorts of all children born EP and contemporaneous term-born controls have been recruited into a research program to evaluate neonatal intensive care since 1991.13 These cohorts provide a unique opportunity to track whether innovations in neonatal and perinatal care have been accompanied by neurodevelopmental improvements. The Victorian Infant Collaborative Study Group recently reported that survival to discharge home in these cohorts for live births free of lethal anomalies had increased from 53% in 1991-1992 to 73% in 2016-2017.2
The aim of the present study was to determine the changes in neurodevelopment at 2 years’ corrected age in children born EP from 1991-1992 to 2016-2017, both overall and within each completed week of gestation. We hypothesized that compared with earlier eras, the 2016-2017 cohort would have lower rates of cerebral palsy (CP) and similar rates of developmental delay and disability. Both overall and within each week of gestation, we expected survival free of major disability would increase over time.
Methods
The Victorian Infant Collaborative Study is a statewide collaboration of all 4 tertiary neonatal units, government data collection agencies, and the statewide newborn emergency retrieval service. This analysis is based on 4 prospective longitudinal cohorts of all infants born EP in Victoria, Australia, and matched controls during 4 discrete eras: January 1, 1991, to December 31, 1992 (24 months); January 1 to December 31, 1997 (12 months); January 1 to December 31, 2005 (12 months); and April 1, 2016, to March 31, 2017 (12 months). The total number of live births in Victoria has increased from approximately 66 000 in 1990 to 80 000 in 2016, and the proportion of live births at 22 to 27 weeks’ gestation was 0.32% in 1991-1992, increasing to 0.37% in 2016.14 The studies were approved by the human research ethics committees at the Royal Women’s Hospital, the Mercy Hospital for Women, Monash Health, and the Royal Children’s Hospital, Melbourne. Written informed consent was obtained from the parents of all controls and the parents of the EP children born in 2005 and 2016-2017. Follow-up to 2 years was considered routine care for EP children in the 2 earlier cohorts. The present data analysis was conducted from September 17, 2020, to April 15, 2021. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
For each era, all EP live births (22-27 completed weeks’ gestation) free of lethal anomalies and contemporaneous controls were recruited at birth. Controls (birth weight ≥2500 g and gestation at birth ≥37 weeks) were recruited from maternity units affiliated with the 3 tertiary perinatal centers in the state and matched with the EP infants according to sex, expected date of birth, the mother’s health insurance status (private or public, as a proxy for social class), and the primary language spoken in her country of birth (English or other).
Data collection methods have been published.2 Gestational age was confirmed by obstetric ultrasonography before 20 weeks (available for >90% of pregnancies) or by menstrual history. Birth weight z scores were computed relative to the British Growth Reference.15 Sociodemographic data collected included years of maternal education (dichotomized into lower and higher levels around the median years of schooling for that cohort), socioeconomic status (based on the occupation of the primary income earner in the family and categorized as lower [unskilled or unemployed] or higher [semiskilled, skilled, or professional]), and the primary language spoken at home (English only vs other).
At 2 years’ corrected age, children were evaluated by trained assessors (M.C., E.K.J., and N.D.) blinded to clinical history and gestational age at birth for CP, blindness (visual acuity <20/200 in the better eye), deafness (hearing loss requiring amplification or a cochlear implant, or worse), and developmental delay. Cerebral palsy was determined by abnormal tone and reflexes and a loss of motor function, with severity determined by a functional classification (1991-1992 and 1997 cohorts) or the Gross Motor Function Classification System (2005 and 2016-2017 cohorts).16 Development was assessed with the contemporary versions of the Bayley Scales for each cohort: Bayley Scales of Infant Development17 (1991-1992), Bayley Scales of Infant Development, 2nd edition18 (1997), and Bayley Scales of Infant & Toddler Development, 3rd edition (Bayley-III)19 (2005 and 2016-2017). To account for the different versions of the Bayley Scales assessment used, developmental delay was classified according to the developmental quotient computed as SD scores relative to the weighted mean and SD for controls for each of the respective cohorts on the Mental Development Index of the first 2 editions of the Bayley Scales,17,18 and according to the mean of the Cognitive and the Language Composite scores on Bayley-III.19,20 For children without a language score, only the cognitive score was used. The mean for the controls in each era was weighted to reflect the distribution of social variables of the corresponding EP cohorts given the known bias of recruiting lower social risk controls6 and assuming the EP group more accurately reflected the sociodemographics of the general population. Developmental delay was defined as less than −1 SD relative to the mean of the controls and categorized as mild (−2 SDs to <−1 SD), moderate (−3 SDs to <−2 SDs), or severe (<−3 SDs). A child who was unable to complete psychological testing because of severe developmental delay was assigned a score of −4 SDs
Neurodevelopmental disability was defined as having CP, blindness, deafness, or any developmental delay, and categorized as mild (mild CP [walking at age 2 years, Gross Motor Function Classification System level I] or mild developmental delay), moderate (moderate CP [not walking at age 2 years but expected to walk eventually, Gross Motor Function Classification System level II or 3], deafness, or moderate developmental delay), or severe (severe CP [unlikely ever to walk; Gross Motor Function Classification System level IV or V], blindness, or severe developmental delay). Major neurodevelopmental disability comprised either the moderate or severe category.
