Abstract
Neuroinflammation is associated with many neurodegenerative diseases. Abnormal activation of microglial cells in the central nervous system (CNS) is a major characteristic of neuroinflammation. Nitric oxide (NO) free radicals are produced by activated microglia and prolonged presence of large quantities of NO in the CNS can lead to neuroinflammation and disease. Hispidin is a polyphenol derived from Phellinus linteus (a valuable medicinal mushroom) with strong antioxidant, anticancer and antidiabetic properties. A previous study demonstrated that hispidin significantly inhibited NO production via lipopolysaccharide (LPS)-induced RAW264.7 macrophages. Therefore, the present study used MTT assay was used to detect the effect of hispdin on cell viability. Griess reagent analysis was used to measure NO production. Reverse transcription-semi quantitative PCR and western blotting were used to evaluate the effects of hispdin on iNOS mRNA and MAPK/ERK/JNK protein levels. Fluorescence microscopy and flow cytometry were used to detect the effects of hispdin on the production of ROS and phagocytosis of cells. The present results indicated that hispidin could significantly inhibit the increase of NO production and iNOS expression in BV-2 microglial cells stimulated by LPS. The inhibitory effect of hispidin on NO production was similar to that of S-methylisothiourea sulfate, an iNOS inhibitor. Signaling studies demonstrated that hispidin markedly suppresses LPS-induced mitogen activated protein kinases and JAK1/STAT3 activation, although not the NF-κB signaling pathway. The present observations in LPS-stimulated BV-2 microglial cells indicated that hispidin might serve as a therapeutic candidate for the treatment of NO-induced neuroinflammation and, potentially, as a novel iNOS inhibitor.
Keywords: hispidin, lipopolysaccharide, nitric oxide, microglia, neuroinflammation
Introduction
Neuroinflammation is a protective mechanism of the central nervous system (CNS) following injury. However, if neuroinflammation persists, it can lead to many neurodegenerative diseases, such as Alzheimer's disease (AD) (1), Huntington's disease (HD) (2) and Parkinson's disease (PD) (3). Neuroinflammation is characterized by abnormal activation of glial cells and the presence of inflammatory mediators in the CNS microenvironment (4). Microglia are tissue macrophages in the CNS, which have unique origins and functions (5). Under normal conditions, microglia protect neurons by immune defense, phagocytic activity, antigen presentation and secretion of immunomodulatory factors (6). However, in response to injury, infection or inflammation, microglia are readily activated and release various pro-inflammatory factors, including IL-6, TNF-α, C-C motif chemokine ligand 2 and reactive oxygen species (ROS), which increase the expression of inducible nitric oxide synthase (iNOS) and cause accumulation of excessive nitric oxide (NO) (7).
As a neurotransmitter and second messenger molecule, NO mediates a variety of neuronal functions in the brain (8). NO is synthesized by iNOS, which is detected at low levels in healthy brains and spinal cords (8). However, continuous activation of microglia induces abnormal expression of iNOS proteins, leading to a large accumulation of NO (9). The latter can damage the cell membrane structure, affect DNA transcription and protein synthesis, and directly damage neurons (10); moreover, it can be further oxidized by oxygen free radicals to form highly toxic peroxynitrite, which can cause nitration of tyrosine residues in cells and damage neurons (11). In addition, NO causes neuronal degeneration by changing the intracellular Fe2+ concentration (12). Knockdown of iNOS genes in primary glial cells inhibits the production of NO, thus reducing the degeneration of CNS (13). A previous study suggested that iNOS- and NO-induced neuroinflammatory responses in the CNS could play an important role in the development of neurodegenerative lesions (13).
Hispidin is a polyphenolic substance that was first isolated from the fruiting body of hispidus in 1889 by Zopf, where its structure was later identified by Edwards et al (14) as 6-(3,4-dihydroxyphenyl)-4-hydroxy-2-pyrone in 1961(15). Recent, hispidin were isolated from ethanolic extracts and fermentation products of medicinal mushroom Phellinus linteus (16). In addition, a previous study demonstrated that hispidin has anti-inflammatory, anti-bacterial, anti-oxidant, anti-cancer and hypoglycemic regulatory functions (17). However, the inhibitory effect of hisplidin on NO production by the lipopolysaccharide (LPS)-induced microglia remains unclear. Therefore, the present study aimed to investigate the effects of hispidin on NO production and iNOS expression in BV-2 microglial cells, along with its underlying mechanism.
