Abstract
Liquorice is a traditional medicine. Triterpenoids such as glycyrrhizin and glycyrrhetinic acid are the main active constituents of liquorice. Studies have revealed that these compounds exert inhibitory effects on several viruses, including SARS-CoV-2. The main mechanisms of action of these compounds include inhibition of virus replication, direct inactivation of viruses, inhibition of inflammation mediated by HMGB1/TLR4, inhibition of β-chemokines, reduction in the binding of HMGB1 to DNA to weaken the activity of viruses, and inhibition of reactive oxygen species formation. We herein review the research progress on the antiviral effects of glycyrrhizin and its derivatives. In addition, we emphasise the significance of exploring unknown antiviral mechanisms, structural modifications, and drug combinations in future studies.
Keywords: liquorice, triterpenoid, antiviral activity, glycyrrhizin, glycyrrhetinic acid
Introduction
Liquorice has been documented as a herbal medicine in ancient medical books of China, India, and Greece and has been used for thousands of years (Zeng et al., 1988). Primarily, it is used in clinics to treat diseases of the respiratory, digestive, and immune systems (Deng et al., 2017; Sun et al., 2018; Yan et al., 2018; Yao and Sun, 2019; Paudel et al., 2020; Yang et al., 2020). Liquorice has favourable preventive and therapeutic effects on various diseases, with fewer side effects (Kwon et al., 2020); therefore, research and mining of potential pharmacological activities of liquorice have been a hot topic in the field of pharmacology. Currently, many experimental results have shown that liquorice and its extract have antibacterial, antiviral, anti-inflammatory, anticancer, antioxidant, liver protection, neuroprotection, skin whitening, hypoglycaemic, memory-enhancing, and other biological activities (Petramfar et al., 2020), indicating that liquorice holds a great developmental and application prospect in cosmetic production and in the treatment of liver diseases, diabetes, ischaemia–reperfusion injury, Alzheimer’s disease, Parkinson’s disease, epilepsy, depression, and cancer (Pastorino et al., 2018; Li et al., 2019; Zhang et al., 2021).
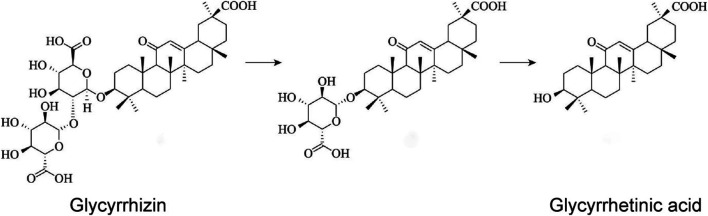
Glycyrrhizin (also known as glycyrrhizic acid or glycyrrhizinic acid) (Drugs B.I., 2006) and glycyrrhetinic acid, which are the most important chemical constituents of liquorice, belong to the class triterpenoids. Among all saponins in liquorice, glycyrrhizin is present in the highest amount (>2%), and its content in wild, high-quality liquorice can be as high as 7% (Zhang and Ye, 2009). Glycyrrhizin is metabolised through the gastrointestinal tract to produce glycyrrhetinic acid (Figure 1) (Yang, 2020), which also possesses many pharmacological properties, and its most interesting property is its antiviral activity. Glycyrrhizin exerts inhibitory effects on hepatitis C virus (HCV) (Zhang et al., 2020), herpes simplex type 1 (HSV-1) (Hirabayashi et al., 1991), influenza virus (Wolkerstorfer et al., 2009), and severe acute respiratory syndrome (SARS)-associated coronaviruses (Cinatl et al., 2003). This article reviews the research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice to provide a reference for further scientific research and for guiding clinical applications. Although only a few reports are available on the role of glycyrrhizin in herpes virus and human immunodeficiency virus (HIV) infections, we present information about herpes virus and HIV based on studies by Pu et al. (2013) and Huan et al. (2020), and these aspects form the basis of the present review. Overall, our review discusses advances in research on the antiviral properties and mechanism of action of glycyrrhizin.
FIGURE 1.
The structure and metabolic relationship between glycyrrhizin and glycyrrhetinic acid.
Anti-Herpes Virus Activity
Herpes virus is one of the most common viruses infecting animals and humans. More than 90% adults have been infected with at least one or more types of herpes viruses, and the viruses usually establish a latent infection (Chayavichitsilp et al., 2009). Some herpes viruses lead to lifelong infections, which are common worldwide (Bradley et al., 2014; Okonko and Cookey, 2015; Reward et al., 2019).
In 1979, a study reported that glycyrrhizic acid could directly inactivate HSV [multiplicities of infection (MOI) = 5], and this effect was found to be irreversible (Pompei et al., 1979). Subsequently, some researchers detected the anti-HSV effects of glycyrrhetinic acid derivatives, sodium carbenoxolone sodium (CBX), and cicloxolone sodium (CCX) (Dargan and Subak-Sharpe, 1986). Through in vivo and in vitro studies, researchers found that 500 μM CBX and 300 μM CCX have anti-HSV-1 and anti-HSV-2 (MOI = 5) activities, and thus can significantly inhibit virus replication and reduce the number of infectious virus particles by 10,000–100,000 times. Glycyrrhizin inhibits HSV-1 replication in vitro, and its IC50 is 0.5 mM (Hirabayashi et al., 1991). Sekizawa et al. (2001) revealed that glycyrrhizin could increase the survival rate of HSV-1-infected mice. Ikeda et al. (2005) found that glycyrrhizin and glycyrrhetic acid inhibited HSV-1 replication, and the effect of glycyrrhetic acid was 10 times greater than that of glycyrrhizin. Glycyrrhizin was also shown to reduce adhesion between cerebral capillary vessel endothelial cells and polymorphonuclear leukocytes in the treatment of HSV infection (Huang et al., 2012).
Curreli et al. (2005) found that latent infection with kaposi sarcoma-associated herpesvirus (KSHV) in B lymphocytes could be terminated using glycyrrhizic acid. Glycyrrhizic acid was found to block the latent KSHV infection by upregulating the expression of viral cyclin (ORF72) and downregulating the expression of latency-associated nuclear antigen (LANA), thereby selectively inducing death of KSHV-infected cells. Glycyrrhizic acid was also reported to inhibit KSHV replication in patients with hepatitits B virus (HBV) infection (Xie et al., 2011). In addition, Kang and Lieberman (2011) found that glycyrrhizic acid decreases transcription during KSHV latency to inhibit virus replication. They confirmed that glycyrrhizic acid disrupts the RNA polymerase II (RNAPII) complex and alters the enrichment of the RNAPII-pausing complex. Furthermore, glycyrrhizic acid was shown to reduce the interaction between SMC3 and RAD21 and between SPT5 and RNAPII. Moreover, it abrogatated RNAPII to pause at intragenic CTCF-cohesion binding sites, leading to reduced mRNA production and a defect in sister chromatid cohesion.
Lin (2003) proved that glycyrrhizic acid dose-dependently inhibits the Epstein–Barr virus (EBV) replication. Glycyrrhizic acid mainly plays an antiviral role in the early stage of the EBV replication cycle; however, it does not affect the adsorption and inactivation of virus particles. Lin et al. (2008) found that seven derivatives of glycyrrhizic acid could inhibit EBV infection in a dose-dependent manner, and the antiviral activity of these derivatives was enhanced when amino acid residues were introduced into the carbohydrate part of glycyrrhizic acid. The authors also reported that 18β-glycyrrhetinic acid (the metabolic product of glycyrrhizic acid) is 7.5-fold more active against EBV than glycyrrhizic acid (Lin et al., 2008).
Cellular SUMOylation processes are the proposed targets for antiviral therapies. Bentz et al. (2019) found that glycyrrhizic acid inhibits SUMOylation processes, limits cell growth, and induces apoptosis in multiple cell lines. Glycyrrhizic acid acts on the first step of the SUMOylation process and leads to low levels of spontaneous EBV reactivation. Glycyrrhizic acid was not found to affect the induced reactivation of the virus; however, its extract was shown to reduce the ability of the virus to infect the remaining cells (Lin et al., 2008). Therefore, glycyrrhizic acid may be beneficial in the treatment of EBV-related malignant tumours and other diseases caused by the SUMOylation process.