Statistical Analysis
Data were analyzed using Stata, release 16 (StataCorp LLC). All comparative analyses were restricted to EP participants, with controls used to determine developmental delay. Participant characteristics were compared between those who were assessed at 2 years and those who were not, using means and SDs and proportions. For 2-year outcomes, multiple imputation was used to account for missing data, carried out using a single imputation model fitted with chained equations, including sociodemographic, perinatal, and neonatal data, to improve prediction of the missing values. Forty imputed data sets were created and the resulting inference was combined using Rubin rules.21 Outcomes were compared between eras (using a 4-level independent categorical variable with the 2016-2017 cohort as the reference) using logistic regression, adjusted for potential confounding perinatal (gestational age at birth, sex, and birth weight z score), and sociodemographic variables (maternal educational level, socioeconomic status, and language spoken at home). We also restricted the analysis to participants with complete data to provide a benchmark for comparison with the multiple imputation results. In addition, complete case analyses were used to compare survival free of major neurodevelopmental disability and survival with major disability across eras via logistic regression. It was not possible to conduct imputation in these analyses owing to excessive amounts of missing data for participants who died. Era was included as a continuous variable in the regression model as years after 1991-1992 (assigned a value of 0). All regression models were fitted using generalized estimating equations and reported with robust (sandwich) estimates of SEs to account for clustering of multiple births within the same family. A threshold of statistical significance was not defined, although the importance of findings are based on magnitude of ORs and 95% CIs.
Results
Participant characteristics are summarized in Table 1 and eTable 1 in Supplement 1. Among 1208 live births during the periods studied, data were available for analysis of 2-year outcomes in 1152 children: 422 (1991-1992), 215 (1997), 263 (2005), 252 (2016-2017). Survival to 2 years was highest in the 2016-2017 cohort: 225 of 428 (53%) for 1991-1992, 151 of 217 (70%) for 1997, 170 of 270 (63%) for 2005, and 215 of 293 (73%) for 2016-2017. Follow-up rates were high for the first 3 cohorts but lower in 2016-2017. Gestation, sex, and birth weight z scores were similar across eras. Of those seen at 2 years, the 2016-2017 cohort had the lowest rates of unfavorable sociodemographic variables compared with the cohorts from earlier eras.
Table 1. Characteristics of Participants.
| Characteristic | Era | |||
|---|---|---|---|---|
| 1991-1992 | 1997 | 2005 | 2016-2017 | |
| Live births free of lethal anomalies, No. | 428 | 217 | 270 | 293 |
| Alive at 2 y, No. (% live births free of lethal anomalies) | 225 (53) | 151 (70) | 170 (63) | 215 (74) |
| Perinatal and neonatal characteristics of those alive at 2 y | ||||
| Born in a tertiary center, No. (%) | 206 (92) | 143 (95) | 148 (87) | 186 (87) |
| Multiple birth, No. (%) | 73 (32) | 30 (20) | 37 (22) | 65 (30) |
| Antenatal corticosteroids, No. (%) | 160 (71) | 134 (89) | 145 (85) | 191/214 (89) |
| Antenatal magnesium sulfate, No. (%)a | NA | NA | NA | 147/213 (69) |
| Cesarean delivery, No. (%) | 60 (27) | 83 (55) | 95 (56) | 131 (61) |
| Male, No. (%) | 113 (50) | 82 (54) | 86 (51) | 11 (52) |
| Gestation at birth (completed weeks), mean (SD) | 25.9 (1.1) | 25.6 (1.2) | 25.8 (1.2) | 25.7 (1.2) |
| Birth gestation groups (completed weeks), No. (%), wkb | ||||
| 23 | 5 (2) | 9 (6) | 7 (4) | 11 (5) |
| 24 | 21 (9) | 12 (8) | 22 (13) | 29 (14) |
| 25 | 51 (23) | 41 (27) | 30 (18) | 44 (21) |
| 26 | 71 (32) | 46 (31) | 47 (28) | 58 (27) |
| 27 | 77 (34) | 42 (28) | 63 (37) | 73 (34) |
| Birth weight, mean (SD), g | 891 (176) | 824 (177) | 867 (195) | 848 (188) |
| Birth weight z score, mean (SD) | 0.11 (0.93) | −0.19 (0.93) | −0.01 (0.94) | −0.07 (0.97) |
