Abstract
Background
Recent studies conducted in several OECD countries have shown that chronic exposure to elevated levels of air pollutants (especially PM2.5, PM10 and NOx), might negatively impact COVID-19 morbidity and mortality rates. The aim of this study was to examine the association between chronic exposure to air pollution in Israeli cities and towns, their demographic and socioeconomic status, and COVID-19 morbidity, during the three local morbidity waves.
Methods
We examined the associations between: (a) annual average concentrations of NOx, CO, PM10, PM2.5 and SO2 in 2016–2019, and demographic and socioeconomic parameters, and (b) COVID-19 positive cases in 279 Israeli cities and towns, in the four state-wide morbidity peaks: 1st wave peak: March 31st, 2020; 2nd wave peaks: July 24th and September 27th, 2020, and the 3rd wave peak: January 17th, 2021, which occurred after the beginning of the nationwide vaccination campaign. These associations were calculated using both Spearman correlations and multivariate linear regressions.
Results
We found statistically significant positive correlations between the concentrations of most pollutants in 2016–19 and COVID-19 morbidity rate at the first three timepoints but not the 4th (January 17th, 2021). Population density and city/town total population were also positively associated with the COVID-19 morbidity rates at these three timepoints, but not the 4th, in which socioeconomic parameters were more dominant - we found a statistically significant negative correlation between socioeconomic cluster and COVID-19 morbidity. In addition, all multivariate models including PM2.5 concentrations were statistically significant, and PM2.5 concentrations were positively associated with the COVID-19 morbidity rates in all models.
Conclusions
We found a nationwide association between population chronic exposure to five main air pollutants in Israeli cities and towns, and COVID-19 morbidity rates during two of the three morbidity waves experienced in Israel. The widespread morbidity that was related to socioeconomic factors during the 3rd wave, emphasizes the need for special attention to morbidity prevention in socioeconomically vulnerable populations and especially in large household communities. Nevertheless, this ecological study has several limitations, such as the inability to draw conclusions about causality or mechanisms of action. The growing body of evidence, regarding association between exacerbated COVID-19 morbidity and mortality rates and long-term chronic exposure to elevated concentrations of air pollutants should serve as a wake-up call to policy makers regarding the urgent need to reduce air pollution and its harmful effects.
Keywords: COVID-19, PM2.5, PM10, NOx, Environmental exposure, Population density, Socioeconomic-cluster, Vaccination campaign
Abbreviations: World Health Organization, (WHO); Particulate matter, (PM); Fine PM with diameter less than 2.5 μm, (PM2.5); Fine PM with diameter less than 10 μm, (PM10); Nitrogen oxides, (NOx); Sulfur dioxide, (SO2); The novel coronavirus disease, (COVID-19); The novel coronavirus, (SARS-CoV-2); Israel Central Bureau of Statistics, (CBS); Greenhouse gases, (GHGs)
1. Introduction
Worldwide exposure to ambient air pollution is related to 4.2 million premature deaths per year (World Health Organization, 2020) and is associated with a variety of adverse health outcomes, such as respiratory and cardiovascular morbidity and premature mortality.
Ambient air pollutants such as PM with diameter less than 10 μm (PM10) and ambient fine PM with diameter less than 2.5 μm (PM2.5), nitrogen oxides (NOx), sulfur dioxide (SO2), and carbon monoxide (CO) are associated with adverse health outcomes. These effects include respiratory diseases such as asthma, chronic obstructive pulmonary disease, pneumonia, and cardiovascular morbidities such as enhanced thrombosis, elevated arterial blood pressure, and enhanced atherosclerosis (Khafaie et al., 2016; World Health Organization, 2006; World Health Organization, 2013).
Furthermore, exposure to air pollution is associated with increased oxidative stress and can increase human sensitivity to respiratory pathogens, which might be an additional factor influencing COVID-19 morbidity and mortality (Mudway et al., 2020). Several studies have shown that exposure to ambient air pollution (such as PM, NOx, SO2 and O3) can create abnormalities in the cilia structure and affect its proper function in the human respiratory tract. In some cases, the cilia might become shorter or even missing, reducing mucociliary clearance ability, hence, reducing the removal of inhaled particles and pathogens from the respiratory tract. These negative impacts on the respiratory system's first line of defense may increase sensitivity to respiratory pathogens such as bacteria and viruses in people who are chronically exposed to air pollution (Blomberg et al., 1999; Houtmeyers et al., 1999; Cao et al., 2020).
Several recent studies conducted in the US, in northern Italy, in the Netherlands, in China and in London (England) have showed that chronic exposure to high levels of air pollutants (in particular PM2.5, PM10 and NOx) might be an additional factor for the recorded elevated morbidity and mortality rates caused in these regions by the novel coronavirus (SARS-CoV-2) (Cole et al., 2020; Conticini et al., 2020; Sasidharan et al., 2020; Tian et al., 2020; Wu et al., 2020; Zoran et al., 2020; De Angelis et al., 2021). A rise of 1 μg/m3 in ambient PM2.5 was associated with an increase of 8% in COVID-19 death rates in the US, while in the Netherlands the same increase in ambient PM2.5 concentrations in 355 municipalities was associated with 9.4 additional COVID-19 cases, 3 more hospital admissions, and 2.3 more deaths per municipality (Cole et al., 2020; Wu et al., 2020). In another study, we recently analyzed air pollution and COVID-19 morbidity and mortality data from 36 OECD countries. We found that long-term exposure to air pollutants such as PM2.5 and NOx at concentrations exceeding WHO guidelines, might exacerbate morbidity and mortality rates from COVID-19 (Barnett-Itzhaki and Levi, 2021).
Recently, it was reported that the entire Israeli population is exposed to PM2.5 concentrations that exceed local target values according to Israel's Clean Air Law and the WHO annual mean (10 μg/m3), with an annual average of 17.8 μg/m3 (Levy et al., 2020). Furthermore, in a study that modeled and estimated the link between PM2.5 exposure in Israel and the risk for respiratory and cardiovascular morbidities, type 2 diabetes in adults and low birth weight, it was estimated that in 2015 PM2.5 exposure was the cause of 1,908 premature deaths (Ginsberg et al., 2016).
