Abstract
Animal models of rheumatoid arthritis (RA) are essential for studying the pathogenesis of RA in vivo and determining the efficacy of anti-RA drugs. During the past decades, numerous rodent models of arthritis have been evaluated as potential models and the modeling methods are relatively well-developed. Among these models, the collagen-induced arthritis (CIA) mouse model is the first choice and the most widely used because it may be generated rapidly and inexpensively and is relatively similar in pathogenesis to human RA. To date, there have been numerous classic studies and reviews discussing related pathogeneses and modeling methods. Based on this knowledge, combined with the latest convenient and effective methods for CIA model construction, the present review aims to introduce the model to beginners and clarify important details regarding its use. Information on the origin and pathogenesis of the CIA model, the protocol for establishing it, the rate of successful arthritis induction and the methods used to evaluate the severity of arthritis are briefly summarized. With this information, it is expected that researchers who have recently entered the field or are not familiar with this information will be able to start quickly, avoid unnecessary errors and obtain reliable results.
Keywords: rheumatoid arthritis, mouse model, collagen-induced arthritis, protocol, applicability
1. Introduction
Rheumatoid arthritis (RA) is a multisystem inflammatory autoimmune disease that destroys the surrounding joints. The basic pathological change that occurs in RA is chronic synovitis, which is accompanied by synovial cell proliferation, inflammatory cell infiltration and the formation of vasospasms that invade the cartilage and bone tissue of the subsynovial layer, causing joint destruction (1-4). When the disease progresses to an advanced stage, the joint tissue is severely damaged and all joint function is lost. Furthermore, to a certain extent, the lungs (5), cardiovascular system (6,7), nervous system (8) and other organs (9,10) are selectively affected, which seriously impairs the patients' quality of life. RA is a disease that occurs globally. It may occur in adults of any age but is mainly diagnosed in middle-aged females (11,12). For the treatment of RA, timely and effective control of disease progression are urgently required. Early diagnosis and treatment may prevent bone and joint damage, as well as reduce disability and suffering, which lays a foundation for improving the quality of life of affected patients (13).
To date, the etiology and pathogenesis of RA have remained to be fully elucidated. Due to its complex pathogenesis, there is currently no ideal drug that is able to completely cure RA. Accordingly, animal models of RA are an important resource for studying and exploring the pathogenesis of RA, as well as developing effective anti-inflammatory drugs. These animal models may be classified in several ways according to species (mainly rat and mouse), disease type (genetically engineered, induced or spontaneous) and inciting agent (chemicals, collagen or exogenous polysaccharides/proteins/proteoglycans) (14). Commonly used models include collagen-induced arthritis (CIA) (15), proteoglycan-induced arthritis (16), Staphylococcus aureus-induced arthritis (17,18) and genetically engineered arthritis mice (such as K/BxN mice) (19,20). In addition, chimera models are frequently used to test drugs with specific targets by transferring corresponding human tissue samples to nonobese diabetes (NOD)/severe combined immunodeficiency (SCID) mice. For instance, the human RA synovium-cartilage-NOD/SCID mouse chimera model (arthritis/SCID mouse chimera model) (21,22) may be used to test the mechanism of synovial invasion of cartilage and bone and the efficacy of related drugs. General practical considerations in the use of various rodent disease models have been reviewed by Bolon et al (14) and Williams (23). Caplazi et al (24) discussed mouse models of RA, providing a wider perspective regarding systemically induced mouse models of RA as well as the value of polyarthritis and spontaneously occurring and genetically engineered models of RA.
The present review mainly focused on providing a detailed introduction to the CIA mouse model. This model is always the first choice and the most widely used, as it may be generated rapidly and inexpensively and is similar in pathogenesis to human RA (25). However, this model has several critical features that are frequently overlooked by researchers. For instance, the use of the model has a limited scope and the arthritis induction rate varies depending on the genetic background of the mice (26). Furthermore, there are several controversies regarding the modeling process, such as when drugs should ideally be administered and how to choose the best administration method. The present review provides detailed knowledge related to these topics. With this information, it is expected that researchers who are new to the field or unfamiliar with this knowledge are able to avoid unnecessary errors and select the appropriate model to obtain reliable results.
2. Currently, the CIA model best reproduces the clinical symptoms of RA
In 1977, Trentham et al (27) reported for the first time that the immunization of rats with a human, chicken or rat type II collagen (CII) emulsion in complete Freund's adjuvant (CFA) led to the development of erosive polyarthritis, accompanied by an autoimmune response to cartilage. This CIA model was reproduced in mice and monkeys in 1980 and 1986, respectively (28,29). Since CII is the main protein in articular cartilage, the immune response generated by CII mainly targets the joints. The production of CII-specific antibodies in mice is an important feature that has also been reported in RA (30). The clinical and histological appearance of CII-induced arthritis in mice indicates that this is an ideal animal model for the investigation of various immunogenetic traits in RA (31). For instance, mice immunized with CII also produce rheumatoid factors (26,32). Furthermore, the pathological features of both RA and CIA consist of marked synovitis with cartilage degradation and bone erosion (32).
Susceptibility to RA is closely related to certain allelic subtypes of human leukocyte antigen (HLA)-DR and HLA-DQ (23,33). The DR and DQ molecules expressed by these subtypes are able to present autoantigen peptides such as CII 260-273 and may be recognized by a specific T-cell receptor (TCR), thereby activating T cells (34). The susceptibility of mice to CIA is also closely related to major histocompatibility complex (MHC) class II molecules. The mouse histocompatibility 2, class II antigen A, beta 1 (IA) gene is homologous to HLA-DQ, and the mouse histocompatibility 2, class II antigen E alpha (IE) gene is homologous to the HLA-DR gene (35). It has been indicated that polymorphisms of the β1 chain of the IA molecule determine susceptibility to CIA and that the IE molecule is also involved in the regulation of CIA-related pathological processes, which are related to the incidence and severity of CIA (36). Sequence analysis of IA, IE, DQ and DR indicated that in humans and mice, the MHC class II molecule β1 chain expressed by the RA/CIA allelic subtypes frequently had the conserved sequence glutamine-lysine/arginine-arginine-alanine-alanine, known as the shared epitope. The spatial structure formed by the side chain molecules of these adjacent amino acid residues is the key binding site of the antigenic peptide. The MHC II molecules expressed by H-2q mice are IAq and IEq, and the MHC class II molecule that recognizes the presented CII antigen is IAq; therefore, IAq mice (such as DBA/1 and B10.Q mice) are frequently used to generate CIA animal models (31,37).