| Intraventricular hemorrhage, grade 3 or 4, No. (%) | 17 (8) | 5 (3) | 16 (9) | 14 (7) |
| Cystic periventricular leukomalacia, No. (%) | 16 (7) | 5 (3) | 6 (4) | 2 (1) |
| Necrotizing enterocolitis, No. (%)c | 17 (8) | 8 (5) | 18 (11) | 30 (14) |
| Oxygen at 36 weeks’ postmenstrual age, No. (%) | 104 (46) | 66 (44) | 96 (56) | 104 (48) |
| Evaluated at 2 y, No. (%) | 219 (97) | 149 (99) | 163 (96) | 174 (81) |
| Sociodemographic variables at 2 y | ||||
| No. | 219 | 149 | 163 | 174 |
| Maternal age, mean (SD), y | 28.6 (5.8) | 29.8 (5.9) | 30.5 (5.5) | 32.0 (5.7) |
| Lower maternal educational level, No. (%)d | 107/206 (52) | 76/149 (51) | 63/144 (44) | 21/144 (15) |
| Lower socioeconomic status, No. (%)e | 69/206 (33) | 40/136 (29) | 58/162 (36) | 34/162 (21) |
| Family English-speaking only, No. (%) | 166/208 (80) | 112/149 (75) | 124/146 (85) | 116/174 (67) |
Abbreviations: EP, extremely preterm; NA, not applicable.
Data for magnesium sulfate not recorded for the 1991-1992, 1997, and 2005 eras.
Numbers for 22 weeks were fewer than 5 and not reported for deidentification purposes.
Bell stage 2 or greater.
Dichotomized around the median years of schooling for that era.
Categorized as lower (unskilled or unemployed) and higher (semi-skilled, skilled, or professional) based on the primary income earner in the family.
Perinatal characteristics were similar between those assessed at 2 years and those who were not, with few exceptions (eTable 2 in Supplement 1). In the 2005 cohort, 49% (n = 79) of those who were assessed were male, compared with 100% (n = 7) of those who were not. In the 2016-2017 cohort, those who were assessed were less mature and more likely to be born in a tertiary center than those who were not.
2-Year Outcomes
The rate of CP was lowest in the 2016-2017 cohort compared with earlier eras, but evidence for these differences was weak. Rates of blindness and deafness were low in all eras (<3%). There was little evidence for a statistical difference in rates of any developmental delay or any neurodevelopmental disability between 2016-2017 and each of the earlier eras (Table 2; Figure 1). A complete case analysis did not alter any conclusions (eTable 3 in Supplement 1).
Table 2. 2-Year Outcomes of Participants Contrasted Between 2016-2017 and Earlier Eras.
| Neurodevelopmental outcome | 1991-1992 | 1997 | 2005 | 2016-2017 (reference) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic, No. (%) | Adjusted OR (95% CI)a | P value | Characteristic, No. (%) | Adjusted OR (95% CI)a | P value | Characteristic, No. (%) | Adjusted OR (95% CI)a | P value | ||
| No. | 219 | 149 | 163 | 174 | ||||||
| Cerebral palsy | ||||||||||
| Any | 24 (11) | 1.57 (0.71-3.51) | .27 | 18 (12) | 1.79 (0.75-4.27) | .19 | 16 (10) | 1.39 (0.59-3.29) | .45 | 11/172 (6) |
| Mild | 9 (4) | NA | NA | 6 (4) | NA | NA | 5 (3) | NA | NA | 6 (3) |
| Moderate | 6 (3) | NA | NA | 8 (5) | NA | NA | 9 (5) | NA | NA | 3 (2) |
| Severe | 9 (4) | NA | NA | 4 (3) | NA | NA | 2 (1) | NA | NA | 2 (1) |
| Blindness | 5 (2) | 6.40 (0.67-61.4) | .11 | 4 (3) | 4.98 (0.51-48.9) | .17 | 0 | NA | 1 (0.6) | |
| Deafness | 2 (1) | 0.45 (0.05-4.18) | .48 | 2 (1) | 0.56 (0.07-4.53) | .59 | 4 (2) | 1.05 (0.21-5.27) | .95 | 3 (2) |
| Developmental delay | ||||||||||
| Any | 79 (36) | 0.81 (0.50-1.31) | .39 | 68 (46) | 1.03 (0.62-1.72) | .91 | 56 (34) | 0.88 (0.53-1.47) | .63 | 70/170 (41) |
| Mild | 40 (18) | NA | NA | 35 (23) | NA | NA | 44 (27) | NA | NA | 44 (26) |
| Moderate | 27 (12) | NA | NA | 13 (9) | NA | NA | 9 (6) | NA | NA | 18 (11) |
| Severe | 12 (5) | NA | NA | 20 (13) | NA | NA | 3 (2) | NA | NA | 8 (5) |
| Neurodevelopmental disability | ||||||||||
| Any | 88 (40) | 0.95 (0.59-1.53) | .84 | 74 (50) | 1.23 (0.74-2.03) | .43 | 63 (39) | 0.92 (0.55-1.52) | .75 | 70/169 (41) |
| Mild | 43 (20) | NA | NA | 35 (23) | NA | NA | 39 (24) | NA | NA | 44 (26) |
| Moderate | 31 (14) | NA | NA | 18 (12) | NA | NA | 19 (12) | NA | NA | 18 (11) |
| Severe | 14 (6) | NA | NA | 21 (14) | NA | NA | 5 (3) | NA | NA | 8 (5) |
Abbreviations: NA, not applicable; OR, odds ratio.
