Abstract
Background:
Rotavirus incidence remains relatively high in low-income countries (LICs) compared to high-income countries (HICs) after vaccine introduction. Ghana introduced monovalent rotavirus vaccine in April 2012 and despite the high coverage, vaccine performance has been modest compared to developed countries. The predictors of low vaccine effectiveness in LICs are poorly understood, and the drivers of subnational heterogeneity in rotavirus vaccine impact are unknown.
Methods:
We used mathematical models to investigate variations in rotavirus incidence in children <5 years old in Ghana. We fit models to surveillance and case-control data from three different hospitals: Korle-Bu Teaching Hospital in Accra, Komfo Anokye Teaching Hospital in Kumasi, and War Memorial Hospital in Navrongo. The models were fitted to both pre- and post-vaccine data to estimate parameters describing the transmission rate, waning of maternal immunity, and vaccine response rate.
Results:
The seasonal pattern and age distribution of rotavirus cases varied among the three study sites in Ghana. Our model was able to capture the spatio-temporal variations in rotavirus incidence across the three sites and showed good agreement with the age distribution of observed cases. The rotavirus transmission rate was highest in Accra and lowest in Navrongo, while the estimated duration of maternal immunity was longer (∼5 months) in Accra and Kumasi and shorter (∼3 months) in Navrongo. The proportion of infants who responded to the vaccine was estimated to be high in Accra and Kumasi and low in Navrongo.
Conclusions:
Rotavirus vaccine impact varies within Ghana. A low vaccine response rate was estimated for Navrongo, where rotavirus is highly seasonal and incidence limited to a few months of the year. Our findings highlight the need to further explore the relationship between rotavirus seasonality, maternal immunity, and vaccine response rate to determine how they influence vaccine effectiveness and to develop strategies to improve vaccine impact.
Keywords: Ghana, Rotavirus, Vaccine impact, Vaccine response rate, Maternal immunity, Transmission rate
1. Introduction
Rotavirus-associated gastroenteritis (RVGE) remains a worldwide public health problem, but the majority of severe morbidity and mortality occurs in infants in low-income countries (LICs) particularly in sub-Saharan Africa [1]. Globally, Tate et al. [2] estimated that rotavirus caused 528,000 deaths among children <5 years of age in 2000, decreasing to 215,000 deaths in 2013; sub-Saharan Africa accounted for about 47% and 56% of deaths in 2000 and 2013, respectively. Vaccination has proven to be a successful intervention strategy against severe RVGE. However, while rotavirus vaccine efficacy is high (85–99%) in high-income countries (HICs), it tends to be considerably lower (50–64%) in LICs [3–8]. Despite this disparity in vaccine efficacy, globally there has been an estimated 44% decrease in rotavirus mortality among children <5 years old between 2005 and 2015, which can at least in part be attributed to vaccine protection [9].
In Ghana, prior to rotavirus vaccine introduction, RVGE was responsible for greater than 25% of all diarrhea-caused hospitalizations and deaths occurring among children <5 years old [10–13]. In an effort to reduce the burden of RVGE-associated morbidity and mortality in the country, Ghana introduced the monovalent Rotarix vaccine (RV1) into its Expanded Program on Immunization (EPI) in April 2012, with a 2-dose schedule given at ages 6 and 10 weeks [14]. Vaccine coverage has increased substantially from 42–56% in 2012 to above 90% in 2016, [15,16]. Early vaccine evaluations of RV1 have shown a moderate and varied reduction in both rotavirus and all-cause diarrhea hospitalizations among <5-year-olds [15–17]. Additional benefits may be derived from rotavirus vaccination if strategies are identified to improve vaccine effectiveness. Consequently, it is important to understand the factors that account for this moderate vaccine impact and identify new strategies to help improve vaccine performance in Ghana as well as other LICs.
Since the introduction of rotavirus vaccine, dynamic mathematical models have been used to evaluate the impact of rotavirus vaccine on severe RVGE, rotavirus-associated deaths and all-cause diarrhea across different socio-economic settings and to assess the cost-effectiveness of the vaccine [18–23]. These modeling studies have assessed the drivers of intra-country transmission dynamics, revealed important factors that affect overall vaccine performance, and suggested strategies that could improve vaccine impact. For instance, it has been shown that failure to complete the full-dose schedule could significantly reduce vaccine impact [20].
While both modeling and observational studies indicate variation in vaccine effectiveness and rotavirus transmission dynamics within and between HICs and LICs, the predictors of low vaccine impact in LICs are poorly understood. However, factors such as season of birth [24], time of first infection [25,26], infant gut microbiome composition [27], malnutrition and co-infections [28] have been found to influence vaccine performance. While breastfeeding is unlikely to explain poor vaccine performance in LICs [29–31], transplacental maternal antibodies may interfere with vaccine take [25,32]. On the other hand, it has been demonstrated that infants exposed to early natural infection are less likely to respond to rotavirus vaccine [25]; thus, knowing the duration of maternal immunity is important to determine the best time to administer the first dose of the vaccine (within manufacturers’ guidelines). Concerns have also been raised that vaccine-induced immunity may wane in LICs, prompted by the shift in the age distribution of RVGE towards older children following vaccine introduction [33,34]. However, more information about possible waning of vaccine-induced immunity is required to determine if a booster dose may be needed [35].
We assessed variations in the estimated duration of maternal immunity, rotavirus transmission rate, duration of vaccine-induced immunity and vaccine response rate as potential driving factors accounting for intra-country heterogeneity in RVGE incidence before and after vaccine introduction in Ghana using surveillance and case-control data from three different hospitals. A better understanding of the factors that account for this variability could help to identify strategies to improve vaccine effectiveness and impact.