Accordingly, the establishment of CIA requires heterogeneous (bovine or chicken) CII to immunize susceptible mice two times. The disease-related CII antigenic peptide must contain a core peptide. CII is a homotrimer composed of three A1 chains containing 1,018 amino acids each. Within these chains, the CII 260-270 segment contains functional amino acids that are able to bind to MHC II molecules and be recognized by the TCR; they are known as the core antigen peptides and are related to the occurrence of RA and CIA. The core peptide is easily degraded at room temperature, which is why it is necessary to work at 4˚C when emulsifying collagen. As the H-2q haplotype has strong susceptibility to CII core peptides, it is able to induce strong T-cell proliferation and CIA production (38). Excessive activation of T cells, increased secretion of cytokines and production of CII-specific antibodies are the major factors involved in the pathogenesis of CIA (39). The CIA mouse model is mainly induced by CD4+ T cells and MHC class II-restricted T cells (40). In this disease model, T helper type 1 (Th1) cytokines are secreted and damage is mediated by the cellular immune response. T cells interact with antigen-presenting cells, activate lymphocytes and stimulate monocytes/macrophages to release numerous inflammatory factors, such as IL-1β and TNF-α, leading to CIA inflammation, hyperplasia of the synovial lining, neoangiogenesis, pannus formation and the destruction of cartilage and bone (41,42).
Of note, Bolon et al (14) and Williams (23), among others, reported that immunization of mice with heterologous type II collagen usually leads to a relatively acute and self-remitting form of arthritis. By contrast, immunization with autologous collagen results in more severe and prolonged arthritis that is probably more reminiscent of human RA. However, due to the low affinity of a specific epitope of murine collagen (CII256-270) for IAq, autologous type II collagen is less arthritogenic than heterologous collagen, resulting in a low level of CII-specific T-cell activation. The use of heterologous CII protein is still recommended as the first choice from a comprehensive perspective (43). In addition, CII-responsive T cells can only be induced when the amount of heterologous CII is large enough. An appropriate amount of CII should be used when immunizing mice; the optimal CII concentration is specified further below.
The CIA mouse model also has certain drawbacks. The joints of mice are small and CIA in mice exhibits a variable disease pattern. By contrast, rat arthritis models offer much larger specimen sizes and the distribution and extent of inflammatory changes in rat CIA joints are more reproducible (14). Therefore, modeling with mice requires more standardized modeling protocols and operations to ensure the replicability of the model as much as possible. Mice have the advantage of costing less than rats (44); thus, it is prudent to use a CIA mouse model in the initial screening of anti-RA drugs unless the purpose of the experiment has specific requirements for specimen size or other restrictions that would preclude mice.
In general, among the various mouse models, the CIA model is most similar to RA in terms of pathogenesis and clinical characteristics. It is relatively stable and is an ideal and internationally recognized arthritis model for studying the pathogenesis of RA and screening drugs for RA treatment.
3. Establishment of the CIA mouse model
Selecting the sex and age of animals
Both females and males may be used for the CIA model (42,45,46). However, it is reported that in mice, CIA tends to be more severe in males than in females (45,47). Overall, the choice of sex in mice depends on the purpose of the experiment. If the study requires to exclude estrogen-related factors, male mice must be selected. If there are no special requirements in the experiment, the use of female mice is also possible. Additionally, there is currently no report clearly stating that female mice cannot be used. In addition, the prevalence of RA is significantly higher in females than in males (48). In the CIA model, the incidence of arthritis may reach 100% in male mice (49) and >80% in female mice.
Mice older than 7-8 weeks may be used to construct the CIA model. The immune system of mice does not mature until the mice reach 7-8 weeks of age. It has been reported that 8-12 weeks of age is the optimal age for starting mouse experiments (42). However, certain studies suggested that the use of older mice (10-14 weeks of age) is important, as the incidence of CIA is higher in older mice (36,50). Of note, the incidence and severity of disease in aged mice also decreases when the mice are too old (36). The ideal compromise should be to use mice aged approximately 10-12 weeks for these experiments.
Preparation of related materials
The main reagents required are as follows: Complete Freund's adjuvant (CFA; 5 ml); incomplete Freund's adjuvant (IFA; 5 ml); and immunization-grade chick type II collagen (10 mg), lyophilized. In the first immunization, it is recommended to use an emulsion of CFA and chicken CII. In the second immunization, an emulsion of IFA and chicken CII is recommended.
Freund used a water-in-mineral oil emulsion containing Mycobacterium tuberculosis (Mtb) cells; this was termed CFA. CFA is primarily used to help activate immune cells and produce antibodies that target the desired antigen. A possible adverse reaction to CFA is the formation of an epithelioid granuloma at the injection site. Conversely, the use of IFA, which lacks the mycobacterial component, does not cause such acute granulomatous adverse reactions (51). It is the CII antigen, not Mtb, that must be constantly present after activation of the immune cells. CFA is not necessary after the first immunization because the immune cells are already active at this point. Thus, it is better to use IFA in the second immunization.
Glacial acetic acid (0.1 M) should be prepared one day in advance, filtered and sterilized. Glacial acetic acid should be added to the chicken CII reagent bottle and a pipette should be used to gently and evenly mix the reagents (final concentration, 4 g/l). The mixed solution should be stored at 4˚C overnight to fully dissolve the chicken CII. In addition, the following supplies should be prepared in advance: A shaver, mouse holders, a homogenizer, 1-ml syringes, an ice box, a bottle of sterile saline, medical cotton balls, 6-well plates and marker pens. In order to produce the immunizing emulsion, CII should be combined with an equal volume of CFA or IFA (the final concentration of CII is 2 g/l in 0.05 M glacial acetic acid solution) by mixing them in short bursts using an homogenizer. Specific instructions for emulsification are as follows: Freund's adjuvant (2.5 ml) is added to the syringe, followed by the addition of an equal volume of collagen solution and stirring at a low speed while adding the collagen solution in a dropwise manner. Subsequently, mixing at full power (~1,000 g) for 2-3 min at a time with intervals for 0.5 min is performed until a stable emulsion is obtained. The whole emulsification process requires to be performed on ice to prevent heating. The final emulsion should be sufficiently thick not to drip out of the vessel when it is inverted (36). A good way to test its quality is to place a drop onto a surface of water. If the emulsion does not disperse, it has the correct consistency [for more specific directions, please refer to this published protocol (36)].