Odds ratios presented relative to the 2016-2017 cohort adjusted for gestational age at birth, sex, birth weight z score, sociodemographic variables (maternal educational level, English only spoken at home, socioeconomic status). Regression analyses presented following multiple imputations for missing data.
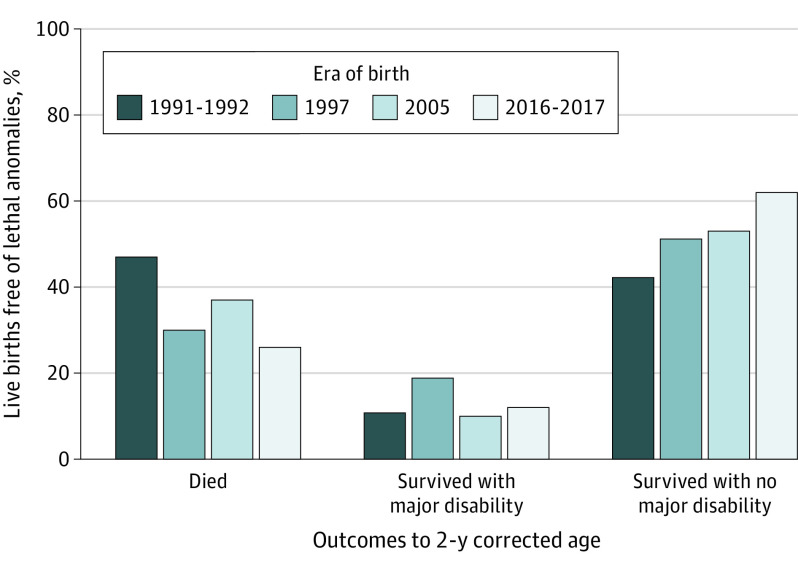
Figure 1. Outcomes to 2 Years’ Corrected Age in Each Cohort.
The annual numbers of children with major neurodevelopmental disability were 22 (20%; 1991-1992), 39 (26%: 1997), 24 (2005), and 26 (15%; 2016-2017). Survival free of major neurodevelopmental disability increased with time, from 42% (1991-1992), 51% (1997), 53% (2005), to 62% (2016-2017) (odds ratio, 1.30 per decade; 95% CI, 1.15-1.48; P < .001) (Figure 1). Survival with major neurodevelopmental disability did not vary significantly with time (odds ratio, 0.94 per decade; 95% CI, 0.77-1.14; P = .50).
Mortality mostly decreased and survival free of major neurodevelopmental disability mostly increased over successive eras across all gestational ages, but the proportions of children with major disability at 2 years were more variable across eras with increasing gestational age (Figure 2; eTable 4 and eTable 5 in Supplement 1).
Figure 2. Outcomes to 2 Years’ Corrected Age by Gestation at Birth in Each Cohort.
