Abstract
Tuberous sclerosis complex (TSC) is a rare genetic disorder caused by mutations in the TSC1 or TSC2 genes, which encode proteins that antagonise the mammalian isoform of the target of rapamycin complex 1 (mTORC1) – a key mediator of cell growth and metabolism. TSC is characterised by the development of benign tumours in multiple organs, together with neurological manifestations including epilepsy and TSC-associated neuropsychiatric disorders (TAND). Epilepsy occurs frequently and is associated with significant morbidity and mortality; however, the management is challenging due to the intractable nature of the seizures. Preventative epilepsy treatment is a key aim, especially as patients with epilepsy may be at a higher risk of developing severe cognitive and behavioural impairment. Vigabatrin given preventatively reduces the risk and severity of epilepsy although the benefits for TAND are inconclusive. These promising results could pave the way for evaluating other treatments in a preventative capacity, especially those that may address the underlying pathophysiology of TSC, including everolimus, cannabidiol and the ketogenic diet (KD). Everolimus is an mTOR inhibitor approved for the adjunctive treatment of refractory TSC-associated seizures that has demonstrated significant reductions in seizure frequency compared with placebo, improvements that were sustained after 2 years of treatment. Highly purified cannabidiol, recently approved in the US as Epidiolex® for TSC-associated seizures in patients ⩾1 years of age, and the KD, may also participate in the regulation of the mTOR pathway. This review focusses on the pivotal clinical evidence surrounding these potential targeted therapies that may form the foundation of precision medicine for TSC-associated epilepsy, as well as other current treatments including anti-seizure drugs, vagus nerve stimulation and surgery. New future therapies are also discussed, together with the potential for preventative treatment with targeted therapies. Due to advances in understanding the molecular genetics and pathophysiology, TSC represents a prototypic clinical syndrome for studying epileptogenesis and the impact of precision medicine.
Keywords: Cannabidiol, epilepsy, epileptogenesis, everolimus, ketogenic diet, mTORC1, tuberous sclerosis complex; TSC-associated neuropsychiatric disorders
Introduction
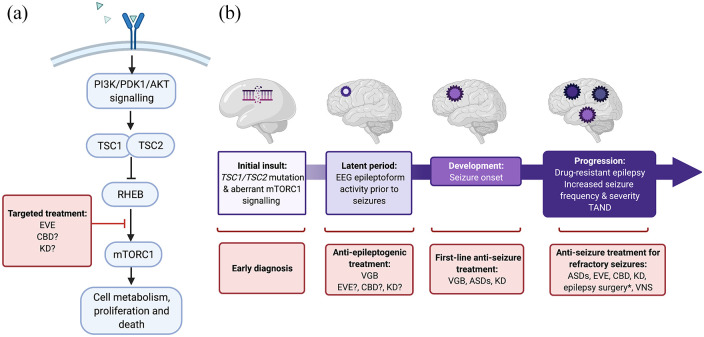
Tuberous sclerosis complex (TSC) is a rare genetic disorder caused by mutations in either the TSC1 or TSC2 tumour suppressor genes. TSC1 and TSC2 encode the proteins hamartin and tuberin, respectively, which form an intracellular complex that antagonises the mammalian isoform of the target of rapamycin complex 1 (mTORC1) through activation of the GTPase activity of RHEB (Ras homolog enriched in brain) (Figure 1a). 1 mTORC1 plays a central role in a whole range of fundamental cell processes, including lipid and nucleotide synthesis, protein synthesis and autophagy. 2 Mutations in TSC1 or TSC2 lead to aberrant activation of this signalling pathway, resulting in the development of benign tumours (hamartomas or tubers) typically in the brain, kidneys, lungs, heart or skin. 3 Given the numerous functions surrounding mTOR signalling, the full mechanisms underlying the pathogenesis of TSC are still being elucidated.
Figure 1.
Simplified schematic of (a) targeted treatment in the mTOR pathway (b) the concept of epileptogenesis and the potential for early intervention in the treatment of TSC. Epileptogenesis describes the complex process by which the normal brain undergoes molecular, structural and functional changes that result in abnormal neuronal activity that promotes seizures (Jozwiak et al., 2020) 4 ; it encompasses the evolution from the initial insult (in the case of TSC, mutation of the TSC1 or TSC2 gene), through what is known as a ‘latent period’ that occurs before the onset of seizures, to the development and progression of seizures. *Epilepsy surgery is indicated for carefully selected patients that underwent presurgical diagnostics; ? = possible, being/to be investigated. Created with BioRender.com.
AKT, protein kinase B; ASD, anti-seizure drugs; CBD, cannabidiol; EEG, electroencephalogram; EVE, everolimus; KD, ketogenic diet, including the medium-chain triglyceride (MCT) KD; mTOR, mammalian target of rapamycin; mTORC1, mammalian/mechanistic target of rapamycin complex 1; PDK1, phosphoinositide-dependent protein kinase-1; PI3K, phosphoinositide 3-kinase; RHEB, Ras homolog enriched in brain; TAND, TSC-associated neuropsychiatric disorders; TSC, tuberous sclerosis complex; TSC1/TSC2, hamartin-tuberin complex; VGB, vigabatrin; VNS, vagus nerve stimulation.
