ABSTRACT
The emergence of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected billions of lives globally, and the world hopes to end this epidemic by effective vaccination. In this review, we depict the latest panorama of global COVID-19 vaccine research and development based on different technology platforms, and summarize key characteristics and available evidence on vaccines authorized for emergency use, in order to provide insights into improve coordination in the COVID-19 outbreak response for related stakeholders.
KEYWORDS: Clinical trial, COVID-19, vaccine, lanscape
COVID-19, an infectious disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has caused substantial morbidity and mortality worldwide. The COVID‐19 vaccine research and development (R&D) landscape is developing at an unprecedented speed and scale, and the achievements are bringing us hope in the midst of this global public health crisis. Herein, the purpose of this study is to depict the latest panorama, key characteristics, and progress of global COVID-19 vaccine R&D, and thus to provide insights into improve coordination in the COVID-19 outbreak response for related stakeholders.
Widely co-sponsorship for Covid-19 vaccines development
As of 26 May, 2021, a total of 442 clinical trials on 185 COVID-19 vaccine candidates have been registered world-wide, with 102 (24.5%) trials in efficacy confirmatory stage and 20 (4.8%) trials under post-marketing surveillance. More than half of the trials (53.8%) were industry-sponsored, another 15.6% and 13.3% trials were respectively sponsored by the academia and government independently (Figure 1). It is worth noting that co-sponsorship from two or even three of these sectors were also observed in over one-sixth of all COVID-19 vaccine trials, indicating the whole society collaborated closely to integrate forces in order to cope with the disease under urgent public need. That is one of the reasons that the development period of COVID-19 vaccine could be shortened substantially to less than a year, another being accelerated review and approval, while vaccine development typically takes up to 10 y.
Figure 1.
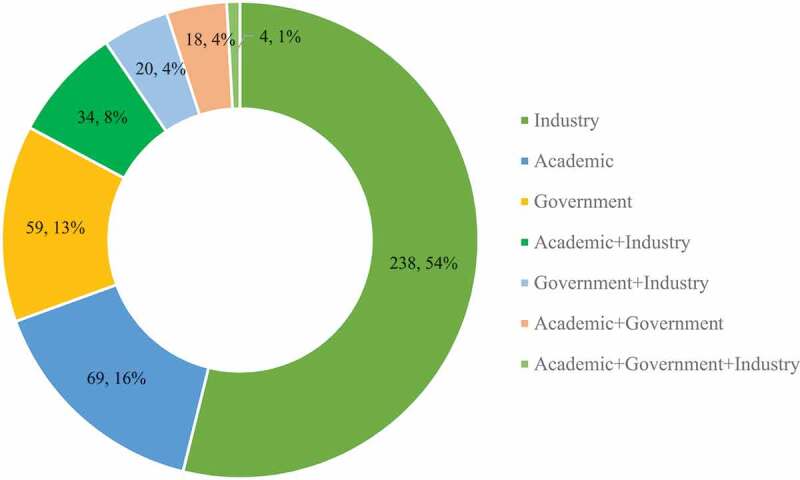
Sponsorship of Covid-19 vaccine clinical trials worldwide (n = 442)
Unbalanced development of Covid-19 vaccine across continents
Uneven geographic distribution of COVID-19 vaccine trials and investigated products were also observed across continents (Table 1). Among all 428 clinical trials clarified regions, 162 clinical trials and 82 vaccine products were investigated or developed in Asia, accounting for a large proportion (38% and 44%) of all trials and products. The continents with the second and third most trials and products were Europe and North America, with 103 (24%) and 87 (20%) ongoing trials and 53 (29%) and 58 (31%) developing or developed vaccine products respectively. The high concentration of vaccine researches in these continents is possibly associated with the large local healthcare demand, considering those regions have relatively higher incidence of cases and number of deaths caused by Covid-19.
Table 1.
Distribution of COVID-19 vaccine trials and products by continent
| Continent | Trial |
Product |
Incidence cases caused by Covid-19, million | Death cases caused by Covid-19, thousand | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Asia | 162 | 38% | 82 | 44% | 50 | 661 |
| Europe | 103 | 24% | 53 | 29% | 46 | 1063 |
| North America | 87 | 20% | 58 | 31% | 39 | 869 |
| South America | 33 | 8% | 23 | 12% | 28 | 756 |
| Africa | 24 | 6% | 15 | 8% | 4.8 | 129 |
| Oceania | 13 | 3% | 13 | 7% | <0.1 | 1 |
All related data has updated to May 26, 2021.