Induction of the mouse CIA model
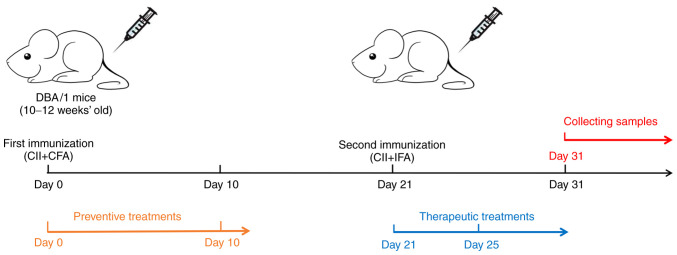
As mentioned above, equal volumes of CFA (4 mg/ml) and chicken CII should be mixed and fully emulsified at low temperatures with a homogenizer until the obtained liquid has a milky white appearance and is insoluble in water. Subsequently, the emulsifier should be dispensed into a 1-ml syringe (the dispensed emulsifier requires to be kept at 4˚C and should be used within 6 h). The mouse should be placed in the holder and the hair should be removed from the base of the tail with a shaver or depilatory cream. The tail of the mouse should be disinfected with alcohol on a cotton ball. Each mouse should be subcutaneously injected with 100 µl of the emulsion. After the injection, the injection site of the mouse should be disinfected with alcohol on a cotton ball. A bulge should form at the base of the tail due to the accumulation of the drug and the operator must gently rub the bulge with their fingers until the bulge is no longer present; this step promotes the complete absorption of the emulsifier. The mouse is then removed from the holder and placed back in the cage. On the 21st day after the first immunization, the same method is used to inject the emulsion of IFA and chicken CII (100 µl per mouse). The mental state, activity, food/water intake and body weight of the mice, as well as the presence of redness and swelling of their paws, should be recorded everyday starting on day 0 (D0) (36). A flowchart for the generation of CIA model mice is provided in Fig. 1.
Figure 1.
Flowchart for the generation of collagen-induced arthritis model mice. CII, collagen type II; CFA, complete Freund's adjuvant; IFA, incomplete Freund's adjuvant.
4. Evaluation indices for the CIA model
Success rate of model establishment
The development of CIA depends on the mouse strain. The DBA/1 strain (H-2q) haplotypes exhibit the greatest susceptibility (52) and are the most commonly used strain for the CIA model in the preclinical testing of potential antiarthritic drugs (42,53).
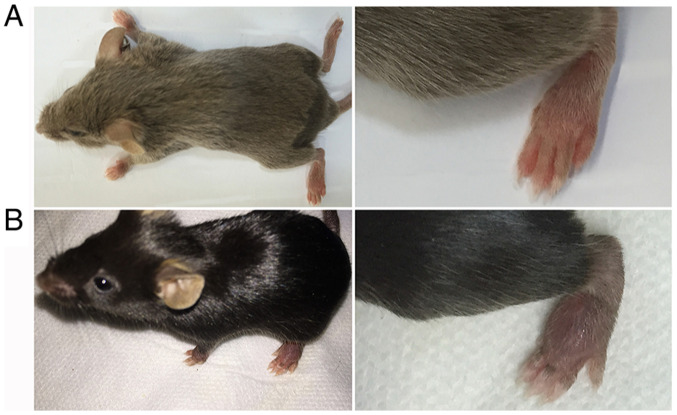
Mice with a C57BL/6 (H-2b) background may also be used for the CIA model (54). For example, certain studies may use transgenic mice to assess the impact of a specific gene on the pathogenesis of arthritis. As most transgenic mice are C57BL/6, those that are C57BL/6 may be used to construct CIA models. However, the C57BL/6 (H-2b) strain is relatively resistant to CIA. After repeated trials, it was verified that the induction rate of mice with a C57BL/6 background was low (approximately 15-65%), while that of mice with a DBA/1 background reached 80-100% (Fig. 2). Therefore, the use of mice with a DBA/1 background is recommended for experiments without special requirements.
Figure 2.
Images of (A) the DBA/1 mouse model of CIA and (B) the C57BL/6 mouse model of CIA. CIA, collagen-induced arthritis.
CIA mouse arthritis score
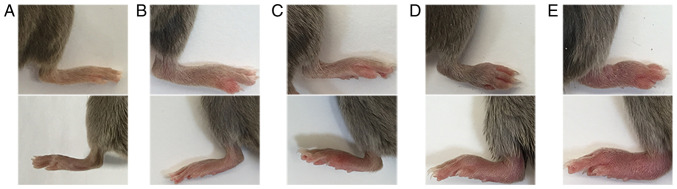
Arthritis scores are determined on the extremities of each mouse and the score for each mouse is the sum of the limb scores (range, 0-16 points, with a maximum score in each mouse of 16). The scoring criteria were adapted from multiple references (55,56), as presented in Table I. Representative images demonstrating the specific changes in the joints for each score are presented in Fig. 3. The mean arthritis index of each group of mice is calculated as follows: Mean arthritis index = (total arthritis score of all mice in the group)/(the number of mice in the group).
Table I.
Scoring criteria for collagen-induced arthritis in mice.
| Severity score | Degree of inflammation |
|---|---|
| 0 | No erythema or swelling |
| 1 | Erythema and mild swelling confined to the tarsals, ankle, or paw joint, with mild swelling at single limb |
| 2 | Erythema and mild swelling extending from the ankle to the tarsals or erythema and mild swelling of more than one toe |
| 3 | Erythema and moderate swelling extending from the ankle to the metatarsal joints or the whole paw with swelling and obvious erythema |
| 4 | Erythema and the whole paw with severe swelling encompass the ankle, foot and digits, or ankylosis of the limb, and dysfunction of the above joints |
Figure 3.
Representative images of hind paws for different clinical scores in mice with collagen-induced arthritis. The upper and lower panels represent the right and left hind paws of different mice, respectively. (A) Normal hind paw (clinical score 0). (B) Erythema and mild swelling confined to the tarsals, ankle joint or paw, with mild swelling in a single limb (clinical score 1). (C) Erythema and mild swelling extending from the ankle to the tarsals or erythema and mild swelling of more than one toe (clinical score 2). (D) Erythema and moderate swelling extending from the ankle to the metatarsal joints, or swelling and obvious erythema of the entire paw (clinical score 3). (E) Erythema and severe swelling of the whole paw, including the ankle, foot and digits; ankylosis of the limb; and dysfunction (clinical score 4).
