Abstract
An increasing number of studies indicate that microRNAs (miRNAs/miRs) are involved in diverse biological signaling pathways and play important roles in the progression of various diseases, including both oncological and non-oncological diseases. These small non-coding RNAs can block translation, resulting in a low expression level of target genes. miR-129 is an miRNA that has been the focus of considerable research in recent years. A growing body of evidence shows that the miR-129 family not only functions in cancer, including osteosarcoma, nasopharyngeal carcinoma, and ovarian, prostate, lung, breast and colon cancer, but also in non-cancerous diseases, including heart failure (HF), epilepsy, Alzheimer's disease (AD), obesity, diabetes and intervertebral disc degeneration (IVDD). It is therefore necessary to summarize current research progress on the role of miR-129 in different diseases. The present review includes an updated summary of the mechanisms of the miR-129 family in oncological and non-oncological diseases. To the best of our knowledge, this is the first review focusing on the role of miR-129 in non-cancerous diseases such as obesity, HF, epilepsy, diabetes, IVDD and AD.
Keywords: microRNA-129-5p, microRNA-129-3p, cancer, non-cancerous diseases, mechanism
1. Introduction
MicroRNAs (miRNAs/miRs) are an evolutionarily conserved class of small non-coding RNAs of 18-25 nucleotides in length, which are considered to be involved in the majority of known physiological and pathological processes, including oncological and non-oncological diseases (1). miRNAs function as target gene expression repressors through base-pairing with the 3'-untranslated region (UTR) of endogenous mRNAs. The miR-129 family consists of two members: miR-129-1 and miR-129-2. miR-129-1 (gene ID, 406917) is located on chromosome 7q32.1, a region that is frequently deleted in multiple tumors. miR-129-2 (gene ID, 406918) is located on chromosome 11p11.2. Each of these miRNAs produces two mature miRNA strands that are denoted with a -3p or -5p suffix (2). The biogenesis of mature miR-129 undergoes the following stages: Pri-miR-129 (the non-coding primary transcript of the miR-129 gene transcribed by RNA polymerase II), pre-miR-129 (the 70-nucleotide stem-loop precursor miR-129 obtained by cleaving pri-miR-129 through Drosha ribonuclease III enzyme), duplex (the complex composed of mature miRNA and antisense miRNA, and obtained by cleaving pre-miR-129 by the cytoplasmic Dicer ribonuclease) and the mature miR-129 strand (one of the strands from the duplex that will be incorporated into Argonaute and then into the RNA-induced silencing complex) (3). It has been reported that the expression of the miR-129 family is aberrant in several types of oncological and non-oncological diseases; it is downregulated in prostate cancer (miR-129-5p and miR-129-3p) (4), osteosarcoma (miR-129-5p) (5), lung cancer (miR-129-5p) (6), breast cancer (miR-129-5p) (7), nasopharyngeal carcinoma (miR-129-5p) (8), ovarian cancer (miR-129-5p and miR-129-3p) (9), colon cancer (miR-129-5p and miR-129-3p) (10), heart failure (HF; miR-129-5p) (11), epilepsy (miR-129-5p) (12), intervertebral disc degeneration (IVDD; miR-129-5p) (13) and Alzheimer's disease (AD; miR-129-5p) (14), but upregulated in obesity (miR-129-5p and miR-129-3p) (15) and diabetes (miR-129-5p and miR-129-3p) (16). In different diseases, the miR-129 family has different targets for modulating various biological processes (Tables I and II) and involves multiple signaling pathways (Figs. 1 and 2), thus playing an important role in promoting or blocking disease progression (Tables III and IV). Members of the miR-129 family have been reported as potentially promising biomarkers in certain diseases, such as diabetes and epilepsy (12,16). Therefore, the aim of the present review was to summarize the functions of the miR-129 family in the development of oncological and non-oncological diseases, focusing on the role of miR-129 as a potential therapeutic target and biomarker in future clinical applications.
Table I.
Targets of microRNA-129 in cancer.
| Cancer type | Target | miRNA regulatory element | (Refs.) |
|---|---|---|---|
| Prostate cancer | Wnt | cgUuCGggguCugGCGUUUU | (4) |
| ETV1 | gucuggcGUUUUU | (20) | |
| Smad3 | cgaaaaaccccaUUCCCGA | (21) | |
| Osteosarcoma | HIF1A-AS2 | guUCGGGUCuggcGUUUUUc | (5) |
| LHX2 | cguucgggucuggCGUUUUU | (25) | |
| Lung cancer | YWHAB | cguucgggucuggCGUUUUUc | (35) |
| ZEB2 | cguucgggucuggCGUUUUUc | (37) | |
| Breast cancer | CBX4 | guucggGgUcuggCGUUUUU | (7) |
| MALAT1 | cguucgggucuggCGUUUUUc | (40) | |
| Nasopharyngeal | ZIC2 | gUuCGggucUggCGUUUUUc | (8) |
| carcinoma | WEE1 | / | (48) |
| Ovarian cancer | BZW1 | gaaaaaccccaUUCCCGAa | (50) |
| SOX4 | / | (53) | |
| PCAT-1 | ucgggucuggCGUUUUUc | (49) | |
| ABCB1 | ucgggucuggcGUUUUUc | (9) | |
| YAP | CgUucggGUCugGcGUUUUUc | (59) | |
| TAZ | cgUucgggUcUggCGUUUUUC | (59) | |
| Colon cancer | HMGB1 | tcgggtctggCGTTTTTc | (10) |
| Pirh2 | / | (64) |
Bases written in upper case of miRNA regulatory elements can form complementary pairs with the bases in 3'-UTR of endogenous mRNAs. miRNA, microRNA; ETV1, ETS variant transcription factor 1; Smad3, SMAD family member 3; HIF1A-AS2, HIF1A antisense RNA 2; LHX2, LIM homeobox 2; YWHAB, tyrosine 3-plus monooxygenase/tryptophan 5-plus monooxygenase activation protein β; ZEB2, Zinc finger E-box binding homeobox 2; CBX4, chromobox 4; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; ZIC2, Zic family member 2; WEE1, WEE1 G2 checkpoint kinase; BZW1, basic leucine zipper and W2 domains 1; SOX4, SRY-box transcription factor 4; PCAT-1, prostate cancer-associated transcript 1; ABCB1, ATP-binding cassette subfamily B member 1; YAP, yes-associated protein; TAZ, tafazzin; HMGB1, high-mobility group box-1; Pirh2, ring finger and CHY zinc finger domain containing 1.
Table II.
Targets of microRNA-129 in non-cancerous diseases.
| Disease type | Target | miRNA regulatory element | (Refs.) |
|---|---|---|---|
| Obesity | ATG7 | cguucgggucuggcGUUUUUUc | (79) |
| Heart failure | HMGB1 | cguucgggucuggCGUUUUUc | (11) |
| GRIN2D | gaaaaccccaUUCCCGAa | (92) | |
| NEAT1 | cguucgggucUGGCGUUUUUc | (97) | |
| Epilepsy | c-Fos | cguucgggUCUGGCGUUUUUc | (105) |
| Diabetes | Casp6 | aAGCCCUUaccaaaaagcau | (121) |
| CCR2 | aACCCCUUAccccaaaaagcau | (121) | |
| Sp1 | / | (122) | |
| Intervertebral disc | FADD | cguucgggucuggCGUUUUUc | (133) |
| degeneration | BMP2 | / | / |
| Beclin-1 | cguucgggucuggcGUUUUUc | (135) | |
| Alzheimer's disease | EP300 | / | (14) |
| SOX6 | CguucgggucuggCGUUUUUc | (140) |
Bases written in upper case of miRNA regulatory elements can form complementary pairs with the bases in 3'-UTR of endogenous mRNAs. ATG7, autophagy-related gene 7; HMGB1, high-mobility group box-1; GRIN2D, glutamate ionotropic receptor N-methyl-D-aspartate type subunit 2D; NEAT1, nuclear paraspeckle assembly transcript 1; c-Fos, c-Fos proto-oncogene; Casp6, caspase 6; CCR2, C-C chemokine receptor type 2; Sp1, specificity protein-1; FADD, Fas-associated death domain; BMP2, bone morphogenetic protein 2; EP300, E1A binding protein P300; SOX6, SRY-box transcription factor 4.
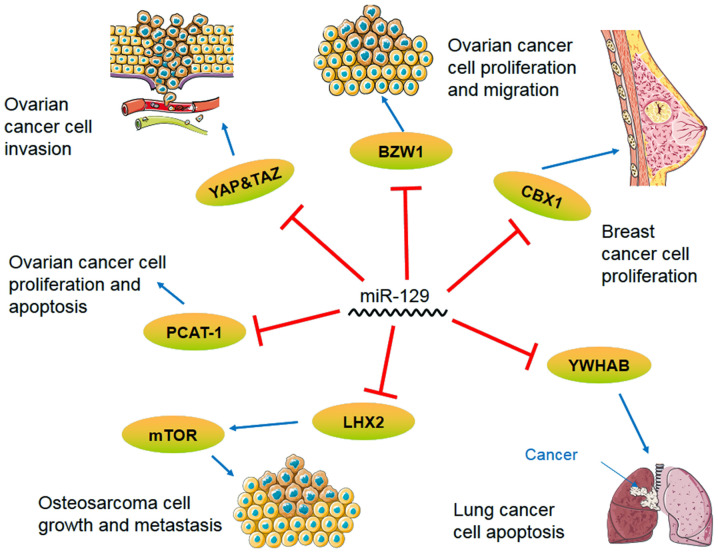
Figure 1.
Signaling pathway axis of miR-129 in lung cancer, breast cancer, ovarian cancer and osteosarcoma. miR, microRNA; BZW1, basic leucine zipper and W2 domains 1; YAP, yes-associated protein; TAZ, tafazzin; mTOR, mechanistic target of rapamycin; CBX1, chromobox 4; YWHAB, tyrosine 3-plus monooxygenase/tryptophan 5-plus monooxygenase activation protein β; LHX2, LIM homeobox 2; PCAT-1, prostate cancer-associated transcript 1.