2. Methods and data
2.1. Data and study area description
Data were obtained from surveillance and case-control studies conducted at three different hospitals in Ghana (Fig. 1): Korle-Bu Teaching Hospital in Accra, Komfo Anokye Teaching Hospital in Kumasi, and War Memorial Hospital in Navrongo. The sites in Accra and Kumasi are both teaching hospitals, while the one in Navrongo is a district hospital. These datasets have been described elsewhere [16]. Briefly, the surveillance data were collected from all children <2 (Navrongo) and <5 (Accra and Kumasi) years of age who presented with acute gastroenteritis. A case was defined as three or more watery stools per day and lasting <7 days. Stool samples were collected and tested for rotavirus using ELISA. After vaccine introduction, rotavirus immunization status was identified for all cases from the children’s immunization cards. For each hospital, the data were separated into pre- and post-vaccine periods: August 2007 to March 2012 (pre-vaccine) and April 2012 to April 2015 (post-vaccine) in Accra; January 2009 to March 2012 (pre-vaccine) and April 2012 to December 2015 (post-vaccine) in Kumasi; and July 2007 to May 2011 (pre-vaccine) and January 2013 to September 2016 (post-vaccine) in Navrongo. There were missing data from Navrongo between June 2011 and December 2012. The confirmed monthly rotavirus-positive cases from each hospital were aggregated into three age groups (0–11 months, 12–23 months, and 24–59 months) for Accra and Kumasi and two age groups (0–11 months and 12–23 months, since only children <24 months were enrolled) for Navrongo.
Fig. 1.
Map of Ghana showing the three hospitals.
We also obtained data on rotavirus vaccine coverage by year and region from the Ministry of Health (Table S1) [36]. We assumed that vaccine coverage at each site was equal to the coverage for the corresponding region (Greater Accra for Accra; Ashanti for Kumasi; and Upper East for Navrongo).
2.2. Model structure description
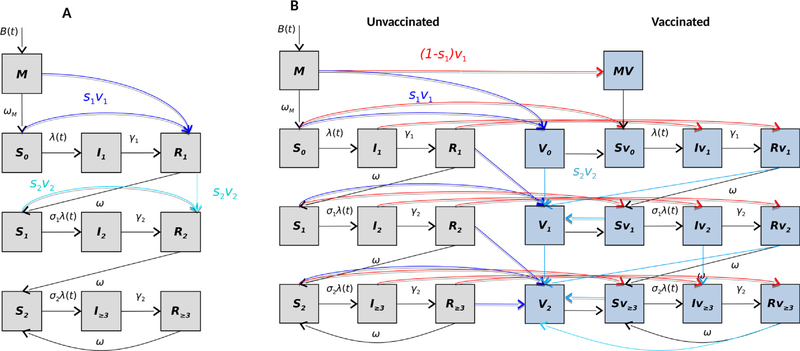
We adapted and modified a SIRS-like model developed by Pitzer et al. [18], which consists of multiple susceptible (S), infective (I), and recovered (R) compartments (Fig. 2A); the full model equations for the base model are given in Supplementary Material S1. Briefly, the model assumes individuals are born into the maternal class, M, at a rate equal to the annual birth rate B. All infants in M are protected by maternally-acquired immunity, which gradually wanes over time at a rate ωm, such that individuals become fully susceptible to first infection (S0). Individuals in S0 are subject to primary infection at a rate λ, and infected individuals are moved to the I1 compartment. They remain infectious for average period given by 1/γ1, with only a fraction (d1) of infected individuals developing severe RVGE. Following primary infection, we assume individuals gain temporary immunity to reinfection (R1) that wanes at a rate ω, after which individuals enter the S1 compartment and become susceptible to secondary infection at a reduced rate σ1λ. Individuals with their second infection (I2) are assumed to have infectiousness lowered by a factor (ρ2), are less likely to develop severe RVGE (d2), and recover at a faster rate γ2 into the R2 compartment. Again, individuals are assumed to have temporary immunity that wanes at the same rate ω, after which they enter the partially-immune susceptible compartment (S2). Individuals in S2 are subject to subsequent infections that are mostly mild or asymptomatic at a further reduced rate σ2λ. A proportion d3 of infected individuals (I≥3) could develop severe RVGE, infectiousness is reduced by a factor (ρ≥3), and infected individuals recover at a faster rate γ2. The recovered individuals are transferred to the temporary immune compartment R≥3 and this immunity wanes at the same rate ω, upon which inviduals return to the S2 compartment.
Fig. 2. Schematic of the model structures:
(A) the base model that assumes the waning of vaccine-acquired immunity is comparable to waning temporary immunity from natural infection; (B) an alternative model allows vaccine-acquired immunity to fully wane at a rate that differs from immunity derived from natural infections. The boxes indicate the various model states and lines indicate rates of movements between compartments. The red lines indicate individuals who fail to respond to the first vaccine dose, while the dark and light blue lines indicate transition of individuals who respond to the first and second doses of the vaccine, respectively.
The force of infection (transmission rate per fully susceptible individual) at time t in months,
λ(t), is given by:
| (1) |
where the definitions of the parameters are given in Table 1. We assume homogeneous mixing in the population.
Table 1.
Definition and values of fixed and estimated model parameters.
| Parameter | Symbol | Value | Reference |
|---|---|---|---|
| Basic reproductive number | R0 | =β0/γ1 | Derived |
| Baseline transmission rate | β0 | Estimated | |
| Duration of maternal immunity (months) | 1/ωm | Estimated | |
| Amplitude of annual seasonal forcing | b1 | Estimated | |
| Annual seasonal offset (months) | Φ1 | Estimated | |
| Amplitude of biannual seasonal forcing | b2 | Estimated | |
| Biannual seasonal offset (months) | Φ2 | Estimated | |
| Proportion of severe diarrhea cases hospitalized and reported | h | Estimated | |
| Proportion of subsequent infections that are severe | d3 | Estimated | |
| Proportion who respond to each vaccine dose | sc | Estimated | |
| Duration of vaccine-induced immunity (months) | 1/ωv | Estimated | |
| Duration of primary infection | 1/γ1 | 1 week | [37] |
| Duration of secondary infection | 1/γ2 | 0.5 week | [38,39] |
| Duration of temporary immunity | 1/ω | 13 weeks | [40] |
| Relative risk of second infection | σ1 | 0.62 | [41,42] |
| Relative risk of third infection | σ2 | 0.35 | [41,42] |
| Relative infectiousness of secondary infection | ρ2 | 0.5 | [43] |
| Relative infectiousness of mild/asymptomatic infections | ρ≥3 | 0.1 | [43] |
Vaccination is incorporated in the model by assuming a dose of vaccine is equivalent to a natural rotavirus infection among those who respond and are immunized. Thus, individuals who are vaccinated and "respond" (i.e. seroconvert) to each vaccine dose are transferred from either the maternal immunity or susceptible or recovered compartments to the next recovered compartment. For instance, the proportion of vaccinated individuals in the M and S0 compartments who respond (Sc) move directly into the R1 compartment after the first dose (with coverage v1). For the models to be consistent with the vaccine schedule in Ghana [14], we assume that infants receive their first dose upon aging into the 2-month age group and the second dose upon aging into the 3-month age group. Thus, children born on or after February 2012 were considered as vaccine-eligible for the post-vaccine simulations.