Materials and methods
Chemicals and antibodies
Hispidin (cat. no. H5257), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and LPS (cat. no. L4391; from Escherichia coli serotype 0111:B4) were purchased from Sigma-Aldrich; Merck KGaA. Fetal bovine serum (FBS, cat. no. SH30070.03) and DMEM (cat. no. SH30003.01) were purchased from HyClone; Cytiva. Hoechst 33258 (cat. no. B8030), penicillin/streptomycin (P/S, cat. no. P1400), N-acetylcysteine (NAC, cat. no. IA0050) were purchased from Beijing Solarbio Science & Technology Co., Ltd. PD98059 (ERK signal inhibitor; cat. no. S1805), SB203580 (P38 signal inhibitor; cat. no. S1863), SP600125 (JNK signal inhibitor; cat. no. S1876), CM-H2DCFDA (cat. no. S0033S) and Dihydroethidium (DHE; cat. no. S0063) were obtained from Beyotime Institute of Biotechnology. Rabbit monoclonal antibodies for phosphorylated (p)-STAT3 (cat. no. bsm-52210R, dilution: 1:1,000), STAT3 (cat. no. bsm-52235R, dilution: 1:500), p-JNK (cat. no. bsm-52462R, dilution: 1:1,000), IκB-α (cat. no. bsm-52169R, dilution: 1:500) and rabbit polyclonal antibodies for p-JAK1 (cat. no. bs-3238R dilution: 1:1,000), JAK1 (cat. no. bs-1439R, dilution: 1:1,000), p-ERK (cat. no. bs-3238R, dilution: 1:1,000), JNK (cat. no. bs-10562R, dilution: 1:1,000), p-P38 (cat. no. bs-5477R, dilution: 1:1,000) and P38 (cat. no. bs-0637R, dilution: 1:1,000) were purchased from BIOSS. Mouse monoclonal antibodies for β-actin (cat. no. ab8226, dilution: 1:1,000) were purchased from Abcam. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no. D111018, dilution: 1:5,000) and anti-mouse IgG (cat. no D110097, dilution: 1:5,000) were purchased from Sangon Biotech Co., Ltd.. Cell culture dishes were obtained from Wuxi NEST Biotechnology Co., Ltd. TRIzol® reagent (cat. no. 10296028) was purchased from Invitrogen; Thermo Fisher Scientific, Inc. PrimeScript™ reagent Kit with gDNA Eraser (cat. no. RR047A) was purchased from Takara Bio, Inc. The primers for iNOS and GAPDH were synthesized by Sangon Biotech Co., Ltd. ECL Western Blotting Substrate (cat. no. PE0010) was obtained from Beijing Solarbio Science & Technology Co., Ltd.
Cell culture
BV-2 microglial cells were obtained from the American Type Culture Collection and cultivated in DMEM supplemented with 10% FBS and 1% P/S (100 U/ml and 100 µg/ml, respectively) at 37˚C and 5% CO2 under saturated humidity. Cells were passaged when they were ~80% confluent. According to different experimental arrangements, cells were inoculated into the cell culture dish based on the required number of cells, and a follow-up study was conducted after the cells adhered to the wall.
Cell viability assay
BV-2 microglial cells were seeded in 96-well plates at a concentration of 4x103 cells per well and cultured in DMEM. The cells were treated with various concentrations of hispidin (0-20 µg/ml) for 24 h. A solution of 5 mg/ml MTT was subsequently added to each well and the cells were incubated for 4 h at 37˚C in an atmosphere containing 5% CO2. Subsequently, the supernatant was removed and formazan was dissolved in DMSO. Absorbance was measured at 490 nm using a UV Max Kinetic Microplate Reader (Molecular Devices, LLC).
Detection of NO production by Griess reagent
NO production was assessed based on the accumulation of nitrite in the medium using a colorimetric reaction with Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 0.1% sulfanilamide, and 2.5% H3PO4]. Culture supernatants were collected and mixed with an equal volume of Griess reagent. Absorbance was measured at 540 nm using the UV Max Kinetic Microplate Reader.
Detection of iNOS mRNA expression by reverse transcription-semi quantitative PCR
Total cellular RNA was prepared using the TRIzol® reagent, followed by cDNA synthesis using by the reverse transcription kit in accordance with the manufacturer's protocols. The reverse transcription reaction conditions were set at 37˚C for 15 min, followed by 85˚C for 5 sec and finally reduced to 4˚C. The cDNA was amplified (SuperScript™ IV One-Step RT-PCR System with ezDNase™; cat. no. 12595025; Thermo Fisher Scientific, Inc.) using the following PCR primers: iNOS forward, 5'-CCCTTCCGAAGTTTCTGGCAGCAGC-3' and reverse, 5'-GGCTGTCAGAGCCTCGTGGCTTTGG-3', GAPDH forward, 5'-TGTGTCCGTCGTGGATCTGA-3' and reverse, 5'-CCTGCTTCACCACCTTCTTGA-3'. Thermocycling was performed using an initial 94˚C hold step for 5 min. This hold step was followed by 25-30 cycles of 94˚C for 30 sec, 65˚C (iNOS) or 52˚C (GAPDH) for 30 sec and 72˚C for 30 sec and a final extension step for 5 min at 72˚C. The amplified samples were separated in 1% agarose gels with ethidium bromide and images were taken with the Alpha Gel Imaging System (Alpha Imager HP; Version 3.5.0; Protein Simple).