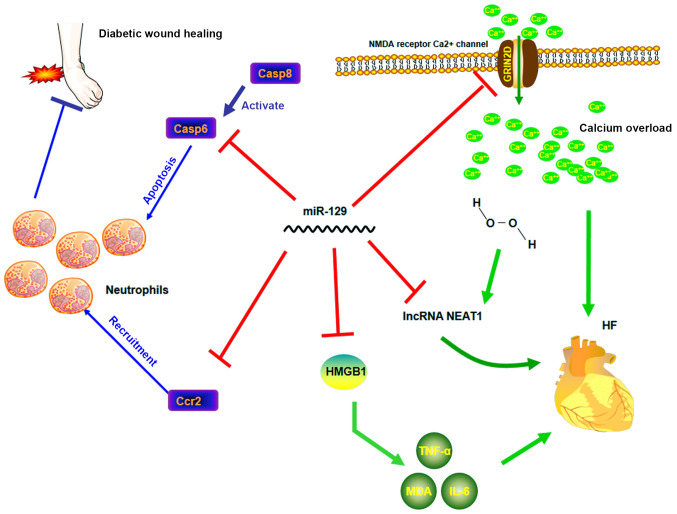
Figure 2.
Molecular regulatory network of miR-129 in HF and diabetes (different colors). miR-129 acts as a beneficial factor by inhibiting functions of neutrophils in diabetes and by inhibiting inflammation and maintaining cellular calcium balance in HF. miR, microRNA; Casp, caspase; Ccr2, C-C chemokine receptor type 2; HMGB1, high-mobility group box-1; NEAT1, nuclear paraspeckle assembly transcript 1; NMDA, N-methyl-D-aspartate; GRIN2D, glutamate ionotropic receptor N-methyl-D-aspartate type subunit 2D; HF, heart failure.
Table III.
Differential expression and functions of the miR-129 family with confirmed targets in cancer.
| Cancer types | Axis | Differential expression | Functions | (Refs.) |
|---|---|---|---|---|
| Prostate cancer | miR-129/Wnt/ZIC2 | Downregulated | Inhibit epithelial-mesenchymal transition, angiogenesis, tumorigenesis, migration and apoptosis | (4) |
| miR-129-5p/ETV1/ Hippo/YAP | Inhibit the growth of prostate cancer cells | (20) | ||
| miR-129-3p/SMAD3/ Bcl-2(BAX) | Promote apoptosis and inhibit the proliferation and invasion of prostate cancer | (21) | ||
| Osteosarcoma | HIF1A-AS2/miR-129 | Downregulated | Inhibit osteosarcoma cell proliferation and cancer cell invasion | (5) |
| miR-129/LHX2/ mTOR | Inhibit tumors and autophagy | (32) | ||
| Lung cancer | miR-129/YWHAB | Downregulated | Increase the apoptosis of lung cancer cells | (35) |
| miR-129/ZEB2 | Inhibit NSCLC cell proliferation, migration and invasion | (37) | ||
| Breast cancer | miR-129-5p/CBX4 | Downregulated | Inhibit the proliferation of cancer cells | (7) |
| MALAT1/miR-129-5p | Inhibit cell proliferation, migration and invasion | (40) | ||
| Nasopharyngeal carcinoma | miR-129-5p/ZIC2 | Downregulated | Inhibit lymphangiogenesis, lymph node metastasis and the apoptosis of NPC | (8) |
| miR-129-3p/WEE1 | Decrease cisplatin resistance | (48) | ||
| Ovarian cancer | miR-129-3p/BZW1 | Downregulated | Inhibit the proliferation and the migration of ovarian cancer cells | (50) |
| SNHG12/miR-129/ SOX4 | Inhibit proliferation and migration ability | (53) | ||
| PCAT-1/miR-129 | Inhibit growth and promote apoptosis of cancer cells | (49) | ||
| UCA1/miR-129/ABCB1 | Inhibit PTX resistance in OC | (9) | ||
| miR-129-5p/ YAP&TAZ | Inhibit the invasiveness of ovarian cancer | (59) | ||
| Colon cancer | MALAT1/miR-129-5p/ HMGB1 | Downregulated | Inhibit the progression of colon cancer | (10) |
| miR-129-3p/Pirh2/p53 | Induce cell senescence | (64) |
miR, microRNA; NSCLC, non-small cell lung cancer; NPC, nucleus pulposus cell; PTX, paclitaxel; OC, ovarian cancer; ETV1, ETS variant transcription factor 1; HIF1A-AS2, HIF1A antisense RNA 2; LHX2, LIM homeobox 2; mTOR, mechanistic target of rapamycin; YWHAB, tyrosine 3-plus monooxygenase/tryptophan 5-plus monooxygenase activation protein β; ZEB2, Zinc finger E-box binding homeobox 2; CBX4, chromobox 4; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; ZIC2, Zic family member 2; WEE1, WEE1 G2 checkpoint kinase; BZW1, basic leucine zipper and W2 domains 1; SOX4, SRY-box transcription factor 4; PCAT-1, prostate cancer-associated transcript 1; ABCB1, ATP-binding cassette subfamily B member 1; YAP, yes-associated protein; TAZ, tafazzin; HMGB1, high-mobility group box-1; Pirh2, ring finger and CHY zinc finger domain containing 1.
Table IV.
Differential expression and functions of the miR-129 family with confirmed targets in non-cancerous diseases.
| Disease type | Axis | Differential expression | Functions | (Refs.) |
|---|---|---|---|---|
| Obesity | miR-129-5P/ATG7 | Upregulated | Downregulate autophagy pathways, resulting in inhibition of white adipogenesis | (79) |
| Heart failure | miR-129-5P/HMGB1 | Downregulated | Inhibit inflammation and decrease levels of TNF-a and IL-6 | (11) |
| miR-129-3p/GRIN2D/Ca2+ | Inhibit calcium overload to avoid damage from high concentration of Ca2+ | (92) | ||
| NEAT1/miR-129-5p | Inhibit H2O2-induced apoptosis in cardiomyocytes | (97) | ||
| Epilepsy | miR-129-5p/c-Fos/MAPK | Downregulated | Inhibit MAPK signaling transduction to decrease the frequency of epilepsy seizures | (105) |
| Diabetes | miR-129-3p/Casp6 | Upregulated | Inhibit caspase 8 signaling pathway to decrease apoptosis of neutrophils | (121) |
| miR-129-3p/Ccr2 | Inhibit recruitment of neutrophils | (121) | ||
| miR-129-5p/Sp1 | Inhibit degradation of ECM components | (122) | ||
| Intervertebral disc | miR-129-5p/ FADD&BMP2 | Upregulated | Facilitate the proliferation and inhibit the apoptosis of NPCs | (133) |
| degeneration | miR-129-5p/Beclin-1 | Inhibit autophagy and thus promote apoptosis of NPCs | (135) | |
| Alzheimer's disease | miR-129-5p/EP300 | Downregulated | Influence histone acetylation and chromatin remodeling to alter expression of β-amyloid and τ-protein | (14) |
| miR-129-5p/SOX6 | Inhibit apoptosis, inflammatory reactions and increase proliferation of rat hippocampal neurons | (140) |
miR, microRNA; ECM, extracellular matrix; NPC, nucleus pulposus cell; ATG7, autophagy-related gene 7; HMGB1, high-mobility group box-1; GRIN2D, glutamate ionotropic receptor N-methyl-D-aspartate type subunit 2D; NEAT1, nuclear paraspeckle assembly transcript 1; c-Fos, c-Fos proto-oncogene; MAPK, mitogen-activated protein kinase; Casp6, caspase 6; CCR2, C-C chemokine receptor type 2; Sp1, specificity protein-1; FADD, Fas-associated death domain; BMP2, bone morphogenetic protein 2; EP300, E1A binding protein P300; SOX6, SRY-box transcription factor 4.
2. Role of miR-129 in oncological phenotypes
Prostate cancer
Prostate cancer is one of the most commonly diagnosed diseases among elderly men (4). The Wnt/β-catenin signaling pathway is one of the major signaling pathways of epithelial-mesenchymal transition (EMT), and its target genes mediate cancer initiation and progression by regulating cell proliferation and apoptosis (17). In prostate cancer cells, the Wnt protein can enhance resistance to treatment (18) and the EMT (4). miR-129-5p has a decreased expression level in prostate cancer tissues (19). In a previous study, the overexpression of miR-129 decreased Zic family member 2 (ZIC2) expression and thus inhibited Wnt/β-catenin signaling pathway, that was the expression of phosphorylated Wnt, β-catenin, N-cadherin and vimentin decreased, but that of E-cadherin increased, demonstrating that the overexpression of miR-129-5p might hinder the activation of the Wnt/β-catenin signaling pathway by targeting (ZIC2 to inhibit EMT, angiogenesis, tumorigenesis, migration and apoptosis in prostate cancer (4). ETS variant transcription factor 1 (ETV1) encodes a number of the E-twenty-six family of transcription factors and accelerates prostate cancer cell proliferation through the transcriptional activation of yes-associated protein (YAP) (20). One study has identified a negative targeting association between the ETV1 gene and miR-129-5p, based on reverse transcription (RT)-qPCR, bioinformatics and dual-luciferase reporter assay experiments (20). In addition, Hippo/YAP signaling has been shown to be suppressed through ETV1(20). Therefore, when miR-129-5p is overexpressed, the proliferation of prostate cancer cells is suppressed through the ETV1/Hippo/YAP axis (20). SMAD family member 3 (SMAD3) is a protein-coding gene; the SMADs protein family plays a key role in transforming growth factor-β (TGF-β) signaling from cell surface receptors to the nucleus, and different SMADs mediate the signal transduction of different TGF-β family members (19). A negative correlation has also been observed between miR-129-3p and its target gene SMAD3, which inhibits the proliferation and invasion of prostate cancer cells by decreasing the expression of anti-apoptosis protein Bcl-2 and increasing that of the pro-apoptotic protein BAX to promote apoptosis (19,21). The level of miR-129 in patients with prostate cancer before and after chemotherapy increase following treatment, indicating that a high miR-129 expression is beneficial to patient survival (22). Therefore, the increased expression of miR-129 plays an important role in inhibiting prostate cancer cells.
Osteosarcoma
Osteosarcoma is the most common primary bone tumor and it occurs most frequently in adolescents (23). HIF1A antisense RNA 2 (HIF1A-AS2) is an RNA gene affiliated with the long non-coding RNA (lncRNAs) class of RNAs. HIF1A-AS2 lncRNA has been shown to play a vital role in bladder cancer, colorectal cancer and osteosarcoma (24-26), among others. The expression of miR-129 has also been found to be altered in osteosarcoma. Certain studies detected the expression of HIF1A-AS2 and miR-129-5p in osteosarcoma cells, and used dual-luciferase reporter assays and other methods to determine the interaction between the two (24-26); the results showed that the increased expression of HIF1A-AS2 inhibited osteosarcoma cell proliferation and cancer cell invasion by altering miR-129-5p expression (5). LIM homeobox 2 (LHX2) is a protein-coding gene; its protein, consisting of two zinc finger domains, participates in cell differentiation and embryonic development (27,28). LHX2 has been shown to play a vital role in nasopharyngeal carcinoma by regulating Wnt signaling in breast and lung cancer (29-31). A previous study showed that in osteosarcoma, LHX2 silencing did not alter the total amount of Akt or mechanistic target of rapamycin (mTOR), but decreased the level of phosphorylated-Akt and -mTOR, and that miR-129-5p knockdown in osteosarcoma promoted tumorigenesis and inhibited autophagy through the transcription factor LHX2/Akt/mTOR signaling axis (32). Therefore, miR-129 is associated with osteosarcoma cell proliferation, cancer cell invasion and autophagy.