To incorporate waning of vaccine-acquired immunity in the model, we included separate compartments for vaccinated (blue) and unvaccinated (grey) individuals (Fig. 2B). The vaccinated individuals who failed to seroconvert (red lines) remain in their current state in the vaccinated compartment. For individuals who responded to the vaccine, we assumed that vaccination offers temporary protection against rotavirus infection (Vi) and wanes at a rate ωv, which is the same for both doses. Following waning of vaccine-acquired immunity, vaccinated individuals either become susceptible to primary (after first dose) or secondary (after second dose) infection. However, individuals who responded to both doses of the vaccine remain protected and enter the next vaccinated-and-protected compartment.
2.3. Model-fitting approach
We fit the models to the data on the age-stratified monthly number of rotavirus-positive cases from each hospital via maximum likelihood. The best-fit model parameters were estimated by minimizing the negative log-likelihood (LL) assuming that the observed number of rotavirus cases in each month and age-group was Poisson distributed with mean equal to the model-predicted number of severe RVGE cases times an estimated reporting fraction (h) (see Supplementary Material S1). We used profile likelihoods to estimate 95% confidence intervals for the model parameters of interest (R0, ωm, Sc, and ωv), varying each parameter across a range of values while fitting the remaining model parameters. We adjusted for variations in h over time by multiplying it by the 24-month moving average of the number of confirmed rotavirus-negative cases at each site divided by the average number of rotavirus-negative cases over the entire study period. The estimated and fixed model parameters are listed in Table 1.
In order to predict the post-vaccine transmission dynamics, we made the following assumptions: (1) the proportion of vaccinated children who responded to each dose of the vaccine was equal and independent (i.e. infants who responded to the first dose were no more or less likely to respond to the second dose); and (2) vaccine coverage was the same for both doses (based on Fig. S1).
We explored different approaches to fit the data. First, we fitted models to the pre-vaccine data only (Model A), and used the best-fit pre-vaccine parameters to predict post-vaccination cases while estimating only the proportion of individuals who responded to the vaccine and assuming infants who respond to each dose receive immunity comparable to a natural infection. Model B was similar to Model A, with the exception that we fitted models to the post-vaccination cases while estimating both the rate of waning vaccine-induced immunity and the proportion of individuals who responded to the vaccine. We then fitted models to both pre- and post-vaccination cases assuming vaccine-induced immunity is comparable to immunity from natural infection (Model C) or estimating the rate of waning vaccine-induced immunity (Model D). For Models A and C, the rate of waning of vaccine-induced immunity (ωv) is assumed to be the same as the waning rate of temporary immunity from natural infection ω. We compared the fit of the four different models in each site using the Bayesian information criterion (BIC).
2.4. Model evaluation
We further evaluated the predictive performance of the models for Navrongo by comparing to data collected between October 2016 and February 2019. This dataset from Navrongo was not used in the fitting of the model.
3. Results
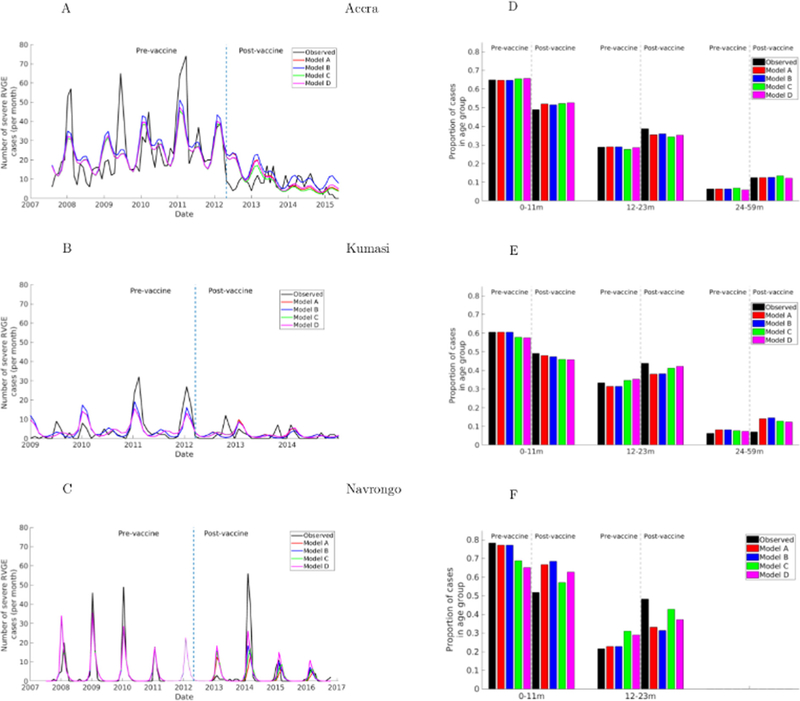
The transmission patterns and age distribution of reported rotavirus cases varied across the three sites (Fig. 3). In the pre-vaccination period, Accra exhibited year-round transmission with a biannual seasonal pattern; the highest number of cases typically occurred during the dry season (October-March) with occasional peaks in the wet season (May-August, e.g. in 2009) and year-to-year variation in the month of peak incidence (Fig. 3A). Rotavirus incidence in Kumasi (Fig. 3B) had a shorter peak transmission period occurring primarily during the dry season (December-February) and minor peaks during the wet season (June-August), and months with zero rotavirus cases. In Navrongo, transmission was strongly seasonal, occurring from December to March with peaks either in January or February (Fig. 3C). Outside this period, there were either zero or a few isolated cases per month. Interestingly, the major peak months in Navrongo coincide with those in Kumasi. The proportion of rotavirus incidence across the age groups was similar for the three sites (Fig. 3D–F), with the majority of cases (>60%) in the 0–11-month age group. However, cases tended to be slightly younger in Navrongo and older in Kumasi.