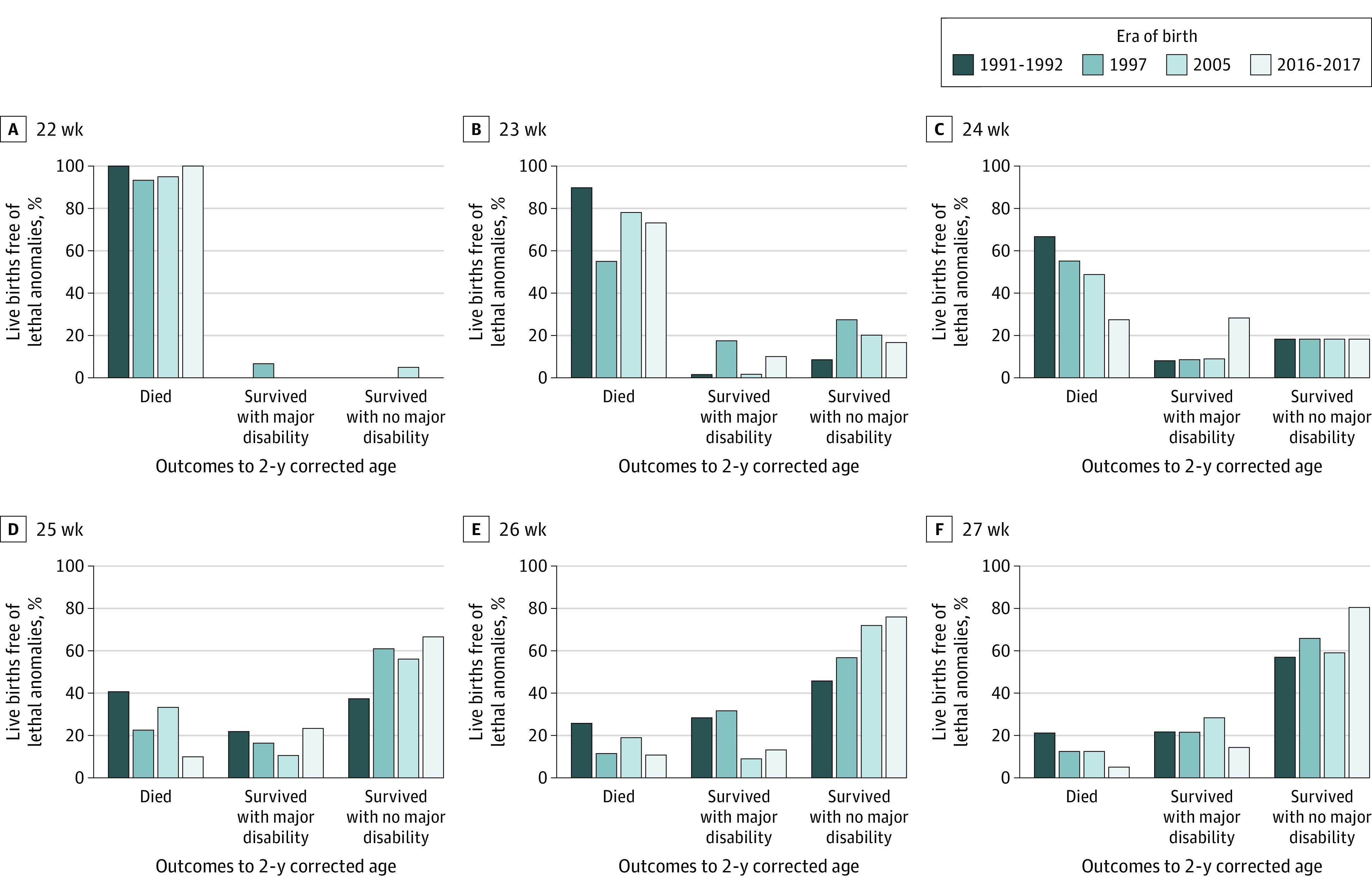
Outcomes are shown for corrected gestational age.
Discussion
The present study provides data suggesting that survival free of major neurodevelopmental disability in infants born EP has improved over 25 years within a discrete geographic population, without a corresponding increase in major neurodevelopmental disability rates at 2 years’ corrected age.
In a previous report from the Victorian Infant Collaborative Study Group, rates of any developmental delay or neurodevelopmental disability at 2 years’ corrected age in EP children were unchanged from the early 1990s to 2005.22 The present study extends these findings by reporting outcomes of a contemporary birth cohort of 2016-2017 births from the same geographic region. There are subtle differences in reported outcomes for both reports. The present study used a revised definition of developmental delay, where the mean of both cognitive and language composite scores was used rather than either the cognitive or language composite score on the Bayley-III Scale.20 Combining both scales was more in line with the Mental Development Index from the first 2 editions of the Bayley Scales used for the 1991-1992 and 1997 cohorts. We also adjusted the present comparisons to reflect the changing sociodemographic status between the cohorts, which was not done previously. The key message nonetheless is that the rates of developmental delay and disability in the latest 2016-2017 cohort were similar to those of previous cohorts, not offsetting any survival advantage that was observed.