Lung cancer
Lung cancer is the second leading cause of mortality in the rural population of China and is known to cause severe health problems worldwide (33). Tyrosine 3-plus monooxygenase/tryptophan 5-plus monooxygenase activation protein β (YWHAB) interacts with a variety of proteins and regulates various functions of the human body through both dependent or independent serine phosphorylation (6). For example, YWHAB is involved in signal transduction, proliferation and apoptosis, and is crucial to the occurrence and development of tumors (34). Research has shown that miR-129-5p overexpression could increase the apoptosis of lung cancer cells following treatment with miR-129-5p mimics in etoposide-induced lung cancer cells. Using the enzyme reporting method, western blot analysis and RT-qPCR, miR-129-5p was proven to be negatively correlated with YWHAB, and miR-129-5p regulated YWHAB in lung cancer tissues (35). Zinc finger E-box binding homeobox 2 (ZEB2) is a member of the ZEB family of transcription factors and is known to help mediate EMT (36). In non-small cell lung cancer (NSCLC), miR-129 was found to directly target ZEB2 to inhibit NSCLC cell proliferation (37). miR-129 also regulates the Wnt/β-catenin signaling pathway by decreasing the expression of activated β-catenin, c-Myc and cyclin D1. Therefore, these studies have concluded that miR-129 inhibits NSCLC cell proliferation, migration and invasion through the ZEB2 axis, EMT and the Wnt/β-catenin axis. miR-129 has an important regulatory role in the occurrence and development of lung cancer through different signaling pathways.
Breast cancer
The deregulation of miRNA is closely linked to cancer development and progression, and miR-129 plays an important role in breast cancer through different signaling pathways (7). A previous study confirmed that miR-129-5p could inhibit proliferation and metastasis in breast cancer (38). Chromobox 4 (CBX4), also known as polycomb 2, is a special chromobox protein that it is not only a transcriptional repressor, but also a small ubiquitin-like modifier E3 ligase, which has been identified as an oncogene (38). The expression of CBX4 in MCF7, T47D, MDA-MB-468, MDA-MB-231, SKBR3 and normal MCF10A breast cancer cells was detected by RT-qPCR and western blot analysis, and it was found that the overexpression of CBX4 could promote cell proliferation, while the depletion of CBX4 could arrest cells in the G2/M phase. The results demonstrated the regulatory relationship between the CBX4 gene and miR-129-5p by showing that the increase of miR-129-5p depleted CBX4, which inhibited cancer cell proliferation. This correlation may provide new targets and ideas for the treatment of breast cancer (7).
Triple-negative breast cancer has high invasive potential and a high recurrence rate, and its successful treatment remains a challenge (39). Silencing lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was shown to halt triple-negative breast cancer cells at the G0/G1 stage through an miR-129-5p-mediated mechanism, and could also markedly decrease cell proliferation, migration and invasion (40). The continuous improvement of the diagnosis and treatment of breast cancer, and the development of novel treatments, is accompanied by drug resistance. Trastuzumab is a drug specifically targeted to human epidermal growth factor receptor 2 (Her-2)-positive breast cancer; it prevents the combination of Her-2 and human epidermal growth factor, thus leading to a therapeutic effect (41). In trastuzumab-resistant Her-2-positive breast cancer cells, miR-129-5p was shown to be downregulated, and a dual-luciferase reporter assay experiment showed that miR-129-5p enhanced breast cancer sensitivity to trastuzumab by targeting ribosomal protein S, which is crucial to combating Her-2 trastuzumab resistance (42). The response to hormonal therapy is affected by the progesterone receptor (PR)-status of patients with breast cancer. The overexpression of miR-129-2 stimulated the effect of progesterone treatment by downregulating PR, and the inhibition of miR-129-2 repealed its interaction with PR in breast cancer cells (43). To the best of our knowledge, there have been no specific experimental studies on luminal A and B breast cancer; however, clinically, the expression of miR-129 in the serum of patients with breast cancer is lower than that of healthy individuals (44,45).
Nasopharyngeal carcinoma
The etiology of nasopharyngeal carcinoma involves several factors and lacks obvious characteristics (8). ZIC2 is a transcription factor encoded by the ZIC2 gene, which has been proven to play a role in a variety of cancer types (46). A direct targeting relationship between miR-129-5p and ZIC2 has been confirmed, which eliminates the activation of the Hedgehog signaling pathway, thereby inhibiting lymphangiogenesis and lymph node metastasis in nasopharyngeal carcinoma. It has also been shown that miR-129-5p can decrease the expression of associated genes smoothened, frizzled class receptor and GLI family zinc finger 1 in the Hedgehog signaling pathway, thus inhibiting the apoptosis of nucleus pulposus cells (NPCs) (8). In addition, WEE1 G2 checkpoint kinase (WEE1; molecular weight, 96 kDa) is a member of the serine/threonine-protein kinase family, which can phosphorylate threonine 14 and tyrosine 15 of cyclin-dependent kinase 1 (CDK1) to inhibit CDK1 kinase activity, thereby inhibiting cell mitosis (47). The expression level of miR-129-3p in cisplatin-resistant nasopharyngeal carcinoma cells was found to be significantly lower than that in non-resistant nasopharyngeal carcinoma cells, and a negative targeting relationship was identified between miR-129-1-3p and WEE1. miR-129-1-3p was found to decrease cisplatin resistance by inhibiting WEE1 in cisplatin-resistant cells (48). In conclusion, miR-129 plays an important role in the apoptosis and drug resistance of nasopharyngeal carcinoma cells.
Ovarian cancer
Ovarian cancer is one of the most common types of cancer and the leading cause of cancer-associated mortality in women, according to global statistics in 2019(49). Basic leucine zipper and W2 domains 1 (BZW1) is a member of the bZIP superfamily of transcription factors (50). The overexpression of miR-129-3p targets the cell cycle regulator BZW1 in SKOV3 cells, which arrests most cells in the G0/G1 phase and only permits a few cells to aggregate in the S phase, suggesting that miR-129-3p inhibits ovarian cancer cell proliferation (50). In addition, studies have confirmed that miR-129-3p could inhibit ovarian cancer cell migration, indicating that miR-129-3p also plays an important role in the development of ovarian cancer (4,9,50). Small nucleolus RNA host gene 12 (SNHG12) is an RNA gene associated with the lncRNA class of RNAs. SNHG12 lncRNA has been shown to play a vital role in gastric and colorectal cancer (51,52). miR-129 expression was downregulated by SNHG12 overexpression in ovarian cancer cells, and it could regulate ovarian cancer development by promoting proliferation and enhancing the migratory ability of cells through the downregulation of SRY-box transcription factor 4 (SOX4) (53). The biological functions of prostate cancer-associated transcript 1 (PCAT1), which was initially shown to play oncogenic roles in prostate cancer (54), have been documented in multiple types of human cancer, including gastric cancer (55), hepatocellular carcinoma and colorectal cancer (56,57). PCAT-1 expression was also increased in ovarian cancer and could promote cancer cell proliferation and inhibit cancer cell apoptosis, the opposite trend of that observed for miR-129 expression and the phenotype in ovarian cancer cells. Bioinformatics predictions and dual-luciferase reporter assay results showed that PCAT-1 and miR-129 were negatively correlated, and that the overexpression of PCAT-1 inhibited miR-129, promoting ovarian cancer development (49).
lncRNA overexpression of urothelial carcinoma-associated protein 1 (UCA1) is associated with resistance to chemotherapeutics and plays a key role in anticancer drug resistance (58). A previous study showed that lncRNA UCA1 was highly expressed in paclitaxel (PTX)-resistant ovarian cancer, while its knockdown weakened that resistance (9). It was further demonstrated that UCA1 competitively bound to miR-129, which triggered the release of ATP-binding cassette subfamily B member 1 (ABCB1) mRNA and increased the resistance of ovarian cancer cells to PTX. Briefly, ABCB1 upregulation via the UCA1/miR-129 axis promoted PTX resistance in ovarian cancer (9). YAP and tafazzin (TAZ) transcription coactivators are key oncogenes in mammalian cells. The levels of these two transcription coactivators were decreased in SKOV3 cells transfected with miR-129-5p. Low miR-129-5p expression was shown to increase YAP and TAZ expression, thus increasing the aggressiveness of ovarian cancer and leading to a poor prognosis (59). Among all the changes in the expression of breast- and ovarian-related genes and their regulatory miRNA expression and the methylation of promoter CpG islands, the methylation of miR-129-3p and SEMA3B has been found at a high frequency in ovarian cancer. In addition, a correlation between SEMA3B promoter hypermethylation and miR-129 gene downregulation was identified in ovarian cancer and breast cancer (60). In conclusion, miR-129 plays a significant role in ovarian cancer cell proliferation and migration.
Colon cancer
MALAT1 has been proven to be an important regulator of miR-129 in breast cancer (10), and high-mobility group box-1 (HMGB1) is a vital member of the HMG family, which has been shown to play a crucial role in pancreatic (61), breast (62) and prostate (63) cancer. In colon cancer cells, miR-129-5p was downregulated, and the expression of HMGB1 and MALAT1 was increased. The MALAT1/miR-129-5p/HMGB1 axis was also found to be an important target axis for regulating the progression of colon cancer (10). p53 is a tumor suppressor protein and transcription factor that can regulate cell division, prevent DNA mutations or division of damaged cells, and transmit apoptotic signals to those cells through transcriptional regulation, thereby preventing tumor formation. The active ingredient, Avenanthramide A, which is present in oats, was found to exert a protective mechanism against colon cancer by elevating miR-129-3p to inhibit ring finger and CHY zinc finger domain containing 1 (Pirh2), resulting in a significant increase in the protein levels of p53 and its downstream target p21, ultimately leading to a significant increase in senescent cells (64). In other words, Avenanthramide A induces cell senescence through the miR-129-3p/Pirh2/p53 axis (64). The occurrence and development of colon cancer are closely linked to the downregulation of miR-129. Experiments using a new miR-129 mimic (mimic-1) found that, compared with natural miR-129, mimic-1 had a stronger inhibitory effect on colon cancer and induced colon cancer cell apoptosis. Furthermore, the half-life of mimic-1 was longer than that of natural miR-129, and so has a more profound impact as a therapeutic candidate in colon cancer treatment (65).