Western blotting
Following treatment with LPS or hispidin, cells were washed twice with PBS, lysed with protein lysis buffer [20 mM HEPES-OH (pH 7.0), 50 mM NaCl, 10% glycerol and 0.5% Triton X-100] and incubated with 0.5 µg/ml leupeptin, 0.7 µg/ml pepstatin A, 0.1 mM 4 (2 aminoethyl) benzenesulfonyl fluoride and 2 µg/ml aprotinin for 30 min at 4˚C. The concentration of the obtained proteins was determined using Coomassie blue staining. The protein assay system was prepared (800 µl DDW + 200 µl Coomassie Blue + 1 µl protein sample). The OD value was detected at 595 nm using a UV spectrophotometer and the obtained OD value was subsequently substituted into a standard curve to obtain the protein concentration. The proteins were then denatured for 5 min at 100˚C and 20 µg protein was separated using 12% SDS-PAGE and transferred onto nitrocellulose membranes (EMD Millipore). The membranes were blocked with 5% skimmed milk for 30 min at room temperature. They were then incubated with primary antibodies at 4˚C overnight. Membranes were washed five times with Tris buffered saline (TBS) containing Tween [10 mM Tris HCl (pH 7.5), 150 mM NaCl and 0.2% Tween-20] and subsequently incubated with HRP-conjugated goat anti-rabbit IgG or anti-mouse IgG (dilution, 1:5,000) for 1 h at room temperature. After five washes with TBS to remove excess antibody, an appropriate amount of ECL Plus (cat. no. PE0010; from Beijing Solarbio Science & Technology Co., Ltd.) was added to each membrane and specific binding was then detected using a chemiluminescence detection system (Amersham Imager 600; Cytiva). All quantification of bands was done using ImageJ 1.52a (National Institutes of Health).
Measurement of ROS by flow cytometry and fluorescence microscopy
To determine ROS levels, the BV-2 microglial cells were incubated at 37˚C for 15 min with 10 mM CM-H2DCFDA. The CM-H2DCFDA fluorescence intensity of 10,000 cells was evaluated by flow cytometry (BDFACSCalibur™, BD Biosciences). The results were analyzed using the WinMDI (Version 2.9, BD Biosciences) software.
Measurement of ROS by fluorescence microscopy
Changes in cellular ROS levels were determined using 1 µM DHE and 2 µg/ml Hoechst 33258 (to observe the nucleus) at 37˚C for 15 min and washed with PBS to detect changes in cellular ROS levels. After washing with PBS, the magnification of the microscope was adjusted to x200 and images were taken with a fluorescent core cell culture microscope (EVOS® XL core cell culture microscope; Thermo Fisher Scientific, Inc.) to qualitatively observe the fluorescence intensity.
Statistical analysis
Data are presented as the means ± standard error of the mean. Differences between experimental groups were analyzed by one-way analysis of variance and a Tukey's test. GraphPad Prism software version 4.0 (GraphPad Software, Inc.) was used to analyze all results and P<0.05 was considered to indicate a statistically significant difference.
Results
Hispidin inhibits the production of NO in LPS-treated BV-2 microglial cells
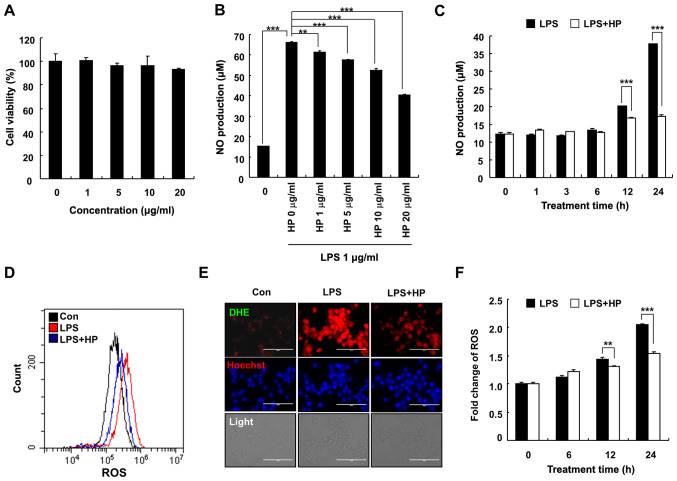
To determine the cytotoxic effects of hispidin on BV-2 microglial cells, the BV-2 microglial cells were treated with various concentrations (0, 1, 5, 10 and 20 µg/ml) of hispidin for 24 h. As presented in Fig. 1A, 20 µg/ml hispidin had no effect on the viability of BV-2 microglial cells, hence indicating that the inhibitory effect of hispidin on LPS-induced inflammation was not due to cytotoxicity. Therefore, in subsequent experiments, 20 µg/ml was used as the maximum treatment concentration. To determine whether hispidin could exert any anti-inflammatory effect, the levels of NO and ROS were measured in BV-2 microglial cells after LPS treatment. Cells were pretreated with hispidin (1, 5, 10 or 20 µg/ml) for 30 min and subsequently treated with LPS for the indicated times (0, 1, 3, 6, 12 or 24 h). The results demonstrated that hispidin decreased the production of NO in LPS-treated BV-2 microglial cells in a dose- and time-dependent manner (Fig. 1B and C). Intracellular ROS levels were detected by flow cytometry and fluorescence microscopy, and the results demonstrated that LPS treatment increased the ROS level of BV-2 microglial cells, while hispidin pretreatment significantly reduced the intracellular ROS levels (Fig. 1D-F). Together, the results indicated that hispidin regulates the activity of BV-2 microglial cells by inhibiting the production of inflammatory mediators.
Figure 1.
HP inhibits the production of NO in LPS-treated BV-2 microglial cells. (A) Cells were treated with various concentrations of HP and cultivated for 24 h. Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (B) The level of NO was analyzed in the culture medium using Griess reagent. (C) Cells were pre-treated with designated concentrations of HP for 30 min prior to LPS treatment (1 µg/ml) and were incubated for 24 h. The cells were pretreated with 20 µg/ml HP for 30 min, followed by treatment with LPS (1 µg/ml) for the indicated durations. Cells were pre-treated with 20 µg/ml HP for 30 min, followed by treatment with LPS (1 µg/ml) for 24 h. The level of cellular ROS was detected using (D) flow cytometry when stained with CM-H2DCFDA and (E) fluorescence microscopy when stained with Hoechst/DHE (blue/red). Scale bar, 200 µm. (F) Following treatment with LPS (1 µg/ml) for different durations, the level of cellular ROS was detected using flow cytometry after staining with CM-H2DCFDA. Data are presented as the means ± standard error of the mean for three different samples. **P<0.01, ***P<0.001. HP, hispidin; NO, nitric oxide; LPS, lipopolysaccharide; ROS, reactive oxygen species; DHE, dihydroethidium; Con, control.