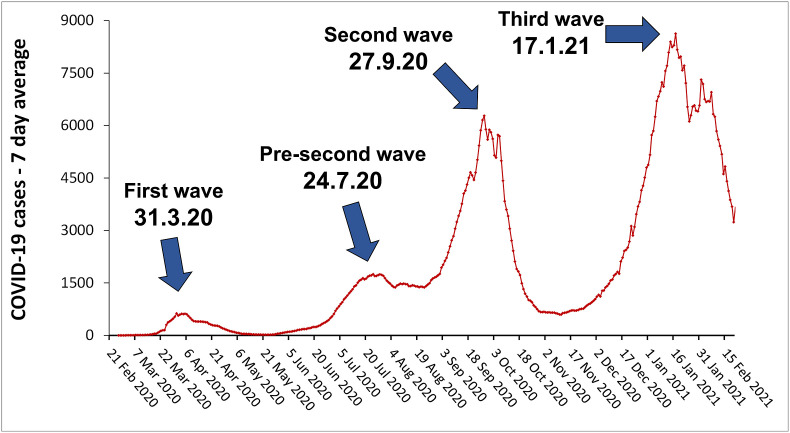
The first COVID-19 cases were detected in Israel at the end of February 2020. Since then, a first wave of COVID-19 outbreak peaked at the end of March, with up to 624 new cases per day (7-day average) (Fig. 1 ) and a total amount of 9,800 active cases. At the end of May, following the first lockdown (from Mid-March until the third week of April), the number of active cases dropped to about 1,900 and limitations on public gathering and movement and on economic and commercial activity were completely removed. Then, again the number of active cases started to rise during June and July with up to 1,746 new cases per day (7-day average) and about 36,300 active cases by the end of July (a pre-second wave). Following local lockdowns and limitations introduced in June and July in COVID-19 highly infected neighborhoods and towns, numbers went down to about 20,300 active cases at the end of August. Despite this brief hiatus, the second major COVID-19 wave kept gaining momentum reaching about 50,000 active cases on September 19th, and 72,400 active cases on October 1st, with up to 6,276 new cases per day (7-day average) in this period. COVID-19 morbidity then decreased to under 7,800 active cases on November 13th, due to a second statewide lockdown. At that point, some of the limitations on public gathering and movement were gradually removed and a moderate rise in morbidity rate began, soon becoming a fast-rising trend, forming the third COVID-19 wave. From the beginning of December 2020 to mid-January 2021, the number of active cases went up from 10,000 to over 83,600 with up to 8,624 new cases per day (7-day average) (Fig. 1). A third complete lockdown was issued after the first week of January and until the end of the month, resulting in a moderate decline of morbidity rate by the end of January followed by a steep decline after the first week of February (Worldometersinfo coronavirus-country-Israel, 2021). Of note, in a nationwide vaccination campaign which began on December 20th, 3 million Israelis were vaccinated with Pfizer's first dose and 1.7 million with the second dose by the end of January 2021 (prioritized by age and other COVID-19 high-risk populations) (COVID-19 data dashboard-health, 2021).
Fig. 1.
Daily new confirmed COVID-19 cases in Israel presented as 7-day average. Source: European CDC - situation update worldwide –last update Feb 20th. (Roser et al., 2020).
While social distancing has been recognized as one of the most effective measures of spread attenuation, household overcrowding in the UK and the US was recently linked to a greater risk for COVID-19 morbidity (Raisi-Estabragh et al., 2020; Wadhera et al., 2020). Moreover, additional demographic and socioeconomic factors were recently shown to have an impact on COVID-19 morbidity and mortality. These include population size and density (De Angelis et al., 2021), higher risks for immigrant communities (Clark et al., 2020) and for African American communities in the US, due to widespread shared accommodations of the youth and elderly family members (Doumas et al., 2020). Similarly, in Israel, ultra-orthodox Jews and some Israeli Arab communities tend to live in crowded households. Additionally, in some of the Arab communities the youth and the elderly tend to be in close contact and live in the same house or compound.
The aim of this study was to examine the association between population chronic exposure to air pollution in Israeli cities and towns, their populations’ demographic and socioeconomic status, and COVID-19 morbidity. We examined the association between the annual average concentrations of five air pollutants: NOx, CO, PM10, PM2.5 and SO2 in 2016–2019 and COVID-19 positive cases in Israeli cities and towns at the state-wide peak of each of the three morbidity waves (March 31st, September 27th −2020 and January 17th− 2021) and in the pre-second mini-wave on July 24th−2020 (Fig. 1).
We hypothesized that populations living in cities and towns chronically suffering from higher air pollution would have higher morbidity rates compared with populations from municipalities with relatively low air pollution. In addition, we hypothesized that high density and low socioeconomic status would also contribute to morbidity rates.
2. Methods
2.1. Data collection
Demographic information per Israeli city/town was collected from the Israeli Central Bureau of Statistics (CBS) website (CBS, 2019a): city/town's total population (in 2019), natural growth per 100,000 capita (2016), density - population per km2 (2016). Socioeconomic cluster per Israeli city/town was also collected from the Israeli CBS website (CBS, 2017a) and updated to 2019. Data on infant mortality rate per thousand live births (2012–2016), and percentage of 12th grade students entitled to a matriculation certificate (2015–2016) were collected from the Israeli Ministry of Health website (Community health map 2016).
Data on COVID-19 morbidity rates during 2020–2021 four state-wide morbidity peaks (2020: March 31st, July 24th, September 27th and 2021: January 17th) in 279 Israeli cities, towns, villages etc., with a total population of 9,070,289 out of 9,136,000 Israeli citizens in 2019 (~99%), were collected from Israel's government COVID-19 dataset website (DataGov, 2021). To reduce the impact of delays in COVID-19 test results (especially on the weekends), the four national morbidity peaks were determined via a 7-day average of COVID-19 daily new confirmed cases (Fig. 1). Of note, at those four timepoints most of the large cities in Israel, including the bulk of the population, experienced local morbidity peaks. For each city/town, at each of these timepoints, we calculated the number of daily verified cases by subtracting the cumulative values of the previous day from the cumulative values on the specific date. Then, we calculated the total COVID-19 positive cases ratio by dividing the COVID-19 positive cases detected in each city/town at that time point, by its total population.
Population exposures to air pollutants were estimated by a hybrid model provided by the Israeli Ministry of Environmental Protection's (IMoEP). The hybrid model is based on the annual averages of the CHIMERE (Monteiro et al., 2007), which are merged with the annual mean of air pollutants measured by the air quality monitoring network measurements. CHIMERE is a multi-scale photochemical and transport model run by the IMoEP on a daily basis as a forecast model and provides hourly forecasts at a spatial resolution of 3 km over the entire area of the state of Israel. The hourly forecasts were averaged separately for each pollutant (NOx, CO, PM10, PM2.5 and SO2) and each year (2016, 2017, 2018, 2019), and residual correction was applied to each of the resulting 20 concentration maps. For further details on the hybrid model see Levy et al. (2020). The pollutant population weighted mean per city/town was calculated by extracting the pollutant's average concentrations in the statistical areas attributed to the city/town in each year (Monteiro et al., 2007; Levi et al., 2020).
Pollutant concentrations per city/town in the studied years (2016–2019) were defined as the pollutant's average concentrations in the statistical areas attributed to the city/town in each specific year.
2.2. Statistical analyses
We calculated Spearman correlations between COVID-19 rates (morbidity per population ratios) and the demographic, socioeconomic, and environmental data. Spearman correlations were used to find associations that are not necessarily linear.
To better understand the associations between the exposure to air pollutants on COVID-19 morbidity, we compared the pollutant concentrations in cities/towns with low COVID-19 morbidity rates to those concentrations in cities/towns with high COVID-19 morbidity rates. This was done by calculating the tertiles of the rates of confirmed cases on each date (2020: March 31st, July 24th, September 27th and 2021: January 17th), and dividing the cities/towns into: “red” cities – cities in the upper tertile, and “green” cities – cities in the lower tertile. We used the nonparametric Wilcoxon test to compare the pollutant concentrations between these two groups of cities/towns.