Other cancer types
miR-129-5p also plays an important role in esophageal carcinoma (66), hepatocellular carcinoma (67), uterine fibroid (68) and cervical cancer (69). The expression of miR-129 has been reported to be low in most types of cancer (4-10). miR-129 has also been shown to play an important role in the proliferation, migration and drug resistance of cancer cells through various molecular axes, such as miR-129/CBX4(7), miR-129/ZEB2(37) and miR-129/ABCB1(4). In combination, these results indicated the potential of miR-129 for use in cancer treatment.
3. Role of miR-129 in non-oncological phenotypes
Obesity
Obesity is a metabolic disease and an important inducing factor of metabolic disorders (70,71), such as type II diabetes and cardiovascular disease (15).
An eicosapentaenoic acid-regulating differentially expressed gene analysis of miRNAs in brown adipose tissue (BAT) from diet-induced obese mice indicated that miR-129-5p and miR-129-3p were significantly upregulated, which was subsequently validated by RT-qPCR (15). Obesity is defined as redundant fat accumulation in adipose tissue without a clearly known pathogenesis (72). Another analysis of autophagy-related genes showed that key adipocyte differentiation pathways, including mTOR and insulin, and adipocytokine signaling pathways may be upregulated by autophagy-related gene 7 (ATG7)-mediated autophagy (73,74). Autophagy is a cellular recycling pathway that delivers cytoplasmic cargo into lysosomes for the maintenance of cellular quality control (75), which may be associated with obesity (76). ATG7 has been identified as one of the principal molecular executors of autophagy (77). By removing ubiquitinated AMP-activated protein kinase and thus stimulating mTOR signaling pathways (78), the activation of ATG7-mediated autophagy, which has been confirmed to lead to increased white adipose tissue (WAT), is vital for adipocyte differentiation. miR-129-5p may directly target ATG7 to downregulate autophagy pathways, resulting in the inhibition of white adipogenesis (79). Consistently, when transfecting miR-129-5p mimics in the subcutaneous fat tissues of mice, the expression of key effectors in adipocyte differentiation, fatty acid-binding protein 4, CCAAT/enhancer-binding protein and peroxisome proliferator-activated receptor γ, were decreased (79).
In addition, the overexpression of miR-129-5p was found to lead to the inhibition of brown adipogenesis, which may be associated with the decrease protein expression of adipogenic genes and specific markers of brown mature adipocytes, such as uncoupling protein 1, PR/SET domain 16 and cell death-inducing DFFA-like effector-a (79). The use of miR-129-5p inhibitors led to an increase in the number of mature brown adipocytes compared with the use of negative control inhibitors (79). However, the direct target of miR-129-5p in BAT remains unknown. Considering that BAT activation abnormality may contribute to obesity, exploring the regulatory mechanism of miR-129-5p in brown adipogenesis is worthy of consideration.
An excessive body intake of triacylglycerols was shown to lead to adipocyte hyperplasia and subsequent accumulation of lipids in WAT, consequently promoting the development of obesity (80). A series of predicted target genes of miR-129 have been found to be associated with triacylglycerol metabolism and thus, miR-129 may regulate the progression of obesity (81). However, the exact mechanism of the miR-129-mediated alteration of triacylglycerol metabolism in obesity requires further study.
The miR-129 family negatively regulates the formation of obesity using at least three different approaches, involving white and brown adipogenesis and triacylglycerol metabolism. To the best of our knowledge, miR-129 can directly target and inhibit ATG7 to decrease the accumulation of WAT, while its other targets remain uncharted or unsubstantiated in brown adipogenesis and triacylglycerol metabolism.
HF
HF occurs in ~1/5 individuals during their lifetime, particularly individuals aged >40 years, decreasing their quality of life (82). As shown by a longitudinal study, HF is associated with inflammatory reactions (83). HMGB1 is an omnipresent nuclear protein that is commonly discharged by dead or dying cells (84). A previous study showed that HMGB1 was an effective biomarker for predicting heart transplantation in patients with HF (85). The persistence of HMGB1 in the microenvironment is one of the main contributing factors to the increase in inflammation and more severe symptoms (86). HMGB1 inhibition can alleviate inflammation, thus affecting the pathogenesis and progression of HF (11). miR-129-5p was found to complementarily bind to the 3'-UTR of HMGB1 mRNA with its seed region, which led to a decrease in the protein levels of HMGB1, followed by a decrease in the levels of TNF-α and IL-6 to ameliorate HF (11).
Anthracyclines are highly effective in the treatment of a large number of malignancies (87). However, HF, a well-known side effect of these drugs, limits its use in chemotherapy (88-90). As shown by Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis, Ca2+ pathway dysregulation is an important cause of anthracycline-induced HF (91,92). Ca2+ can trigger mitochondrial swelling in anthracycline-damaged cardiac tissues (93) and Ca2+ overload may result in the degradation of the myofilament protein titin, causing sarcomere disruption and cell necrosis (91). Glutamate ionotropic receptor N-methyl-D-aspartate (NMDA) type subunit 2D (GRIN2D) is an essential subunit of the NMDA receptor Ca2+ channel, which involves the formation of ligand-gated ion channels with high Ca2+ permeability (94). Since GRIN2D is considered a key regulator of Ca2+ influx, its expression is closely linked to cytoplasmic Ca2+ content (10). Calmodulin 1 (CALM1) and Ca2+/calmodulin-dependent protein kinase II δ (CaMKIIδ), the key downstream activators of the Ca2+ pathway in cardiomyocytes, have been linked to myocardial cell death, cardiomyopathy and HF (95,96). Therefore, GRIN2D overexpression caused Ca2+ overload, leading to excessive activation of CALM1 and CaMKIIδ, and eventually deteriorating HF (92). By targeting GRIN2D and thus alleviating the GRIN2D-mediated Ca2+ signaling pathway activity (92), miR-129-3p can downregulate Ca2+ overload to prevent pirarubicin-induced cardiomyocyte death and HF (92).
In addition, a study reported that the clearly increased serum levels of lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) in patients with HF were negatively correlated with miR-129-5p levels (97). Further research showed that the improvement in H2O2-induced apoptosis mediated by a lncRNA NEAT1 mimic in H2C9 immortalized cardiomyocytes could be strongly attenuated by a miR-129-5p mimic, which indicated that the interaction between lncRNA NEAT1 and miR-129-5p in myocardiocytes may have implications in HF (97). In conclusion, the miR-129 family, including miR-129-5p and miR-129-3p, modulates the progression of HF through various mechanisms, and current evidence suggests it has a favorable effect on HF.
Epilepsy
Epilepsy is a neurodegenerative disease that affects >50 million individuals worldwide (98); its long-term predisposition to seizures is caused by the hyperexcitability and hypersynchrony of brain neurons (99,100). To date, there is a lack of effective therapeutic targets or specific biomarkers for epileptogenesis (101,102). Mitogen-activated protein kinases (MAPKs) are serine-threonine kinases that regulate various cellular activities, including proliferation, differentiation, survival and death, through intracellular signaling (103). Therefore, MAPK pathway abnormality is closely correlated with multiple types of cancer and neurodegenerative diseases, including Parkinson's disease, AD and epilepsy. At present, MAPK is considered an important regulator of synaptic excitability, whose overactivation gives rise to epileptic seizures (104). c-Fos is considered a molecular sensor for MAPK signaling duration (105). Inhibiting c-Fos can block MAPK signaling transduction. A previous study reported that miR-129-5p could negatively target c-Fos (105), suggesting that miR-129-5p may repress epilepsy seizures (103). Therefore, miR-129-5p targets the sensor c-Fos to inhibit MAPK signaling and then apoptosis, and promotes the proliferation of hippocampal neurons, thereby suppressing the occurrence and development of epilepsy (105). However, the protective role of miR-129-5p is controversial due to its synaptic downscaling function. Synaptic downscaling is a homeostatic mechanism that decreases the firing rates of neurons during chronically elevated network activity (106,107). Although synaptic downscaling is crucial in epilepsy, the related pathways are poorly understood (106,107). Following a small RNA profiling of a synaptic downscaling model of picrotoxin-induced hippocampal neurons, miR-129-5p was found to be increased in response to picrotoxin (106,107). miR-129-5p inhibition led to the inhibition of synaptic downscaling in vitro and decreased epileptic seizure severity in vivo, indicating the potential malignant influence of miR-129-5p on epilepsy (106,107).
By contrast, a different study reported the opposite conclusion, supporting the beneficial function of miR-129-5p. The study found that a high concentration of miR-129-5p significantly enhanced the expression of pro-inflammatory factors such as IL-6, cyclooxygenase-2 and TNF-α in epilepsy cell models, which increased the frequency of epileptic seizures (108). The explanation of these two opposite conclusions may be explained by the fact that miR-129-5p plays different roles in each stage of epilepsy development; therefore, its underlying mechanism requires further study.
Diabetes
Although diabetes has various symptoms, different subtypes of diabetes share the same pathophysiological abnormality, that is, damage to the islets (109). The timely detection of islet destruction may benefit the protection of islet β cell function (16). Exosomes, as newly discovered essential conveyers of cellular information, have attracted considerable attention as disease biomarkers in recent years (110,111). Exosomes are extracellular small membrane vesicles (30-100 nm) that contain proteins and nucleic acids, including miRNA (111); they can be secreted by various types of cells into the blood circulation and then seized into recipient cells (110), which renders them efficient intercellular information transmitters. Information in these vesicles varies based on the status of the cells (110). As a consequence, the detection of changes in functional biomolecules in exosomes may reveal cell characteristics. miRNA profiles of exosomes derived from streptozotocin- or mixed cytokine [TNF-α, IL-1β and interferon (IFN-γ)]-induced injured islets demonstrated that six miRNAs, including miR-129-5p, were differentially expressed at the intersection between two injured conditions (112). Although debates on the mechanisms of metformin in different tissues continue to this day (16), metformin has become a commonly used drug in the treatment of type 2 diabetes since French scientist Jean Sterne first produced it in 1957(113). One previous study reported that 3 months of metformin treatment for type 2 diabetes significantly attenuated miR-129-3p expression, which may be a result of improved islet function (16). This finding showed that the differential expression of miR-129-5p and five other miRNAs in islet-derived exosomes specifically indicated islet β cell injury, regardless of the cause of injury.