Diversity of COVID-19 vaccine technology platforms
Notably, the technology platforms used on COVID-19 vaccines were abundant and could be classified into 11 types in summary (Figure 2). Among all vaccine trials, the most common technology platforms, in descending order of frequency, were protein subunit (PS), RNA, viral vector non-replicating (VVNR), inactivated virus (IV), DNA, virus-like particle (VLP), viral vector replicating (VVR), VVR combined with antigen presenting cell (APC), live attenuated virus (LAV), dendritic cell vaccine (DCV) and T cell-based vaccine (TCV).
Figure 2.
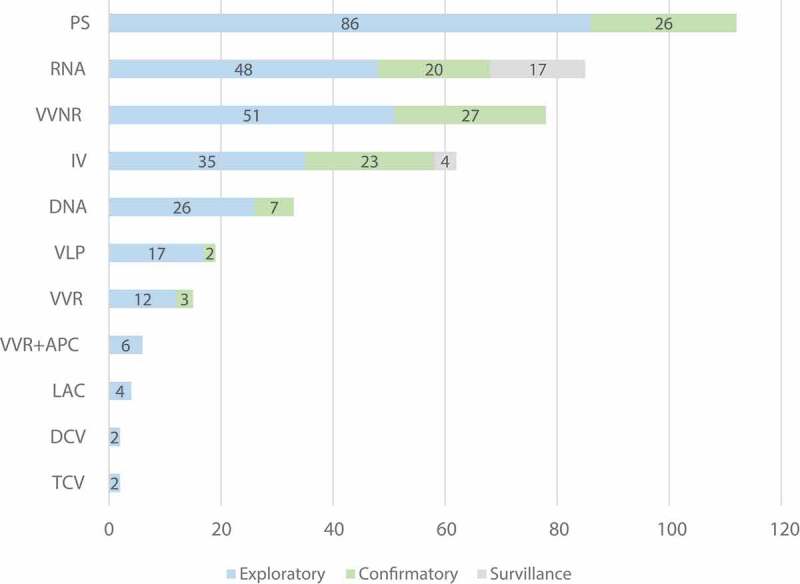
Pipeline of trials on Covid-19 vaccine candidates worldwide (n = 418)
Footnote: Among the 417 Covid-19 vaccine trials, technology platform for 396 trials was clarified. The above figure displayed the technology platform combined with its clinical progress. Abbreviation: Protein subunit (PS); Viral vector non-replicating (VVNR); Inactivated virus (IV); Virus-like particle (VLP); Viral vector replicating (VVR); Viral vector replicating combined with antigen presenting cell (VVR+APC); Live attenuated virus (LAV); Dendritic cell (DCV), T cell-based (TCV).
Latest progress of COVID-19 by vaccine technology platform
In the perspective of R&D process, vaccine types including TCV, LAV, DCV, VVR+APC, were still in its initial phase that few products were designed for each type, and no product has been confirmed with adequate safety up to this date, let alone efficacy. Notwithstanding no effectiveness confirmed on status quo, VVR, VLP and DNA have more products arranged and part of involved products have already entered efficacy confirmatory phase. It is gratifying that there were two RNA vaccines, two PS vaccines, four VVNR vaccines and seven IV vaccines authorized for emergency use. Among those, Gam-COVID-Vac Lyo was the first approved vaccine while BNT162b2, currently authorized by 84 countries, is the most widely licensed vaccine. Among the newest approved products, there were two single-dosed VVNR vaccine, one three-dosed PS vaccine. For detailed information, refer to Table 2.
Table 2.