Sample collection
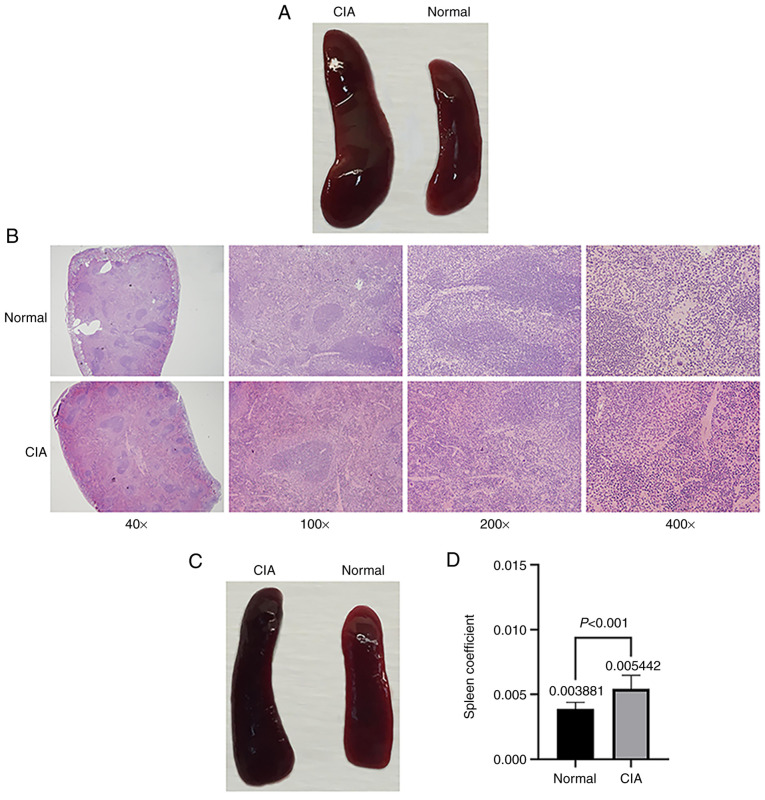
Samples and data are collected to detect and evaluate the severity of arthritis in mice with CIA. In general, mouse body weight, joint tissues, serum, peripheral blood lymphocytes and spleen samples are routinely collected (57). The following results are very clear and have been verified in published studies (36,57). On D31, the spleens of CIA mice are significantly larger than those of normal mice and the color of the spleens is dark red (Fig. 4A and B). It is worth noting that even in the absence of arthritis symptoms in CIA mice, obvious spleen enlargement may be observed (Fig. 4C), and the spleen coefficient of mice in the CIA group is significantly higher than that of the mice in the normal control group (Fig. 4D). Compared with normal mice, CIA mice were also reported to have an increased thymus index (58). Severe synovial hyperplasia, inflammatory cell infiltration and destruction of bone and cartilage were observed in the joint space and bone tissue of CIA mice (Fig. 5A and B). The levels of the cytokines TNF-α, IFN-γ, IL-1β, IL-6, IL-17A and IL-18 in the serum of CIA mice were significantly increased; by contrast, the levels of the cytokines IL-4 and IL-10 were significantly decreased (59). Accordingly, the Th1/Th2 and Th17/T-regulatory cell ratios exhibited significant increases (60).
Figure 4.
Comparison of the spleens of normal mice and mice with CIA. (A) Representative images of spleens on day 31. The spleens of CIA mice were significantly larger than those of normal mice and the color of the spleen was dark red. (B) Immunohistochemical images of spleen sections. Mouse spleens were fixed in 4% formalin for 24 h. Histological analysis of the spleens was performed by staining 5-mm sections of paraffin-embedded tissues with H&E (magnification, x40, 100, 200 and 400 as indicated). (C) Representative images of spleens of asymptomatic model animals and controls; in the absence of arthritis symptoms in CIA mice, the spleens of CIA mice were still significantly larger than those of normal mice. (D) Statistical analysis of spleen coefficients in CIA mice (DBA/1 background) and normal mice (n=10 for each group). CIA, collagen-induced arthritis.
Figure 5.
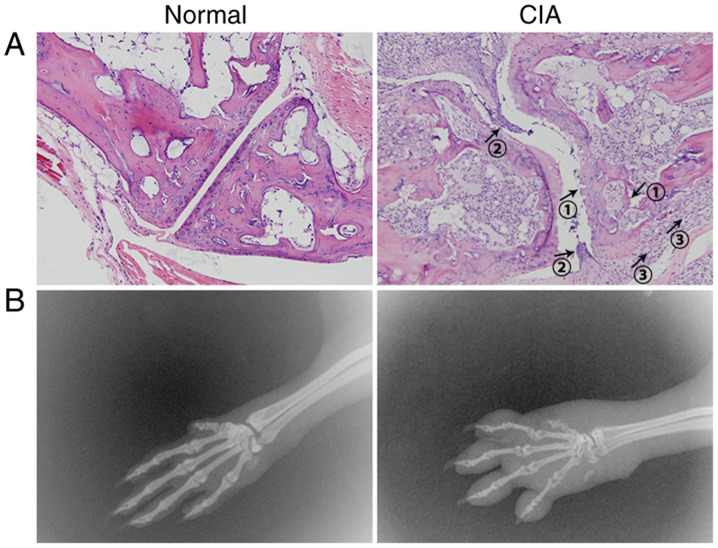
(A) Histology images of the normal group and the arthritis group reveal the main histological structure of the knee joint. Mouse joints were dissected and cleaned, and the samples were fixed in paraformaldehyde for one week prior to being decalcified for 1 month. When the bone became soft, the joints were embedded in paraffin for sectioning. The 5-mm sections were then stained with H&E for histopathological examination. In the arthritis group, typical cartilage and bone damage (arrow 1), synovial lining hyperplasia (arrow 2) and infiltration of inflammatory cells (arrow 3) were observed (magnification, x200). (B) Representative radiographs of the effects in the normal group and the arthritis group. Joint damage is visible at 31 days after arthritis induction, while normal joints were complete and exhibited no evidence of bone destruction. The joint space was clear and smooth. The joints of the mice with CIA displayed narrow spaces and diffuse soft tissue swelling. The ankle and toe joint spaces were hazy and narrow, and the bone displayed cystic changes and signs of apparent erosion and degradation. CIA, collagen-induced arthritis.
5. Choice of intervention time and administration method
There are three commonly used methods for administration: Intraperitoneal administration (61), intravenous administration (62) and oral administration (63). The medication route is selected according to the purpose of the experiment and the characteristics of the drug. In general, Traditional Chinese Medicine is administered orally by dissolving the medicine in drinking water or by gavage (64).