Anti-Hepatitis Virus Activity
Glycyrrhizic acid exhibits direct anti-hepatitis virus activity and low toxicity in host cells (Romero et al., 2005). It has been used to treat chronic hepatitis for many years in Japan. Glycyrrhizin can inhibit the secretion of HBV surface antigen (HBsAg) in PLC/PRF/5 cells in vitro, which leads to its accumulation in the cytoplasmic vacuoles of the Golgi apparatus, changes in the intracellular transport of HBsAg, and inhibition of its sialylation in a dose-dependent manner (Takahara et al., 1994). Sato et al. (1996) revealed that glycyrrhizin could bind to hepatocytes at a certain concentration, change the expression of HBV-related antigens on hepatocytes, and inhibit the sialylation of HBsAg. Matsuo et al. (2001) revealed that glycyrrhizin in combination with lamivudine may inhibit HBV replication in an HBV carrier with non-Hodgkin lymphoma.
Glycyrrhizin can not only effectively inhibit HBV infection but also exhibit a strong anti-HCV activity. Ashfaq et al. (2011) used a nontoxic dose of glycyrrhizin to detect its antiviral activity in HCV-infected hepatocytes. The study results revealed that glycyrrhizin can decrease the titre of hepatitis C virus (2 × 105 copies of HCV infection) by 50% at a concentration of 7 ± 1 μg/ml. Additionally, its inhibitory effect increased when it was used in combination with interferon. In addition to interferon (Fujisawa, 1991), glycyrrhizin is used in combination with various drugs to treat hepatitis virus infection, and its use in combination with other drugs has yielded superior clinical outcomes (Tandon et al., 2001; Tarao, 2001; Hidaka et al., 2007; Chen et al., 2017). A glycyrrhizin-containing preparation, Stronger Neo-Minophagen CTM (SNMC), protects mitochondria by reducing oxidative stress during HCV treatment in transgenic mice (Korenaga et al., 2011). Matsumoto et al. (2013) demonstrated that glycyrrhizin can decrease the release of infectious HCV particles by inhibiting PLA2G1B.
A recent study reported that glycyrrhetinic acid could inhibit the release of high-mobility group box 1 (HMGB1), block the cytokine activity of HMGB1, and significantly improve the liver inflammatory injury induced by mouse hepatitis virus (1 × 104 PFU/mouse) through the HMGB1-TLR4 signalling pathway. This protective effect is related to a substantial reduction in IL-17 and IL-22 levels rather than the direct inhibition of intracellular virus replication (Shi et al., 2020).
Anti-Influenza Virus Activity
The effect of glycyrrhizic acid on influenza virus was reported in the early 1980s. In vitro experiments by Pompei et al. (1983) demonstrated that glycyrrhizic acid could inhibit the replication of influenza virus in chicken embryos by reducing the haemagglutinin level. The mechanism of inhibition of influenza virus replication by glycyrrhizic acid has been reported (Wolkerstorfer et al., 2009; Michaelis et al., 2010; Sakai-Sugino et al., 2017). A combination of HMGB1 with influenza virus nucleoprotein can promote the growth of influenza virus and enhance the activity of virus polymerase (Moisy et al., 2012). Glycyrrhizin can reduce the activity of influenza virus polymerase by antagonising this binding effect, thus inhibiting influenza virus replication (Paudel et al., 2020). Michaelis et al. (2011) found that 200 μg/ml glycyrrhizin could significantly reduce the cytopathic effect (CPE) caused by H5N1 influenza virus infection at an MOI of 0.01, 0.1, or 1 in A549 cells. The therapeutic concentration of glycyrrhizin interferes with the replication of highly pathogenic H5N1 influenza A virus at least partly by interfering with the formation of reactive oxygen species (ROS) induced by H5N1, thereby reducing the activation of p38, JNK, and NF-kB in lung cells. Glycyrrhizin was also found to inhibit H5N1-induced production of CXCL10, IL-6, and CCL5 (MOI = 2) and H5N1-induced apoptosis without affecting virus replication and NK cell activity (Michaelis et al., 2010).
The combination of glycyrrhizic acid with various drugs produces a synergistic effect, which protects against influenza virus infection. Smirnov et al. (2012) studied the regulatory effects of glutamyl-tryptophan (EW) and glycyrrhizic acid and their combination on influenza A (H3N2) virus infection in mice (1 or 10 LD50). The results revealed that compared with EW alone, glycyrrhizic acid (10 mg/kg body weight) in combination with EW (0.1 μg/kg, 10 μg/kg, and 1,000 μg/kg) produced significant antiviral effects, which alleviated lung oedema and inflammatory cell infiltration. Use of glycyrrhizic acid in combination with ribavirin, a broad-spectrum antiviral drug, significantly inhibited lung consolidation in mice infected with H1N1 influenza virus. A combination of 50 mg kg−1 d−1 glycyrrhizin and 40 mg kg−1 d−1 ribavirin was found to provide 100% protection to infected mice (5 times LD50 of influenza H1N1 virus infection), suggesting that the combination of ribavirin and glycyrrhizin has a potential clinical value (Chen et al., 2015b).
Anti-Human Immunodeficiency Virus Activity
Studies have shown that glycyrrhizin inhibits HIV replication in a dose-dependent manner (Ito et al., 1987; Ito et al., 1988; Hattori et al., 1989). Ito et al. (1988) found that glycyrrhizin at a concentration of 0.6 mM completely inhibited HIV-induced (MOI = 0.002) MT-4 cell plaque formation as well as HIV-induced cytopathogenicity in MT-4 and MOLT-4 cells in vitro (Hirabayashi et al., 1991).
In addition, glycyrrhizin increases the number of OKT4 lymphocytes and improves liver dysfunction and thus prevents the progression of haemophilia to AIDS in HIV-positive patients with haemophilia (Mori et al., 1989). Glycyrrhizin can not only inhibit HIV replication but also affect the entry of HIV into cells. HIV requires chemokine receptors or chemokine receptor-like molecules to enter cells (Cocchi et al., 1996; Feng et al., 2011). Sasaki et al. (2002) found that glycyrrhizin could induce the production of CC chemokine ligand (CCL) 4 and CCL5 in cultures of peripheral blood mononuclear cells from HIV-infected patients. These β-chemokines can compete with HIV to bind with chemokine receptors, thus inhibiting the entry of NSI-HIV. CC chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4) are the coreceptors necessary for HIV-1 entry into cells (Alkhatib et al., 1996; Dragic et al., 1996). Yoshida et al. (2009) and Takei et al. (2005) confirmed that glycyrrhizin can greatly inhibit the production of CCL2 and interleukin 10 (IL-10). Consequently, CCR5 expression, which is mediated by CCL2 or IL-10, reduces significantly, resulting in effective inhibition of the entry of HIV into cells.
Glycyrrhizin can also reduce the fluidity of the cell membrane, which reduces intercellular fusion, thus inhibiting the spread of HIV across cells. Harada (2005) washed the cells treated with glycyrrhizin, added the replacement medium lacking glycyrrhizin, and observed a time-dependent increase in the fluidity of the membrane as well as in the sensitivity of the cells to infection and fusion. This finding provides a novel strategy for the prevention and treatment of enveloped viruses.
Anti- SARS-CoV Activity
Researchers began investigating the role of glycyrrhizin in protection against SARS-associated coronavirus (SARS-CoV) infection after the outbreak of SARS in 2003. Cinatl et al. (2003) evaluated the antiviral effects of five drugs, including ribavirin and glycyrrhizin, on SARS-CoV, and glycyrrhizin was found to exhibit the strongest inhibitory effect on SARS-CoV replication in Vero cells. Furthermore, a study found that glycyrrhizin can inhibit the early stages of the virus replication cycle, namely adsorption and penetration, and the effect of glycyrrhizin addition on virus adsorption was not found to be as good as that of glycyrrhizin addition after virus adsorption. However, Chen et al. (2004) could not detect the inhibitory effect of glycyrrhizin on SARS-CoV in the fRhK-4 cell line. The modification of the glycyrrhizin structure, particularly the production of amide derivatives and amino acid conjugates, can significantly improve its anti-SARS-CoV activity, although this modification increases its cytotoxicity (Hoever et al., 2005). In clinical trials, clinical symptoms such as dyspnoea improved rapidly (Lu et al., 2003), the average time for lung lesion improvement from the most severe to 50% decreased, and no side effects were observed in the glycyrrhizin treatment group (Wu et al., 2004).