Continuous accumulation of neutrophils and macrophages is commonly detected in the wound sites of diabetic patients (114), which can delay wound healing and complications, such as foot ulcers (114,115). Previous studies have also suggested that the caspase family is the main contributor of both spontaneous and Fas receptor-mediated apoptosis in neutrophils (116,117). Fas ligand and its death receptors are the activators of caspase 8 (116,118), which mainly contribute to the activation of death-inducing signaling complex by activating its downstream caspases and can stimulate neutrophil apoptosis (116). C-C chemokine receptor type 2 (CCR2) is a chemokine receptor commonly expressed in lymphocytes and monocytes, but not in neutrophils (88). However, specific inflammatory stimuli in wounds have been confirmed to elevate the expression of CCR2 in neutrophils (119,120). In diabetic wound sites, CCR2 expression was increased, which promoted wound recruitment in neutrophils (120); accordingly, CCR2 expression may play a key role in chronic inflammation in diabetic patients (121). miR-129-3p was previously found to directly regulate the translation of caspase 6, the downstream activator of caspase 8, and CCR2 to decrease apoptosis and neutrophil recruitment and consequently accelerate diabetic wound healing (121). Similar to miR-129-3p, miR-129-5p plays a beneficial role in diabetic wound healing (122).
Matrix metalloproteinases (MMPs) are widely known to inhibit tissue remodeling by degrading extracellular matrix (ECM) components (123). A high level of MMP-9 expression causes excessive degradation of ECM components, contributing to the imbalance between ECM synthesis and degradation (120). The high level of MMP-9 is therefore usually indicative of poor wound healing, as well as the existence of diabetic wounds (120). MMP expression and activity can be tightly regulated by methylation at specific DNA loci in the MMP-9 promoter region (124). Thus, specificity protein-1 (Sp1), a transcription factor found to bind to the MMP-9 promoter to initiate MMP-9 gene expression, can be an important regulatory target for MMP-9(122). Through dual-luciferase reporter assays, miR-129-5p was confirmed to directly regulate Sp1 protein levels and significantly decrease the expression of MMP-9. miR-129-5p thus modulates the SP1/MMP-9 axis and removes an important obstacle in diabetic wound healing (122).
IVDD
IVDD is a cause of lower back pain, which affects >50% of the world's population during their lifetime, and is a severe social burden (125-127). Accumulating evidence shows that the apoptosis and proliferation of fibrocartilaginous tissues on the intervertebral disc are implicated in the progression of IVDD (128). NPCs, a type of chondroid cells that exist in the intervertebral disc, lead the deposition of fibrocartilage lamellas and fibers in a centripetal direction, thus converting notochordal nucleus pulposus into fibrocartilaginous nucleus pulposus (129). It has been demonstrated that one of the main characteristics of IVDD is the decrease in the number of NPCs, which leads to severe disc deformation and dysregulation of its function (130).
Bone morphogenetic protein 2 (BMP2), first reported as the anabolic growth factor regulating proteoglycan release (131), was expressed at low levels in NPCs collected from patients with IVDD, and its low expression was accompanied by increased miR-129-5p expression (13). Previous reports confirmed that BMP2 was involved in the mediation of apoptosis, with its role being dependent on cellular context and cell type (13). Yang and Sun (13) validated BMP2 as a promoter of apoptosis and an inhibitor of NPC proliferation in IVDD, although it was also found to stimulate proteoglycan secretion to prevent the degradation of the ECM, which may help delay IVDD progression (13). Fas-associated death domain (FADD), an important agent for the triggering of cell death without the enrollment of death receptors, was also demonstrated to facilitate NPC apoptosis (132,133). Both BMP2 and FADD were confirmed to be targeted by miR-129-5p, and the overexpression of miR-129-5p was found to significantly facilitate the proliferation and suppress the apoptosis of NPCs (13). Furthermore, high miR-129-5p expression alleviated disc inflammation by promoting the apoptosis of macrophages and CD8+ cells. Collectively, miR-129-5p exerts effects not only on NPCs, but also on inflammatory cells, although its targets in the latter have not yet been verified (13,134). Contrary to the aforementioned results, one study reported that miR-129-5p increased NPC apoptosis by suppressing autophagy (135). Specifically, autophagy progresses through the Beclin-1-dependent pathway; therefore, Beclin-1 is essential for autophagy (136). Beclin-1 may promote autophagy, while autophagy can decrease cathepsin B release into the cytoplasm (135). In light of the positive correlation between the cytoplasmic level of cathepsin B and apoptosis, the Beclin-1-targeting capability of miR-129-5p increased cytoplasmic release of cathepsin B from lysosomes and resulted in the inhibition of autophagy and promotion of apoptosis. This death-inducing effect to NPCs rendered miR-129-5p harmful to the human intervertebral disc in that study (135). In conclusion, miR-129-5p appears to modulate apoptosis in NPCs by targeting different factors, but its implications in IVDD require further study.
AD
As a significant neurodegenerative disease, AD is characterized by the accumulation of β-amyloid plaques and the dysfunction of τ-protein in the brain (137). In a longitudinal cohort study of aging (based on 700 autopsy samples), miR-129-5p was shown to be downregulated in the human dorsolateral prefrontal cortex of patients with AD (14), and its expression was found to be associated with the two characteristics of AD, as shown by miRNA expression profiling (14,138). In the study, miR-129-5p appeared to be involved in a variety of pathways, the most significant of which was transcription regulation (14). Notably, miR-129-5p coordinated with another miRNA, miR-132, to target E1A binding protein P300 (EP300), the encoding gene of the histone acetyltransferase protein E1A-associated cellular p300 transcriptional co-activator, and each miRNA was responsible for part of the variation in EP300 expression (14). A reasonable speculation is that miR-129-5p influences histone acetylation and chromatin remodeling through the regulation of EP300 to alter the expression of β-amyloid and τ-protein in patients with AD.
The SOX gene family contains transcription factor-coding genes that possess highly conserved HMG-box sequences (138). A principal function of SOX transcription factors is to regulate neurogenesis in the embryonic and adult nervous system (139). It has been reported that the expression of SOXB transcription factors may be involved in the early disability of hippocampal neurogenesis in 5xFAD mouse models of AD (139). A previous study demonstrated that miR-129-5p overexpression decreased the expression of its target SOX6(140), leading to the decrease of apoptosis, the suppression of inflammatory reactions and the increased proliferation of rat hippocampal neurons (140).
Other non-cancerous diseases
Certain studies have reported the role of the miR-129 family in other diseases. For example, miR-129 was found to target beclin-1 in atherosclerosis (141), HMGB1 in spinal cord injury (142), spleen-associated tyrosine kinase in ischemic stroke (143), fibroblast growth factor 23 in chronic kidney disease (144) and NF-κB in hepatic fibrosis (145).
4. Conclusion
Increasing evidence indicates the important functions of the miR-129 family in various diseases, highlighting its potential as a therapeutic candidate or prognostic biomarker. In the present review, it was concluded that the dysregulated expression of miR-129 is a common feature in several types of cancer. In addition, it was shown that miR-129 plays different roles in various types of cancer, depending on the disease type. In most types, miR-129 regulates genes involved in a variety of biological processes, including cell proliferation, migration, invasion, cell apoptosis induction and drug resistance. In conclusion, miR-129 family can be potentially used as a drug-tool in the treatment for some of the aforementioned diseases with little if any harm to the normal tissue. With regards to non-cancerous diseases, previous research has supported the roles of miR-129 in the treatment of HF and AD, and confirmed that miR-129 has the potential to be a biomarker in diabetes and epilepsy. It is worth noting that the suppressive effect of miR-129 on apoptosis has been consistently reported by multiple research groups, providing a direction for future research. However, since miR-129 could induce or suppress NPC apoptosis through different signaling axes, its role in IVDD remains controversial and requires further study. The present review systematically summarized the role of the miR-129 family in oncological and non-oncological diseases. To the best of our knowledge, this is the first review to summarize the functions of miR-129 in non-cancerous diseases, including obesity, HF, epilepsy, diabetes, IVDD and AD.
Acknowledgements
Not applicable.
Funding Statement
Funding: Funding was provided by the Natural Science Foundation of Hunan Province, China (grant no. 2019JJ40392) and the Undergraduate Innovation and Entrepreneurship Training Program Supportive Project (grant no. 2020105330143).
Availability of data and materials
Not applicable.