The time of administration should be given special consideration and depends on whether the researcher aims to detect the preventive or therapeutic effect of the drug. Normally, the time of the first immunization is referred to as D0 and the time of the second immunization of DBA/1 mice is D21. In most studies, mice are administered injections as preventive treatments on D0-10 and as therapeutic treatments on D21-25 (65-69). Based on the pathogenesis of the CIA model as described above, the specific development of RA pathology began after the first immunization. The second immunization only enhanced the symptoms (63). Approximately 10% of CIA mice develop symptoms of joint swelling, which can be visually observed between the first and second immunizations. It further verifies that the onset of pathological signs occurs during this period. Accordingly, the other 90% were observed 4-5 days after the second immunization (D25-D26 of the experiment) (70). Consequently, it is recommended that mice are administered preventive treatments from D0 and therapeutic treatments from D21; regardless of the drug's preventive or therapeutic effects, earlier administration of the drug is more efficient. Furthermore, the observation time should not be too long. If the lymphocyte reaction is being monitored, the best time-point is D31. If joint damage and deformation are being monitored, D31-D41 is an appropriate range. When the observation time exceeds 42-60 days, the mouse heals and symptoms of arthritis, such as redness and swelling, disappear (26).
6. Should the CIA mouse model be used?
Study of RA pathogenesis
The CIA mouse model may be used to study the mechanisms by which cytokines and lymphocytes, including T cells and particularly CD4+ T cells and B cells, are involved in the pathogenesis of RA. In addition, it may be used to study the pathogenesis of bone and cartilage destruction in RA (23,71). Typically, mouse CIA is characterized by symmetrical joint involvement and the peripheral joints are affected (38). Infiltration of cells (T cells, B cells, macrophages and neutrophils) into the joint space leading to pannus formation, hyperplastic synoviocyte membranes, marginal erosion, and bone and cartilage destruction are similar to the processes observed in human RA (26).
Study of the efficacy of anti-RA drugs
For small-molecule drugs, the mouse CIA model is preferred. For antibodies, the mouse arthritis model is not ideal due to issues with species specificity. With the development of antibody technology, humanized chimeric antibodies and fully humanized antibodies are gradually becoming available. However, the technical development of a corresponding humanized animal model has not kept up. Therefore, in studies on the efficacy of such antibody drugs, the absence of a suitable animal model will lead to limitations. The existing solutions are not perfect. If the antigen targeted by the antibody is homologous and highly conserved between humans and mice, the animal model may be used to preliminarily verify the efficacy of the antibody (72,73).
Although largely similar, RA and murine CIA also differ in certain aspects. For instance, the joint pathology of RA is chronic and symmetrical, whereas that of CIA in mice is transient and asymmetrical. In addition, CIA is self-resolving (after ~60 days in mice). The similarities and differences between human RA and CIA are summarized in Table II (26,74,75). Overall, the CIA mouse model is currently the closest to the pathogenesis of human RA, however, researchers must consider their individual experimental aims when deciding whether this model should be used.
Table II.
Non-exhaustive list of similarities and differences in clinical disease course and pathologic mechanisms between RA and CIA.
| Parameter | RA | CIA |
|---|---|---|
| Type | Polyarticular | Polyarticular |
| Predisposition association | With certain MHC-II haplotypes | With certain H-2 complexes |
| Joint pathology | Similar | Similar |
| Hyperplasia of synovial membrane | Present | Present |
| Synovial immune infiltration | Present | Present |
| Neutrophils in synovial fluid | Present | Present |
| Pannus formation | Present | Present |
| Bone destruction by osteoclasts | Present | Present |
| Serological markers | Present | Present |
| Rheumatoid factor | Present | Present |
| Anti-CII antibodies | Present | Present |
| Anticyclic citrullinated peptide antibodies | Present | Present |
| Changes in body composition and increased resting energy expenditure found in cachexia | Present | Present |
| Joint involvement | Symmetric | Nonsymmetric |
| Disease progression | Chronic, almost impossible to self-heal | Transient, able to self-heal |
| Systemic manifestations | Systemic/extra-articular manifestations reported | None reported |
7. Additional considerations
Consideration should be given to the extent of animal suffering (76). In the process of CIA modeling, mice exhibit ulceration and inflammatory reactions in the tail skin due to stimulation from the emulsion at the base of the tail. Therefore, it is necessary to perform the injections as slowly as possible and the depth of the needle should be appropriate. If the needle is placed too superficially, it may penetrate the skin. This leads to leakage of the emulsion, which may increase the possibility of ulceration in the tail of the mouse. In general, mice experience substantial pain due to the process of CIA model generation and the symptoms of inflammation itself. Therefore, the researcher should be as gentle as possible with the experimental animals, provide stable keeping conditions and minimize errors and pain during the operations. Humane end-points with severity limits should be incorporated into protocols to limit suffering (76). When taking samples from sacrificed mice, researchers should keep their workspace away from the cages of the remaining animals to prevent panic and psychological tension caused by the mice witnessing the suffering of their peers. This panic may even cause changes in immune cells (77). In addition, it is worth mentioning that numerous small animals lose their lives in research and researchers should respect the animals. Operators should strictly follow the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. In fact, one of the intentions of the present review is to help experimenters minimize errors and reduce the number of experimental animals used. The experiments should therefore be carefully planned and designed together with a primary investigator or supervisor, ensuring that each individual experiment is ethically approved beforehand.
8. Conclusion and outlook
The CIA mouse model has been used in numerous studies on RA etiology, pathogenesis, drug screening, transgenic mice and immunotherapy worldwide. Research on CIA and the standardized application of CIA animal models are of great significance in these fields. However, in certain studies, irregularities still exist in the establishment and application of this model. The present review examined the molecular basis and limitations of the CIA model in detail and aimed to provide a reference for the investigation and use of CIA. All of the small and easily overlooked but important details, including the reagents and protocol required for modeling, are provided in this report.
It is worth mentioning that scientists are also exploring humanized RA animal models, which may be used to verify the efficacy and mechanisms of anti-rheumatic drugs that involve humanized antibodies. At present, it is thought that the most stable model animals for humanized RA are humanized bone marrow/liver/thymus mice. In this model, 6- to 8-week-old NOD.Cg-Prkdcscid Il2rgtm1wjl/SzJ (NSG) immunocompromised mice receive a thymus/liver implant, as in the SCID-hu mouse model, followed by a second human hematopoietic stem cell transplant (78). The advantage of this system is the full reconstitution of the human immune system in the periphery. This model is stable for 12 to 18 weeks (79,80). Accordingly, in theory, it is most desirable to induce CIA in BLT mice and then assess the efficacy of humanized antibodies in them. However, several problems were encountered in the experiment. Frist, as the genetic background of NSG mice is NOD (MHC II H-2g), the success rate of CIA establishment is unknown. Second, the emergence of BLT mice is still relatively novel, meaning that the development of the model itself is not very mature, and that there are uncertainties associated with it. In addition, the cost of BLT mice is quite high right now. Therefore, much work remains to be performed to overcome the challenges associated with this model.