SARS-CoV-2 is a novel coronavirus, and the coronavirus disease 2019 pandemic caused by SARS-CoV-2 was named coronavirus disease-2019 (COVID-2019) by the World Health Organisation (WHO) (Coronaviridae Study Group of the International Committee on Taxonomy of, 2020; Li et al., 2020; Zhu et al., 2020). SARS-CoV-2 and SARS-CoV gene sequences share 79.5% homology (Zhou et al., 2020), and many similarities have been observed in the clinical symptoms of the infections caused by these two viruses (Wang et al., 2020). Therefore, whether glycyrrhizin shows antiviral activity against SARS-CoV-2 similar to SARS-CoV remains to be investigated (Bailly and Vergoten, 2020). Luo et al. (2020) explored the potential pharmacological effects of glycyrrhizin in COVID-19 treatment. The authors found that glycyrrhizin exerts various pharmacological effects such as angiotensin-converting enzyme II (ACE2) binding, proinflammatory cytokine downregulation, endogenous interferon induction, inhibition of intracellular R accumulation and thrombin, and excessive production of airway exudates. These findings suggest that glycyrrhizin may be a promising drug for COVID-19 treatment (Ding et al., 2020; Murck, 2020; Chrzanowski et al., 2021).
In a recent study, glycyrrhizin significantly inhibited SARS-CoV-2 (MOI = 0.01) replication in Vero E6 cells in a dose-dependent manner and exhibited no significant cytotoxicity (Gowda et al., 2021). Glycyrrhizin can also prevent virus replication (100 TCID50 of SARS-CoV-2) by inhibiting the viral main protease Mpro (Sinha et al., 2021; van de Sand et al., 2021). Using computer-aided drug design and biological verification, Yu et al. (2021) found that glycyrrhizin is the most effective and nontoxic broad-spectrum anti-coronavirus molecule in vitro, particularly against SARS-CoV-2.
Effects on Some Animal Viruses
Glycyrrhizin has demonstrated antiviral activity against some animal viruses. Li et al. (2009) studied the effect of glycyrrhizin diammonium on infectious bronchitis virus (IBV) cell infection (MOI = 0.001) through CPE observation, plaque reduction test, and RT-PCR. The results revealed that glycyrrhizin diammonium has a direct antiviral activity and that it can completely inhibit cell infection. Glycyrrhizin alone or in combination with duck hepatitis virus (DHV) vaccine has demonstrated good immune stimulant and antiviral effects against DHV (Soufy et al., 2012; Okda et al., 2013). Dipotassium glycyrrhizinate can directly inactivate and/or interfere with infectious bursal disease virus (IBDV) replication and effectively inhibit IBDV infection (100 TCID50, 1 × 10−1.5) in vitro (Sun et al., 2013). Li et al. (2014) found that diammonium glycyrrhizinate (DG) exerts an antiviral effect on porcine testicular (ST) cells infected with PPV (100 TCID50), and DG was found to have a strong inhibitory effect on PPV when the virus was treated before incubation. DG also demonstrated its antiviral activity against Marc-145 cells infected with porcine reproductive and respiratory syndrome virus (PRRSV). PRRSV could be effectively controlled through inhibition of viral replication and N gene expression and through reduction of apoptosis (Wang et al., 2013). Studies have also reported that glycyrrhizin mainly inhibits the penetration of PRRSV and has a slight effect on the adsorption or release of PRRSV during its life cycle (Duan et al., 2015). By using carbon dots (CDs) with high biocompatibility and glycyrrhizic acid, Tong et al. (2020) synthesised Gly-CDs through the hydrothermal method. These Gly-CDs could inhibit the invasion and replication of PRRSV, stimulate the innate immune response of antivirus, and inhibit the accumulation of intracellular ROS caused by PRRSV infection (MOI = 1). Glycyrrhizin can also inhibit the entry and replication of porcine epidemic diarrhoea virus (PEDV) but does not affect the assembly and release of the virus (Huan et al., 2017). Inhibition of PEDV infection (MOI = 0.1) and secretion of proinflammatory cytokines occurred mainly through the HMGB1/TLR4-MAPK p38 pathway (Gao et al., 2020).
Discussion
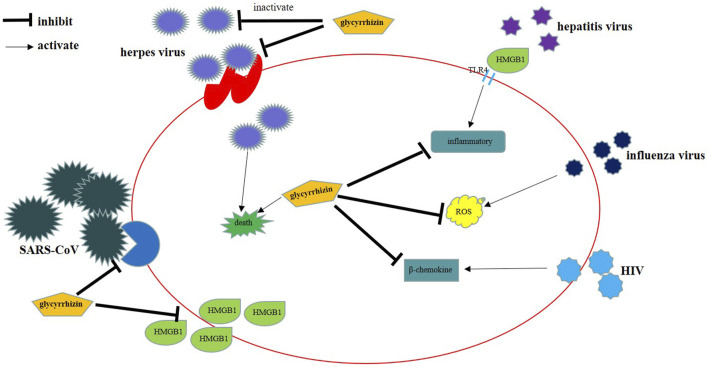
Glycyrrhizin and its derivative protect against many viruses through their antiviral activity (Table 1). The main mechanisms of action of these compounds include inhibition of virus replication, direct inactivation of viruses, inhibition of the replication and expression of viral genes, increase in cell death, inhibition of inflammation mediated by HMGB1/TLR4, inhibition of β-chemokines, reduction in HMGB1 release, reduction in the fluidity of the cell membrane, reduction in the binding of HMGB1 to DNA to weaken the activity of viruses, and inhibition of the formation of ROS (Figure 2). The mechanisms of action of glycyrrhizin against viruses are complex; however, many mechanisms remain unknown. Therefore, elucidation of the antiviral mechanism of glycyrrhizin may help in developing strategies to enhance its antiviral activity and is a subject worthy of further studies. In our further studies, we aim to focus on exploring the mechanisms through which glycyrrhizin induces direct inactivation of viruses and inflammatory and immune responses.
TABLE 1.
The antiviral activity and mechanism of actions of glycyrrhizin and its derivatives.