Multivariate linear regressions were used to examine the associations between demographic, socioeconomic and air pollution features, and the rate of COVID-19 positive cases in the aforementioned dates. Every regression model included: the city/town's density (people per km2), total population, percentage of matriculation certificate eligibility among 12th grade students, and socioeconomic cluster. Every model also included the average concentration (2016–2019) of one of the three air pollutants: NOx, PM10, and PM2.5. We used the logarithm value of the numbers of cases per population in the multivariate linear regression. For each model we calculated its statistical significance, its root mean square error (RMSE), and the coefficient and p-value of the air pollution parameter.
All statistical analyses were performed using Matlab© version R2019b.
3. Results and discussion
The aim of this study was to examine the association between population chronic exposure to air pollution (during 2016–2019), demographic and socioeconomic factors, and COVID-19 morbidity during the three major waves of morbidity (including four morbidity peaks) experienced in Israel during 2020 and the first quarter of 2021 COVID-19 pandemic.
3.1. Air pollutant concentrations in Israel during 2016–2019
We focused on five air pollutants: NOx, CO, PM10, PM2.5 and SO2 and calculated their annual average concentrations in 2016, 2017, 2018 and 2019 (see supplementary figure S2A-E). We found a significant trend of reduction in SO2 concentrations in these years (from 1.33 μg/m3 in 2016 to 0.67 μg/m3 in 2019) (see supplementary figure S2E). This decline can be attributed to a national policy of gradually switching from coal dominated electricity production, to natural gas as the main fuel in Israeli power plants. While in 2012 the coal share was 34.2% and the natural gas share was only 8.2% of Israel's primary energy mix, in 2019 the coal share dropped to 18.6% and the natural gas rose to 34.4% (Bdal et al., 2019). This change is attributed to a significant reduction in specific air pollutants emitted by coal power plants, and in particular SO2 and NOx (Venkatesh et al., 2012). Of note, this reduction was later linked to a statistically significant reductions in cardiovascular mortality in three Israeli major cities (Yinon and Thurston, 2017).
NOx and CO showed a mild reduction during 2016–2019, while no such trend was found for PM10 and PM2.5. (see Supplementary Figs. S2A–D). Although the sharp reduction in coal usage was expected to generate a significant NOx decline, NOx levels only moderately declined during this period, probably due an increase in emissions from the transportation sector. Indeed, between 2016 and 2019 there was an annual 3–4.8% rise in the number of registered motor vehicles in Israel (CBS, 2019b).
Concentrations of each of the air pollutants in Israeli cities and towns were positively correlated (p < 0.0001) between the studied years (2016–2019).
3.2. Association between air pollutant concentrations and rates of positive cases
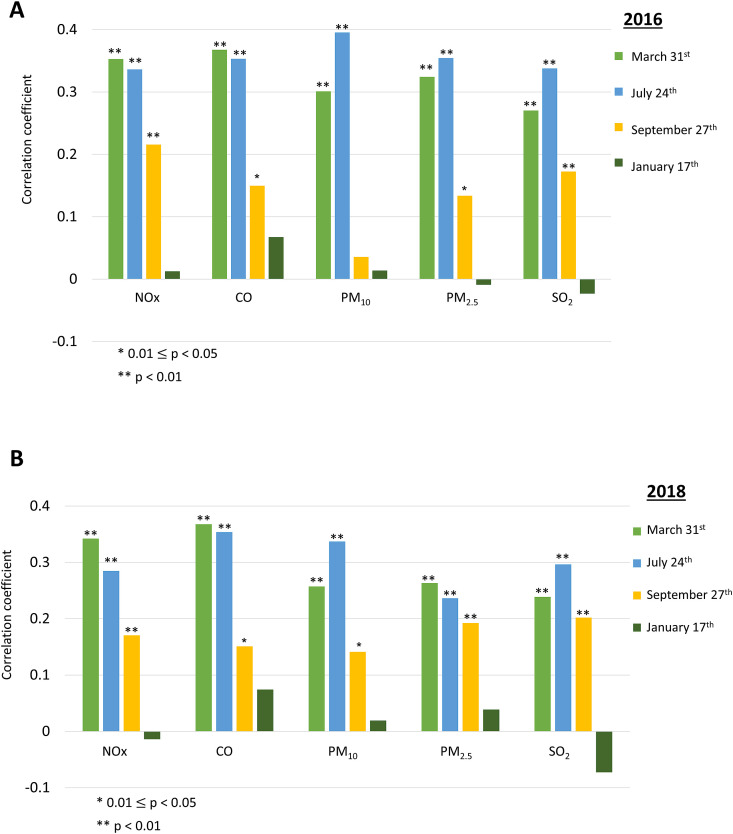
We calculated Spearman correlations between pollutant concentrations in 2016–2019, and the rates of COVID-19 positive cases of the city/town population (“COVID-19 rate”). We found statistically significant positive correlations between all pollutants in 2016 and COVID-19 rate on March 31st and on July 24th (p < 0.01). COVID-19 rates on September 27th were statistically significantly positively correlated with the concentrations of NOx (p < 0.01), CO (p = 0.014), PM2.5 (p = 0.03) and SO2 (p < 0.01). Of note, no statistically significant correlation was found between the air pollutant concentrations and COVID-19 rates on January 17th. (Fig. 2A ).
Fig. 2.
Spearman correlations between air pollution concentrations parameters and rates of COVID-19 positive cases in the four selected timepoints (2020: March 31st, July 24th, September 27th and 2021: January 17th) in Israeli cities and towns in (A) 2016 and (B) 2018.
We found statistically significant positive correlations between all pollutants in 2018 and COVID-19 rate on March 31st and July 24th (p < 0.01). COVID-19 rates on September 27th were statistically significantly positively correlated with the concentrations of NOx (p < 0.01), CO (p = 0.013), PM2.5 (p < 0.01), PM10 (p = 0.021) and SO2 (p < 0.01). Again, no statistically significant correlation was found between air pollutant concentrations and COVID-19 rates on January 17th. (Fig. 2B).
Additionally, we found similar associations between COVID-19 rates on these timepoints and air pollutant concentrations in 2017 and 2019 (Supplementary figures S1A and S1B, respectively).
Our findings that long-term exposure to air pollutants (NOx, CO, PM10, PM2.5 and SO2) was associated with COVID-19 morbidity rates during Israel's COVID-19 first wave, pre-second wave and second wave (March 31st, July 24th and September 27th, respectively) (Fig. 2A and B) are consistent with findings of previous recent studies, especially for NOx, PM10 and PM2.5. Studies conducted in the US, in northern Italy, in the Netherlands, in China and in London (England), showed that chronic exposure to high levels of these air pollutants might be an additional factor for COVID-19 higher morbidity rates (Cole et al., 2020; Conticini et al., 2020; Sasidharan et al., 2020; Tian et al., 2020; Wu et al., 2020; Zoran et al., 2020; De Angelis et al., 2021). Furthermore, in our recently published study, we revealed statistically significant multistate associations between the concentrations of PM2.5 and NOx in 36 OECD countries, to morbidity and mortality from COVID-19. We concluded that concentrations of those pollutants exceeding WHO guidelines, might exacerbate COVID-19 morbidity and mortality (Barnett-Itzhaki and Levi, 2021).