The findings from the present Victorian Infant Collaborative Study Group (eTable 6 in Supplement 1) are consistent with reports by the EPICure cohorts in the UK in which outcomes at 2½ to 3 years for infants born at 22 to 25 weeks’ gestation were compared between 1995 and 2006, with imputation for missing data owing to a high proportion of missing values (45%) in the 2006 cohort.3 Although rates of severe disability, which comprised nonambulant CP, deafness not improved with amplification, blindness, or a developmental quotient less than −3 SDs were 18% in 1995 and 19% in 2006, the proportion of infants surviving with no disability increased from 23% to 34% over that period. The EPIPAGE cohorts in France reported improvement in outcomes at 2 years’ corrected age of infants born at 22 to 31 weeks’ gestation; survival without sensory or neuromotor disabilities increased from 74.5% in 1997 to 80.5% in 2011, and CP decreased from a mean of 9.0% to 5.4%.23 Outcomes reported in EPIPAGE were derived from questionnaires completed by the referring physician (for blindness, deafness, and CP) and parents (Ages and Stages Questionnaire for development) rather than independent blinded direct assessments, as in the present study. Neither the EPICure nor EPIPAGE studies reported outcomes dating back to the early 1990s, which spanned the duration of the present study, and neither group has reported outcomes for births as recent as 2016-2017, which our study does.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, comprising 11 academic centers in the US, reported improvement in outcomes at 18 to 22 months of infants born at 22 to 24 completed weeks of gestational age over 3 consecutive eras over a narrower time period than the present study (2000-2003, 2004-2007, and 2008-2011).4 Survival without neurodevelopmental impairment increased from 16% (95% CI, 14%-18%) in 2000-2003 to 20% (95% CI, 18%-23%) in 2008-2011. Our data for the same gestational age range showed a larger increase in survival without major disability, but over a longer period (eTable 6 in Supplement 1). Reasons for the different rates reported in our study and those reported elsewhere include the different gestational age inclusion criteria, geographic vs neonatal network hospital populations, different sociodemographic characteristics, and possibly different care practices between countries.
The lower rate of CP in the 2016-2017 cohort than in the previous 3 cohorts seen in the present study mirrors changes worldwide.24,25,26 Several factors may have contributed to this observation. Magnesium sulfate, which has been shown to reduce CP,10 was used widely for fetal neuroprotection in the latest cohort. Within the EP 2016-2017 cohort, children whose mothers received magnesium sulfate had a lower rate of CP compared with those whose mothers did not (4% vs 13%).27 The rate of cystic periventricular leukomalacia, a well-established risk factor for CP,28 was lowest in the 2016-2017 cohort compared with earlier cohorts.
The rate of blindness remained low in the present study, despite higher oxygen saturation targeting, a longer duration of supplemental oxygen, and a higher rate of treated retinopathy of prematurity in the 2016-2017 cohort compared with earlier cohorts.2 Higher oxygen saturation targeting was shown in a large prospective individual participant data meta-analysis to be associated with a lower risk of death but a higher rate of retinopathy of prematurity, without an increase in blindness.29 Although the meta-analysis was published after the recruitment of the 2016-2017 cohort for our study, the practice of higher oxygen saturation targeting had been introduced in Victoria before 2016 following outcomes of the individual international randomized clinical trials, one of which involved Australia, that were included in the overall meta-analysis.30,31 However, central visual impairment is the commonest cause of visual loss following EP birth.
Comparing outcomes across different eras has inherent challenges of changes in sociodemographics and different developmental assessment tools. The sociodemographic characteristics in the EP cohort were more favorable in the latest cohort compared with previous eras, with decreased rates of lower maternal educational level and social class, owing in part to a governmental push to increase educational levels across society between the 1990-1991 and 2016-2017 eras. We accounted for this difference in sociodemographic characteristics across the eras by weighting the mean for the controls in each era to reflect the distribution of social variables of the corresponding EP cohort. To overcome the different versions of the Bayley Scales and for the bias associated with underestimation of developmental delay related to the Bayley-III Scale,32 we computed means and SDs relative to contemporaneous controls.
Strengths and Limitations
Strengths of the present study include the geographic cohorts comprising all EP births in the state, which reduces selection biases associated with hospital or network cohorts. We had contemporaneous term-born controls with which to standardize our results, and we adjusted the results to reflect the changes in sociodemographic status over the different eras. This adjustment enabled us to overcome issues such as reliance on published normative data, which may not reflect our population and may underestimate developmental delay.32 The follow-up assessments were conducted face-to-face by trained assessors blinded to clinical history, which reduces bias associated with parent- or clinician-reported developmental questionnaires. Our cohorts span the 25 years since the introduction of surfactant and other key innovations in perinatal and neonatal intensive care.