Hispidin inhibits the expression of iNOS protein in LPS-treated BV-2 microglial cells
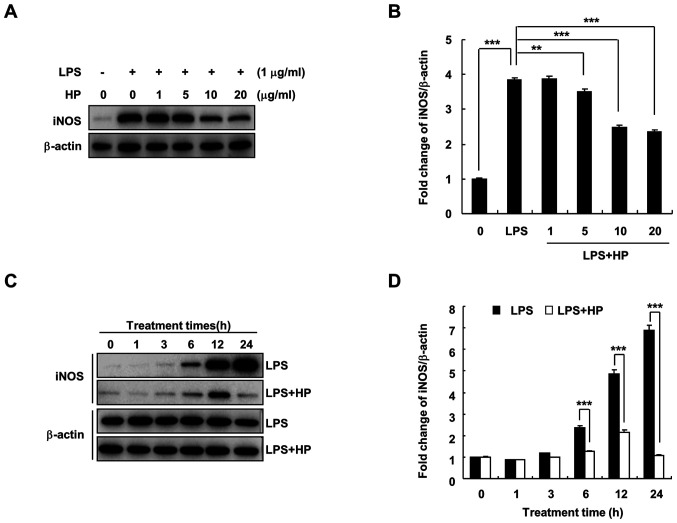
Under the pathological conditions of inflammation and hypoxia and in the presence of tumors, iNOS expression is increased and catalyzes the production of NO (7). When BV-2 microglial cells are stimulated by substances, such as LPS, the expression of iNOS increases, which in turn induces the production of large quantities of NO and promotes the development of inflammation and other diseases (18). As shown in Fig. 2, hispidin treatment was found to significantly decrease LPS-induced iNOS protein expression in a dose- and time-dependent manner.
Figure 2.
HP inhibits the expression of iNOS protein in LPS-treated BV-2 microglial cells. The level of iNOS protein expression was evaluated by western blotting. (A and B) Cells were pre-treated with designated concentrations of HP for 30 min prior to LPS treatment (1 µg/ml) and were incubated for 24 h. (C and D) Cells were pretreated with 20 µg/ml HP for 30 min, followed by treatment with LPS (1 µg/ml) for the indicated durations. Data are presented as the means ± standard error of the mean for three different samples. **P<0.01 and ***P<0.001. HP, hispidin; NO, nitric oxide; iNOS, inducible NO synthase; LPS, lipopolysaccharide.
Hispidin inhibits NO production and iNOS expression by inhibiting MAPK signaling pathway in LPS-treated BV-2 microglial cells
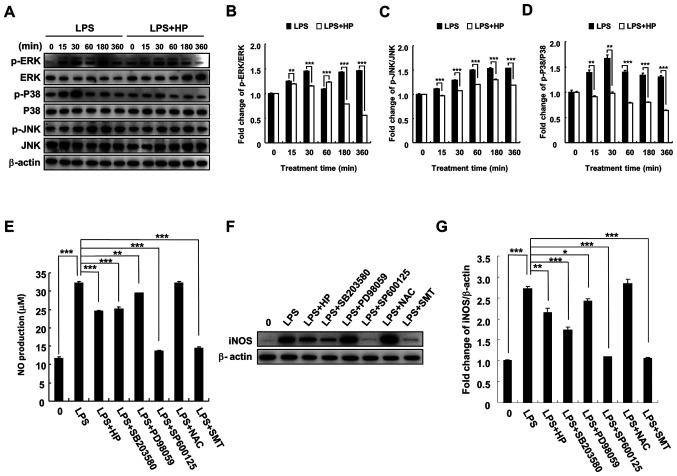
To investigate the mechanism of inhibition of LPS-induced NO production and iNOS expression by hispidin in BV-2 microglial cells, phosphorylation of MAPK signaling pathway-related proteins (JNK, P38 and ERK) by hispidin was investigated. BV-2 microglial cells were pretreated with 20 µg/ml hispidin for 30 min and then treated with LPS (1 µg/ml) for the indicated times. As expected, LPS treatment increased the phosphorylation levels of JNK, P38 and ERK. However, after pretreatment with hispidin, the phosphorylation levels were downregulated and the P38 signaling pathway was inhibited to the greatest extent (Fig. 3A-D). Next, to clarify whether hispidin inhibits LPS-induced NO production in BV-2 microglial cells by inhibiting the MAPK signaling pathway, the inhibitory effects of MAPK inhibitors (P38, SB203580; ERK, PD98059; and JNK, SP600125), ROS scavenger (NAC) and selective inhibitor of iNOS (S-methylisothiourea sulfate; SMT) on LPS-induced NO production and iNOS protein expression were compared. The results indicated that all reagents, except NAC, reduced NO production and iNOS protein expression in LPS-treated BV-2 microglial cells (Fig. 3E-G).