In addition to the well-known negative health outcomes of chronic exposure to PM (WHO, 2013; WHO, 2020), several recent studies suggested that PM aerosols can also serve as a vector for transporting bacteria and viruses through airborne diffusion, allowing them to penetrate deeply into the lungs and surpass the multilayer barriers of the respiratory system, thus enhancing SARS-CoV-2 virus transmission mechanism (Setti et al., 2020; Zoran et al., 2020).
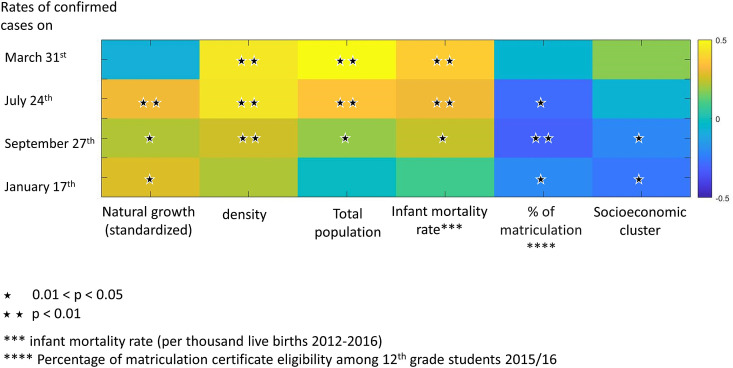
As opposed to the first and the second waves, COVID-19 morbidity rates in the third wave (January 17th 2021) were not statistically significantly correlated with the concentrations of air pollutants in 2016–2019 (Fig. 2A and B and Supplementary Figs. S1A and S1B). At that time point COVID-19 morbidity rate was associated with several socioeconomic factors (Fig. 3 ).
Fig. 3.
Spearman correlations between COVID-19 morbidity (rates of positive cases) in Israeli cities and towns and demographic and socioeconomic parameters, on the four selected timepoints (2020: March 31st, July 24th, September 27th and 2021: January 17th). Stars indicate statistically significant correlations (p < 0.05).
3.3. Association between demographic and socioeconomic parameters and the rates of positive cases
We calculated Spearman correlations between demographic parameters and the rates of COVID-19 positive cases in the local population. Standardized natural growth was statistically significantly positively correlated with COVID-19 rate in July, September, and January (R = 0.31, p < 0.001; R = 0.22, p = 0.01; R = 0.28, p = 0.002, respectively). Density (population/km2) was statistically significantly positively correlated with COVID-19 rate in March, July, and September (R = 0.45, p < 0.001; R = 0.44, p < 0.001; R = 0.26, p = 0.004, respectively), and so were total population and the infant mortality rate (population: March: R = 0.5, p < 0.001; July: R = 0.36, p < 0.001; September: R = 0.19, p = 0.04; infant mortality rate: March: R = 0.38, p < 0.001; July: R = 0.31, p < 0.001; September: R = 0.21, p = 0.007). However, socioeconomic clusters and percentage of matriculation certificate eligibility among 12th grade students were statistically significantly negatively correlated with COVID-19 rates (higher rates of morbidity in cities/towns with lower socioeconomic status and lower matriculation certificate), in September and in January (matriculation certificate: R = −0.31, p < 0.001 in September, R = −0.2, p = 0.002 in January; socioeconomic cluster: R = −0.21, p = 0.02 in September, R = −0.26, p = 0.003 in January). Matriculation certificate eligibility was also statistically significantly negatively correlated with COVID-19 rates in July (R = −0.28, p = 0.0015), (Fig. 3).
It appears that the COVID-19 morbidity dynamic in the third wave (Jan 17th 2021), which was characterized by wide-scale and wide-spread morbidity, was considerably less influenced by past chronic exposure to air pollution and was more associated with demographic and socioeconomic factors such as socioeconomic cluster and the percentage of matriculation certificate eligibility among 12th grade students. (Fig. 3). In fact, the third COVID-19 wave was so pervasive, that high morbidity rates were also detected in previously low morbidity cities and towns and in areas with low air pollution. This will be further discussed later on.
Population density and city/town total population, which were positively associated with the rates of COVID-19 positive cases in the first and the second morbidity waves (during March and July to September 2020), did not significantly affect morbidity rates in the third most massive wave (Jan 17th 2021). This could be attributed to the national extensive morbidity and the initial movement restriction followed by the third complete lockdown initiated in the beginning of January. The impact of density and total population at that time was probably reduced and replaced by inhouse infection chains, in which family members in large households or households where the youth and the elderly tend to share accommodation (multigeneration households) infected each other. Such household properties characterize cities and towns in the low socioeconomic clusters.
This assumption is strengthened by the positively significant correlation observed on January 17th between COVID-19 morbidity and the cities' and towns' natural growth per 100,000 people, and by the observed variability in morbidity properties between similar density cities and towns due to different population characteristics (such as socioeconomic and religious community characteristics). For example, the ultra-orthodox city of Bnei-Brak (socioeconomic cluster 2 - extremely low) with its 205,000 citizens, has a relatively similar high population density to the secular city of Givatayim (socioeconomic cluster 9 - extremely high) with its 61,000 citizens (density of 27,800 and 18,700 citizens per km2, respectively) (CBS, 2017a; CBS, 2019a). However, while Bnei-Brak had 785, 6,372, 17,263 and 35,928 cumulative number of confirmed COVID-19 cases on March 31st, July 24th, September 27th, and January 17th (respectively), Givatayim only had 22, 228, 646 and 1,362 cumulative number of confirmed COVID-19 cases, respectively (DataGov, 2021). Although Givatayim has a population density and a total population about 1.5 and 3.4 smaller (respectively) than Bnei-Brak, its cumulative number of confirmed COVID-19 cases on January 17th was 26.4 times lower.
Additionally, these socioeconomic and religious community characteristics, can be reflected in the large variability in morbidity rates among similar population sizes in various Israeli large and medium sized cities. For example, the two largest Israeli cities have very distinct populations: Tel Aviv - a secular city with population of ~460,000 (2019) situated in socioeconomic cluster 8, and Jerusalem - a city with large ultra-orthodox and Arab communities and a secular minority, with population of ~940,000 located in socioeconomic cluster 3. On January 17th, the cumulative number of COVID-19 positive cases in Tel-Aviv (17,196) was only a fifth of the number of positive cases in Jerusalem (89,553) (DataGov, 2021).