| Virus | Mechanisms of action | Glycyrrhizin and its derivatives | References | |
|---|---|---|---|---|
| Herpes virus | HSV-1 | Directly inactivates herpes simplex virus | Glycyrrhizic acid (8 mM) | Pompei et al. (1979) |
| HSV-1, HSV-2 | Inhibition of virus replication | Carbenoxolone sodium (500 μM) and cicloxolone sodium (300 μM) | Dargan and Subak-Sharpe, (1986) | |
| KSHV | Upregulation of the expression of viral cyclin and downregulation of the expression of latency-associated nuclear antigen, selectively inducing cell death in KSHV-infected cells | Glycyrrhizic acid (3 mM, 4 mM) | Curreli et al. (2005) | |
| EBV | Inhibit virus invasion into host cells in the early stages of virus replication; inhibit the sumerization process, prevent cell proliferation, increase cell death, and prevent the resulting virus from infecting new cells | Glycyrrhizic acid (IC50 = 0.04 mM); glycyrrhizic acid (0.5, 1.0, 2.0, 3.0, 4.0 mM) | (Lin, 2003; Bentz et al., 2019) | |
| Hepatitis virus | HBV | Inhibition of hepatitis B surface antigen secretion, sialylation, and intracellular transpo | Glycyrrhizin (0.5 mg/ml, 1 mg/ml, 2 mg/ml) | Takahara et al. (1994) |
| HCV | Inhibition of HCV3a core gene expression at mRNA and protein levels | Glycyrrhizin (2.5 μg/ml, 5 μg/ml, 10 μg/ml, 20 μg/ml) | Ashfaq et al. (2011) | |
| Mouse hepatitis virus | Improvement of liver inflammatory injury through the high-mobility group box 1 (HMGB1)–TLR4 signal pathway | Glycyrrhetinic acid (10 μg/ml, 100 μg/ml, 1,000 μg/ml) | Shi et al. (2020) | |
| Influenza virus | H5N1 | Inhibits the formation of ROS induced by H5N1 and then decreases the activation of NFκB, JNK, and p38 | Glycyrrhizin (25 μg/ml, 50 μg/ml, 100 μg/ml, 200 μg/ml) | Michaelis et al. (2011) |
| Influenza virus | Antagonises the binding of HMGB1 to influenza virus nucleoprotein and reduces the activity of influenza virus polymerase, thus inhibiting the replication of influenza virus | Glycyrrhizin | Moisy et al. (2012) | |
| H5N1 | Inhibits H5N1-induced CXCL10, IL-6 and CCL5 production, inhibits H5N1-induced apoptosis but does not interfere with H5N1 replication | Glycyrrhizin (50 μg/ml, 100 μg/ml, 200 μg/ml) | Michaelis et al. (2010) | |
| HIV | HIV-1 | Inhibition of virus replication by inhibiting protein kinase C | Glycyrrhizin (0.075, 0.15, 0.3, 0.6, 1.2, 2.4 mM) | Ito et al. (1988) |
| NSI-HIV | Inhibition of NSI-HIV replication in PBMC by inducing the production of β-chemokines (CCL4 and CCL5) | Glycyrrhizin (0.1 μg/ml, 1 μg/ml, 10 μg/ml, 100 μg/ml) | Sasaki et al. (2002) | |
| HIV-1 | Reduces the fluidity of cell membrane, resulting in a decrease in intercellular fusion, thus inhibiting the transmission of HIV between cells | Glycyrrhizin (0.06 mg/ml, 0.13 mg/ml, 0.25 mg/ml, 0.5 mg/ml, 1 mg/ml) | Harada, (2005) | |
| SARS-CoV | SARS-CoV | Inhibits virus replication; inhibits the adsorption and infiltration of viruses | Glycyrrhizin (after virus adsorption, EC50 = 600 μg/ml; during and after virus adsorption, EC50 = 300 μg/ml; during virus adsorption, EC50 = 2,400 μg/ml) | Cinatl et al. (2003) |
| SARS-CoV-2 | Inhibition of HMGB1 release; inhibition of virus replication | Glycyrrhizin (10 mM, 250 mM, 1,000 μM) | Gowda et al. (2021) | |
| Animal viruses | IBV | Direct antiviral effect, inhibit cell infection; decrease apoptosis of infected cells | Glycyrrhizin diammonium (0.0225, 0.09, 0.36, 1.44 mM) | Li et al. (2009) |
| IBDV | Inhibition of virus replication, direct inactivation of virus, and inhibition of virus adsorption | Dipotassium glycyrrhizinate (EC50 = 663.2 ± 268.4 μg/ml) | Sun et al. (2013) | |
| PPV | Directly inactivates the virus | Diammonium glycyrrhizinate (250 μg/ml) | Li et al. (2014) | |
| PRRSV | Directly inactivates PRRSV, inhibits PRRSV invasion and replication, stimulates cells to produce interferon, and inhibits PRRSV infection-induced reactive oxygen species production | Glycyrrhizic-acid-based carbon dots (0.30 mg/ml) | Tong et al. (2020) | |
| PEDV | Inhibition of PEDV infection and secretion of proinflammatory cytokines through the HMGB1/TLR4-MAPKp38 pathway | Glycyrrhizin (0.1, 0.2, 0.4, 0.8 mM) | Gao et al. (2020) | |
FIGURE 2.
The mechanisms of action of glycyrrhizin against viruses.
COVID-19 has become a pandemic. Although vaccines have been approved, they have not been widely used and no specific treatment for COVID-19 is available to date (Al-Kamel and Grundmann, 2021). Glycyrrhizin exerts extensive antiviral effect and obvious inhibitory effects on SARS-CoV; therefore, glycyrrhizin may be an effective drug against COVID-19. Ding et al. provided the clinical data of a critically ill patient with COVID-19 who was cured by a combination of DG and vitamin C (Ding et al., 2020). SARS-CoV-2 has been shown to enter cells through angiotensin-converting enzyme 2 (ACE2) receptors (Letko et al., 2020; Wan et al., 2020; Xu et al., 2020). Therefore, targeting ACE2 could be a promising strategy to prevent SARS-CoV-2 infection. Glycyrrhizin was recently shown to have the potential to bind to ACE2 (Zhou and Huang, 2020). All these findings suggest that the use of glycyrrhizin could be promising in COVID-19 treatment.
Structural modification of natural products is one of the approaches to discover novel drugs. Through structural modification, the potency and solubility of natural products can be increased, pharmacokinetic properties such as absorption and distribution can be improved, and side effects can be eliminated or reduced (Chen et al., 2015a). Many artificially modified natural product derivatives, such as artemisinin (antimalaria) and paclitaxel (anticancer), have been successfully used in clinics (Yao et al., 2017). Some glycyrrhizin derivatives have demonstrated stronger antiviral activity than glycyrrhizin, although with higher cytotoxicity (Hoever et al., 2005; Baltina et al., 2019; El-Senduny et al., 2019). Therefore, structural modification of glycyrrhizin in the future could be valuable for identifying glycyrrhizin derivatives with the highest antiviral activity and least cytotoxicity. The use of a combination of two or more drugs is another approach to improve efficacy and reduce side effects of drugs. Since the approval of the first batch of combination drugs by FDA in the 1940s, combination drugs have become invaluable for clinical application (Das et al., 2019). The combinations of glycyrrhizin with entecavir, lamivudine, and other drugs have been reported to yield superior clinical outcomes (Ide et al., 2005; Chen et al., 2020; Minsart et al., 2020; Yu et al., 2020); therefore the potential of combining glycyrrhizin with other drugs must be explored in future studies.
The United States Food and Drug Administration, Council of Europe, and Joint FAO/WHO Expert Committee on Food Additives have allowed the use of liquorice extract and glycyrrhizin in food (Pastorino et al., 2018). Although liquorice is believed to be a healthy natural herb without any serious adverse effects (Kwon et al., 2020), it should be used with caution, particularly in patients with hypertension, considering its side effects. Side effects reported in literature include hypokalaemia (Sontia et al., 2008; Flores-Robles et al., 2013), hypertension (Smedegaard and Svart, 2019), hypertensive encephalopathy (Russo et al., 2000), rhabdomyolysis (Alaygut et al., 2017), and cardiac arrest (Attou et al., 2020). A good correlation has been observed between the content of glycyrrhizin and the incidence of side effects, which indicates that the content of glycyrrhizin is a suitable index to avoid adverse reactions (Nose et al., 2017). However, the upper safe limit of glycyrrhizin remains to be determined. The European Union recommends an upper limit of 100 mg of glycyrrhizin per day (Murphy et al., 2009). Based on in vivo results and clinical evidence, Isbrucker et al. recommended that the acceptable daily intake of glycyrrhizin is 0.015–0.229 mg/kg body weight/day (Isbrucker and Burdock, 2006). This range is consistent with the 0.2 mg/kg body weight/day proposed by van Gelderen et al. (2000). Considering the well-known biological functions and side effects of glycyrrhizin, the use of an appropriately controlled dosage of liquorice can lead to its health benefits outweigh its side effects.
Acknowledgments
We thank Xiang Mao for editing the English text of a draft of this manuscript.