Fig. 3. Fit of models to the observed data.
(Left) Model-predicted and observed monthly incidence of severe RVGE cases among children during both pre- and post-vaccine periods. The vertical line indicates the date of vaccine introduction (April 2012). (Right) Model-predicted and observed proportion of severe RVGE in each age group before and after vaccine introduction. The grey dashed lines separate the pre- and post-vaccination distribution for each age group. The light line region of C indicates model predictions for Navrongo for the period with missing data.
Table 2 summarizes the estimated parameter values that provided the best fit to the data. The models were mostly able to capture the spatio-temporal variations in rotavirus incidence across the three sites prior to vaccine introduction, and all of the models were able to reproduce the proportion of rotavirus cases in each age group, particularly in the pre-vaccination period (Fig. 3A–C). While the models predicted the biannual transmission patterns in Accra and Kumasi, they were not able to capture the variation in the major and minor peaks in different years, in particular the major peak in June 2009 in Accra and July 2009 in Kumasi (Fig. 3A and B). In Navrongo, the models accurately predicted the observed strong seasonal transmission (Fig. 3C). However, the models tended to under- or over-estimate the peak values in all sites. All the models provided an acceptable estimate of the proportion of rotavirus cases in each age group, particularly in the pre-vaccination period across the sites (Fig. 3D–F).
Table 2. Model estimated parameters for each study site.
The symbols used in this table are explained in Table 1. Values in parentheses are 95% confidence intervals based on the profile likelihood. An example of the profile likelihoods for Model D in Kumasi is provided in Fig. S2.
| Parameter | R0 | ωm months |
b1 | Φ1 months |
b2 | Φ2 months |
h | d3 | sc | ωv months |
BIC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accra | |||||||||||
| Model A | 37.861 (36.22–39.54) |
4.838 (4.46–5.23) |
0.077 | 7.203 | 0.132 | 1.037 | 0.107 | 9.54E-06 | 0.989 (0.87–1.0) |
1473.093 | |
| Model B | 37.861 (36.22–39.54) |
4.838 (4.46–5.23) |
0.077 | 7.203 | 0.132 | 1.037 | 0.107 | 9.54E-06 | 0.989 (0.71–1.0) |
24.102 (11.11–48.38) |
1467.183 |
| Model C | 35.69 (34.24–37.52) |
4.276 (3.72–4.68) |
0.068 | 7.185 | 0.126 | 1.042 | 0.099 | 2.92E-05 | 0.987 (0.82–1.0) |
1471.098 | |
| Model D | 38.815 (37.35–40.36) |
4.848 (4.32–5.29) |
0.073 | 7.205 | 0.121 | 1.545 | 0.1 | 3.11E-05 | 0.961 (0.87–1.0) |
26.342 (15.94–52.92) |
1466.643 |
| Kumasi | |||||||||||
| Model A | 33.661 (29.5–36.2) |
4.714 (3.46–5.75) |
0.167 | 4.127 | 0.498 | 1.055 | 0.017 | 3.09E-07 | 0.734 (0.35–0.96) |
656.518 | |
| Model B | 33.661 (29.5–36.2) |
4.714 (3.46–5.75) |
0.167 | 4.127 | 0.498 | 1.055 | 0.017 | 3.09E-07 | 0.649 (0.42–0.88) |
5.33 (1.23–14.29) |
657.557 |
| Model C | 36.951 (33.2–40.4) |
5.706 (4.56–6.72) |
0.143 | 4.012 | 0.376 | 1.101 | 0.016 | 1.75E-07 | 0.81 (0.49–1.0) |
615.916 | |
| Model D | 38.589 (36.0-41.75) |
5.989 (4.89–6.9) |
0.144 | 4.034 | 0.373 | 1.104 | 0.016 | 1.88E-07 | 0.733 (0.38–0.93) |
4.591 (1.4–10) |
617.498 |
| Navrongo | |||||||||||
| Model A | 31.529 (30.36–32.78) |
1.890 (0.39–3.18) |
0.999 | 1.050 | 6.63E-08 | 5.125 | 0.012 | 2.54E-05 | 0.243 (0.08–0.36) |
585.837 | |
| Model B | 31.529 (30.36–32.78) |
1.890 (0.39–3.18) |
0.999 | 1.050 | 6.63E-08 | 5.125 | 0.012 | 2.54E-05 | 0.203 (0.08–0.32) |
5.26 (2.46–8.4) |
585.266 |
| Model C | 30.91 (29.87–31.71) |
3.565 (2.64–4.44) |
0.999 | 1.044 | 1.92E-08 | 4.755 | 0.013 | 3.46E-05 | 0.177 (0.10–0.37) |
576.391 | |
| Model D | 31.348 (30.48–32.18) |
3.554 (2.71–4.53) |
0.999 | 1.045 | 4.2E-08 | 3.574 | 0.013 | 2.23E-05 | 0.18 (0.04–0.32) |
6.281 (2.7–11.76) |
575.599 |
Following vaccine introduction, seasonal transmission patterns of rotavirus cases remained relatively the same, particularly in Navrongo where dry-season epidemics continued to occur most years, with only a small peak in January 2013 (Fig. 3C). In Accra, there was a significant reduction in cases compared to the pre-vaccination period. Also, transmission remained stable throughout the year, with only slight differences between the dry and wet seasons (Fig. 3A). Similarly, in Kumasi, there was a significant decrease in cases and transmission tends to be intermittent with more months with zero cases, particularly in the wet season (Fig. 3B). Interestingly, in Accra and Kumasi, transmission tends to be relatively stable with only small differences in incidence between major and minor seasons. On the other hand, in Navrongo, a post-vaccination epidemic occurred in 2014, with incidence exceeding that of the pre-vaccination peaks; smaller epidemics also occurred in 2015 and 2016 (Fig. 3C). In terms of age distribution, there was a marked shift away from rotavirus cases occurring in the 0–11 m age group towards older age groups relative to the pre-vaccination period (Fig. 3D–F). This shift in the age distribution was variable across sites, with a greater shift occurring in Navrongo.