Medium to high population sized cities such as Haifa and Be'er-Sheva (socioeconomic cluster 7, 5, respectively) had on January 17th only 0.51–0.65 (respectively) of Ashdod's (socioeconomic cluster 4), cumulative number of COVID-19 positive cases (11,548, 9,117 and 17,785, respectively), although the three of them are of similar population sizes (~285,000, 210,000 and 226,000, in 2019, respectively). (CBS, 2017a; DataGov, 2021).
These vast variabilities in population density and total population size may explain the nonsignificant correlations with COVID-19 positive rates, at the peak of the third wave morbidity. To overcome these limitations, there is a need for further research with higher resolution data (when it becomes available), such as: city quarters or neighborhoods each with their specific socioeconomic, religious, ethnic and morbidity rate parameters.
Another important element that needs to be taken into account regarding the factors that influence the morbidity in the third wave (Jan 17th 2021), is the nationwide vaccination campaign that began on December 20th, in which adult Israeli citizens were vaccinated with two doses of the Pfizer vaccine (BNT162b2 mRNA vaccine), prioritized during the first stages of the campaign in December and January by age and COVID-19 high risk populations (Dagan et al., 2021). By Jan 17th over 2.23 million Israelis had received the first vaccine dose and more than 333,500 had already received the second dose. Due to the age priority policy these numbers included: 513,000 and 106,600 vaccinated citizens between the ages of 60–69; 405,500 and 98,000 between the ages of 70–79; 180,000 and 33,600 between the ages of 80–89 and 42,100 and 6,100 above the age of 90 (first and second dose, respectively), (see supplementary Figure S3). The growing COVID-19 immunity that the Israeli population 60 years of age and older gained during this period (Dec 20th to Jan 17th), reduced their susceptibility to COVID-19 morbidity. The growing immunity may also play a part in the lack of association between chronic air pollution exposure and COVID-19 morbidity on Jan 17th.
As the elderly population is one of the most vulnerable groups regarding chronic air pollution related morbidities (Khafaie et al., 2016), their growing COVID-19 immunity, due to the vaccination campaign, could also have had an impact on this observation (no association between chronic exposure to all five air pollutants and COVID-19 morbidity on Jan 17th). Nevertheless, the optimal protection of the vaccine (92–94%) is established only at least seven days after the second dose, while a partial protection of 46–57% is established 14–20 days following the first dose (Dagan et al., 2021). The number of Israelis that were already vaccinated by two doses at least seven days prior to Jan 17th and thus, optimally protected, was approximately only 6,000 people. The number of partially protected Israelis was between 526,000 and 1.292 million, out of them approximately 811,000 and over 374,000 (14 and 20 days after the first dose, respectively) were 60 years old or older (DataGov, 2021). Therefore, we assume that at the peak morbidity point of the third wave (Jan 17th), the vaccination campaign already had some impact on elderly morbidity rates, but the vaccines were probably not the main cause for the lack of association between COVID-19 morbidity rates and all five air pollution parameters.
Socioeconomic factors were shown to have a significant impact on COVID-19 morbidity and mortality in several other cases due to several factors, including structural racism, crowded living conditions, multi-generational homes, limited access to health care and a healthy diet, employment conditions requiring close interaction with others and a history of exposure to elevated air pollution levels of these communities (Brandt et al., 2020). According to Clark et al. (2020), vulnerable populations such as immigrant communities in the US, are at risk of a disproportionate negative impact from the COVID-19 pandemic. According to Oronce et al. (2020), states in the US with higher income inequality (state-level Gini index) experienced a higher number of deaths from COVID-19. Additionally, Brandt et al. (2020) reported that although population density is considered to promote viral spread, it fails to explain why until April 2020 the Bronx (with 30% of residence below poverty line and a majority of black and Latino among them) had twice the number of COVID-19 cases and deaths than Manhattan. In Several other US cities significantly higher rates of COVID-19 cases and deaths were reported in the black community compared to their share in the local population, while white residence deaths share was dramatically smaller than their demographic share. In total, US black residents' COVID-19 death rates were reported to be more than twice their share in the US population.
The effect of household overcrowding was found to be linked to greater odds of COVID-19 morbidity both in the UK and the US, where the highest number of cases occurred in areas with the largest average household size (Brandt et al., 2020; Raisi-Estabragh et al., 2020; Wadhera et al., 2020).
Furthermore, according to Doumas et al. (2020) and to Brandt et al. (2020), the family structure of African Americans, where multigenerational family members are more likely to share accommodation, resulting in close contact between the young and the elderly family members, could increase the risk for COVID-19 morbidity and reduce the effectiveness of social distancing, which has been recognized as one of the most effective measures to attenuate spread and protect the elderly from COVID-19.
In Israel, ultra-orthodox Jews and Israeli Arabs tend to live in a large average household and in some Israeli Arab communities the youth and the elderly tend to live in close contact in the same house or compound. In fact, members of these two communities (ultra-orthodox Jews and Israeli Arabs) comprise the majority of the households in the cities and towns classified in the lowest socioeconomic clusters (1–3) (CBS, 2017a). According to the Israeli Central Bureau of Statistics, in 2016 the average number of persons per household in the Jewish sector was 5.5 for the ultra-orthodox and 4.5 for very religious communities, compared with 2.97 for traditional communities and 2.7 for secular communities (CBS, 2017b). In the Arab sector, the average number of persons per household was 4.53 and in Arab communities in Israel's biggest city – Jerusalem – 5.21 persons per household (CBS, 2016).
In the peak morbidity of the 3rd wave (Jan 17th 2021), under severe gathering limitations and lockdowns, COVID-19 spread to almost every city and town in the country. At that timepoint, the local spread of the highly transmissible SARS-CoV-2 variant B.1.1.7 (Sah et al., 2021) also contributed to that process. At this point in time, the association between COVID-19 morbidity to chronic air pollution exposure and to demographic parameters weakened substantially, due to a much stronger socioeconomic impact driven especially by large household communities. We assume that in those large households, the transfer of the virus from infected house members to other family members, raised the morbidity rate more than any other factor. In these cases, moving COVID-19 patients as soon as possible to a quarantine or treatment facility, may reduce the number of infected family members especially in small apartments where self-quarantine is not possible.