Acknowledgements
The authors would like to thank Professor Xingchun Gao from the Youth Innovation Team of Shaanxi Universities for his suggestions for the writing of the current review.
Funding Statement
Funding: This work was supported by the Research Fund of Shaanxi Provincial Education Department (grant no. 20JS136), the Xi'an Weiyang District Science and Technology Information Bureau (grant no. 201933), the Natural Science Basic Research Plan of Shaanxi Province in China (grant no. 2020JQ-877) and the Science Research Program of Xi 'an Medical University (grant no. 2020DOC25).
Availability of data and materials
Not applicable.
Authors' contributions
JL and ZH wrote the manuscript. JL, JC, RZ, PY, HG and GN performed the experiments, analyzed the data and revised the manuscript. NG and XG supervised the current study, proofread the manuscript and confirmed the authenticity of all the raw data. All authors read and approved the final version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bird P, Kirkham B, Portek I, Shnier R, Joshua F, Edmonds J, Lassere M. Documenting damage progression in a two-year longitudinal study of rheumatoid arthritis patients with established disease (the DAMAGE study cohort): Is there an advantage in the use of magnetic resonance imaging as compared with plain radiography? Arthritis Rheum. 2004;50:1383–1389. doi: 10.1002/art.20165. [DOI] [PubMed] [Google Scholar]
- 2.Døhn UM, Ejbjerg BJ, Hasselquist M, Narvestad E, Møller J, Thomsen HS, Østergaard M. Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res Ther. 2008;10(R25) doi: 10.1186/ar2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32:174–187. doi: 10.1016/j.berh.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Østergaard M. The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: A comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum. 2005;52:2300–2306. doi: 10.1002/art.21207. [DOI] [PubMed] [Google Scholar]
- 5.Bendstrup E, Møller J, Kronborg-White S, Prior TS, Hyldgaard C. Interstitial Lung Disease in Rheumatoid Arthritis Remains a Challenge for Clinicians. J Clin Med. 2019;8(E2038) doi: 10.3390/jcm8122038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandyopadhyay D, Banerjee U, Hajra A, Chakraborty S, Amgai B, Ghosh RK, Haddadin FI, Modi VA, Sinha K, Aronow WS, et al. Trends of Cardiac Complications in Patients With Rheumatoid Arthritis: Analysis of the United States National Inpatient Sample; 2005-2014. Curr Probl Cardiol. 2021;46(100455) doi: 10.1016/j.cpcardiol.2019.100455. [DOI] [PubMed] [Google Scholar]
- 7.Zulfiqar AA, Niazi R, Pennaforte JL, Andres E. Rheumatoid arthritis and cardiovascular risk factor: Literature review. Rev Med Liege. 2018;73:634–639. (In French) [PubMed] [Google Scholar]
- 8.Atzeni F, Talotta R, Masala IF, Gerardi MC, Casale R, Sarzi-Puttini P. Central nervous system involvement in rheumatoid arthritis patients and the potential implications of using biological agents. Best Pract Res Clin Rheumatol. 2018;32:500–510. doi: 10.1016/j.berh.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Nakao E, Mitsunaga A, Hamano T, Shirato M, Shirato I, Nishino T. Case report of rheumatoid arthritis associated with type A gastritis and Hashimoto thyroiditis. Nihon Shokakibyo Gakkai Zasshi. 2010;107:1927–1932. (In Japanese) [PubMed] [Google Scholar]
- 10.Lora V, Cerroni L, Cota C. Skin manifestations of rheumatoid arthritis. G Ital Dermatol Venereol. 2018;153:243–255. doi: 10.23736/S0392-0488.18.05872-8. [DOI] [PubMed] [Google Scholar]
- 11.Kurmann RD, Mankad R. Atherosclerotic Heart Disease in Women With Autoimmune Rheumatologic Inflammatory Conditions. Can J Cardiol. 2018;34:381–389. doi: 10.1016/j.cjca.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Favalli EG, Biggioggero M, Crotti C, Becciolini A, Raimondo MG, Meroni PL. Sex and Management of Rheumatoid Arthritis. Clin Rev Allergy Immunol. 2019;56:333–345. doi: 10.1007/s12016-018-8672-5. [DOI] [PubMed] [Google Scholar]
- 13.Law ST, Taylor PC. Role of biological agents in treatment of rheumatoid arthritis. Pharmacol Res. 2019;150(104497) doi: 10.1016/j.phrs.2019.104497. [DOI] [PubMed] [Google Scholar]
- 14.Bolon B, Stolina M, King C, Middleton S, Gasser J, Zack D, Feige U. Rodent preclinical models for developing novel antiarthritic molecules: Comparative biology and preferred methods for evaluating efficacy. J Biomed Biotechnol. 2011;2011(569068) doi: 10.1155/2011/569068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose J, Tanaka S. Animal models for bone and joint disease. CIA, CAIA model. Clin Calcium. 2011;21:253–259. (In Japanese) [PubMed] [Google Scholar]
- 16.Hanyecz A, Olasz K, Tarjanyi O, Nemeth P, Mikecz K, Glant TT, Boldizsar F. Proteoglycan aggrecan conducting T cell activation and apoptosis in a murine model of rheumatoid arthritis. BioMed Res Int. 2014;2014(942148) doi: 10.1155/2014/942148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrado A, Donato P, Maccari S, Cecchi R, Spadafina T, Arcidiacono L, Tavarini S, Sammicheli C, Laera D, Manetti AG, et al. Staphylococcus aureus-dependent septic arthritis in murine knee joints: Local immune response and beneficial effects of vaccination. Sci Rep. 2016;6(38043) doi: 10.1038/srep38043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40:193–200. doi: 10.1080/08923973.2018.1434793. [DOI] [PubMed] [Google Scholar]
- 19.Christensen AD, Haase C, Cook AD, Hamilton JA. K/BxN Serum-Transfer Arthritis as a Model for Human Inflammatory Arthritis. Front Immunol. 2016;7(213) doi: 10.3389/fimmu.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sønderstrup G. Development of humanized mice as a model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:35–45. doi: 10.1007/s00281-003-0129-z. [DOI] [PubMed] [Google Scholar]
- 21.Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N, Tanaka S, Katsuki I, Matsushita S, Tanaka Y. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2011;186:3745–3752. doi: 10.4049/jimmunol.1002475. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Hasegawa H, Takemasa E, Suzuki Y, Oka K, Kiyoi T, Takeda H, Ogasawara T, Sawasaki T, Yasukawa M, et al. Efficiency and Safety of CRAC Inhibitors in Human Rheumatoid Arthritis Xenograft Models. J Immunol. 2017;199:1584–1595. doi: 10.4049/jimmunol.1700192. [DOI] [PubMed] [Google Scholar]