Generally, the models were able to predict the observed reduction in the reported incidence of rotavirus in both Accra and Kumasi during the post-vaccination period. However, the models missed some noticeable peaks and, in addition, predicted a slight shift in the peak season in these two sites. Furthermore, the models overestimated the number of cases occurring in Accra during the first year following vaccine introduction. In Navrongo, while the models underestimated the post-vaccination epidemic intensity in 2014, they slightly overestimated the observed cases in 2013 and subsequent years. The models were able to capture the observed shift in the age distribution of cases to some extent, but slightly over- or under-estimated the proportion of cases in each age group relative to the observations in Accra and Kumasi (Fig. 3D and E), and were not able to fully capture the shift towards older cases during the post-vaccination period in Navrongo (Fig. 3F). The models assuming waning of vaccine-induced immunity tended to better predict the shift in cases to 12–23-month-olds and provided a slightly better fit to the data (according to BIC).
The estimated seasonal transmission parameters (b1, b2, φ1, φ2) were similar across the models for each site, but varied between sites (Table 2). In Accra, the model predicted transmission peaks in July and January with maximum amplitude occurring in the dry season (January). The model estimated peaks in Kumasi in April and January with maximum amplitude in the dry season (January). The model was able to capture the annual transmission in Navrongo, with transmission peaking in January, whereas the observed cases peaked either in January or February.
The other important model parameters that showed the greatest variation across the three locations were the rotavirus transmission rate, duration of maternal immunity, waning of vaccine-induced immunity, and proportion who responded to the vaccine (Table 2). The estimated rotavirus transmission rate was highest in Accra (R0 = 35.69–38.82) and lowest in Navrongo (R0 = 30.91–31.53), while the mean estimated duration of maternal immunity was longer (∼5 months) in Accra and Kumasi and shorter (∼3 months) in Navrongo. The proportion who responded to each vaccine dose was higher in Accra (Sc = 0.96–0.99) and Kumasi (Sc = 0. 65–0.81), but was estimated to be very low in Navrongo (Sc = 0.1 8–0.24). Furthermore, the estimated duration of vaccine-induced immunity was longer in Accra (>20 months), but shorter and comparable (4–6 months) in Kumasi and Navrongo (Table 2).
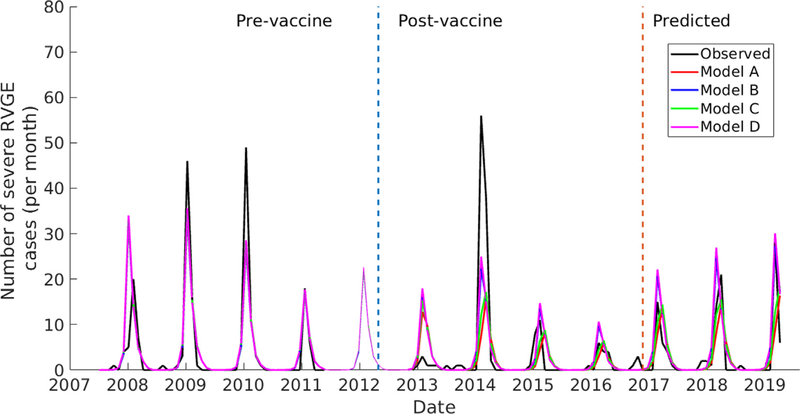
In Navrongo, transmission remains high and strongly seasonal 6 years following vaccine introduction, and there is a slightly increasing trend in intensity from 2016 (Fig. 4). There is satisfactory agreement between the model-predicted and observed post-vaccination rotavirus incidence for the evaluation period (October 2016-February 2019).
Fig. 4. Comparison of model and observed rotavirus incidence in Navrongo showing both models fitting and evaluation periods.
The brown vertical line separates the model fitting period from the evaluation period.
4. Discussion
While numerous studies have documented low vaccine effectiveness in LICs relative to HICs [21], the possible drivers accounting for this are poorly understood. Rotavirus seasonality also varies greatly between countries [44,45], but no research has attempted to investigate the potential drivers of intra-country variations in rotavirus vaccine effectiveness and its possible relationship with rotavirus seasonality. In this study, we used mathematical models to describe and quantify the variation in rotavirus transmission parameters across three sites in Ghana. Our results suggest the existence of intra-country variations in rotavirus transmission. In addition, the estimated vaccine response rate and duration of vaccine-induced immunity correlate with variation in other model parameters such as transmission seasonality, duration of maternal immunity, and transmission rates across the sites. Understanding how these different drivers of rotavirus transmission influence vaccine effectiveness could help to identify optimal strategies to improve vaccine performance across different settings, particularly in LICs.
Clear seasonal variations exist in rotavirus incidence across the three sites in Ghana (Fig. 3A–C), which are similar to those observed across countries in West Africa. Year-round rotavirus transmission with peaks during the dry season, as we and others have observed in Accra and Kumasi [13,46], has also been reported in other parts of West Africa, including Abidjan (the capital of Ivory Coast) [47] and Lome (the capital of Togo) [48]. Our model was able to reproduce this pattern, although we could not explain the increased incidence observed in both Accra and Kumasi during the 2009 wet season (Fig. 3A and B). Our models predicted a relatively stable transmission in Accra and Kumasi especially in the second year following vaccine introduction, which agrees with the observations. However, the models failed to predict the spontaneous reduction in Accra around the time vaccine was introduced, which is unlikely to be attributable to vaccination (Fig. 3A). Rotavirus vaccine was introduced in April, which coincides with the end of the rotavirus season, and only a small cohort of infants would be eligible for vaccination at that time.
In contrast, a strong seasonal pattern of rotavirus cases was observed for Navrongo for both the pre- and post-vaccine periods (Fig. 3C). Similar trends have been observed in the same study location since at least the late 1990s [49], as well as elsewhere in northern Ghana [50]. These seasonal patterns have also been observed in other West African countries. Fischer et al. [51] found the majority of cases in Guinea-Bissau occur from January to April, with few to zero cases outside these months. Another study in Enugu, Nigeria also reported this observed strong seasonal transmission, with greater than 90% of cases occurring between December and March over the six-year study period [52]. Similarly, in Ouagadougou (the capital of Burkina Faso), 72% of acute viral gastroenteritis cases among children aged <5 years were rotavirus-positive during the dry season (November to May) compared to 6% rotavirus-positive during the wet season (June to October); other viral pathogens did not vary seasonally [53]. Similar patterns were also found in the rural town of Gaoua, Burkina Faso [54]. Interestingly, Ouagadougou is an urban metropolis located about 180 km from Navrongo, which is a peri-urban center located >500 km from Kumasi and >800 km from Accra. Despite the differences in population and economic development across study sites in West Africa, they experience a similar climate regime characterized by a prolonged dry season. While climate could be a possible driver of these dynamics, further research is required to support this hypothesis.