3.4. Comparison between highly and lowly COVID-19 infected cities/towns
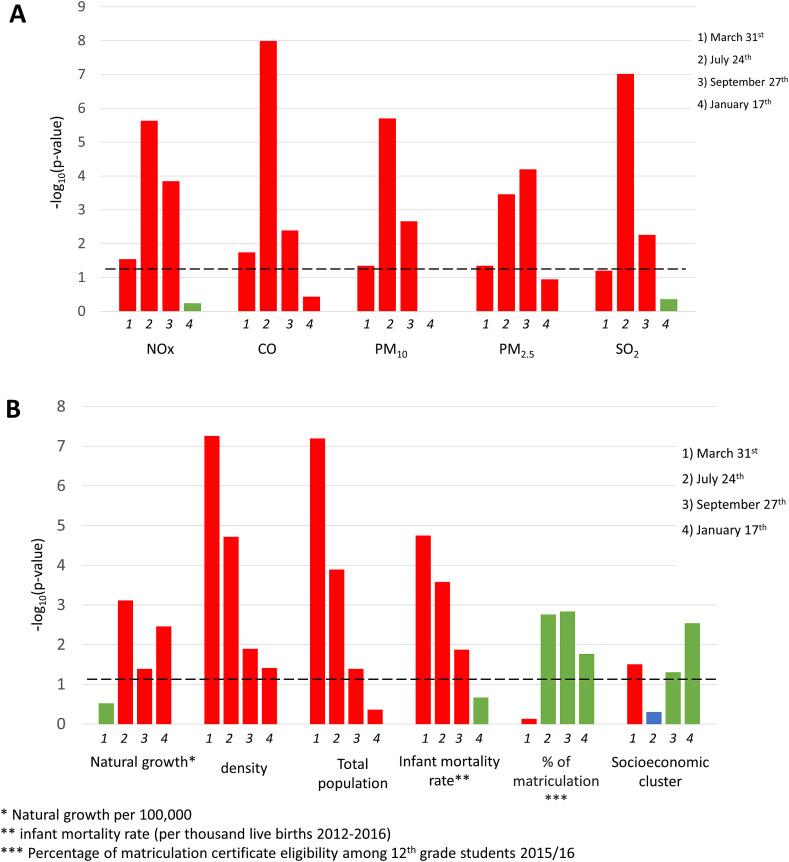
To better understand the effect of air pollutants on COVID-19 positive rates in each of the aforementioned timepoints (March 31st, July 24th, September 27th and January 17th), we divided the cities/towns into tertiles according to their COVID-19 positivity rates on these dates. Next, we defined two lists of cities/towns: those with high COVID-19 rates, in the upper tertile (“red list”) and those with low rates, in the lowest tertile (“green list”).
In general, lower median levels of pollutants were found in cities/towns in the green list, in comparison to those in the red list, as can be seen in Fig. 4A . All air pollutants measured in 2017 were statistically significantly higher in red cities/towns in comparison to green cities/towns, during March, July, and September 2020.
Fig. 4.
Comparison between “red” and “green” cities and towns in the four selected timepoints (2020: March 31st, July 24th, September 27th and 2021: January 17th): log10 (Wilcoxon p-value): (A) Air pollution levels in 2017. (B) demographic and socioeconomic features. Red: higher median value in the red cities and towns, in comparison to the green ones. Green: higher median value in the green cities and towns, in comparison to the red ones. Blue: same medians. The dashed line represents the threshold for statistically significance (p = 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
On March 31st, red cities were denser, more populated, from higher socioeconomic clusters, and were characterized by higher rates of infant mortality, these results were statistically significant (p < 0.001). On July 24th, red cities were denser, more populated, were characterized by higher rates of infant mortality and lower eligibility for matriculation certificate, these results were statistically significant (p = 0.001, p < 0.001, p < 0.001, p < 0.001, p = 0.002, accordingly). Similar results were found on September 27th, in addition to higher socioeconomic clusters of green cities (p = 0.05). As seen earlier, on January 17th no significant link was found between COVID-19 morbidity to air pollution in the green or red cities and towns. Nevertheless, red cities were denser (p = 0.04), with higher natural growth (p < 0.001), with lower eligibility for matriculation certificates (p = 0.02), and in lower socioeconomic clusters (p = 0.003), (Fig. 4B). These results strengthen the assumption that socioeconomic factors had a wider impact on COVID-19 morbidity in the third wave in January 2021, and emphasize the role of factors such as large households’ in-house infection chains and their difficulties with in-house quarantine as reflected by the higher natural growth per 100,000 people in the red cities and towns.
3.5. Multivariate linear regressions
Multivariate linear regressions were used to predict the number of COVID-19 cases per population on March 31st, July 24th, September 27th (2020) , and January 17th (2021), as a function of demographic parameters, socioeconomic parameters, and exposure to air pollution (2016–2019 average concentrations). Due to the significant correlations between many of the collected parameters, we used specific parameters that were not correlated with each other in the linear regressions.
On March 31st, exposures to all three of the analyzed air pollutants (NOx, PM10 and PM2.5) were positively associated with higher rates of COVID-19 positive cases, however these associations were not statistically significant, probably due to the relatively low numbers of COVID-19 positive cases during the first morbidity wave. On July 24th, exposures to all pollutants were statistically significantly positively associated with higher rates of COVID-19 morbidity rates: (NOx: β = 0.086, PM10: β = 0.285, and PM2.5: β = 0.403, and p-values were all <0.05). On September 27th only exposure to PM2.5 was statistically significantly positively associated with COVID-19 rates (β = 0.403, p = 0.024) and on January 17th, none of the air pollutant concentrations were statistically significantly associated with rates of COVID-19 (Table 1 ).
Table 1.
Associations between NOx, PM10 and PM2.5 (average concentrations in the years 2016–2019), and COVID-19 rates in cities and towns in the four morbidity peaks in Israel 2020: March 31st, July 24th, September 27th and 2021: January 17th – results of multivariate regressions (statistically significant values in bold).
| Pollutant | March 31st |
July 24th |
September 27th |
January 17th |
||||
|---|---|---|---|---|---|---|---|---|
| beta | p-value | beta | p-value | beta | p-value | beta | p-value | |
| NOx | 0.049 | 0.25 | 0.086 | 0.015 | 0.038 | 0.211 | 0 | 0.88 |
| PM10 | 0.123 | 0.15 | 0.285 | <0.001 | 0.006 | 0.919 | −0.037 | 0.487 |
| PM2.5 | 0.263 | 0.086 | 0.403 | 0.001 | 0.245 | 0.024 | 0.147 | 0.124 |
Of note, all the multivariate models we built including PM2.5 concentrations were statistically significant (p < 0.05). These models, together with the PM2.5 models in our previous study of 36 OECD countries (Barnett-Itzhaki and Levi, 2021), emphasize the importance of reducing the ambient concentrations of this dangerous air pollutant in the struggle for decreasing the morbidity and mortality rates of COVID-19.
3.6. Study limitations and strengths
In this study we show an association between chronic exposure to NOx, CO, PM2.5, PM10 and SO2 and higher rates of COVID-19 morbidity, in addition to the impact of demographic and socioeconomic parameters.
As this is an ecological study, there might be inherent limitations including the use of grouped data instead of personalized data, under-reporting of health outcomes, inadequate control for confounding factors, inadequate resolution, not referring to outliers or analyzing them and conducting the analyses in the middle of an active pandemic (Villeneuve et al., 2020). Ecological studies are effective for testing initial hypotheses but further studies such as cohort or case control studies are needed to determine causation and point at mechanisms of action. Other limitations of the study include: (a) The available data collected on the chronic exposure levels of all five air pollutants are updated for the years 2016–2019 only. Further analysis of an additional more current database (including the years 2020 and the first quarter of 2021), might strengthen our findings. (b) There might be some discrepancies between the actual air pollution exposure and the calculated exposure attributed to residents, since residents do not spend their entire day in their city or town of residence. This is especially relevant to working Israeli citizens ages of 18–67 who do not live and work in the same city or town as was demonstrated by Shafran-Nathan et al. (2017), who concluded that assigning NOx exposure of adults only at their home address might result in larger exposure estimation errors. Nevertheless, as more and more people work indoor in a controlled airconditioned environment, and as telecommuting expands in recent years to many new sectors and fields, it is not clear how much impact this phenomenon might have on our results, if at all. (c) It is probable that some residents of cities and towns during the COVID-19 epidemic lived in a different area between the years 2016–2019, when air quality data for this study was collected.