Overview of authorized COVID-19 vaccines worldwide
| Vaccine | Sponsor | Vaccine type | Administration |
Approval information |
Efficacy evidence |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage | Dosage | Date | Age range | Approval countries | Confirmatory evidence | Sample size | Efficacy | |||
| Gam-COVID-Vac Lyo | Gamaleya Research Institute | VVNR | stable at 2–8°C | 2 doses, 0/21d | 2020/8/10 | Unclarified | 68 | Yes | 21,977 | 92% |
| BBIBP-CorV* | Sinopharm | IV | stable at 2–8°C | 2 doses, 0/21d | 2020/8/12 | Unclarified | 41 | Yes | 25,463 | 78.1% |
| EpiVacCorona | Federal Budgetary Research Institution | PS | Unclarified | 2 doses, 0/21-28d | 2020/10/13 | ≥18 y old | 2 | Unreported | / | / |
| BNT162b2 | Pfizer/BioNtech/ Fosun Pharma | RNA | Stable at −80 ~ −60°C; 2 ~ 8°C for 1 month | 2 doses, 0/21d | 2020/11/20 | ≥16 y old | 84 | Yes | 43,548 | 95% |
| mRNA-1273 | Moderna/NIAID | RNA | Stable at −50 ~ −15°C; 2 ~ 8°C for 30 d; 8 ~ 25°C for 24 hours | 2 doses, 0/28d | 2020/12/18 | ≥18 y old | 46 | Yes | 30,420 | 94% |
| AZD-1222 | Oxford Universty/ AstraZeneca | VVNR | stable at 2–8°C | 2 doses, 0/4-12 w | 2020/12/30 | ≥18 y old | 98 | Yes | 11,636 8534 |
70% 70% |
| COVAXIN | Bharat Biotech | IV | stable at 2–8°C | 2 doses, 0/28d | 2021/1/3 | Unclarified | 9 | Yes | 25,800 | 81% |
| CoronaVac* | Sinovac | IV | stable at 2–8°C | 2 doses, 0/14d | 2021/1/11 | Unclarified | 25 | Unreported | / | / |
| QAZCOVID-IN | Unclarified | IV | Unclarified | Unclarified | 2021/1/113 | Unclarified | 1 | Unreported | / | / |
| CoviVac | Russian Academy of Sciences | IV | Unclarified | Unclarified | 2021/2/20 | Unclarified | 1 | Yes | 12,396 7371 | 51% 91% |
| Unclarified* | Sinopharm | IV | stable at 2–8°C | 2 doses, 0/21d | 2021/2/25 | Unclarified | 1 | Yes | 25,480 | 72.8% |
| Ad5-nCoV* | CanSino BIO | VVNR | stable at 2–8°C | 1 dose | 2021/2/25 | Unclarified | 5 | Unreported | / | / |
| Ad26.COV2.S | Janssen | VVNR | Stable at −20°C; 2 ~ 8°C for 3 months | 1 dose | 2021/3/1 | ≥18 y old | 41 | Unreported | / | / |
| ZF2001 | Zhifei/Chinese Academy of Sciences | PS | stable at 2–8°C | 3 doses, 0/30/60d | 2021/3/1 | Unclarified | 2 | Unreported | / | / |
| KCONVAC | Beijing Minhai Biotechnology Co | IV | Unclarified | 2 doses, 0/28d | 2021/5/14 | ≥18 y old | 1 | Unreported | / | / |
* Vaccines with an asterisk were conditionally approved in China, and the others were approved for emergency use authorization only. Viral vector non-replicating is abbreviated as VVNR; Inactivated virus is abbreviated as IV; Protein subunit is abbreviated as PS.
Safety and efficacy evidence on approved COVID-19 vaccines
For those vaccines developed under vaccine platforms that have not yet received any approval for emergency use, neither safety nor efficacy evidence has been found from randomized controlled trials with sufficient sample size. The safety and efficacy of three IV vaccines (Cronavac, QAZCOVID-IN and KCONVAC), two VVNR vaccines (Ad5-nCoV and Ad26.COV2.S) and two PS vaccines (EpiVacCorona and ZF2001) have not been confirmed by any confirmatory trial yet. Overall, compared to statistical success criterion and 50% efficacy goal set for a placebo-controlled vaccine trial by FDA,1 the efficacy of the six vaccines with reported confirmatory trials was all acceptable, ranging from 51% to 95%.2–10 Additionally, severe, serious, and medically attended adverse events occurred at low levels (less than 0.5%) and were balanced between vaccine and placebo groups.