In addition to seasonal variations, our models also predict variation in the duration of maternal immunity, which we estimated to be shorter in Navrongo and longer in Accra and Kumasi (Table 2). The strong seasonal transmission in Navrongo may have an adverse impact on naturally acquired immunity among mothers, resulting in shorter maternal immunity duration. Consequently, infants are exposed to early rotavirus infections in Navrongo. A previous study in this same study area reported a high rotavirus incidence in infants <2 months of age [49]. Several studies have reported neonatal rotavirus infections in both LIC and HIC settings, with most infections being asymptomatic [44,51,55]. Neonatal rotavirus infections have implications for vaccine effectiveness, as studies have shown that infants exposed to natural rotavirus infection prior to vaccination tend to have a lower seroconversion rate [25,26]. Previous rotavirus models have typically treated the duration of maternal immunity as a fixed parameter [18,21–23,56]. However, our analysis reveals that duration of maternal immunity may be highly variable and should be estimated when fitting models to data from different settings.
The estimated transmission rate of rotavirus, as quantified by the basic reproductive number R0, was highest in Accra and lowest in Navrongo (Table 2). Despite this, the observed annual average percentage reduction in rotavirus prevalence between the pre-and post-vaccination periods was highest in Accra (74.2%) followed by Kumasi (64.4%) and Navrongo (34.9%). This differential reduction in incidence could possibly be due to variable vaccine performance, as we also estimated a higher vaccine response rate in Accra and Kumasi (65–99%) compared to Navrongo (18–24%). These results demonstrate that identifying strategies aimed at improving the vaccine response rate could reduce the burden of rotavirus even within high transmission settings.
The lower vaccine response rate seen in Navrongo could be partially explained by the shorter duration of maternal immunity and early exposure to rotavirus infection [57]. By the time the first vaccine dose is administered (6 weeks), some of the infants may have already been exposed to their first rotavirus natural infection and therefore have a lower vaccine response rate. There are studies elsewhere that support this explanation, suggesting that infants who have been infected with rotavirus before receiving vaccine were less likely to respond [25,26]. Neonatal rotavirus vaccines may provide greater protection in such a setting. Reassortant rotavirus tetravalent vaccine with the first dose administered during the neonatal period showed an efficacy of 63.1% in Navrongo [57]. Another possible explanation could be due to significant variations in the gut microbiota composition among responders and non-responder infants in Navrongo [27], thereby influencing the low vaccine response rate. However, additional studies are required to ascertain whether significant differences exist in infant gut microbiome composition across the three sites to support this hypothesis. Furthermore, we estimated a longer duration of maternal immunity and a higher vaccine response rate in Accra and Kumasi, which suggests maternal antibodies are unlikely to interfere with the vaccine response rate, as has been suggested [28,29]. This finding is supported by studies that showed withholding breastfeeding at the time of vaccine administration led to poorer vaccine effectiveness [30,57].
Comparing the pre- and post-vaccine periods provides important insights into the occurrence of rotavirus cases in various age groups (Fig. 3D–F). Both observations and our models show a similar increase in the proportion of cases in older age groups following vaccine introduction. This shift in age distribution of rotavirus cases towards >1-year-olds after vaccine introduction has been observed in both LIC and HIC settings, and for both the Rotarix and RotaTeq vaccines [23,58–60]. Before vaccine introduction, 90% of rotavirus hospitalizations occurred in infants aged between 3 and 12 months across five African countries [45], and in Nigeria, 66% of rotavirus cases occurred among infants aged <1 year [52]. In Belgium, Zeller et al. [59] found consistently greater than 55% of cases in the 0–11-month age group over a seven-year period prior to vaccine introduction, decreasing to 32–43% after vaccine introduction. The almost equal proportion of infections between the 0–11 m and 12–23 m age groups after vaccine introduction in all the sites in Ghana is similar to observations in Burkina Faso [54]. In Malawi, there was a shift in the mean age of rotavirus caused diarrhea from 9.3 months in pre-vaccine to 11.8 months following vaccination [61].
Note that our models were able to reproduce the shift in the age distribution of cases even when we assumed the waning of vaccine-induced immunity was only partial and comparable to that of natural immunity. There are two factors that contribute to this: (1) as the vaccination program rolled out, only <1-year-olds will be directly protected by the vaccine in the first year of the program; and (2) vaccination reduces the rate of rotavirus transmission (through herd immunity), thereby delaying the time to infection among individuals who are unvaccinated, did not respond to the vaccine, or are only partially protected by vaccination. Nevertheless, waning of vaccine-induced immunity may help to explain the poor vaccine performance in Navrongo, as models in which we estimated the duration of vaccine-induced immunity provided a slightly better fit to the data and were better able to reproduce the shift in the age distribution of cases in this setting. Our findings thus highlight the need to identify vaccine strategies aimed at prolonging vaccine-induced protection in addition to the overall response rate in such settings.
The problem of low vaccine performance still persists in Navrongo 6 years following vaccine introduction. The models were able to predict the peaks of the outbreaks as well as the increasing intensity over the evaluation period. This indicates that the models provide satisfactory predictions of vaccine impact and can be used to assess the potential effectiveness of different vaccination strategies, particularly in settings with strong seasonal transmission.
5. Conclusions
In summary, our results highlight the existence of marked variations in rotavirus incidence within Ghana, similar to what has been observed between countries in West Africa and other parts of the world, as well as the potential for rotavirus vaccine to reduce RVGE incidence with sustained vaccination in Ghana. However, the low vaccine impact in Navrongo presents a challenge requiring further investigation to understand how the strong seasonal transmission and shorter duration of maternal immunity might interfere with vaccine performance. While missing data in the post-vaccination period might have affected the estimated parameters for Navrongo, our models reliably predicted rotavirus incidence during the evaluation period. Our findings highlight the need to further explore the relationship between rotavirus seasonality, maternal immunity duration, and vaccine response rate and how they impact vaccine effectiveness to develop strategies to improve vaccine impact in settings with highly seasonal rotavirus transmission.