TSC is rare, affecting an estimated 1 in 5000 persons worldwide, 5 and an estimated annual incidence rate of 1:27,312 live births. 6 As the largest clinical case series of TSC patients, the International multicentre ‘TuberOus SClerosis registry to increase disease Awareness (TOSCA)’ has been pivotal in characterising the natural history of TSC: in 2093 patients, the most common manifestations of TSC included epilepsy, cortical tubers, subependymal nodules, intellectual disability, subependymal giant cell astrocytomas (SEGA), renal angiomyolipomas (AML), lymphangioleiomyomatosis (LAM), cardiac rhabdomyomas, facial angiofibromas, forehead plaque, ⩾3 hypomelanotic macules and shagreen patches. 7 Broadly speaking, TSC2 mutations are associated with a more severe phenotype than TSC1 mutations, including being more likely to have intractable focal epilepsy or infantile spasms (IS), SEGAs and intellectual disability,8,9 although TSC is highly clinically heterogeneous, with both inter- and intra-familial phenotypic variability.
TSC has a substantial negative impact on the quality of life (QoL) of patients, that is worse than some other chronic conditions, affecting psychosocial factors with negative consequences for education and career.10–13 TSC is also associated with increased mortality, predominantly due to complications from seizures and renal complications. 5 In addition, caregivers report negative impacts on family, social and work-related dynamics. 11 TSC is associated with a high burden of illness with consequent cost and resource implications for healthcare systems.5,14,15
TSC-associated epilepsy and neuropsychiatric disorders
Epilepsy is one of the most common manifestations of TSC, associated with significant morbidity and mortality and, as such, the management of seizures is an important treatment goal.16–18 The TOSCA registry reported that 84% of TSC patients had epilepsy: 39% had IS and 68% had focal seizures. 19 TSC is associated with almost all seizure types and most patients develop multiple types including focal aware and focal impaired awareness seizures, tonic, atonic, tonic-clonic seizures, myoclonic and atypical absences. 20 Onset of seizures in TSC typically occurs in the first 2 years of life, although they can also develop in adulthood.17,20 Treatment is particularly challenging because seizures are generally refractory to standard anti-seizure drugs (ASDs).
TSC is a common cause of West syndrome, an epileptic syndrome characterised by IS, hypsarrhythmia in an electroencephalogram (EEG) and developmental delay, that generally presents in the first year of life. 21 Of note, IS can also occur in TSC patients independently of West syndrome, without signs of hypsarrhythmia. 22 In either case, a TSC diagnosis should be investigated in infants with IS.16,17 Indeed, TSC is often diagnosed due to the presence of IS and other seizures,16,17 although there is now an increasing emphasis on diagnosing TSC before the onset of seizures by the early identification of other prevalent features including cardiac rhabdomyomas or hypomelanotic skin macules, with prenatal diagnosis even being possible in cases with cardiac rhabdomyomas that can be detected on ultrasound.23,24 TSC is also associated with Lennox-Gastaut syndrome (LGS), another epileptic syndrome that generally begins in children aged 3–5 years, characterised by a clinical triad consisting of specific slow spike-and-wave EEG pattern, multiple seizure types and cognitive impairment/behavioural difficulties. 25
TAND (TSC-Associated Neuropsychiatric Disorders) is a term that encompasses the neuropsychiatric comorbidities associated with TSC that includes a range of behavioural, psychiatric, intellectual, academic, neuropsychological and psychosocial manifestations including autism spectrum disorder and intellectual disability.18,26,27 The pathogenetic mechanisms of TAND are poorly understood, and it is still not clear to what extent the underlying mechanisms of mTORC1 dysregulation contribute to their development and to what extent is the result of the epilepsy itself.28–31 Seizures clearly have an important influence because a history of seizures, IS, early seizure onset, and refractory seizures are strongly associated with poor cognitive outcome.19,20,32–34 Overall, the aim is to have targeted therapies that can reduce both seizure burden as well as the development of TAND.