- 23.Williams RO. Collagen-induced arthritis in mice. Methods Mol Med. 2007;136:191–199. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 24.Caplazi P, Baca M, Barck K, Carano RA, DeVoss J, Lee WP, Bolon B, Diehl L. Mouse Models of Rheumatoid Arthritis. Vet Pathol. 2015;52:819–826. doi: 10.1177/0300985815588612. [DOI] [PubMed] [Google Scholar]
- 25.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Schurgers E, Billiau A, Matthys P. Collagen-induced arthritis as an animal model for rheumatoid arthritis: Focus on interferon-γ. J Interferon Cytokine Res. 2011;31:917–926. doi: 10.1089/jir.2011.0056. [DOI] [PubMed] [Google Scholar]
- 27.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cathcart ES, Hayes KC, Gonnerman WA, Lazzari AA, Franzblau C. Experimental arthritis in a nonhuman primate. I. Induction by bovine type II collagen. Lab Invest. 1986;54:26–31. [PubMed] [Google Scholar]
- 29.Courtenay JS, Dallman MJ, dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 30.Nandakumar KS. Pathogenic antibody recognition of cartilage. Cell Tissue Res. 2010;339:213–220. doi: 10.1007/s00441-009-0816-8. [DOI] [PubMed] [Google Scholar]
- 31.Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarkowski A, Holmdahl R, Klareskog L. Rheumatoid factors in mice. Monogr Allergy. 1989;26:214–229. [PubMed] [Google Scholar]
- 33.Benson RA, McInnes IB, Garside P, Brewer JM. Model answers: Rational application of murine models in arthritis research. Eur J Immunol. 2018;48:32–38. doi: 10.1002/eji.201746938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers LK, Tang B, Rosloniec EF, Stuart JM, Chiang TM, Kang AH. Characterization of a peptide analog of a determinant of type II collagen that suppresses collagen-induced arthritis. J Immunol. 1998;161:3589–3595. [PubMed] [Google Scholar]
- 35.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 36.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 37.Stuart JM, Townes AS, Kang AH. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- 38.Joe B, Wilder RL. Animal models of rheumatoid arthritis. Mol Med Today. 1999;5:367–369. doi: 10.1016/s1357-4310(99)01528-2. [DOI] [PubMed] [Google Scholar]
- 39.Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005;12:167–181. doi: 10.1016/j.pathophys.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Hegen M, Keith JC Jr, Collins M, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis. 2008;67:1505–1515. doi: 10.1136/ard.2007.076430. [DOI] [PubMed] [Google Scholar]
- 41.Petersen F, Yu X. A novel preclinical model for rheumatoid arthritis research. Arthritis Res Ther. 2010;12(148) doi: 10.1186/ar3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 43.Huang JC, Vestberg M, Minguela A, Holmdahl R, Ward ES. Analysis of autoreactive T cells associated with murine collagen-induced arthritis using peptide-MHC multimers. Int Immunol. 2004;16:283–293. doi: 10.1093/intimm/dxh039. [DOI] [PubMed] [Google Scholar]
- 44.Buglak NE, Bahnson ESM. doi: 10.3791/60473. A Rat Carotid Artery Pressure-Controlled Segmental Balloon Injury with Periadventitial Therapeutic Application. J Vis Exp 161: 10.3791/60473, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- 46.Boissier MC, Feng XZ, Carlioz A, Roudier R, Fournier C. Experimental autoimmune arthritis in mice. I. Homologous type II collagen is responsible for self-perpetuating chronic polyarthritis. Ann Rheum Dis. 1987;46:691–700. doi: 10.1136/ard.46.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilder RL. Hormones and autoimmunity: Animal models of arthritis. Baillieres Clin Rheumatol. 1996;10:259–271. doi: 10.1016/s0950-3579(96)80017-3. [DOI] [PubMed] [Google Scholar]
- 48.Vandenbroucke JP, Valkenburg HA, Boersma JW, Cats A, Festen JJ, Huber-Bruning O, Rasker JJ. Oral contraceptives and rheumatoid arthritis: Further evidence for a preventive effect. Lancet. 1982;2:839–842. doi: 10.1016/s0140-6736(82)90809-1. [DOI] [PubMed] [Google Scholar]
- 49.Lee YK, Choi KH, Kwak HS, Chang YH. The preventive effects of nanopowdered red ginseng on collagen-induced arthritic mice. Int J Food Sci Nutr. 2018;69:308–317. doi: 10.1080/09637486.2017.1358359. [DOI] [PubMed] [Google Scholar]
- 50.Holmdahl R, Jansson L, Gullberg D, Rubin K, Forsberg PO, Klareskog L. Incidence of arthritis and autoreactivity of anti-collagen antibodies after immunization of DBA/1 mice with heterologous and autologous collagen II. Clin Exp Immunol. 1985;62:639–646. [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart-Tull DE. Freund's complete and incomplete adjuvants, preparation, and quality control standards for experimental laboratory animals use. Methods Mol Biol. 2010;626:59–72. doi: 10.1007/978-1-60761-585-9_5. [DOI] [PubMed] [Google Scholar]
- 52.Wooley PH, Luthra HS, Griffiths MM, Stuart JM, Huse A, David CS. Type II collagen-induced arthritis in mice. IV. Variations in immunogenetic regulation provide evidence for multiple arthritogenic epitopes on the collagen molecule. J Immunol. 1985;135:2443–2451. [PubMed] [Google Scholar]
- 53.Chen HH, Chen DY, Chao YH, Chen YM, Wu CL, Lai KL, Lin CH, Lin CC. Acarbose Decreases the Rheumatoid Arthritis Risk of Diabetic Patients and Attenuates the Incidence and Severity of Collagen-induced Arthritis in Mice. Sci Rep. 2015;5(18288) doi: 10.1038/srep18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, Williams RO. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res Ther. 2007;9(R113) doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Yang J, Zu B, Wang J, Sheng K, Zhao L, Xu W. Acacetin regulated the reciprocal differentiation of Th17 cells and Treg cells and mitigated the symptoms of collagen-induced arthritis in mice. Scand J Immunol. 2018;88(e12712) doi: 10.1111/sji.12712. [DOI] [PubMed] [Google Scholar]