Nevertheless, our results are supported by many recent studies conducted on a global and local scale and this study has several additional strengths: First, the study focused on 279 Israeli cities and towns comprising a vast majority of the Israeli population and produced a high-resolution statewide picture. Second, the Ministry of Health open COVID-19 database reports are indeed grouped data (due to medical confidentiality) but are considered extremely reliable, and include almost the entire local epidemic period, so far (except only part of the late decline in morbidity at the end of the third morbidity wave). Third, the Israeli population long-term data of exposure to the major five air pollutants (2016–19) was obtained from a highly reliable multi-scale photochemical and transport model, which provides hourly forecasts at a high spatial resolution of 3 km over the entire area of the state of Israel, and run on a daily basis as a forecast model by the Israeli Ministry of Environmental Protection. Fourth, the demographic and socioeconomic data was obtained from the Israeli Central Bureau of Statistics databases and is highly accurate and in a high resolution.
All in all, despite of the above limitations, statistically significant correlations and models showed an association between long-term chronic exposure of the Israeli population to NOx, CO, PM2.5, PM10 and SO2 concentrations, demographic and socioeconomic parameters, and the rate of COVID-19 morbidity in 279 Israeli cities and towns. Altogether, the geographic high resolution, the large number of cities and towns, including the bulk of the Israeli population, the precise COVID-19 morbidity data, and long-term exposure data to all five major air pollutants strengthen our findings. Further research on this association should be explored locally and globally, to establish causation and to identify mechanisms of air pollution damage that may impacts COVID-19 morbidity and mortality.
4. Conclusion
In this study, we show statistically significant nationwide associations between population chronic exposure (2016–2019) to five main air pollutants: NOx, CO, PM10, PM2.5 and SO2, in 279 Israeli cities and towns, and COVID-19 morbidity rates during two of the three major waves of morbidity experienced in Israel during 2020–2021. We also show that in the third morbidity wave, during which morbidity was very high and spatially widespread, socioeconomic factors became more dominant while the previous association to chronic air pollution exposure was weakened. The nationwide vaccination campaign that began a month earlier, probably impacted third wave morbidity of the elderly population, a highly sensitive population regarding chronic air pollution exposure and its related morbidities.
The association between air pollution and COVID-19 morbidity should be further investigated in cohort and/or case-control studies, worldwide and locally in Israel, including analyses of air pollutant levels around and during the COVID-19 pandemic and in a higher geographic resolution as data becomes available. The associations found in this study between chronic exposure to air pollutants and COVID-19 morbidity on a nationwide scale, strengthen the findings of many recently published studies (local and multi-national) during the first global morbidity wave, which pointed to chronic air pollution exposure as a force multiplier to the SARS-CoV-2 induced morbidity.
The scientific community and decision makers worldwide have long recognized that population long-term chronic exposure to various air pollutants causes adverse health outcomes (including chronic respiratory and cardiovascular morbidities). However, according to WHO, severe ambient air pollution is still responsible for the premature deaths of 4.2 million people around the world annually. The growing body of evidence, regarding exacerbated COVID-19 morbidity and mortality rates due to long-term chronic exposure to elevated concentrations of air pollutants, should serve as a wake-up call among policy makers and world leaders regarding the urgent need to reduce air pollution and its harmful effects.
Additionally, low-level socioeconomic populations, immigrants, and minority communities are especially vulnerable to COVID-19. In each nation or municipality experiencing COVID-19 outbreak, special care should be taken of these vulnerable populations. Morbidity spread within communities that are characterized by large households or households where the youth and the elderly tend to share accommodation, can create inhouse infection chains, further expanding morbidity and the burden on the healthcare system, even under quarantine conditions. Moving COVID-19 patients as soon as possible from these weakened communities to quarantine or treatment facilities, may help reduce the number of infected family members, especially in small apartments where proper self-quarantine is virtually impossible.
As vaccine campaigns worldwide continue to gain momentum and the endpoint of the pandemic might be seen in the horizon, it is becoming clear that only uncompromising global and local policies for dramatic reduction of air pollution can lead to improved post pandemic public health, reducing chronic respiratory and cardiovascular morbidities. The central steps required on this road map include an extensive transition to renewable energy sources, acceleration of the electrification of transportation systems, combined with stricter enforcement of air quality regulations and restrictions on pollutants emissions, especially in densely populated areas. These steps could significantly improve public health during and post pandemic, but also help mitigate with global climate change. This opportunity to reduce populations’ air pollution exposure alongside with cutting GHGs (Greenhouse gases) emissions can be beneficial to both global public health and the health of our planet.
Contribution
A.L. and Z.B.I: A.L – Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing - Original Draft, Writing - Review & Editing. Z.B.I. – Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing - Original Draft, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Ilan Levy for his assistance in the collection of the air pollution data.