Limitations of the study include the lower follow-up rate in the 2016-2017 cohort that may have underestimated the magnitude of differences between eras.33 We performed multiple imputations to account for missing data in the analysis of the neurodevelopmental outcomes and conducted a complete case analysis as a sensitivity analysis, which resulted in similar conclusions. Our follow-up for the 2016-2017 cohort has been to 2 years of age, which precludes reliable assessment of other important outcomes, such as IQ, attention, executive function, and academic achievement. We know from experience that favorable outcomes at 2 years in the 2005 cohort compared with the 1991-1992 and 1997 eras did not translate to similar conclusions when the cohorts were reassessed at 8 years.6,7,8
Conclusions
The findings of this study suggest that survival free of major disability at age 2 years for EP infants has steadily improved since the early 1990s, which may reflect innovations introduced to clinical care. Major disability rates were static across eras. Because development in early childhood does not reliably estimate later development,34,35,36 it is important to evaluate these cohorts at older ages to determine whether a similar pattern of development persists into school-aged years and beyond.
eTable 1. Characteristics of Term Controls in Each of the 4 Eras
eTable 2. Comparisons Between Participants Born EP Who Were Assessed at 2 Years and Those Who Were Not
eTable 3. Two-Year Outcomes of Participants Born EP Contrasted Between 2016-17 and Each of the Earlier Eras (Complete Case Analysis)
eTable 4. Two-Year Outcomes of Participants Born EP by Gestational Age at Birth
eTable 5. Live Births Free of Lethal Anomalies in Each Era by Gestation at Birth
eTable 6. Supplementary Data for International Comparisons
Nonauthor Collaborators. The Victorian Infant Collaborative Study Group
References
- 1.Doyle LW; Victorian Infant Collaborative Study Group . Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: I— effectiveness. Pediatrics. 2004;113(3, pt 1):505-509. doi: 10.1542/peds.113.3.505 [DOI] [PubMed] [Google Scholar]
- 2.Cheong JLY, Olsen JE, Huang L, et al. ; Members of the Victorian Infant Collaborative Study Group . Changing consumption of resources for respiratory support and short-term outcomes in four consecutive geographical cohorts of infants born extremely preterm over 25 years since the early 1990s. BMJ Open. 2020;10(9):e037507. doi: 10.1136/bmjopen-2020-037507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younge N, Goldstein RF, Bann CM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376(7):617-628. doi: 10.1056/NEJMoa1605566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchen WH, Richards A, Ryan MM, et al. A longitudinal study of very low-birthweight infants; II—results of controlled trial of intensive care and incidence of handicaps. Dev Med Child Neurol. 1979;21(5):582-589. doi: 10.1111/j.1469-8749.1979.tb01673.x [DOI] [PubMed] [Google Scholar]
- 6.Cheong JLY, Anderson PJ, Burnett AC, et al. ; Victorian Infant Collaborative Study Group . Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics. 2017;139(6):e20164086. doi: 10.1542/peds.2016-4086 [DOI] [PubMed] [Google Scholar]
- 7.Burnett AC, Anderson PJ, Lee KJ, Roberts G, Doyle LW, Cheong JLY; Victorian Infant Collaborative Study Group . Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics. 2018;141(1):e20171958. doi: 10.1542/peds.2017-1958 [DOI] [PubMed] [Google Scholar]
- 8.Spittle AJ, Cameron K, Doyle LW, Cheong JL; Victorian Infant Collaborative Study Group . Motor impairment trends in extremely preterm children: 1991-2005. Pediatrics. 2018;141(4):e20173410. doi: 10.1542/peds.2017-3410 [DOI] [PubMed] [Google Scholar]
- 9.Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet. 2017;389(10079):1649-1659. doi: 10.1016/S0140-6736(17)30312-4 [DOI] [PubMed] [Google Scholar]
- 10.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1):CD004661. doi: 10.1002/14651858.CD004661.pub3 [DOI] [PubMed] [Google Scholar]
- 11.Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;(2):CD000144. doi: 10.1002/14651858.CD000144 [DOI] [PubMed] [Google Scholar]
- 12.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. doi: 10.1002/14651858.CD004454.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle LW, Gultom E, Chuang SL, James M, Davis P, Bowman E. Changing mortality and causes of death in infants 23-27 weeks’ gestational age. J Paediatr Child Health. 1999;35(3):255-259. doi: 10.1046/j.1440-1754.1999.00349.x [DOI] [PubMed] [Google Scholar]
- 14.Australian Institute of Health and Welfare . Australia’s Mothers and Babies 2018: In Brief— Perinatal Statistics Series No. 36. Cat. No. PER 108. Australian Institute of Health & Welfare ; 2020. [Google Scholar]
- 15.Cole TJ, Williams AF, Wright CM; RCPCH Growth Chart Expert Group . Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol. 2011;38(1):7-11. doi: 10.3109/03014460.2011.544139 [DOI] [PubMed] [Google Scholar]