Authors' contributions
BD and XT wrote the paper, and YW revised the manuscript. All authors have read and approved the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang HD, Jiang LH, Sun DW, Li J, Ji ZL. The role of miR-130a in cancer. Breast Cancer. 2017;24:521–527. doi: 10.1007/s12282-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 2.Gao Y, Feng B, Han S, Lu L, Chen Y, Chu X, Wang R, Chen L. MicroRNA-129 in human cancers: From tumorigenesis to clinical treatment. Cell Physiol Biochem. 2016;39:2186–2202. doi: 10.1159/000447913. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Z, Zhang Y, Chen X, Wu P, Chen D. Inactivation of the Wnt/β-catenin signaling pathway underlies inhibitory role of microRNA-129-5p in epithelial-mesenchymal transition and angiogenesis of prostate cancer by targeting ZIC2. Cancer Cell Int. 2019;19(271) doi: 10.1186/s12935-019-0977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Peng L, Gong X, Zhang X, Sun R. LncRNA HIF1A-AS2 promotes osteosarcoma progression by acting as a sponge of miR-129-5p. Aging (Albany NY) 2019;11:11803–11813. doi: 10.18632/aging.102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obsil T. 14-3-3 proteins-a family of universal scaffolds and regulators. Semin Cell Dev Biol. 2011;22:661–662. doi: 10.1016/j.semcdb.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Meng R, Fang J, Yu Y, Hou LK, Chi JR, Chen AX, Zhao Y, Cao XC. miR-129-5p suppresses breast cancer proliferation by targeting CBX4. Neoplasma. 2018;65:572–578. doi: 10.4149/neo_2018_170814N530. [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Han GH, Zhao X, Liu X, Xue K, Wang D, Xu CB. MicroRNA-129-5p suppresses nasopharyngeal carcinoma lymphangiogenesis and lymph node metastasis by targeting ZIC2. Cell Oncol (Dordr) 2020;43:249–261. doi: 10.1007/s13402-019-00485-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Ye C, Liu J, Hu Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501:1034–1040. doi: 10.1016/j.bbrc.2018.05.104. [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Meng WY, Jie Y, Zhao H. LncRNA MALAT1 induces colon cancer development by regulating miR-129-5p/HMGB1 axis. J Cell Physiol. 2018;233:6750–6757. doi: 10.1002/jcp.26383. [DOI] [PubMed] [Google Scholar]
- 11.Xiao N, Zhang J, Chen C, Wan Y, Wang N, Yang J. miR-129-5p improves cardiac function in rats with chronic heart failure through targeting HMGB1. Mamm Genome. 2019;30:276–288. doi: 10.1007/s00335-019-09817-0. [DOI] [PubMed] [Google Scholar]
- 12.Hübner A, Jaeschke A, Davis RJ. Oncogene addiction: Role of signal attenuation. Dev Cell. 2006;11:752–754. doi: 10.1016/j.devcel.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Sun P. Downregulation of microRNA-129-5p increases the risk of intervertebral disc degeneration by promoting the apoptosis of nucleus pulposus cells via targeting BMP2. J Cell Biochem. 2019;120:19684–19690. doi: 10.1002/jcb.29274. [DOI] [PubMed] [Google Scholar]
- 14.Patrick E, Rajagopal S, Wong HA, McCabe C, Xu J, Tang A, Imboywa SH, Schneider JA, Pochet N, Krichevsky AM, et al. Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer's disease. Mol Neurodegener. 2017;12(51) doi: 10.1186/s13024-017-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahlavani M, Wijayatunga NN, Kalupahana NS, Ramalingam L, Gunaratne PH, Coarfa C, Rajapakshe K, Kottapalli P, Moustaid-Moussa N. Transcriptomic and microRNA analyses of gene networks regulated by eicosapentaenoic acid in brown adipose tissue of diet-induced obese mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1523–1531. doi: 10.1016/j.bbalip.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demirsoy IH, Ertural DY, Balci Ş, Çınkır Ü, Sezer K, Tamer L, Aras N. Profiles of circulating MiRNAs following metformin treatment in patients with type 2 diabetes. J Med Biochem. 2018;37:499–506. doi: 10.2478/jomb-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arend RC, Londoño-Joshi AI, Straughn JM Jr, Buchsbaum DJ. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecol Oncol. 2013;131:772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Murillo-Garzón V, Kypta R. WNT signalling in prostate cancer. Nature reviews. Urology. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 19.Tang PM, Zhou S, Meng XM, Wang QM, Li CJ, Lian GY, Huang XR, Tang YJ, Guan XY, Yan BP, et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat Commun. 2017;8(14677) doi: 10.1038/ncomms14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, Xiu D, Yang B, Sun D, Wei X, Ding Y, Ma Y, Wang Z. miR-129-5p inhibits prostate cancer proliferation via targeting ETV1. OncoTargets and Ther. 2019;12:3531–3544. doi: 10.2147/OTT.S183435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Gao Y, Dou J. Effects of miR-129-3p on biological functions of prostate cancer cells through targeted regulation of Smad3. Oncol Lett. 2020;19:1195–1202. doi: 10.3892/ol.2019.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Guo J, Zhao M, Jiang T, Yang X. Predictive values of miR-129 and miR-139 for efficacy on patients with prostate cancer after chemotherapy and prognostic correlation. Oncol Lett. 2019;18:6187–6195. doi: 10.3892/ol.2019.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang RM, Tang T, Yu HM, Yao XD. LncRNA DLX6-AS1/miR-129-5p/DLK1 axis aggravates stemness of osteosarcoma through Wnt signaling. Biochem Biophys Res Commun. 2018;507:260–266. doi: 10.1016/j.bbrc.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Liu M, Meng F, Sun B, Jin X, Jia C. The long noncoding RNA HIF1A-AS2 facilitates cisplatin resistance in bladder cancer. J Cell Biochem. 2019;120:243–252. doi: 10.1002/jcb.27327. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Shi Z, Yu Z, He Z. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and DNMT3A. Biomed Pharmacother. 2018;98:433–439. doi: 10.1016/j.biopha.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Zhao Z, Hao Y, He J, He J. Long noncoding RNA HIF1A-AS2 facilitates cell survival and migration by sponging miR-33b-5p to modulate SIRT6 expression in osteosarcoma. Biochem Cell Biol. 2020;98:284–292. doi: 10.1139/bcb-2019-0171. [DOI] [PubMed] [Google Scholar]
- 27.Hou PS, Chuang CY, Kao CF, Chou SJ, Stone L, Ho HN, Chien CL, Kuo HC. LHX2 regulates the neural differentiation of human embryonic stem cells via transcriptional modulation of PAX6 and CER1. Nucleic Acids Res. 2013;41:7753–7770. doi: 10.1093/nar/gkt567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomann P, Paus R, Millar SE, Scheidereit C, Schmidt-Ullrich R. Lhx2 is a direct NF-κB target gene that promotes primary hair follicle placode down-growth. Development. 2016;143:1512–1522. doi: 10.1242/dev.130898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38(97) doi: 10.1186/s13046-019-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Kuzmanov A, Hopfer U, Marti P, Meyer-Schaller N, Yilmaz M, Christofori G. LIM-homeobox gene 2 promotes tumor growth and metastasis by inducing autocrine and paracrine PDGF-B signaling. Mol Oncol. 2014;8:401–416. doi: 10.1016/j.molonc.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F, Gou S, Xiong J, Wu H, Wang C, Liu T. Oncogenicity of LHX2 in pancreatic ductal adenocarcinoma. Mol Biol Rep. 2014;41:8163–8167. doi: 10.1007/s11033-014-3716-2. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Liu J, Wu X, Zhou Y, Chen X, Chen J, Deng K, Mao C, Huang S, Liu Z. LHX2 promotes malignancy and inhibits autophagy via mTOR in osteosarcoma and is negatively regulated by miR-129-5p. Aging (Albany NY) 2019;11:9794–9810. doi: 10.18632/aging.102427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Xie J, Wang J. Tumor suppressor function of miR-129-5p in lung cancer. Oncol Lett. 2019;17:5777–5783. doi: 10.3892/ol.2019.10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong BH, Jin HT, Choi EK, Carp RI, Kim YS. Lack of association between 14-3-3 beta gene (YWHAB) polymorphisms and sporadic Creutzfeldt-Jakob disease (CJD) Mol Biol Rep. 2012;39:10647–10653. doi: 10.1007/s11033-012-1954-8. [DOI] [PubMed] [Google Scholar]
- 35.Xu C, Du Z, Ren S, Liang X, Li H. MiR-129-5p sensitization of lung cancer cells to etoposide-induced apoptosis by reducing YWHAB. J Cancer. 2020;11:858–866. doi: 10.7150/jca.35410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob S, Nayak S, Fernandes G, Barai RS, Menon S, Chaudhari UK, Kholkute SD, Sachdeva G. Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer. Endocr Relat Cancer. 2014;21:473–486. doi: 10.1530/ERC-13-0514. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Li C, Bi H, Bai S, Zhao L, Zhang J, Qi C. Targeting ZEB2 By microRNA-129 in non-small cell lung cancer suppresses cell proliferation, invasion and migration via regulating Wnt/beta-catenin signaling pathway and epithelial-mesenchymal transition. Onco Targets Ther. 2019;12:9165–9175. doi: 10.2147/OTT.S217536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Li L, Wu Y, Zhang R, Zhang M, Liao D, Wang G, Qin G, Xu RH, Kang T. CBX4 suppresses metastasis via recruitment of HDAC3 to the Runx2 promoter in colorectal carcinoma. Cancer Res. 2016;76:7277–7289. doi: 10.1158/0008-5472.CAN-16-2100. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhary LN, Wilkinson KH, Kong A. Triple-negative breast cancer: Who should receive neoadjuvant chemotherapy? Surg Oncol Clin N Am. 2018;27:141–153. doi: 10.1016/j.soc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Zuo Y, Li Y, Zhou Z, Ma M, Fu K. Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed Pharmacother. 2017;95:922–928. doi: 10.1016/j.biopha.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Del Mastro L, De Laurentiis M. Clinical applications of trastuzumab in the management of HER-2-positive breast cancer. Recenti Prog Med. 2019;110:594–603. doi: 10.1701/3278.32518. (In Italian) [DOI] [PubMed] [Google Scholar]
- 42.Lu X, Ma J, Chu J, Shao Q, Zhang Y, Lu G, Li J, Huang X, Li W, Li Y, et al. MiR-129-5p sensitizes the response of Her-2 positive breast cancer to trastuzumab by reducing Rps6. Cell Physiol Biochem. 2017;44:2346–2356. doi: 10.1159/000486122. [DOI] [PubMed] [Google Scholar]
- 43.Godbole M, Chandrani P, Gardi N, Dhamne H, Patel K, Yadav N, Gupta S, Badwe R, Dutt A. miR-129-2 mediates down-regulation of progesterone receptor in response to progesterone in breast cancer cells. Cancer Biol Ther. 2017;18:801–805. doi: 10.1080/15384047.2017.1373216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue J, Zhu X, Huang P, He Y, Xiao Y, Liu R, Zhao M. Expression of miR-129-5p and miR-433 in the serum of breast cancer patients and their relationship with clinicopathological features. Oncol Lett. 2020;20:2771–2778. doi: 10.3892/ol.2020.11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng H, Wang L, Wang J, Chen T, Li H, Zhang K, Chen J, Zhen S, Tuluhong D, Li J, Wang S. microRNA-129-5p suppresses Adriamycin resistance in breast cancer by targeting SOX2. Arch Biochem Biophys. 2018;651:52–60. doi: 10.1016/j.abb.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Yi W, Wang J, Yao Z, Kong Q, Zhang N, Mo W, Xu L, Li X. The expression status of ZIC2 as a prognostic marker for nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2018;11:4446–4460. [PMC free article] [PubMed] [Google Scholar]