Supplementary Material
Acknowledgments
We thank the rotavirus surveillance teams at War Memorial Hospital (Navrongo), Komfo Anokye Teaching Hospital (Kumasi) and the Korle Bu Teaching hospital (Accra) for their contribution to the study.
Funding
The work was supported by the National Institute of Allergy and Infectious Diseases, United State is part of the National Institutes of Health [grant number R01AI112970 to V.E.P.].
Abbreviations:
- EPI
Expanded Program on Immunization
- HICs
high-income countries
- LICs
low-income countries
- RVGE
Rotavirus-associated gastroenteritis
- RV1
monovalent Rotarix vaccine
Footnotes
Declaration of competing interest
VEP has received reimbursement for travel expenses from Merck to attend a Scientific Input Engagement unrelated to the subject of this paper. All other authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.05.057.
References
- [1].Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infectious Diseases 2012;12(2):136–41. [DOI] [PubMed] [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, Network WHOCGRS, Agocs M, et al. Global, regional, and national estimates of rotavirus mortality in children < 5 years of age, 2000–2013. Clin Infect Dis 2016;62(suppl 2):S96–S105. [DOI] [PubMed] [Google Scholar]
- [3].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human—bovine (WC3) reassortant rotavirus vaccine. New England J Medicine 2006;354(1):23–33. [DOI] [PubMed] [Google Scholar]
- [4].Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New England J Medicine 2006;354(1):11–22. [DOI] [PubMed] [Google Scholar]
- [5].Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor J, Cohen R. et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007;370(9601):1757–63. [DOI] [PubMed] [Google Scholar]
- [6].Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376(9741):606–14. [DOI] [PubMed] [Google Scholar]
- [7].Zaman K, Anh DD, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;376(9741):615–23. [DOI] [PubMed] [Google Scholar]
- [8].Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England J Medicine 2010;362(4):289–98. [DOI] [PubMed] [Google Scholar]
- [9].GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infectious Diseases 2017;17(9):909–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arvay ML, Curns AT, Terp S, Armah G, Wontuo P, Parashar UD, et al. How much could rotavirus vaccines reduce diarrhea-associated mortality in northern Ghana? A model to assess impact. J Infectious Dis 2009;200(Supplement 1): S85–91. [DOI] [PubMed] [Google Scholar]
- [11].Enweronu-Laryea CC, Sagoe KW, Glover-Addy H, Asmah RH, Mingle JA, Armah GE. Prevalence of severe acute rotavirus gastroenteritis and intussusceptions in Ghanaian children under 5 years of age. J Infect Dev Ctries 2011;6(02):148–55. [DOI] [PubMed] [Google Scholar]
- [12].Enweronu-Laryea CC, Sagoe KW, Damanka S, Lartey B, Armah GE. Rotavirus genotypes associated with childhood severe acute diarrhoea in southern Ghana: a cross-sectional study. Virol J 2013;10(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Damanka S, Adiku TK, Armah GE, Rodrigues O, Donkor ES, Nortey D, et al. Rotavirus infection in children with diarrhea at Korle-Bu teaching hospital, Ghana. Jpn J Infect Dis 2016;69(4):331–4. [DOI] [PubMed] [Google Scholar]
- [14].Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. J Infectious Diseases 2016;213(11):1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Enweronu-Laryea CC, Boamah I, Sifah E, Diamenu SK, Armah G. Decline in severe diarrhea hospitalizations after the introduction of rotavirus vaccination in Ghana: a prevalence study. BMC Infectious Diseases 2014;14(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Armah G, Pringle K, Enweronu-Laryea CC, Ansong D, Mwenda JM, Diamenu SK, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clinical Infectious Diseases 2016;62(suppl 2): S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adadey SM, Quaye O. The burden of gastroenteritis in the post-rotavirus vaccine era in Ghana: a hospital diagnoses-based study. Int J Med Sci Public Health 2017;6(7):45–9. [Google Scholar]
- [18].Pitzer VE, Viboud C, Simonsen L, Steiner C, Panozzo CA, Alonso WJ, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 2009;325(5938):290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine 2009;27(30):4025–30. [DOI] [PubMed] [Google Scholar]
- [20].de Blasio BF, Kasymbekova K, Flem E. Dynamic model of rotavirus transmission and the impact of rotavirus vaccination in Kyrgyzstan. Vaccine 2010;28(50):7923–32. [DOI] [PubMed] [Google Scholar]
- [21].Lopman BA, Pitzer VE, Sarkar R, Gladstone B, Patel M, Glasser J, et al. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS ONE 2012;7(8):e41720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pitzer VE, Atkins KE, de Blasio BF, Van Effelterre T, Atchison CJ, Harris JP, et al. Direct and indirect effects of rotavirus vaccination: comparing predictions from transmission dynamic models. PLoS ONE 2012;7(8):e42320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park J, Goldstein J, Haran M, Ferrari M. An ensemble approach to predicting the impact of vaccination on rotavirus disease in Niger. Vaccine 2017;35 (43):5835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lopman B, Dahl R, Shah M, Parashar UD. Timing of birth as an emergent risk factor for rotavirus hospitalization and vaccine performance in the post-vaccination era in the United States. Am J Epidemiology 2018;187:1745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Becker-Dreps S, Vilchez S, Velasquez D, Moon SS, Hudgens MG, Zambrana LE, et al. Rotavirus-specific IgG antibodies from mothers’ serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatric Infectious Dis J 2015;34(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chilengi R, Simuyandi M, Beach L, Mwila K, Becker-Dreps S, Emperador DM, et al. Association of maternal immunity with rotavirus vaccine immunogenicity in Zambian infants. PLoS ONE 2016;11(3):e0150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infectious Diseases 2017;215(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens 2017;6(4):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014;32:A134–9. [DOI] [PubMed] [Google Scholar]
- [30].Groome MJ, Moon SS, Velasquez D, Jones S, Koen A, Niekerk Nv, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Org 2014;92:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine-a randomized trial. PLoS ONE 2015;10(6):e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, Koen A, et al. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 2016;62(2):157–65. [DOI] [PubMed] [Google Scholar]
- [33].Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Diseases 2013;208(2):284–94. [DOI] [PubMed] [Google Scholar]
- [34].Zaman K, Fleming JA, Victor JC, Yunus M, Bari TIA, Azim T, et al. Noninterference of rotavirus vaccine with measles-rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J Infectious Diseases 2016;213(11):1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pitzer VE, Bennett A, Bar-Zeev N, Jere KC, Lopman BA, Lewnard JA, et al. Evaluating strategies to improve rotavirus vaccine impact during the second year of life in Malawi. Sci Translational Med 2019;11(505):eaav6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ghana Health Service Facts and Figures, https://www.ghanahealthservice.org/ghs-item-details.php?cid=2&scid=55&iid=135; 2016. [accessed 1 March 2020].