Figure 3.
HP inhibits NO production and iNOS expression via inhibition of the MAPK signaling pathway in LPS-treated BV-2 microglial cells. Cell were pre-treated with 20 µg/ml HP for 30 min, followed by treatment with LPS (1 µg/ml) as well as inhibitors (P38, SB203580; ERK, PD98059; and JNK, SP600125) for the indicated durations. (A) Protein expression levels of p-ERK, ERK, p-P38, P38, p-JNK and JNK were detected by western blotting. (B-D) The associated protein expression levels are represented as the means ± SD. (E) Level of NO was evaluated in the culture medium using Griess reagent. (F) Protein expression of iNOS was detected by western blotting. (G) Associated protein expression levels are presented as means ± SD. *P<0.05, **P<0.01 and ***P<0.001. HP, hispidin; NO, nitric oxide; iNOS, inducible NO synthase; LPS, lipopolysaccharide; p, phosphorylated; NAC, N-acetylcysteine; SMT, S-methylisothiourea sulfate.
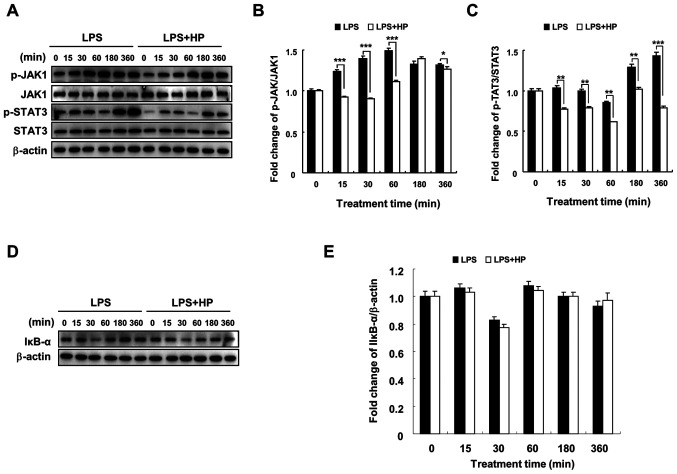
Hispidin inhibits the JAK1/STAT3 signaling pathway, but does not affect the NF-κB signaling pathway in LPS-treated BV-2 microglial cells
In a previous study, hispidin was found to inhibit NF-κB, MAPK and JAK1/STAT3 signaling pathways in RAW264.7 macrophages and played an anti-inflammatory role (17). The current results indicated that hispidin could inhibit the MAPK signaling pathway in LPS-treated BV-2 microglial cells (Fig. 3) and changes were detected in two other signaling pathways by western blotting. Hispidin treatment was observed to downregulate the expression of p-JAK1 and p-STAT3 compared to that in the LPS-treated group; however, it had no significant effect on the protein expression of IκB-α (Fig. 4). These results indicate that although hispidin inhibited the LPS-induced JAK1/STAT3 signaling pathway in BV-2 microglial cells, it had no effect on the NF-κB signaling pathway.
Figure 4.
HP inhibits the JAK1/STAT3 signaling pathway, but has no effect on the NF-κB signaling pathway in LPS-treated BV-2 microglial cells. Cells were pre-treated with 20 µg/ml HP for 30 min, followed by treatment with LPS (1 µg/ml) for the indicated durations. (A) Expression levels of p-JAK1, JAK1, p-STAT3 and STAT3 proteins were detected by western blotting. (B and C) The associated protein expression levels are presented as the means ± SD, *P<0.05, **P<0.01 and ***P<0.001. (D) The expression of IκB-α protein was detected by western blotting. (E) The associated protein expression levels are presented as means ± SD. HP, hispidin; LPS, lipopolysaccharide; p, phosphorylated.
Discussion
Microglia are resident immune cells in the CNS and the literature has confirmed that they have a macrophage-like phenotype, with regard to morphological and quantitative changes during microglial cell activation (5). Microglia form one of the main factors in the occurrence and development of neuroinflammation (19). Undifferentiated, resting microglia are responsible for monitoring and patrolling the brain environment, as well as activating a response to injury and infection (20). Activated microglia trigger the inflammatory immune response in the nervous system by releasing cytokines, including IL-1, IL-6 and TNF-α, which clears damaged and necrotic cells or neurons from the lesion (21). However, when microglial cells remain activated, they secrete a large number of neurotoxic mediators, including inflammatory cytokines, inflammatory chemokines, ROS, NO and the excitotoxic amino acid glutamate, which disrupt synapses and neural connections in the brain, triggering a series of neuroinflammatory responses that result in neurodegenerative diseases, such as AD (22). These cytotoxic mediators interact with each other to amplify their own toxicity, induce neuronal death, damage the nervous system and increase the risk of central neurodegenerative diseases (23). This pathological process further activates the surrounding microglia, stimulates the excessive release of inflammatory mediators, induces central neuroinflammatory responses and forms a vicious cycle of central inflammatory cascade responses (24). Therefore, the aforementioned studies have collectively indicated that inflammation of brain tissue due to continuous activation of microglia is the key to the induction of neurodegenerative diseases.