- 16.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development. Psychological Corp; 1969. [Google Scholar]
- 18.Bayley N. Bayley Scales of Infant Development—Revised. Psychological Corp; 1993. [Google Scholar]
- 19.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. Psychological Corp; 2005. [Google Scholar]
- 20.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75(5):670-674. doi: 10.1038/pr.2014.10 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Multiple Imputation for Non Response in Surveys. Wiley; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 22.Doyle LW, Roberts G, Anderson PJ; Victorian Infant Collaborative Study Group . Outcomes at age 2 years of infants <28 weeks’ gestational age born in Victoria in 2005. J Pediatr. 2010;156(1):49-53.e1. doi: 10.1016/j.jpeds.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Pierrat V, Marchand-Martin L, Arnaud C, et al. ; EPIPAGE-2 writing group . Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448. doi: 10.1136/bmj.j3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galea C, Mcintyre S, Smithers-Sheedy H, et al. ; Australian Cerebral Palsy Register Group . Cerebral palsy trends in Australia (1995-2009): a population-based observational study. Dev Med Child Neurol. 2019;61(2):186-193. doi: 10.1111/dmcn.14011 [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Kinsman SL, Jenkins DD, Hovell MF, Ryan RM. Decreasing prevalence of cerebral palsy in birth cohorts in South Carolina using Medicaid, disability service, and hospital discharge data, 1996 to 2009. Dev Med Child Neurol. 2019;61(5):593-600. doi: 10.1111/dmcn.14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sellier E, Platt MJ, Andersen GL, Krägeloh-Mann I, De La Cruz J, Cans C; Surveillance of Cerebral Palsy Network . Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol. 2016;58(1):85-92. doi: 10.1111/dmcn.12865 [DOI] [PubMed] [Google Scholar]
- 27.Doyle LW, Spittle AJ, Olsen JE, et al. ; Victorian Infant Collaborative Study Group . Translating antenatal magnesium sulphate neuroprotection for infants born <28 weeks’ gestation into practice: a geographical cohort study. Aust N Z J Obstet Gynaecol. 2021. doi: 10.1111/ajo.13301 [DOI] [PubMed] [Google Scholar]
- 28.Volpe JJ, Inder TE, Darras BT, et al. , eds. Volpe’s Neurology of the Newborn. 6th ed.Elsevier; 2018. [Google Scholar]
- 29.Askie LM, Darlow BA, Finer N, et al. ; Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration . Association between oxygen saturation targeting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 2018;319(21):2190-2201. doi: 10.1001/jama.2018.5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarnow-Mordi W, Stenson B, Kirby A, et al. ; BOOST-II Australia and United Kingdom Collaborative Groups . Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med. 2016;374(8):749-760. doi: 10.1056/NEJMoa1514212 [DOI] [PubMed] [Google Scholar]
- 31.Carlo WA, Finer NN, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959-1969. doi: 10.1056/NEJMoa0911781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW; Victorian Infant Collaborative Group . Underestimation of developmental delay by the new Bayley-III scale. Arch Pediatr Adolesc Med. 2010;164(4):352-356. doi: 10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 33.Doyle LW, Anderson PJ, Burnett A, et al. ; Victorian Infant Collaborative Study (VICS) Group . Developmental disability at school age and difficulty obtaining follow-up data. Pediatrics. 2018;141(2):e20173102. doi: 10.1542/peds.2017-3102 [DOI] [PubMed] [Google Scholar]
- 34.Spittle AJ, Spencer-Smith MM, Eeles AL, et al. Does the Bayley-III Motor Scale at 2 years predict motor outcome at 4 years in very preterm children? Dev Med Child Neurol. 2013;55(5):448-452. doi: 10.1111/dmcn.12049 [DOI] [PubMed] [Google Scholar]
- 35.Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III Cognitive and Language scales in preterm children. Pediatrics. 2015;135(5):e1258-e1265. doi: 10.1542/peds.2014-3039 [DOI] [PubMed] [Google Scholar]
- 36.Roberts G, Anderson PJ, Doyle LW; Victorian Infant Collaborative Study Group . The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child. 2010;95(10):786-790. doi: 10.1136/adc.2009.160283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Term Controls in Each of the 4 Eras
eTable 2. Comparisons Between Participants Born EP Who Were Assessed at 2 Years and Those Who Were Not
eTable 3. Two-Year Outcomes of Participants Born EP Contrasted Between 2016-17 and Each of the Earlier Eras (Complete Case Analysis)
eTable 4. Two-Year Outcomes of Participants Born EP by Gestational Age at Birth
eTable 5. Live Births Free of Lethal Anomalies in Each Era by Gestation at Birth
eTable 6. Supplementary Data for International Comparisons
Nonauthor Collaborators. The Victorian Infant Collaborative Study Group