- 56.Deng Y, Luo H, Shu J, Shu H, Lu C, Zhao N, Geng Y, He X, Lu A. Pien Tze Huang alleviate the joint inflammation in collagen-induced arthritis mice. Chin Med. 2020;15(30) doi: 10.1186/s13020-020-00311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luan J, Zhang K, Yang P, Zhang Y, Feng F, Zhu YM, Zhu P, Chen ZN. The combination of FK506 and an anti-CD147 mAb exerts potential therapeutic effects on a mouse model of collagen-induced arthritis. Mol Immunol. 2018;101:1–9. doi: 10.1016/j.molimm.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Fu J, Zhang L, Song S, Sheng K, Li Y, Li P, Song S, Wang Q, Chu J, Wei W. Effect of bone marrow-derived CD11b(+)F4/80 (+) immature dendritic cells on the balance between pro-inflammatory and anti-inflammatory cytokines in DBA/1 mice with collagen-induced arthritis. Inflamm Res. 2014;63:357–367. doi: 10.1007/s00011-014-0707-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X, Zhao X, Tang L, Zhang Y, Ruan H, Pi H, Qiu J, Wu J. Immunomodulatory activity of the rhizomes of Impatiens pritzellii var. hupehensis on collagen-induced arthritis mice. J Ethnopharmacol. 2007;109:505–509. doi: 10.1016/j.jep.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Zhuo F, Chu P, Yang X, Zhao G. Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem Biophys Res Commun. 2019;518:560–564. doi: 10.1016/j.bbrc.2019.08.084. [DOI] [PubMed] [Google Scholar]
- 61.Jia Q, Wang T, Wang X, Xu H, Liu Y, Wang Y, Shi Q, Liang Q. Astragalin Suppresses Inflammatory Responses and Bone Destruction in Mice With Collagen-Induced Arthritis and in Human Fibroblast-Like Synoviocytes. Front Pharmacol. 2019;10(94) doi: 10.3389/fphar.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svetlicky N, Kivity S, Odeh Q, Shovman O, Gertel S, Amital H, Gendelman O, Volkov A, Barshack I, Bar-Meir E, et al. Anti-citrullinated-protein-antibody-specific intravenous immunoglobulin attenuates collagen-induced arthritis in mice. Clin Exp Immunol. 2015;182:241–250. doi: 10.1111/cei.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang P, Qian F, Zhang M, Xu AL, Wang X, Jiang B, Zhou L, Zhou X. Zishen Tongluo formula ameliorates collagen-induced arthritis in mice by modulation of Th17/Treg balance. J Ethnopharmacol. 2020;250(112428) doi: 10.1016/j.jep.2019.112428. [DOI] [PubMed] [Google Scholar]
- 64.Feng ZT, Yang T, Hou XQ, Wu HY, Feng JT, Ou BJ, Cai SJ, Li J, Mei ZG. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed Pharmacother. 2019;113(108759) doi: 10.1016/j.biopha.2019.108759. [DOI] [PubMed] [Google Scholar]
- 65.Fan J, Luo J, Yan C, Hao R, Zhao X, Jia R, He J, Xu D, Miao M, Li X. Methotrexate, combined with cyclophosphamide attenuates murine collagen induced arthritis by modulating the expression level of Breg and DCs. Mol Immunol. 2017;90:106–117. doi: 10.1016/j.molimm.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Wu S, Li Y, Li Y, Yao L, Lin T, Jiang S, Shen H, Xia L, Lu J. Interleukin-35 attenuates collagen-induced arthritis through suppression of vascular endothelial growth factor and its receptors. Int Immunopharmacol. 2016;34:71–77. doi: 10.1016/j.intimp.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 67.Gui H, Liu X, Liu LR, Su DF, Dai SM. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology. 2015;220:817–822. doi: 10.1016/j.imbio.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 69.Guo Y, Xing E, Song H, Feng G, Liang X, An G, Zhao X, Wang M. Therapeutic effect of dioscin on collagen-induced arthritis through reduction of Th1/Th2. Int Immunopharmacol. 2016;39:79–83. doi: 10.1016/j.intimp.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 70.Suszko A, Obmińska-Mrukowicz B. Influence of polysaccharide fractions isolated from Caltha palustris L. on the cellular immune response in collagen-induced arthritis (CIA) in mice. A comparison with methotrexate. J Ethnopharmacol. 2013;145:109–117. doi: 10.1016/j.jep.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 71.Bessis N, Decker P, Assier E, Semerano L, Boissier MC. Arthritis models: Usefulness and interpretation. Semin Immunopathol. 2017;39:469–486. doi: 10.1007/s00281-017-0622-4. [DOI] [PubMed] [Google Scholar]
- 72.Zhong C, Wang J, Li B, Xiang H, Ultsch M, Coons M, Wong T, Chiang NY, Clark S, Clark R, et al. Development and preclinical characterization of a humanized antibody targeting CXCL12. Clin Cancer Res. 2013;19:4433–4445. doi: 10.1158/1078-0432.CCR-13-0943. [DOI] [PubMed] [Google Scholar]
- 73.Huang X, He Y, Han J, Zhuang J, He J, Sun E. Not only anti-inflammation, etanercept abrogates collagen-induced arthritis by inhibiting dendritic cell migration and maturation. Cent Eur J Immunol. 2019;44:237–245. doi: 10.5114/ceji.2019.89595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alabarse PVG, Lora PS, Silva JMS, Santo RCE, Freitas EC, de Oliveira MS, Almeida AS, Immig M, Teixeira VON, Filippin LI, et al. Collagen-induced arthritis as an animal model of rheumatoid cachexia. J Cachexia Sarcopenia Muscle. 2018;9:603–612. doi: 10.1002/jcsm.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Vincent TL, Williams RO, Maciewicz R, Silman A, Garside P. Mapping pathogenesis of arthritis through small animal models. Rheumatology (Oxford) 2012;51:1931–1941. doi: 10.1093/rheumatology/kes035. Arthritis Research UK animal models working group. [DOI] [PubMed] [Google Scholar]
- 77.Marazziti D, Ambrogi F, Abelli M, Di Nasso E, Catena M, Massimetti G, Carlini M, Dell'Osso L. Lymphocyte subsets, cardiovascular measures and anxiety state before and after a professional examination. Stress. 2007;10:93–99. doi: 10.1080/10253890601170563. [DOI] [PubMed] [Google Scholar]
- 78.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 79.Akkina R. Humanized Mice for Studying Human Immune Responses and Generating Human Monoclonal Antibodies. Microbiol Spectr. 2014;2 doi: 10.1128/microbiolspec.AID-0003-2012. [DOI] [PubMed] [Google Scholar]
- 80.Vatakis DN, Bristol GC, Kim SG, Levin B, Liu W, Radu CG, Kitchen SG, Zack JA. Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J Vis Exp. 2012;70(e4181) doi: 10.3791/4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.