- [37].Wilde J, Yolken R, Willoughby R, Eiden J. Improved detection of rotavirus shedding by polymerase chain reaction. Lancet 1991;337(8737): 323–6. [DOI] [PubMed] [Google Scholar]
- [38].Ward RL, Bernstein DI, Young EC, Sherwood JR, Knowlton DR, Schiff GM. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis 1986;154(5):871–80. [DOI] [PubMed] [Google Scholar]
- [39].Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infectious Diseases 2004;4(2):91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].White L, Buttery J, Cooper B, Nokes D, Medley G. Rotavirus within day care centres in Oxfordshire, UK: characterization of partial immunity. J Royal Soc Interface 2008;5(29):1481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Velázquez FR, Matson DO, Calva JJ, Guerrero ML, Morrow AL, Carter-Campbell S, et al. Rotavirus infection in infants as protection against subsequent infections. New England J Medicine 1996;335(14):1022–8. [DOI] [PubMed] [Google Scholar]
- [42].Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. New Engl J Med 2011;365(4):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Koopman J, Monto A, Longini I Jr. The Tecumseh Study. XVI: Family and community sources of rotavirus infection. Am J Epidemiol 1989;130 (4):760. [DOI] [PubMed] [Google Scholar]
- [44].Cunliffe N, Kilgore P, Bresee J, Steele A, Luo N, Hart C, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Org 1998;76(5):525. [PMC free article] [PubMed] [Google Scholar]
- [45].Mwenda JM, Ntoto KM, Abebe A, Enweronu-Laryea C, Amina I, Mchomvu J, et al. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J Infect Diseases 2010;202(Supplement 1):S5–S11. [DOI] [PubMed] [Google Scholar]
- [46].Enweronu-Laryea CC, Sagoe KW, Mwenda JM, Armah GE. Severe acute rotavirus gastroenteritis in children less than 5 years in southern Ghana: 2006–2011. Pediatric Infectious Disease J 2014;33:S9–S13. [DOI] [PubMed] [Google Scholar]
- [47].Akoua-Koffi C, Kouadio VA, Atteby JJY. Hospital-based surveillance of rotavirus gastroenteritis among children under 5 years of age in the Republic of Ivory Coast: a cross-sectional study. BMJ Open 2014;4(1):e003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tsolenyanu E, Seheri M, Dagnra A, Djadou E, Tigossou S, Nyaga M, et al. Surveillance for rotavirus gastroenteritis in children less than 5 years of age in Togo. Pediatric Infectious Disease J 2014;33:S14–8. [DOI] [PubMed] [Google Scholar]
- [49].Group NRR. Incidence and risk factors of paediatric rotavirus diarrhoea in northern Ghana. Tropical Med Int Health 2003;8(9):840–6. [DOI] [PubMed] [Google Scholar]
- [50].Akuffo R, Armah G, Clemens M, Kronmann K, Jones A, Agbenohevi P, et al. Prevalence of enteric infections among hospitalized patients in two referral hospitals in Ghana. BMC Res Notes 2017;10(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fischer TK, Aaby P, Molbak K, Rodrigues A. Rotavirus disease in Guinea-Bissau, West Africa: a review of longitudinal community and hospital studies. J Infectious Diseases 2010;202(Supplement 1):S239–42. [DOI] [PubMed] [Google Scholar]
- [52].Tagbo B, Mwenda J, Eke C, Edelu B, Chukwubuike C, Armah G, et al. Rotavirus diarrhoea hospitalizations among children under 5 years of age in Nigeria, 2011–2016. Vaccine 2018;36:7759–64. [DOI] [PubMed] [Google Scholar]
- [53].Ouedraogo N, Ngangas SMT, Bonkoungou IJO, Tiendrebeogo AB, Traore KA, Sanou I, et al. Temporal distribution of gastroenteritis viruses in Ouagadougou, Burkina Faso: seasonality of rotavirus. BMC Public Health 2017;17(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bonkoungou IJO, Aliabadi N, Leshem E, Kam M, Nezien D, Drabo MK, et al. Impact and effectiveness of pentavalent rotavirus vaccine in children <5 years of age in Burkina Faso. Vaccine 2018;36:7170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Espinoza F, Paniagua M, Hallander H, Svensson L, Strannegård O. Rotavirus infections in young Nicaraguan children. Pediatric Infect Disease J 1997;16 (6):564–71. [DOI] [PubMed] [Google Scholar]
- [56].Atkins KE, Shim E, Pitzer VE, Galvani AP. Impact of rotavirus vaccination on epidemiological dynamics in England and Wales. Vaccine 2012;30(3):552–64. [DOI] [PubMed] [Google Scholar]
- [57].Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infectious Diseases 2013;208(3):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009;123(3): e393–400. [DOI] [PubMed] [Google Scholar]
- [59].Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010;28(47):7507–13. [DOI] [PubMed] [Google Scholar]
- [60].Yoshikawa T, Matsuki T, Sato K, Mizuno M, Shibata M, Hasegawa S, et al. Impact of rotavirus vaccination on the burden of acute gastroenteritis in Nagoya city, Japan. Vaccine 2018;36(4):527–34. [DOI] [PubMed] [Google Scholar]
- [61].Bar-Zeev N, Jere KC, Bennett A, Pollock L, Tate JE, Nakagomi O, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis 2016;62(suppl 2):S213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.