- 47.Matheson CJ, Backos DS, Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol Sci. 2016;37:872–881. doi: 10.1016/j.tips.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Lu J, Jiang C, Lu W. miR-129-1-3p reverses cisplatin resistance of HNE1/CDDP human nasopharyngeal carcinoma cells by targeting inhibition of WEE1 kinase. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35:1014–1019. (In Chinese) [PubMed] [Google Scholar]
- 49.Gu LP, Jin S, Xu RC, Zhang J, Geng YC, Shao XY, Qin LB. Long non-coding RNA PCAT-1 promotes tumor progression by inhibiting miR-129-5p in human ovarian cancer. Arch Med Sci. 2019;15:513–521. doi: 10.5114/aoms.2018.75534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Zhao H, Gong L, Yao L, Li Y, Zhang W. MicroRNA-129-3p functions as a tumor suppressor in serous ovarian cancer by targeting BZW1. Int J Clin Exp Pathol. 2018;11:5901–5908. [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Lu W. LncRNA SNHG12 regulates gastric cancer progression by acting as a molecular sponge of miR-320. Mol Med Rep. 2018;17:2743–2749. doi: 10.3892/mmr.2017.8143. [DOI] [PubMed] [Google Scholar]
- 52.Wang JZ, Xu CL, Wu H, Shen SJ. LncRNA SNHG12 promotes cell growth and inhibits cell apoptosis in colorectal cancer cells. Braz J Med Biol Res. 2017;50(e6079) doi: 10.1590/1414-431X20176079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun D, Fan XH. LncRNA SNHG12 accelerates the progression of ovarian cancer via absorbing miRNA-129 to upregulate SOX4. Eur Rev Med Pharmacol Sci. 2019;23:2345–2352. doi: 10.26355/eurrev_201903_17378. [DOI] [PubMed] [Google Scholar]
- 54.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi M, Yu H, Huang B, Tang C. Long non-coding RNA PCAT-1 over-expression promotes proliferation and metastasis in gastric cancer cells through regulating CDKN1A. Gene. 2017;626:337–343. doi: 10.1016/j.gene.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 56.Wen J, Xu J, Sun Q, Xing C, Yin W. Upregulation of long non coding RNA PCAT-1 contributes to cell proliferation, migration and apoptosis in hepatocellular carcinoma. Mol Med Rep. 2016;13:4481–4486. doi: 10.3892/mmr.2016.5075. [DOI] [PubMed] [Google Scholar]
- 57.Qiao L, Liu X, Tang Y, Zhao Z, Zhang J, Feng Y. Down regulation of the long non-coding RNA PCAT-1 induced growth arrest and apoptosis of colorectal cancer cells. Life Sci. 2017;188:37–44. doi: 10.1016/j.lfs.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Guan Z, He K, Qian J, Cao J, Teng L. LncRNA UCA1 in anti-cancer drug resistance. Oncotarget. 2017;8:64638–64650. doi: 10.18632/oncotarget.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan G, Cao X, Dai Q, Zhang B, Huang J, Xiong S, Zhang Yy, Chen W, Yang J, Li H. A novel role for microRNA-129-5p in inhibiting ovarian cancer cell proliferation and survival via direct suppression of transcriptional co-activators YAP and TAZ. Oncotarget. 2015;6:8676–8686. doi: 10.18632/oncotarget.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pronina IV, Loginov VI, Burdennyy AM, Fridman MV, Kazubskaya TP, Dmitriev AA, Braga EA. Expression and DNA methylation alterations of seven cancer-associated 3p genes and their predicted regulator miRNAs (miR-129-2, miR-9-1) in breast and ovarian cancers. Gene. 2016;576:483–491. doi: 10.1016/j.gene.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 61.Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, et al. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017;27:916–932. doi: 10.1038/cr.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amornsupak K, Jamjuntra P, Warnnissorn M, O-Charoenrat P, Sa-Nguanraksa D, Thuwajit P, Eccles SA, Thuwajit C. High ASMA+ fibroblasts and low cytoplasmic HMGB1+ breast cancer cells predict poor prognosis. Clin Breast Cancer. 2017;17:441–452.e2. doi: 10.1016/j.clbc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Chou YE, Yang PJ, Lin CY, Chen YY, Chiang WL, Lin PX, Huang ZY, Huang M, Ho YC, Yang SF. The impact of HMGB1 polymorphisms on prostate cancer progression and clinicopathological characteristics. Int J Environ Res Public Health. 2020;17(7247) doi: 10.3390/ijerph17197247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu R, Yang P, Sajid A, Li Z. Avenanthramide A induces cellular senescence via miR-129-3p/Pirh2/p53 signaling pathway to suppress colon cancer growth. J Agric Food Chem. 2019;67:4808–4816. doi: 10.1021/acs.jafc.9b00833. [DOI] [PubMed] [Google Scholar]
- 65.Wu N, Fesler A, Liu H, Ju J. Development of novel miR-129 mimics with enhanced efficacy to eliminate chemoresistant colon cancer stem cells. Oncotarget. 2017;9:8887–8897. doi: 10.18632/oncotarget.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang M, Li Y, Liu W, Wang R, Tang A, Hao H, Liu Z, Ou H. miR-129-2 suppresses proliferation and migration of esophageal carcinoma cells through downregulation of SOX4 expression. Int J Mol Med. 2013;32:51–58. doi: 10.3892/ijmm.2013.1384. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Lu J, Zeng G, Pang J, Zheng X, Feng J, Zhang J. MiR-129-5p inhibits liver cancer growth by targeting calcium calmodulin-dependent protein kinase IV (CAMK4) Cell Death Dis. 2019;10(789) doi: 10.1038/s41419-019-1923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu JL, Zhao L, Han SC, Bi JL, Liu HX, Yue C, Lin L. MiR-129 is involved in the occurrence of uterine fibroid through inhibiting TET1. Eur Rev Med Pharmacol Sci. 2018;22:4419–4426. doi: 10.26355/eurrev_201807_15492. [DOI] [PubMed] [Google Scholar]
- 69.Wang YF, Yang HY, Shi XQ, Wang Y. Upregulation of microRNA-129-5p inhibits cell invasion, migration and tumor angiogenesis by inhibiting ZIC2 via downregulation of the Hedgehog signaling pathway in cervical cancer. Cancer Biol Ther. 2018;19:1162–1173. doi: 10.1080/15384047.2018.1491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv X, Zhou W, Sun J, Lin R, Ding L, Xu M, Xu Y, Zhao Z, Chen Y, Bi Y, et al. Visceral adiposity is significantly associated with type 2 diabetes in middle-aged and elderly Chinese women: A cross-sectional study. J Diabetes. 2017;9:920–928. doi: 10.1111/1753-0407.12499. [DOI] [PubMed] [Google Scholar]
- 71.Hruby A, Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu YH. Making sense of metabolic obesity and hedonic obesity. J Diabetes. 2017;9:656–666. doi: 10.1111/1753-0407.12529. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed M, Nguyen HQ, Hwang JS, Zada S, Lai TH, Kang SS, Kim DR. Systematic characterization of autophagy-related genes during the adipocyte differentiation using public-access data. Oncotarget. 2018;9:15526–15541. doi: 10.18632/oncotarget.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang HY, Wang YH, Wang Y, Qu YN, Huang XH, Yang HX, Zhao CM, He Y, Li SW, Zhou J, et al. miR-129-5p regulates the immunomodulatory functions of adipose-derived stem cells via targeting Stat1 signaling. Stem Cells Int. 2019;2019(2631024) doi: 10.1155/2019/2631024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suryawan A, Hu CY. Effect of serum on differentiation of porcine adipose stromal-vascular cells in primary culture. Comp Biochem Physiol Comp Physiol. 1993;105:485–492. doi: 10.1016/0300-9629(93)90424-3. [DOI] [PubMed] [Google Scholar]
- 77.Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J Biol Chem. 1996;271:17621–17624. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed M, Hwang JS, Lai TH, Zada S, Nguyen HQ, Pham TM, Yun M, Kim DR. Co-expression network analysis of AMPK and autophagy gene products during adipocyte differentiation. Int J Mol Sci. 2018;19(1808) doi: 10.3390/ijms19061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu X, Jin L, Han L, Yuan Y, Mu Q, Wang H, Yang J, Ning G, Zhou D, Zhang Z. miR-129-5p inhibits adipogenesis through autophagy and may be a potential biomarker for obesity. Int J Endocrinol. 2019;2019(5069578) doi: 10.1155/2019/5069578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bielawiec P, Harasim-Symbor E, Chabowski A. Phytocannabinoids: Useful drugs for the treatment of obesity? Special focus on cannabidiol. Front Endocrinol (Lausanne) 2020;11(114) doi: 10.3389/fendo.2020.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gracia A, Miranda J, Fernández-Quintela A, Eseberri I, Garcia-Lacarte M, Milagro FI, Martínez JA, Aguirre L, Portillo MP. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016;7:1680–1688. doi: 10.1039/c5fo01090j. [DOI] [PubMed] [Google Scholar]
- 82.Dinatolo E, Sciatti E, Anker MS, Lombardi C, Dasseni N, Metra M. Updates in heart failure: What last year brought to us. ESC Heart Fail. 2018;5:989–1007. doi: 10.1002/ehf2.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 84.Martinotti S, Patrone M, Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101–109. doi: 10.2147/ITT.S58064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volz HC, Laohachewin D, Schellberg D, Wienbrandt AR, Nelles M, Zugck C, Kaya Z, Katus HA, Andrassy M. HMGB1 is an independent predictor of death and heart transplantation in heart failure. Clin Res Cardiol. 2012;101:427–435. doi: 10.1007/s00392-011-0409-x. [DOI] [PubMed] [Google Scholar]
- 86.Gorgulho CM, Romagnoli GG, Bharthi R, Lotze MT. Johnny on the Spot-chronic inflammation is driven by HMGB1. Front Immunol. 2019;10(1561) doi: 10.3389/fimmu.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piorecka K, Smith D, Kurjata J, Stanczyk M, Stanczyk WA. Synthetic routes to nanoconjugates of anthracyclines. Bioorg Chem. 2020;96(103617) doi: 10.1016/j.bioorg.2020.103617. [DOI] [PubMed] [Google Scholar]
- 88.Anakwue R. Cytotoxic-induced heart failure among breast cancer patients in Nigeria: A call to prevent today's cancer patients from being tomorrow's cardiac patients. Ann Afr Med. 2020;19:1–7. doi: 10.4103/aam.aam_24_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R, Amdani S, Lipshultz ER, Lipshultz SE. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology. 2019;5(18) doi: 10.1186/s40959-019-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai F, Luis MAF, Lin X, Wang M, Cai L, Cen C, Biskup E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol Clin Oncol. 2019;11:15–23. doi: 10.3892/mco.2019.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Qin M, Tan Q, Li T, Gu Z, Huang P, Ren L. MicroRNA-129-1-3p protects cardiomyocytes from pirarubicin-induced apoptosis by down-regulating the GRIN2D-mediated Ca2+ signalling pathway. J Cell Mol Med. 2020;24:2260–2271. doi: 10.1111/jcmm.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javadov S, Hunter JC, Barreto-Torres G, Parodi-Rullan R. Targeting the mitochondrial permeability transition: Cardiac ischemia-reperfusion versus carcinogenesis. Cell Physiol Biochem. 2011;27:179–190. doi: 10.1159/000327943. [DOI] [PubMed] [Google Scholar]