NO is a highly unstable biological free radical, which is widely distributed in various tissues of mammals, particularly the nervous tissue (25). It is critical in various important physiological functions and is one of the main neurotransmitters in memory formation (26). NO is catalyzed by NOS to generate L-arginine; there are three main types of NOS in the body, namely, eNOS, present in endothelial cells, nNOS, present in neuronal cells, and iNOS, present in macrophages, hepatocytes and glial cells (27). NOS is present in almost all tissues, and multiple subtypes coexist in the same tissue, for example, all three subtypes are present in the nervous system (28). As an intercellular second messenger, NO plays an important role in cardiovascular, neurological, immunomodulatory, anti-inflammatory and antitumor processes (29). In the CNS, NO plays a dual role as an intercellular messenger for neuroprotection and neurotoxicity (11). NO produced under normal physiological conditions is beneficial to the functioning of the nervous system; studies have shown that low concentrations of NO plays an important role in brain neuromodulation, neurotransmission and synaptic plasticity (8,30). When endogenous or exogenous NO is overproduced and released, it becomes a strong neurotoxic substance that can be involved in a variety of neurodegenerative and inflammatory pathological processes, leading to neuronal apoptosis and disease induction (8). A large volume of evidence has indicated excessive NO and its mediated apoptosis in organs and tissues to be associated with neurological diseases, such as epilepsy, AD, PD, amyotrophic lateral sclerosis and HD, as well as cerebral ischemia/reperfusion injury and Down's syndrome (18,31-34). This is of particular interest to neurodegenerative conditions, such as AD and PD, which increasingly are the focus of research on the pathogenesis and treatment of such diseases (3,35). Therefore, effective inhibition of NO production by activated microglia may be an important strategy to control neuroinflammation. In the present study, hispidin treatment was found to significantly decrease NO production and iNOS protein expression levels in LPS-treated BV-2 microglial cells. The effect of hispidin on iNOS mRNA was also investigated and hispidin treatment did not affect the mRNA content of iNOS (Fig. S1); hence, it was speculated that hispidin inhibits LPS-induced NO production in BV-2 cells by affecting iNOS protein synthesis, although the probable underlying mechanism requires further investigation. The current results indicate that hispidin may play an important role in the suppression of neuroinflammation.
In addition, the present results demonstrated the inhibitory effect of hispidin on ROS levels in LPS-treated microglia. ROS includes oxygen-containing radicals or oxygen atoms involved in oxidation reactions in the body, as well as peroxides and superoxides that produce oxygen radical electrons (36,37). ROS plays a vital role in regulating the process of neurodegenerative diseases (38,39). As an upstream regulator of inflammation, ROS increase the expression of pro-inflammatory genes and induce neurotoxicity through lipid peroxidation (38,40). Moreover, oxygen free radicals catalyze the formation of neurotoxic substances with NO, such as peroxynitrite (36). A pharmacological studies have shown that oxidative stress damage is closely associated with neuroinflammatory response, and jointly promotes the occurrence and development of CNS-degenerative diseases (41,42). The present findings on the inhibitory effect of hispidin on ROS production suggested that hispidin could be a potential anti-inflammatory drug candidate for the treatment of LPS-induced neuroinflammation.
In our previous study, it was reported that hispidin exerts anti-inflammatory effects by extensively inhibiting the activation of three signaling pathways in LPS-induced RAW264.7 macrophages, including NF-κB, MAPK and JAK1/STAT3(17). However, whether hispidin plays a role against neuroinflammation by inhibiting the signaling pathways in microglia remains unknown. The NF-κB signaling pathway is heavily involved in the inflammatory response and consists of p65/p50 and IκB. Of these, IκB inhibits NF-κB, and the transcription of hundreds of genes, such as inflammatory factors, chemical chemokines, enzymes and adhesion molecules can be regulated only when this inhibition is removed (41). Typically, NF-κB, consisting of p65/p50 and IκB, is present in the cytoplasm in an inactive state (43). When a pathogen infects the cell, it acts as a pattern of pathogen-associated molecules that is recognized by a pattern recognition receptor on the surface of the cell membrane and binds to it (44). This receptor-ligand binding transmits an extracellular infection signal into the cell, which in turn recruits signal adapter proteins in the cytoplasm to phosphorylate, ubiquitinate and degrade IκB through activation of the IκB kinase complex (45). It can enter the cell nucleus, bind to DNA, and regulate the transcription and expression of inflammatory cytokines (45). The cytokines produced bind to receptors on the cell membrane, phosphorylate JAK, which in turn phosphorylates downstream molecules of STAT, ultimately inducing a transcriptional response and increasing gene expression of relevant inflammatory mediators (46). Notably, the present results demonstrated that LPS decreased the expression of IκB-α while hispidin had no effect on IκB-α. This result was inconsistent with the previous finding in RAW264.7, in which hispidin increased the expression of IκB-α and inhibited activation of the NF-κB signaling pathway. The appearance of this discrepancy was of interest and subsequently it was noted that hispidin had previously been reported as an inhibitor of cell permeability against protein kinase C (PKC) (47). During macrophage activation, PKC acts as an intermediate mediator of MAPK signaling pathway activation (48); while it has been shown that when PKC binds to a foreign stimulus, such as LPS, it leads to phosphorylation of IκB-α (49). Therefore, the present study speculated that the reason why hispidin has different regulatory effects on the activated signaling pathway in the BV-2 microglial cells and RAW264.7 is most likely due to the difference in the origin of the two cells, which leads to differences in PKC protein expression on the membranes of these two cells. However, this hypothesis requires further investigation. Meanwhile, the present study identified that hispidin significantly downregulated the phosphorylation of JAK1 and STAT3 and inhibited activation of the JAK1/STAT3 signaling pathway. Although the effect of hispidin on cytokine production by LPS-activated BV-2 microglial cells was not examined in the present study, inhibition of the JAK1/STAT3 signaling pathway implied that hispidin influences cytokine production.