Author Contributions
All the authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was funded by the National Natural Science Foundation of China (31902253), the Natural Science Foundation of Jiangsu Province (BK20180921), the individual technology research and development of modern agricultural industry of Jiangsu Province (CX(19)3024), the China Postdoctoral Science Foundation (2018M632399), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the earmarked fund for Jiangsu Agricultural Industry Technology System.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Al-Kamel H., Grundmann O. (2021). Glycyrrhizin as a Potential Treatment for the Novel Coronavirus (COVID-19). Mini. Rev. Med. Chem. 21, 1–5. 10.2174/1389557521666210210160237 [DOI] [PubMed] [Google Scholar]
- Alaygut D., Bayram M. T., Kasap B., Soylu A., Türkmen M., Kavukcu S. (2017). Rhabdomyolysis with Different Etiologies in Childhood. World J. Clin. Pediatr. 6 (4), 161–168. 10.5409/wjcp.v6.i4.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G., Combadiere C., Broder C. C., Feng Y., Kennedy P. E., Murphy P. M., et al. (1996). CC CKR5: A RANTES, MIP-1 , MIP-1 Receptor as a Fusion Cofactor for Macrophage-Tropic HIV-1. Science 272 (5270), 1955–1958. 10.1126/science.272.5270.1955 [DOI] [PubMed] [Google Scholar]
- Ashfaq U. A., Masoud M. S., Nawaz Z., Riazuddin S. (2011). Glycyrrhizin as Antiviral Agent against Hepatitis C Virus. J. Transl Med. 9, 112. 10.1186/1479-5876-9-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attou R., Redant S., Honore P. M., Preseau T., Hantson P., De Bels D. (2020). Liquorice Intoxication Can Lead to Cardiac Arrest!. Case Rep. Emerg. Med. 2020, 1–3. 10.1155/2020/3727682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., Vergoten G. (2020). Glycyrrhizin: An Alternative Drug for the Treatment of COVID-19 Infection and the Associated Respiratory Syndrome?. Pharmacol. Ther. 214, 107618. 10.1016/j.pharmthera.2020.107618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L. A., Tasi Y.-T., Huang S.-H., Lai H.-C., Baltina L. A., Petrova S. F., et al. (2019). Glycyrrhizic Acid Derivatives as Dengue Virus Inhibitors. Bioorg. Med. Chem. Lett. 29 (20), 126645. 10.1016/j.bmcl.2019.126645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz G. L., Lowrey A. J., Horne D. C., Nguyen V., Satterfield A. R., Ross T. D., et al. (2019). Using Glycyrrhizic Acid to Target Sumoylation Processes during Epstein-Barr Virus Latency. PLoS One 14 (5), e0217578. 10.1371/journal.pone.0217578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley H., Markowitz L. E., Gibson T., McQuillan G. M. (2014). Seroprevalence of Herpes Simplex Virus Types 1 and 2--United States, 1999-2010. J. Infect. Dis. 209 (3), 325–333. 10.1093/infdis/jit458 [DOI] [PubMed] [Google Scholar]
- Chayavichitsilp P., Buckwalter J. V., Krakowski A. C., Friedlander S. F. (2009). Herpes Simplex. Pediatr. Rev. 30 (4), 119–130. 10.1542/pir.30-4-119 [DOI] [PubMed] [Google Scholar]
- Chen F., Chan K. H., Jiang Y., Kao R. Y. T., Lu H. T., Fan K. W., et al. (2004). In Vitro susceptibility of 10 Clinical Isolates of SARS Coronavirus to Selected Antiviral Compounds. J. Clin. Virol. 31 (1), 69–75. 10.1016/j.jcv.2004.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li W., Yao H., Xu J. (2015a). Insights into Drug Discovery from Natural Products through Structural Modification. Fitoterapia 103, 231–241. 10.1016/j.fitote.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., et al. (2020). A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients 12 (4), 1193. 10.3390/nu12041193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Chen H., Wang W., Liu J., Liu W., Ni P., et al. (2017). Glycyrrhetic Acid, but Not Glycyrrhizic Acid, Strengthened Entecavir Activity by Promoting its Subcellular Distribution in the Liver via Efflux Inhibition. Eur. J. Pharm. Sci. 106, 313–327. 10.1016/j.ejps.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Chen X. X., Zhou H. X., Qi W. B., Ning Z. Y., Ma Y. J., Li Y. L., et al. (2015b). Antiviral Effects of the Combination of Glycyrrhizin and Ribavirin against Influenza A H1N1 Virus Infection In Vivo . Yao Xue Xue Bao 50 (8), 966–972. [PubMed] [Google Scholar]
- Chrzanowski J., Chrzanowska A., Graboń W. (2021). Glycyrrhizin: An Old Weapon against a Novel Coronavirus. Phytotherapy Res. 35 (2), 629–636. 10.1002/ptr.6852 [DOI] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. (2003). Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of SARS-Associated Coronavirus. The Lancet 361 (9374), 2045–2046. 10.1016/s0140-6736(03)13615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., DeVico A. L., Garzino-Demo A., Cara A., Gallo R. C., Lusso P. (1996). The V3 Domain of the HIV-1 Gp120 Envelope Glycoprotein Is Critical for Chemokine-Mediated Blockade of Infection. Nat. Med. 2 (11), 1244–1247. 10.1038/nm1196-1244 [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of V (2020). The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming it SARS-CoV-2. Nat. Microbiol. 5 (4), 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreli F., Friedman-Kien A. E., Flore O. (2005). Glycyrrhizic Acid Alters Kaposi Sarcoma-Associated Herpesvirus Latency, Triggering P53-Mediated Apoptosis in Transformed B Lymphocytes. J. Clin. Invest. 115 (3), 642–652. 10.1172/JCI2333410.1172/jci200523334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan D. J., Subak-Sharpe J. H. (1986). The Antiviral Activity against Herpes Simplex Virus of the Triterpenoid Compounds Carbenoxolone Sodium and Cicloxolone Sodium. J. Antimicrob. Chemother. 18 (Suppl. B), 185–200. 10.1093/jac/18.supplement_b.185 [DOI] [PubMed] [Google Scholar]
- Das P., Delost M. D., Qureshi M. H., Smith D. T., Njardarson J. T. (2019). A Survey of the Structures of US FDA Approved Combination Drugs. J. Med. Chem. 62 (9), 4265–4311. 10.1021/acs.jmedchem.8b01610 [DOI] [PubMed] [Google Scholar]
- Deng Q.-P., Wang M.-J., Zeng X., Chen G. G., Huang R.-Y. (2017). Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell Physiol. Biochem. 41 (4), 1383–1392. 10.1159/000467897 [DOI] [PubMed] [Google Scholar]
- Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. (2020). Glycyrrhetinic Acid and its Derivatives as Potential Alternative Medicine to Relieve Symptoms in Nonhospitalized COVID‐19 Patients. J. Med. Virol. 92, 2200–2204. 10.1002/jmv.26064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., et al. (1996). HIV-1 Entry into CD4+ Cells Is Mediated by the Chemokine Receptor CC-CKR-5. Nature 381 (6584), 667–673. 10.1038/381667a0 [DOI] [PubMed] [Google Scholar]
- Drugs B.I. (2006). “Licorice,” in Drugs and Lactation Database (LactMed) (Bethesda, MD: National Library of Medicine; ). [Google Scholar]
- Duan E., Wang D., Fang L., Ma J., Luo J., Chen H., et al. (2015). Suppression of Porcine Reproductive and Respiratory Syndrome Virus Proliferation by Glycyrrhizin. Antiviral Res. 120, 122–125. 10.1016/j.antiviral.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Senduny F. F., Zidane M. M., Youssef M. M., Badria F. A. (2019). An Approach to Treatment of Liver Cancer by Novel Glycyrrhizin Derivative. Acamc 19 (15), 1863–1873. 10.2174/1871520619666190411114718 [DOI] [PubMed] [Google Scholar]
- Feng Y., Broder C. C., Kennedy P. E., Berger E. A. (2011). Pillars Article: HIV-1 Entry Cofactor: Functional cDNA Cloning of a Seven-Transmembrane, G Protein-Coupled Receptor. Science. 1996. 272: 872-877. J. Immunol. 186 (11), 6076–6081. [PMC free article] [PubMed] [Google Scholar]