- 94.Hajdú T, Juhász T, Szűcs-Somogyi C, Rácz K, Zákány R. NR1 and NR3B Composed intranuclear N-methyl-d-aspartate receptor complexes in human melanoma cells. Int J Mol Sci. 2018;19(1929) doi: 10.3390/ijms19071929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu XR, Rempel DL, Gross ML. Composite conformational changes of signaling proteins upon ligand binding revealed by a single approach: Calcium-calmodulin study. Anal Chem. 2019;91:12560–12567. doi: 10.1021/acs.analchem.9b03491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwang HS, Baldo MP, Rodriguez JP, Faggioni M, Knollmann BC. Efficacy of flecainide in catecholaminergic polymorphic ventricular tachycardia is mutation-independent but reduced by calcium overload. Front Physiol. 2019;10(992) doi: 10.3389/fphys.2019.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei Q, Zhou HY, Shi XD, Cao HY, Qin L. Long noncoding RNA NEAT1 promotes myocardiocyte apoptosis and suppresses proliferation through regulation of miR-129-5p. J Cardiovasc Pharmacol. 2019;74:535–541. doi: 10.1097/FJC.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 98.Camfield P, Camfield C. Regression in children with epilepsy. Neurosci Biobehav Rev. 2019;96:210–218. doi: 10.1016/j.neubiorev.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 99.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 101.Hunsberger JG, Bennett AH, Selvanayagam E, Duman RS, Newton SS. Gene profiling the response to kainic acid induced seizures. Brain research. Mol Brain Res. 2005;141:95–112. doi: 10.1016/j.molbrainres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Sharma AK, Searfoss GH, Reams RY, Jordan WH, Snyder PW, Chiang AY, Jolly RA, Ryan TP. Kainic acid-induced F-344 rat model of mesial temporal lobe epilepsy: Gene expression and canonical pathways. Toxicol Pathol. 2009;37:776–789. doi: 10.1177/0192623309344202. [DOI] [PubMed] [Google Scholar]
- 103.Gorter JA, Iyer A, White I, Colzi A, van Vliet EA, Sisodiya S, Aronica E. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol Dis. 2014;62:508–520. doi: 10.1016/j.nbd.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 104.Pernice HF, Schieweck R, Kiebler MA, Popper B. mTOR and MAPK: From localized translation control to epilepsy. BMC Neurosci. 2016;17(73) doi: 10.1186/s12868-016-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu DM, Zhang YT, Lu J, Zheng YL. Effects of microRNA-129 and its target gene c-Fos on proliferation and apoptosis of hippocampal neurons in rats with epilepsy via the MAPK signaling pathway. J Cell Physiol. 2018;233:6632–6643. doi: 10.1002/jcp.26297. [DOI] [PubMed] [Google Scholar]
- 106.Rajman M, Metge F, Fiore R, Khudayberdiev S, Aksoy-Aksel A, Bicker S, Ruedell Reschke C, Raoof R, Brennan GP, Delanty N, et al. A microRNA-129-5p/Rbfox crosstalk coordinates homeostatic downscaling of excitatory synapses. EMBO J. 2017;36:1770–1787. doi: 10.15252/embj.201695748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siddoway B, Hou H, Xia H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology. 2014;78:38–44. doi: 10.1016/j.neuropharm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wan Y, Yang ZQ. LncRNA NEAT1 affects inflammatory response by targeting miR-129-5p and regulating Notch signaling pathway in epilepsy. Cell Cycle. 2020;19:419–431. doi: 10.1080/15384101.2020.1711578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: A disease with increasing heterogeneity. Lancet. 2014;383:1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- 110.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu Q, Jiang H, Wang Z, Wang X, Chen H, Shen Z, Xiao L, Guo X, Yang T. Injury factors alter miRNAs profiles of exosomes derived from islets and circulation. Aging (Albany NY) 2018;10:3986–3999. doi: 10.18632/aging.101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collinson P. Laboratory medicine is faced with the evolution of medical practice. J Med Biochem. 2017;36:211–215. doi: 10.1515/jomb-2017-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wicks K, Torbica T, Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol. 2014;26:341–353. doi: 10.1016/j.smim.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 115.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diabet Rep. 2007;7:242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 116.Daigle I, Simon HU. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int Arch Allergy Immuno. 2001;126:147–156. doi: 10.1159/000049506. [DOI] [PubMed] [Google Scholar]
- 117.Pongracz J, Webb P, Wang K, Deacon E, Lunn OJ, Lord JM. Spontaneous neutrophil apoptosis involves caspase 3-mediated activation of protein kinase C-delta. J Biol Chem. 1999;274:37329–37334. doi: 10.1074/jbc.274.52.37329. [DOI] [PubMed] [Google Scholar]
- 118.Zhao R, Guan DW, Zhang W, Du Y, Xiong CY, Zhu BL, Zhang JJ. Increased expressions and activations of apoptosis-related factors in cell signaling during incised skin wound healing in mice: A preliminary study for forensic wound age estimation. Leg Med (Tokyo, Japan) 2009;11 (Suppl 1):S155–S160. doi: 10.1016/j.legalmed.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 119.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 120.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Umehara T, Mori R, Mace KA, Murase T, Abe Y, Yamamoto T, Ikematsu K. Identification of specific miRNAs in neutrophils of type 2 diabetic mice: Overexpression of miRNA-129-2-3p accelerates diabetic wound healing. Diabetes. 2019;68:617–630. doi: 10.2337/db18-0313. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, Liu D, Wang C, Hu MD, Zeng TT, Yan L, Ren M. MicroRNA-129 and -335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes. 2018;67:1627–1638. doi: 10.2337/db17-1238. [DOI] [PubMed] [Google Scholar]
- 123.Jobin PG, Butler GS, Overall CM. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta Mol Cell Res. 2017;1864:2043–2055. doi: 10.1016/j.bbamcr.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 124.Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- 125.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:403–425. doi: 10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 126.Wieser S, Horisberger B, Schmidhauser S, Eisenring C, Brügger U, Ruckstuhl A, Dietrich J, Mannion AF, Elfering A, Tamcan O, Müller U. Cost of low back pain in Switzerland in 2005. Eur J Health Econ. 2011;12:455–467. doi: 10.1007/s10198-010-0258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordoñana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J. 2015;15:1106–1117. doi: 10.1016/j.spinee.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 129.Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003;28:982–990. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- 130.Patterson JE. The pre-travel medical evaluation: The traveler with chronic illness and the geriatric traveler. Yale J Biol Med. 1992;65:317–327. [PMC free article] [PubMed] [Google Scholar]
- 131.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 132.Antunovic M, Matic I, Nagy B, Caput Mihalic K, Skelin J, Stambuk J, Josipovic P, Dzinic T, Paradzik M, Marijanovic I. FADD-deficient mouse embryonic fibroblasts undergo RIPK1-dependent apoptosis and autophagy after NB-UVB irradiation. J Photochem Photobiol B. 2019;194:32–45. doi: 10.1016/j.jphotobiol.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 133.Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis D, Jia LT, Wu SX, Huang J, Chen J, Luo ZJ. Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J Pathol. 2011;225:232–242. doi: 10.1002/path.2931. [DOI] [PubMed] [Google Scholar]
- 134.Li N, Gao Q, Zhou W, Lv X, Yang X, Liu X. MicroRNA-129-5p affects immune privilege and apoptosis of nucleus pulposus cells via regulating FADD in intervertebral disc degeneration. Cell Cycle. 2020;19:933–948. doi: 10.1080/15384101.2020.1732515. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Zhao K, Zhang Y, Kang L, Song Y, Wang K, Li S, Wu X, Hua W, Shao Z, Yang S, Yang C. Methylation of microRNA-129-5P modulates nucleus pulposus cell autophagy by targeting Beclin-1 in intervertebral disc degeneration. Oncotarget. 2017;8:86264–86276. doi: 10.18632/oncotarget.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 137.Pluta R, Ulamek-Koziol M, Januszewski S, Czuczwar SJ. Gut microbiota and pro/prebiotics in Alzheimer's disease. Aging (Albany NY) 2020;12:5539–5550. doi: 10.18632/aging.102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Y, Han Y, Niu L, Li J, Chen Y. MiR-499 inhibited hypoxia/reoxygenation induced cardiomyocytes injury by targeting SOX6. Biotechnol Lett. 2019;41:837–847. doi: 10.1007/s10529-019-02685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaletel I, Schwirtlich M, Perović M, Jovanović M, Stevanović M, Kanazir S, Puškaš N. Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer's disease are associated with altered expression of SOXB transcription factors. J Alzheimer's Dis. 2018;65:963–976. doi: 10.3233/JAD-180277. [DOI] [PubMed] [Google Scholar]
- 140.Zeng Z, Liu Y, Zheng W, Liu L, Yin H, Zhang S, Bai H, Hua L, Wang S, Wang Z, et al. MicroRNA-129-5p alleviates nerve injury and inflammatory response of Alzheimer's disease via downregulating SOX6. Cell Cycle. 2019;18:3095–3110. doi: 10.1080/15384101.2019.1669388. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Geng Z, Xu F, Zhang Y. MiR-129-5p-mediated Beclin-1 suppression inhibits endothelial cell autophagy in atherosclerosis. Am J Transl Res. 2016;8:1886–1894. [PMC free article] [PubMed] [Google Scholar]
- 142.Wan G, An Y, Tao J, Wang Y, Zhou Q, Yang R, Liang Q. MicroRNA-129-5p alleviates spinal cord injury in mice via suppressing the apoptosis and inflammatory response through HMGB1/TLR4/NF-κB pathway. Biosci Rep. 2020;40(BSR20193315) doi: 10.1042/BSR20193315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang S, Lv Z, Wen Y, Wei Y, Zhou L, Ke Y, Zhang Y, Xu Q, Li L, Guo Y, et al. miR-129-2-3p directly targets SYK gene and associates with the risk of ischaemic stroke in a Chinese population. J Cell Mol Med. 2019;23:167–176. doi: 10.1111/jcmm.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu M, Li H, Bai Y, He J, Chen R, An N, Li Y, Dong Y. miR-129 blocks secondary hyperparathyroidism-inducing Fgf23/αKlotho signaling in mice with chronic kidney disease. Am J Med Sci. 2021;361:624–634. doi: 10.1016/j.amjms.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 145.Zhu Y, Hu Y, Cheng X, Li Q, Niu Q. Elevated miR-129-5p attenuates hepatic fibrosis through the NF-κB signaling pathway via PEG3 in a carbon CCl4 rat model. J Mol Histol. 2021;52:491–501. doi: 10.1007/s10735-020-09949-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.