MAPKs play an important regulatory role in the expression and secretion of a variety of inflammatory mediators (50,51). In our previous study, it was shown that inhibition of phosphorylation of JNK inhibits the production of NO and expression of iNOS protein induced by LPS (17). In the present study, hispidin was found to inhibit the phosphorylation of p38, ERK and JNK, with p38 demonstrating the most marked change. Furthermore, the inhibitory effects of MAPK inhibitors, the ROS scavenger (NAC) and the selective inhibitor of iNOS (SMT) on LPS-induced NO production and iNOS protein expression were compared. The results demonstrated that the addition of MAPK signaling pathway inhibitors decreased LPS-induced NO production and western blot results showed the reduction of iNOS protein expression. These results collectively suggested that activation of the MAPK signaling pathway is associated with the activation of BV-2 microglial cells by LPS. Conversely, the inhibition of NO by the addition of other inhibitors may suggest that hispidin could be a potential inhibitor candidate of iNOS; however, the mechanism of this inhibition requires further investigation. The scavenging of ROS by NAC did not inhibit NO production and iNOS protein expression, indicating that ROS produced by LPS-induced BV-2 microglial cells does not promote the production of NO and that they may be relatively independent in the development of neuroinflammation.
Thus, the present study indicated that hispidin significantly inhibits NO production and iNOS expression by suppressing the MAPK signaling pathway, though not the NF-κB signaling pathway, in LPS-activated microglial cells. In addition, hispidin was shown to inhibit activation of the JAK1/STAT3 signaling pathway, which may have an effect on cytokine release. These findings may provide a novel insight into the possibility of hispidin serving as a therapeutic target for clinical treatment of neuroinflammation.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. 2020R1I1A2052417), the Korean Research Institute of Bioscience and Biotechnology Research Information System (grant no. RBM0112112) and also supported by the project Sanzong of Heilongjiang Bayi Agricultural University (grant no. ZRCPY202029).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MHJ, DQC, HNS and TK performed the experiments and analyzed the data. YHJ prepared screen natural product, analyzed the data and took part in the study design. YHH collected, analyzed and interpreted the experimental data. MHJ, DQC and HNS confirm the authenticity of all the raw data. HS and TK conceived and designed the project. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: The devil is in the details. J Neurochem. 2016;139 (Suppl 2):S136–S153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker FO. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, He C, Wu WY, Chen F, Wu YY, Li WZ, Chen HQ, Yin YY. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson's disease. Pharmacol Biochem Behav. 2015;138:96–103. doi: 10.1016/j.pbb.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci. 2020;21:139–152. doi: 10.1038/s41583-020-0263-9. [DOI] [PubMed] [Google Scholar]
- 6.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 7.Sun HN, Jin MH, Han B, Feng L, Han YH, Shen GN, Yu YZ, Jin CH, Lian ZX, Lee DS, et al. 16α,17α-epoxypregnenolone-20-oxime prevent LPS-induced NO production and iNOS expression in BV-2 microglial cells by inhibiting JNK phosphorylation. Biol Pharm Bull. 2014;37:1096–1102. doi: 10.1248/bpb.b13-00706. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: The role of vitagenes. In Vivo. 2004;18:245–267. [PubMed] [Google Scholar]
- 9.Hannibal L. Nitric oxide homeostasis in neurodegenerative diseases. Curr Alzheimer Res. 2016;13:135–149. doi: 10.2174/1567205012666150921101250. [DOI] [PubMed] [Google Scholar]
- 10.Meini A, Sticozzi C, Massai L, Palmi M. A nitric oxide/Ca(2+)/calmodulin/ERK1/2 mitogen-activated protein kinase pathway is involved in the mitogenic effect of IL-1beta in human astrocytoma cells. Br J Pharmacol. 2008;153:1706–1717. doi: 10.1038/bjp.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ijomone OM, Aluko OM, Okoh COA, Ebokaiwe AP. Nω-nitro-L-arginine, a nitric oxide synthase inhibitor, attenuates nickel-induced neurotoxicity. Drug Chem Toxicol. 2021:1–10. doi: 10.1080/01480545.2021.1917382. [DOI] [PubMed] [Google Scholar]
- 12.Majewski M, Kozlowska A, Thoene M, Lepiarczyk E, Grzegorzewski WJ. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J Physiol Pharmacol. 2016;67:3–19. [PubMed] [Google Scholar]
- 13.Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50:633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RL, Lewis DG, Wilson DV. 983. Constituents of the higher fungi. Part I. Hispidin, a new 4-hydroxy-6-styryl-2-pyrone from polyporus hispidus (Bull.) Fr. J Chem Soc: 4995-5002, 1961. [Google Scholar]
- 15.Edwards RL, Wilson DV. 984. Constituents of the higher fungi. Part II. The synthesis of hispidin. J Chem Soc (Resumed) 1961:5003–5004. [Google Scholar]
- 16.Chandimali N, Huynh DL, Jin WY, Kwon T. Combination effects of hispidin and gemcitabine via inhibition of stemness in pancreatic cancer stem cells. Anticancer Res. 2018;38:3967–3975. doi: 10.21873/anticanres.12683. [DOI] [PubMed] [Google Scholar]