- Flores Robles B. J., Hurtarte Sandoval A. R., Penate Dardon J. D., Alonso Blas C. (2013). Lethal Liquorice Lollies (Liquorice Abuse Causing Pseudohyperaldosteronism). Case Rep. 2013, bcr2013201007. 10.1136/bcr-2013-201007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa K. (1991). Interferon Therapy in Hepatitis C Virus (HCV) Induced Chronic Hepatitis: Clinical Significance of Pretreatment with Glycyrhizine. Trop. Gastroenterol. 12 (4), 176–179. [PubMed] [Google Scholar]
- Gao R., Zhang Y., Kang Y., Xu W., Jiang L., Guo T., et al. (2020). Glycyrrhizin Inhibits PEDV Infection and Proinflammatory Cytokine Secretion via the HMGB1/TLR4-MAPK P38 Pathway. Int. J. Mol. Sci. 21 (8), 2961. 10.3390/ijms21082961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda P., Patrick S., Joshi S. D., Kumawat R. K., Sen E. (2021). Glycyrrhizin Prevents SARS-CoV-2 S1 and Orf3a Induced High Mobility Group Box 1 (HMGB1) Release and Inhibits Viral Replication. Cytokine 142, 155496. 10.1016/j.cyto.2021.155496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S. (2005). The Broad Anti-viral Agent Glycyrrhizin Directly Modulates the Fluidity of Plasma Membrane and HIV-1 Envelope. Biochem. J. 392 (Pt 1), 191–199. 10.1042/BJ20051069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Ikematsu S., Koito A., Matsushita S., Maeda Y., Hada M., et al. (1989). Preliminary Evidence for Inhibitory Effect of Glycyrrhizin on HIV Replication in Patients with AIDS. Antivir. Res. 11 (5-6), 255–261. 10.1016/0166-3542(89)90035-1 [DOI] [PubMed] [Google Scholar]
- Hidaka I., Hino K., Korenaga M., Gondo T., Nishina S., Ando M., et al. (2007). Stronger Neo-Minophagen C?, a Glycyrrhizin-Containing Preparation, Protects Liver against Carbon Tetrachloride-Induced Oxidative Stress in Transgenic Mice Expressing the Hepatitis C Virus Polyprotein. Liver Int. 27 (6), 845–853. 10.1111/j.1478-3231.2007.01492.x [DOI] [PubMed] [Google Scholar]
- Hirabayashi K., Iwata S., Matsumoto H., Mori T., Shibata S., Baba M., et al. (1991). Antiviral Activities of Glycyrrhizin and its Modified Compounds against Human Immunodeficiency Virus Type 1(HIV-1) and Herpes Simplex Virus Type 1(HSV-1) In Vitro . Chem. Pharm. Bull. 39 (1), 112–115. 10.1248/cpb.39.112 [DOI] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G. A., et al. (2005). Antiviral Activity of Glycyrrhizic Acid Derivatives against SARS−Coronavirus. J. Med. Chem. 48 (4), 1256–1259. 10.1021/jm0493008 [DOI] [PubMed] [Google Scholar]
- Huan C.-c., Wang H.-x., Sheng X.-x., Wang R., Wang X., Mao X. (2017). Glycyrrhizin Inhibits Porcine Epidemic Diarrhea Virus Infection and Attenuates the Proinflammatory Responses by Inhibition of High Mobility Group Box-1 Protein. Arch. Virol. 162 (6), 1467–1476. 10.1007/s00705-017-3259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan C., Chen C., Xu W., Guo T., Pan H., Gao S. (2020). Study on Antiviral Activities of Glycyrrhizin. Int. J. Biomed. Eng. Clin. Sci. 6 (4), 68–70. 10.11648/j.ijbecs.20200604.11 [DOI] [Google Scholar]
- Huang W., Chen X., Li Q., Li P., Zhao G., Xu M., et al. (2012). Inhibition of Intercellular Adhesion in Herpex Simplex Virus Infection by Glycyrrhizin. Cell Biochem Biophys. 62 (1), 137–140. 10.1007/s12013-011-9271-8 [DOI] [PubMed] [Google Scholar]
- Ide T., Kumashiro R., Kuwahara R., Koga H., Koga Y., Hino T., et al. (2005). Clinical Course of Patients with Chronic Hepatitis B with Viral Breakthrough during Long-Term Lamivudine Treatment. J. Gastroenterol. 40 (6), 625–630. 10.1007/s00535-005-1597-9 [DOI] [PubMed] [Google Scholar]
- Ikeda T., Yokomizo K., Okawa M., Tsuchihashi R., Kinjo J., Nohara T., et al. (2005). Anti-herpes Virus Type 1 Activity of Oleanane-type Triterpenoids. Biol. Pharm. Bull. 28 (9), 1779–1781. 10.1248/bpb.28.1779 [DOI] [PubMed] [Google Scholar]
- Isbrucker R. A., Burdock G. A. (2006). Risk and Safety Assessment on the Consumption of Licorice Root (Glycyrrhiza sp.), its Extract and Powder as a Food Ingredient, with Emphasis on the Pharmacology and Toxicology of Glycyrrhizin. Regul. Toxicol. Pharmacol. 46 (3), 167–192. 10.1016/j.yrtph.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Ito M., Nakashima H., Baba M., Pauwels R., De Clercq E., Shigeta S., et al. (1987). Inhibitory Effect of Glycyrrhizin on the In Vitro Infectivity and Cytopathic Activity of the Human Immunodeficiency Virus [HIV (HTLV-III/LAV)]. Antiviral Res. 7 (3), 127–137. 10.1016/0166-3542(87)90001-5 [DOI] [PubMed] [Google Scholar]
- Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., et al. (1988). Mechanism of Inhibitory Effect of Glycyrrhizin on Replication of Human Immunodeficiency Virus (HIV). Antiviral Res. 10 (6), 289–298. 10.1016/0166-3542(88)90047-2 [DOI] [PubMed] [Google Scholar]
- Kang H., Lieberman P. M. (2011). Mechanism of Glycyrrhizic Acid Inhibition of Kaposi's Sarcoma-Associated Herpesvirus: Disruption of CTCF-Cohesin-Mediated RNA Polymerase II Pausing and Sister Chromatid Cohesion. J. Virol. 85 (21), 11159–11169. 10.1128/JVI.00720-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga M., Hidaka I., Nishina S., Sakai A., Shinozaki A., Gondo T., et al. (2011). A Glycyrrhizin-Containing Preparation Reduces Hepatic Steatosis Induced by Hepatitis C Virus Protein and Iron in Mice. Liver Int. 31 (4), 552–560. 10.1111/j.1478-3231.2011.02469.x [DOI] [PubMed] [Google Scholar]
- Kwon Y.-J., Son D.-H., Chung T.-H., Lee Y.-J. (2020). A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 23 (1), 12–20. 10.1089/jmf.2019.4459 [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. (2020). Functional Assessment of Cell Entry and Receptor Usage for SARS-CoV-2 and Other Lineage B Betacoronaviruses. Nat. Microbiol. 5 (4), 562–569. 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yin J., Sui X., Li G., Ren X. (2009). Comparative Analysis of the Effect of Glycyrrhizin Diammonium and Lithium Chloride on Infectious Bronchitis Virus Infectionin Vitro. Avian Pathol. 38 (3), 215–221. 10.1080/03079450902912184 [DOI] [PubMed] [Google Scholar]
- Li P., Zou H., Ren Y., Zarlenga D. S., Ren X. (2014). Antiviral Effect of Diammonium Glycyrrhizinate on Cell Infection by Porcine Parvovirus. Curr. Microbiol. 69 (1), 82–87. 10.1007/s00284-014-0540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. (2020). Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 382 (13), 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Sun R., Liu R. (2019). Natural Products in Licorice for the Therapy of Liver Diseases: Progress and Future Opportunities. Pharmacol. Res. 144, 210–226. 10.1016/j.phrs.2019.04.025 [DOI] [PubMed] [Google Scholar]
- Lin J.-C., Cherng J.-M., Hung M.-S., Baltina L. A., Baltina L., Kondratenko R. (2008). Inhibitory Effects of Some Derivatives of Glycyrrhizic Acid against Epstein-Barr Virus Infection: Structure-Activity Relationships. Antiviral Res. 79 (1), 6–11. 10.1016/j.antiviral.2008.01.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.-C. (2003). Mechanism of Action of Glycyrrhizic Acid in Inhibition of Epstein-Barr Virus Replication In Vitro . Antiviral Res. 59 (1), 41–47. 10.1016/s0166-3542(03)00030-5 [DOI] [PubMed] [Google Scholar]
- Lu H. Y., Huo N., Wang G. F., Li H. C., Nie L. G., Xu X. Y. (2003). Clinical Observation of Therapeutic Effect of Compound Glycyrrhizin on SARS. China Pharm. (10), 34–36. [Google Scholar]