In the past year, great breakthroughs have been made in the global fight against the epidemic. However, the unprecedented speed has raised potential concerns by the public on safety and efficacy. Though confirmatory evidences have been found in phase III trials of the five vaccines, more attention should be paid to capture real-world data to identify long-term safety and efficacy of authorized products. To date, real-world effectiveness data are only available for BNT162b2 vaccine in nationwide mass vaccination setting in Israel.11
It is also worth noting that the primary endpoint of the five confirmatory studies was inconsistent, despite the regulatory guidance from the FDA.12 Cases in Gam-COVID-Vac Lyo and BNT162b2 trial were defined as confirmed COVID-19 infection by laboratory test, while in AZD-1222, mRNA-1273 and COVAXIN trial, cases were defined as having one and two qualifying symptoms, which indicates the success of vaccines was based on preventing COVID-19 infection of essentially any severity, instead of preventing severe infection or efficacy in frail elderly.
Outlook for next-generation Covid-19 vaccine
An ideal vaccine should be safe, efficacious and cost-effective. In the meantime, it should have good immunogenicity that can induce persistent neutralizing antibody, and have high thermal stability that makes storage and global transportation feasible. It was reported that PS vaccines produced higher neutralizing antibody titers and more complete protection than live-attenuated DNA-based vaccines. The potential advantages of mRNA vaccines include the ability to mimic natural infection to stimulate a more potent immune response as well as the ability to combine multiple mRNAs into a single vaccine.13 However, it is not without any problem: despite that they have been approved and used, RNA-based vaccines are relatively difficult to be stored and distributed since it requires to be stored in a low temperature which cannot be easily implemented with limited budget. The merits of nucleic acid-based vaccines consisting of DNA or mRNA are that they can be adapted quickly when new viruses emerge, which explains why they were among the very first COVID-19 vaccines to enter clinical trials.14
In terms of future development of vaccines, additional collaboration in the areas of antiviral discovery and enhancement of clinical practice is of great importance. Given the prolonged and costly drug development process, a more sophisticated system is needed to accelerated the approval and distribution of vaccines. Besides, more attention should be paid on older patients, as well as those vulnerable population suffering from other diseases in future COVID-19 vaccine research and development.
Conclusion
In summary, the pace of global vaccine R&D has been phenomenal, and we are confident that vaccines will ultimately be able to accommodate the demand of the public within a foreseeable number of years. On the one hand, it is crucial to keep an eye on the long-term efficacy and safety of vaccines that have been authorized in real-world, as well as to explore heterologous prime-boost strategy combining vaccines of different technology platforms, which might increase levels and persistence of neutralizing antibodies. On the other hand, we need to ensure a continuous safe supply of vaccines, especially in under-developed regions. Meanwhile, alternative options for individuals not eligible for vaccination, surveillance and developing targeting vaccines of the most common new strains should also be incorporated in R&D of next-generation COVID-19 vaccines.
Funding Statement
The data analysis and interpretation were supported by the Chinese Academy of Medical Sciences’ Initiative for Innovative Medicine [grant 2020-I2M-2-007].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research . Development and licensure of vaccines to prevent COVID-19: guidance for industry. [accessed 2021 April 25]. https://www.fda.gov/media/139638/download.
- 2.Xia SL, Zhang YT, Wang YX, Wang H, Yang YK, Gao F, Tan WJ, Wu GZ, Xu M, Lou ZY, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;S1473-3099(20)30942–7. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;S0140-6736(21)00234–8. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders RW, de Jong MD.. Pandemic moves and countermoves: vaccines and viral variants. Lancet. 2021;397(10282):1326–27. doi: 10.1016/S0140-6736(21)00730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SINOVAC . Summary of Clinical Trial Data of Sinovac’s COVID-19 Vaccine (CoronaVac®). [accessed 2021 April 28]. http://www.sinovac.com.cn/news/shownews.php?id=1154&lang=en.
- 10.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021May26. doi: 10.1001/jama.2021.8565. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD.. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA Center for Biologics Evaluation and Research . Development and licensure of vaccines to prevent COVID-19. [accessed 2021 March 2]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19.
- 13.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–31. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Riel D, De Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–12. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]