We thank Dr. Tamar Berman for her insightful comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111673.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Barnett-Itzhaki Z., Levi A. Effects of chronic exposure to ambient air pollutants on COVID-19 morbidity and mortality-A lesson from OECD countries. Environ. Res. 2021;195:110723. doi: 10.1016/j.envres.2021.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bdal D., Glas E., Bachar N. Israeli Ministry of Energy (In Hebrew); 2019. Israel's Energy Sector 2019 Report.https://www.gov.il/he/departments/publications/reports/energy_sector_2019 [Google Scholar]
- Blomberg A., Krishna M.T., Helleday R., Söderberg M., Ledin M.C., Kelly F.J., et al. Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am. J. Respir. Crit. Care Med. 1999;159:536–543. doi: 10.1164/ajrccm.159.2.9711068. [DOI] [PubMed] [Google Scholar]
- Brandt E.B., Beck A.F., Mersha T.B. Air pollution, racial disparities, and COVID-19 mortality. J. Allergy Clin. Immunol. 2020;146(1):61–63. doi: 10.1016/j.jaci.2020.04.035. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Chen M., Dong D., Xie S., Liu M. Environmental pollutants damage airway epithelial cell cilia: implications for the prevention of obstructive lung diseases. Thoracic Cancer. 2020;11(3):505–510. doi: 10.1111/1759-7714.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBS- Israel Central Bureau of Statistics Table 20: Arab households by housing density and type of locality of residence. 2016. https://www.cbs.gov.il/he/publications/doclib/2018/households_eco16_1724/t_20.pdf
- CBS- Israel Central Bureau of Statistics Characterization and classification of geographical units by the socio-economic level of the population. Table A -Local authorities in ascending order of the socio-economic index 2017: index value, rank and cluster, and changes compared to. 2017;2015 https://www.cbs.gov.il/he/mediarelease/doclib/2020/403/24_20_403t1.pdf [Google Scholar]
- CBS- Israel Central Bureau of Statistics Table 47: Jewish households by labour force characteristics, of household members, average number of persons per household and religious lifestyle of persons residing in the dwelling. 2017. https://www.cbs.gov.il/he/publications/doclib/2018/households_eco16_1724/7.zip
- CBS- Israel Central Bureau of Statistics Municipalities in Israel - data files for processing 1999-2019. 2019. https://www.cbs.gov.il/he/publications/doclib/2019/hamakomiot1999_2017/2019.xlsx
- CBS- Israel Central Bureau of Statistics Motor Vehicles- percentage of change in the number of motor vehicles each year compared with previous year. 2019. https://www.cbs.gov.il/he/publications/doclib/2020/1806/t01.pdf
- Clark E., Fredricks K., Woc-Colburn L., Bottazzi M.E., Weatherhead J. Disproportionate impact of the COVID-19 pandemic on immigrant communities in the United States. PLoS Neglected Trop. Dis. 2020;14(7):e0008484. doi: 10.1371/journal.pntd.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M., Ozgen C., Strobl E. IZA; 2020. Air Pollution Exposure and COVID-19.https://ssrn.com/abstract=3628242 Discussion Paper No. 13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Community health map The geographical information portal of the Ministry of Health. 2016. https://www.health.gov.il/subjects/equality_in_health/healthmap/Pages/default.aspx
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 data dashboard-health Israeli Government website. 2021. https://datadashboard.health.gov.il/COVID-19/general
- Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DataGov Israel's government COVID-19 dataset website. 2021. https://data.gov.il/dataset/covid-19/resource/8a21d39d-91e3-40db-aca1-f73f7ab1df69 Jan 20th. 2021.
- De Angelis E., Renzetti S., Volta M., Donato F., Calza S., Placidi D., et al. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic, and socioeconomic variables. Environ. Res. 2021:110777. doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumas M., Patoulias D., Katsimardou A., Stavropoulos K., Imprialos K., Karagiannis A. COVID19 and increased mortality in African Americans: socioeconomic differences or does the renin angiotensin system also contribute? J. Hum. Hypertens. 2020;34(11):764–767. doi: 10.1038/s41371-020-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg G.M., Kaliner E., Grotto I. Mortality, hospital days and expenditures attributable to ambient air pollution from particulate matter in Israel. Isr. J. Health Pol. Res. 2016;5(1):1–7. doi: 10.1186/s13584-016-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtmeyers E., Gosselink R., Gayan-Ramirez G., Decramer M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999;13(5):1177–1188. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- Khafaie M.A., Yajnik C.S., Salvi S.S., Ojha A. Critical review of air pollution health effects with special concern on respiratory health. Journal of Air Pollution and Health. 2016;1(2):123–136. [Google Scholar]
- Levy I., Karakis I., Berman T., Amitay M., Barnett-Itzhaki Z. A hybrid model for evaluating exposure of the general population in Israel to air pollutants. Environ. Monit. Assess. 2020;192(1):1–10. doi: 10.1007/s10661-019-7960-8. [DOI] [PubMed] [Google Scholar]
- Monteiro A., Miranda A., Borrego C., Vautard R., Ferreira J., Perez A. Long-term assessment of particulate matter using CHIMERE model. Atmos. Environ. 2007;41(36):7726–7738. [Google Scholar]
- Mudway I.S., Kelly F.J., Holgate S.T. Oxidative stress in air pollution research. Free Radical Biol. Med. 2020;151:2–6. doi: 10.1016/j.freeradbiomed.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronce C.I.A., Scannell C.A., Kawachi I., Tsugawa Y. Association between state-level income inequality and COVID-19 cases and mortality in the USA. J. Gen. Intern. Med. 2020;35(9):2791–2793. doi: 10.1007/s11606-020-05971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisi-Estabragh Z., McCracken C., Bethell M.S., Cooper J., Cooper C., Caulfield M.J., et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25 (OH)-vitamin D status: study of 1326 cases from the UK Biobank. J. Publ. Health. 2020;42(3):451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. "Coronavirus Pandemic (COVID-19)". Published online at OurWorldInData.org. 2020. https://ourworldindata.org/coronavirus Retrieved from:
- Sah P., Vilches T.N., Moghadas S.M., Fitzpatrick M.C., Singer B.H., Hotez P.J., Galvani A.P. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. EClinicalMedicine. 2021;35:100865. doi: 10.1016/j.eclinm.2021.100865. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan M., Singh A., Torbaghan M.E., Parlikad A.K. A vulnerability-based approach to human-mobility reduction for countering COVID-19 transmission in London while considering local air quality. Sci. Total Environ. 2020;741:140515. doi: 10.1016/j.scitotenv.2020.140515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Licen S., Perrone M.G., et al. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ open. 2020;10(9):e039338. doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran-Nathan R., Levy I., Broday D.M. Exposure estimation errors to nitrogen oxides on a population scale due to daytime activity away from home. Sci. Total Environ. 2017;580:1401–1409. doi: 10.1016/j.scitotenv.2016.12.105. [DOI] [PubMed] [Google Scholar]
- Tian H., Liu Y., Song H., Wu C.H., Li B., Kraemer M.U., et al. medRxiv; 2020. Risk of COVID-19 Is Associated with Long-Term Exposure to Air Pollution. [Google Scholar]
- Venkatesh A., Jaramillo P., Griffin W.M., Matthews H.S. Implications of near-term coal power plant retirement for SO2 and NOx and life cycle GHG emissions. Environ. Sci. Technol. 2012;46(18):9838–9845. doi: 10.1021/es3023539. [DOI] [PubMed] [Google Scholar]
- Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128(9):95001. doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera R.K., Wadhera P., Gaba P., Figueroa J.F., Maddox K.E.J., Yeh R.W., Shen C. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. Jama. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2006. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005.https://www.euro.who.int/__data/assets/pdf_file/0005/78638/E90038.pdf [PubMed] [Google Scholar]
- World Health Organization . The WHO European Centre for Environment and Health; Bonn: 2013. Review of Evidence on Health Aspects of Air Pollution–REVIHAAP Project: Final Technical Report.https://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report-final-version.pdf [Google Scholar]
- World Health Organization Air pollution. 2020. https://www.who.int/health-topics/air-pollution#tab=tab_2
- Worldometers.info coronavirus-country-Israel Dover, Delaware, U.S.A. 2021. https://www.worldometers.info/coronavirus/country/israel/
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. medRxiv; 2020. Exposure to Air Pollution and COVID-19 Mortality in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinon L., Thurston G. An evaluation of the health benefits achieved at the time of an air quality intervention in three Israeli cities. Environ. Int. 2017;102:66–73. doi: 10.1016/j.envint.2016.12.025. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci. Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.