- Luo P., Liu D., Li J. (2020). Pharmacological Perspective: Glycyrrhizin May Be an Efficacious Therapeutic Agent for COVID-19. Int. J. Antimicrob. Agents 55 (6), 105995. 10.1016/j.ijantimicag.2020.105995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuura T., Aoyagi H., Matsuda M., Hmwe S. S., Date T., et al. (2013). Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro . PLoS One 8 (7), e68992. 10.1371/journal.pone.0068992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Takenaka K., Shimomura H., Fujii N., Shinagawa K., Kiura K., et al. (2001). Lamivudine and Glycyrrhizin for Treatment of Chemotherapy-Induced Hepatitis B Virus (HBV) Hepatitis in a Chronic HBV Carrier with Non-Hodgkin Lymphoma. Leuk. Lymphoma 41 (1-2), 191–195. 10.3109/10428190109057970 [DOI] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H. W., et al. (2011). Glycyrrhizin Exerts Antioxidative Effects in H5N1 Influenza A Virus-Infected Cells and Inhibits Virus Replication and Pro-inflammatory Gene Expression. PLoS One 6 (5), e19705. 10.1371/journal.pone.0019705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B., et al. (2010). Glycyrrhizin Inhibits Highly Pathogenic H5N1 Influenza A Virus-Induced Pro-inflammatory Cytokine and Chemokine Expression in Human Macrophages. Med. Microbiol. Immunol. 199 (4), 291–297. 10.1007/s00430-010-0155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minsart C., Rorive S., Lemmers A., Quertinmont E., Gustot T. (2020). N-acetylcysteine and Glycyrrhizin Combination: Benefit Outcome in a Murine Model of Acetaminophen-Induced Liver Failure. World J. Hepatol. 12 (9), 596–618. 10.4254/wjh.v12.i9.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisy D., Avilov S. V., Jacob Y., Laoide B. M., Ge X., Baudin F., et al. (2012). HMGB1 Protein Binds to Influenza Virus Nucleoprotein and Promotes Viral Replication. J. Virol. 86 (17), 9122–9133. 10.1128/JVI.00789-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Sakai H., Suzuki S., Sugai K., Akutsu Y., Ishikawa M., et al. (1989). Effects of Glycyrrhizin (SNMC: Stronger Neo-Minophagen C) in Hemophilia Patients with HIV Infection. Tohoku J. Exp. Med. 158 (1), 25–35. 10.1620/tjem.158.25 [DOI] [PubMed] [Google Scholar]
- Murck H. (2020). Symptomatic Protective Action of Glycyrrhizin (Licorice) in COVID-19 Infection?. Front. Immunol. 11, 1239. 10.3389/fimmu.2020.01239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. C., Agger S., Rainey P. M. (2009). Too Much of a Good Thing: a Woman with Hypertension and Hypokalemia. Clin. Chem. 55 (12), 2093–2096. 10.1373/clinchem.2009.127506 [DOI] [PubMed] [Google Scholar]
- Nose M., Tada M., Kojima R., Nagata K., Hisaka S., Masada S., et al. (2017). Comparison of Glycyrrhizin Content in 25 Major Kinds of Kampo Extracts Containing Glycyrrhizae Radix Used Clinically in Japan. J. Nat. Med. 71 (4), 711–722. 10.1007/s11418-017-1101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okda F. A., Yassein S., Ahmed A. R., Soufy H., Nasr S. M. (2013). Some Haematological and Biochemical Investigations on Duck Virus Hepatitis Following Administration of Glycyrrhizin. ISRN Pharmacol. 2013, 1–10. 10.1155/2013/849412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonko I., Cookey T. (2015). Seropositivity and Determinants of Immunoglobulin-G (IgG) Antibodies against Herpes Simplex Virus (HSV) Types -1 and -2 in Pregnant Women in Port Harcourt, Nigeria. Afr. Health. Sci. 15 (3), 737–747. 10.4314/ahs.v15i3.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M. B. P. P. (2018). Liquorice (Glycyrrhiza Glabra ): A Phytochemical and Pharmacological Review. Phytotherapy Res. 32 (12), 2323–2339. 10.1002/ptr.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel Y. N., Angelopoulou E., Semple B., Piperi C., Othman I., Shaikh M. F. (2020). Potential Neuroprotective Effect of the HMGB1 Inhibitor Glycyrrhizin in Neurological Disorders. ACS Chem. Neurosci. 11 (4), 485–500. 10.1021/acschemneuro.9b00640 [DOI] [PubMed] [Google Scholar]
- Petramfar P., Hajari F., Yousefi G., Azadi S., Hamedi A. (2020). Efficacy of Oral Administration of Licorice as an Adjunct Therapy on Improving the Symptoms of Patients with Parkinson's Disease, A Randomized Double Blinded Clinical Trial. J. Ethnopharmacology 247, 112226. 10.1016/j.jep.2019.112226 [DOI] [PubMed] [Google Scholar]
- Pompei R., Paghi L., Ingianni A., Uccheddu P. (1983). Glycyrrhizic Acid Inhibits Influenza Virus Growth in Embryonated Eggs. Microbiologica 6 (3), 247–250. [PubMed] [Google Scholar]
- Pompei R., Flore O., Marccialis M. A., Pani A., Loddo B. (1979). Glycyrrhizic Acid Inhibits Virus Growth and Inactivates Virus Particles. Nature 281 (5733), 689–690. 10.1038/281689a0 [DOI] [PubMed] [Google Scholar]
- Pu J. Y., He L., Wu S. Y., Zhang P., Huang X. (2013). Anti-virus Research of Triterpenoids in Licorice. Bing Du Xue Bao 29 (6), 673–679. [PubMed] [Google Scholar]
- Reward E. E., Muo S. O., Orabueze I. N. A., Ike A. C. (2019). Seroprevalence of Herpes Simplex Virus Types 1 and 2 in Nigeria: a Systematic Review and Meta-Analyses. Pathog. Glob. Health 113 (5), 229–237. 10.1080/20477724.2019.1678938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M. R., Efferth T., Serrano M. A., Castaño B., Macias R. I. R., Briz O., et al. (2005). Effect of Artemisinin/artesunate as Inhibitors of Hepatitis B Virus Production in an "In Vitro" Replicative System. Antiviral Res. 68 (2), 75–83. 10.1016/j.antiviral.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Russo S., Mastropasqua M., Mosetti M. A., Persegani C., Paggi A. (2000). Low Doses of Liquorice Can Induce Hypertension Encephalopathy. Am. J. Nephrol. 20 (2), 145–148. 10.1159/000013572 [DOI] [PubMed] [Google Scholar]
- Sakai-Sugino K., Uematsu J., Kamada M., Taniguchi H., Suzuki S., Yoshimi Y., et al. (2017). Glycyrrhizin Inhibits Human Parainfluenza Virus Type 2 Replication by the Inhibition of Genome RNA, mRNA and Protein Syntheses. Drug Discov. Ther. 11 (5), 246–252. 10.5582/ddt.2017.01048 [DOI] [PubMed] [Google Scholar]
- Sasaki H., Takei M., Kobayashi M., Pollard R. B., Suzuki F. (2002). Effect of Glycyrrhizin, an Active Component of Licorice Roots, on HIV Replication in Cultures of Peripheral Blood Mononuclear Cells from HIV-Seropositive Patients. Pathobiology 70 (4), 229–236. 10.1159/000069334 [DOI] [PubMed] [Google Scholar]
- Sato H., Goto W., Yamamura J., Kurokawa M., Kageyama S., Takahara T., et al. (1996). Therapeutic Basis of Glycyrrhizin on Chronic Hepatitis B. Antivir. Res. 30 (2-3), 171–177. 10.1016/0166-3542(96)00942-4 [DOI] [PubMed] [Google Scholar]
- Sekizawa T., Yanagi K., Itoyama Y. (2001). Glycyrrhizin Increases Survival of Mice with Herpes Simplex Encephalitis. Acta Virol. 45 (1), 51–54. 10.1007/s00122-004-1752-3 [DOI] [PubMed] [Google Scholar]
- Shi X., Yu L., Zhang Y., Liu Z., Zhang H., Zhang Y., et al. (2020). Glycyrrhetinic Acid Alleviates Hepatic Inflammation Injury in Viral Hepatitis Disease via a HMGB1-TLR4 Signaling Pathway. Int. Immunopharmacol. 84, 106578. 10.1016/j.intimp.2020.106578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S. K., Prasad S. K., Islam M. A., Chaudhary S. K., Singh S., Shakya A. (2021). Potential Leads from Liquorice Against SARS-CoV-2 Main Protease Using Molecular Docking Simulation Studies. Comb. Chem. High Throughput. Screen 24 (4), 591–597. 10.2174/1386207323999200817103148 [DOI] [PubMed] [Google Scholar]