- 17.Han YH, Chen DQ, Jin MH, Jin YH, Li J, Shen GN, Li WL, Gong YX, Mao YY, Xie DP, et al. Anti-inflammatory effect of hispidin on LPS induced macrophage inflammation through MAPK and JAK1/STAT3 signaling pathways. Appl Biol Chem. 2020;63(21) [Google Scholar]
- 18.Oh YC, Li W, Choi JG. Saussureae radix attenuates neuroinflammation in LPS-stimulated mouse BV2 microglia via HO-1/Nrf-2 induction and inflammatory pathway inhibition. Mediators Inflamm. 2021;2021(6687089) doi: 10.1155/2021/6687089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takata K, Kitamura Y, Saeki M, Terada M, Kagitani S, Kitamura R, Fujikawa Y, Maelicke A, Tomimoto H, Taniguchi T, Shimohama S. Galantamine-induced amyloid-{beta} clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J Biol Chem. 2010;285:40180–40191. doi: 10.1074/jbc.M110.142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 21.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer's disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 23.González-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 24.Voet S, Srinivasan S, Lamkanfi M, van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 2019;11(e10248) doi: 10.15252/emmm.201810248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Northington FJ, Martin LJ. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct Funct. 2010;214:219–234. doi: 10.1007/s00429-009-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly MEM, Barnes S. Physiology and pathophysiology of nitric oxide in the retina. Neuroscientist. 1997;3:357–360. [Google Scholar]
- 27.Marks JD, Schreiber MD. Inhaled nitric oxide and neuroprotection in preterm infants. Clin Perinatol. 2008;35:793–807. doi: 10.1016/j.clp.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: An overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Jaffrey SR, Snyder SH. Nitric oxide: A neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 30.Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA. Oxidative stress, protein modification and Alzheimer disease. Brain Res Bull. 2017;133:88–96. doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Gjoneska E, Pfenning AR, Mathys H, Quon G, Kundaje A, Tsai LH, Kellis M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: The good, the bad, and the resting. J Neuroimmune Pharmacol. 2009;4:389–398. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- 33.Shaw PR, Haydar TF. Mitigating cognitive deficits in down syndrome by managing microglia activation. Neuron. 2020;108:799–800. doi: 10.1016/j.neuron.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Jiang J, Jiang W, Zhou X, Lu W, Wang J, Luo Y. doi: 10.1007/s11064-021-03360-8. Spata2 knockdown exacerbates brain inflammation via NF-κB/P38MAPK signaling and NLRP3 inflammasome activation in cerebral ischemia/reperfusion rats. Neurochem Res: Jun1, 2021 (Epub ahead of print). doi: 10.1007/s11064-021-03360-8. [DOI] [PubMed] [Google Scholar]
- 35.Law A, Gauthier S, Quirion R. Say NO to Alzheimer's disease: The putative links between nitric oxide and dementia of the Alzheimer's type. Brain Res Brain Res Rev. 2001;35:73–96. doi: 10.1016/s0165-0173(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 36.Estévez AG, Jordán J. Nitric oxide and superoxide, a deadly cocktail. Ann N Y Acad Sci. 2002;962:207–211. doi: 10.1111/j.1749-6632.2002.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong JS. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 40.Farber JL. Mechanisms of cell injury by activated oxygen species. Environ Health Perspect. 1994;102 (Suppl 10):S17–S24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.You MM, Chen YF, Pan YM, Liu YC, Tu J, Wang K, Hu FL. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediators Inflamm. 2018;2018(7834381) doi: 10.1155/2018/7834381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solt LA, May MJ. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Yin W, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y, Pi R. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: Involvement of NF-κB inhibition and Nrf2 pathway activation. Eur J Pharmacol. 2017;806:1–9. doi: 10.1016/j.ejphar.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 45.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 46.Heinrich PC, Behrmann I, MüLler-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonindard C, Bergonzi C, Denier C, Sergheraert C, Klaebe A, Chavant L, Hollande E. Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro. Cell Biol Toxicol. 1997;13:141–153. doi: 10.1023/a:1007321227010. [DOI] [PubMed] [Google Scholar]
- 48.Dann SG, Golas J, Miranda M, Shi C, Wu J, Jin G, Rosfjord E, Upeslacis E, Klippel A. p120 catenin is a key effector of a Ras-PKCε oncogenic signaling axis. Oncogene. 2014;33:1385–1394. doi: 10.1038/onc.2013.91. [DOI] [PubMed] [Google Scholar]
- 49.van der Vorst EPC, Theodorou K, Wu Y, Hoeksema MA, Goossens P, Bursill CA, Aliyev T, Huitema LFA, Tas SW, Wolfs IMJ, et al. High-density lipoproteins exert pro-inflammatory effects on macrophages via passive cholesterol depletion and PKC-NF-κB/STAT1-IRF1 signaling. Cell Metab. 2017;25:197–207. doi: 10.1016/j.cmet.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Wu WY, Wu YY, Huang H, He C, Li WZ, Wang HL, Chen HQ, Yin YY. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int J Mol Med. 2015;35:391–398. doi: 10.3892/ijmm.2014.2020. [DOI] [PubMed] [Google Scholar]
- 51.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.