- Smedegaard S. B., Svart M. V. (2019). Licorice Induced Pseudohyperaldosteronism, Severe Hypertension, and Long QT. Endocrinol. Diabetes Metab. Case Rep. 2019, 1–4. 10.1530/EDM-19-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov V. S., Zarubaev V. V., Anfimov P. M., Shtro A. A. (2012). Effect of a Combination of Glutamyl-Tryptophan and Glycyrrhizic Acid on the Course of Acute Infection Caused by Influenza (H3H2) Virus in Mice. Vopr Virusol 57 (3), 23–27. [PubMed] [Google Scholar]
- Sontia B., Mooney J., Gaudet L., Touyz R. M. (2008). Pseudohyperaldosteronism, Liquorice, and Hypertension. J. Clin. Hypertens. 10 (2), 153–157. 10.1111/j.1751-7176.2008.07470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufy H., Yassein S., Ahmed A., Khodier M., Kutkat M., Nasr S., et al. (2012). Antiviral and Immune Stimulant Activities of Glycyrrhizin against Duck Hepatitis Virus. Afr. J. Trad. Compl. Alt. Med. 9 (3), 389–395. 10.4314/ajtcam.v9i3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zeng H., Wang Q., Yu Q., Wu J., Feng Y., et al. (2018). Glycyrrhizin Ameliorates Inflammatory Pain by Inhibiting Microglial Activation-Mediated Inflammatory Response via Blockage of the HMGB1-TLR4-NF-kB Pathway. Exp. Cel Res. 369 (1), 112–119. 10.1016/j.yexcr.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Sun Y., Song M., Niu L., Bai X., Sun N., Zhao X., et al. (2013). Antiviral Effects of the Constituents Derived from Chinese Herb Medicines on Infectious Bursal Disease Virus. Pharm. Biol. 51 (9), 1137–1143. 10.3109/13880209.2013.781197 [DOI] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. (1994). Effects of Glycyrrhizin on Hepatitis B Surface Antigen: a Biochemical and Morphological Study. J. Hepatol. 21 (4), 601–609. 10.1016/s0168-8278(94)80108-8 [DOI] [PubMed] [Google Scholar]
- Takei M., Kobayashi M., Li X.-D., Pollard R. B., Suzuki F. (2005). Glycyrrhizin Inhibits R5 HIV Replication in Peripheral Blood Monocytes Treated with 1-methyladenosine. Pathobiology 72 (3), 117–123. 10.1159/000084114 [DOI] [PubMed] [Google Scholar]
- Tandon A., Tandon B. N., Bhujwala R. A. (2001). Treatment of Subacute Hepatitis with Lamivudine and Intravenous Glycyrrhizin: a Pilot Study. Hepatol. Res. 20 (1), 1–8. 10.1016/s1386-6346(00)00123-6 [DOI] [PubMed] [Google Scholar]
- Tarao K. (2001). Prevention of Development of Hepatocellular Carcinoma from HCV-Associated Liver Cirrhosis by Multi-Agents Therapy Including Stronger-Neo-Minophagen C. Nihon Rinsho 59 Suppl 6 (Suppl. 6), 769–773. [PubMed] [Google Scholar]
- Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W., et al. (2020). Glycyrrhizic‐Acid‐Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 16 (13), 1906206. 10.1002/smll.201906206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C. S., Steinmann E., et al. (2021). Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 13 (4), 609. 10.3390/v13040609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen C. E. M., Bijlsma J. A., van Dokkum W., Savelkoull T. J. F. (2000). Glycyrrhizic Acid: the Assessment of a No Effect Level. Hum. Exp. Toxicol. 19 (8), 434–439. 10.1191/096032700682694251 [DOI] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R. S., Li F. (2020). Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 94 (7), e00127–20. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323, 1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-W., Sun N., Wu C.-H., Jiang J.-B., Bai Y.-S., Li H.-Q. (2013). In Vitro antiviral Activity and Underlying Molecular Mechanisms of Dipotassium Glycyrrhetate against Porcine Reproductive and Respiratory Syndrome Virus. Antivir. Ther. 18 (8), 997–1004. 10.3851/IMP2662 [DOI] [PubMed] [Google Scholar]
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O. H. J. (2009). Glycyrrhizin Inhibits Influenza A Virus Uptake into the Cell. Antiviral Res. 83 (2), 171–178. 10.1016/j.antiviral.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Xu X. Y., Lu H. Y., ling X. H., Hou F. Q., Yu Y. Y., et al. (2004). Analysis of the Chest X-ray Manifestations in SARS Patients Treated with Compound Glycyrrhizin. China Pharm. (01), 40–42. [Google Scholar]
- Xie Y., Ruan B., Chen Y., Wu N., Hu M., Zhu B. (2011). Kaposi's Sarcoma-Associated Herpesvirus Infection in Chinese Patients with Chronic Hepatitis B. J. Med. Virol. 83 (5), 879–883. 10.1002/jmv.22001 [DOI] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. (2020). Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 63 (3), 457–460. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., Wang H., Cao L., Wang Q., Takahashi S., Yagai T., et al. (2018). Glycyrrhizin Alleviates Nonalcoholic Steatohepatitis via Modulating Bile Acids and Meta-Inflammation. Drug Metab. Dispos 46 (9), 1310–1319. 10.1124/dmd.118.082008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Jiang Y., Zhang Z., Hou J., Tian S., Liu Y. (2020). The Anti-diabetic Activity of Licorice, a Widely Used Chinese Herb. J. Ethnopharmacol. 263, 113216. 10.1016/j.jep.2020.113216 [DOI] [PubMed] [Google Scholar]
- Yang X. W. (2020). Antiviral Effect of Glycyrrhizic Acid. Mod. Chin. Med. 22 (04), 533–541. [Google Scholar]
- Yao H., Liu J., Xu S., Zhu Z., Xu J. (2017). The Structural Modification of Natural Products for Novel Drug Discovery. Expert Opin. Drug Discov. 12 (2), 121–140. 10.1080/17460441.2016.1272757 [DOI] [PubMed] [Google Scholar]
- Yao L., Sun T. (2019). Glycyrrhizin Administration Ameliorates Streptococcus Aureus-Induced Acute Lung Injury. Int. Immunopharmacol. 70, 504–511. 10.1016/j.intimp.2019.02.046 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kobayashi M., Li X. D., Pollard R. B., Suzuki F. (2009). Inhibitory Effect of Glycyrrhizin on the Neutrophil‐dependent Increase of R5 HIV Replication in Cultures of Macrophages. Immunol. Cel Biol. 87 (7), 554–558. 10.1038/icb.2009.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Li Y., Ding Y., Shi Y. (2020). Combination Therapy with Acitretin and Glycyrrhizin in Generalized Pustular Psoriasis with Liver Test Abnormalities: A Case Series. Dermatol. Ther. 33 (3), e13318. 10.1111/dth.13318 [DOI] [PubMed] [Google Scholar]
- Yu S., Zhu Y., Xu J., Yao G., Zhang P., Wang M., et al. (2021). Glycyrrhizic Acid Exerts Inhibitory Activity against the Spike Protein of SARS-CoV-2. Phytomedicine 85, 153364. 10.1016/j.phymed.2020.153364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Li S. H., Lou Z. C. (1988). Morphological and Histological Studies of Chinese Licorice. Yao Xue Xue Bao 23 (3), 200–208. [PubMed] [Google Scholar]
- Zhang K.-X., Wang P.-R., Chen F., Qian X.-J., Jia L., Liu X.-J., et al. (2020). Synthesis and Anti-HCV Activities of 18β-Glycyrrhetinic Acid Derivatives and Their In-Silico ADMET Analysis. Curr. Comput. Aided Drug Des. 16, 1–7. 10.2174/1573409916666200827104008 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ye M. (2009). Chemical Analysis of the Chinese Herbal Medicine Gan-Cao (Licorice). J. Chromatogr. A 1216 (11), 1954–1969. 10.1016/j.chroma.2008.07.072 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang L., Hou J., Tian S., Liu Y. (2021). Molecular Mechanisms Underlying the Anticancer Activities of Licorice Flavonoids. J. Ethnopharmacol. 267, 113635. 10.1016/j.jep.2020.113635 [DOI] [PubMed] [Google Scholar]
- Zhou J., Huang J. (2020). Current Findings Regarding Natural Components with Potential Anti-2019-nCoV Activity. Front. Cel Dev. Biol. 8, 589. 10.3389/fcell.2020.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. (2020). A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 579 (7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 382 (8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]