There is now a growing body of evidence that early intervention before the onset of seizures has positive implications for the treatment of both epilepsy and TAND in TSC patients. To this end, studies have been focussing on elucidating the mechanisms of epileptogenesis, as well as improvements in early diagnosis, identifying biomarkers of later epilepsy, and developing targeted therapies for the prevention of seizure development (Figure 1).17,4,35 In TSC, seizures are believed to arise due to the epileptogenic cortical tubers and the surrounding abnormally developed tissue that result as a consequence of increased mTORC1 signalling that occurs during embryonic brain development, although the full mechanisms are complex and are still being elucidated (Figure 1).28,36 The ‘latent period’ may provide a window for initiating preventative treatment, while early diagnosis, biomarkers of epileptogenesis, and having appropriate treatments are all crucial to exploiting this opportunity (Figure 1). 4
TSC is a prototypic clinical disorder for studying epileptogenesis and the impact of prophylactic interventions: not only are the underlying genetic mechanisms known, but suspected prenatal or early neonatal diagnosis of TSC is possible in some cases due to the visibility of cardiac tumours or cortical tubers on foetal ultrasounds, allowing for early diagnosis. 24 In addition, early abnormal EEG activity, which can be observed before the development of clinical seizures in TSC (i.e. in the latent period), has been determined to be a reliable prognostic biomarker for later epilepsy and indeed neurodevelopmental comorbidities (Figure 1).37–39 Crucially, this discovery has been instrumental in the design of clinical trials focussed on epilepsy prevention, including the EPISTOP and PREVeNT trials that are described below. In addition, in line with current European guidelines for infants and children to receive regular EEG monitoring, 17 a recent study showed that conducting at least one EEG study in TSC patients before epilepsy onset and continuing with regular EEG monitoring is now the standard of care in many centres, while it is becoming more common for clinicians to prescribe ASDs prophylactically when epileptiform discharges occur on EEG before the emergence of clinical seizures. 40
Vigabatrin and other conventional anti-seizure drugs used widely for TSC-associated epilepsy
Conventional treatment with vigabatrin
In the European Union (EU), vigabatrin (VGB) is indicated as monotherapy for IS, and in combination with other ASDs for patients with refractory focal epilepsy, with no age specifications (Table 1). In the US, VGB is indicated for IS as monotherapy in infants 1 month to 2 years of age, and as adjunctive therapy for refractory focal seizures from 2 years of age (Table 1); the latter is a recent welcome expansion whereby until January 2020 the indication for focal seizures was only for patients aged 10 years and older. VGB is the recommended first-line monotherapy for TSC-associated IS,16–18 and for focal seizures in the EU. 17
Table 1.
Summary of conventional ASDs widely used for the treatment of seizures associated with TSC.
| ASD | Generation (year of MA for epilepsy) | MOA via | Epilepsy indication(s) EU | Epilepsy indication(s) US | Studies reporting ⩾50% responder rate in TSC | Other considerations | |
|---|---|---|---|---|---|---|---|
| Study design | ⩾50% responder rate | ||||||
| Vigabatrine | Second generation (1992) | GABA-T | FS b ; IS a | Refractory complex FS (⩾2 y) b ; IS a (infants 1 mo to 2 y) | RCT (N = 22; 11 treated with VGB) 41 | 100% for IS | Risk of visual field defects |
| Retrospective (N = 42) 42 | 73% for IS 34% for FS |
||||||
| Retrospective (N = 670) 43 | 88% c | ||||||
| Retrospective (N = 49) 44 | 31% for FS | ||||||
| Retrospective (N = 21) 45 | 81% for ES and TC | ||||||
| Valproate | First generation (1967) | • Voltage-gated sodium channels • T-type calcium channels • GABA |
FS and GS or other epilepsy | FS*/ b ; Multiple seizure types b | Retrospective (N = 60) 46 | 70% d | Risk of hepatotoxicity and pancreatitis Due to teratogenic effect a Pregnancy Prevention Program is in place |
| Lamotrigine | Second generation (1991) | • Voltage-sensitive sodium channels | FS and GS, including GTC and LGS (⩾ 2 y b ; ⩾ 13 y a ) | FS, GTC and LGS (⩾2 y b ;⩾ 16 s a ) | Retrospective (N = 57) 47 | 79% (42% seizure free + 37% >50% reduction) | Rare risk of life-threatening serious rash; mandatory slow titration reduces the risk |
| Levetiracetam | Second generation (2000) | • Synaptic vesicle protein SV2A | FS in newly diagnosed epilepsy
a
(⩾ 16 y) PS b with epilepsy (⩾ 1 mo); JME (myoclonic seizures) b (⩾ 12 y); IGE (GTC seizures) b (⩾ 12 y). |
FS (⩾1 mo); JME (myoclonic seizures) b (⩾ 12 y); IGE (GTC seizures) b (⩾ 6 y) |
Retrospective (N = 20) 48 | 40% | Risk of psychobehavioural AEs, particularly in children or individuals with cognitive impairment |
| Oxcarbazepine | Second generation (1990) | • Voltage-sensitive sodium channels | FS with or without secondary GTC (⩾ 6 y a / b ) | FS (4–16 y a ; 2–16 y b ) | Retrospective (N = 16) 46 | 67%‡ | – |
| Carbamazepine | First generation (1965) | • Voltage-sensitive sodium channels | GTC and FS | FS with complex symptomatology, GTC and mixed seizure patterns, other partial or generalised seizures. | Retrospective (N = 29) 46 | 67% d | Risk of serious and sometimes fatal dermatologic reactions and aplastic anaemia and agranulocytosis. |
| Topiramate | Second generation (1995) | • Voltage-sensitive sodium channels • GABA-A receptor • AMPA/kainate glutamate receptors |
FS and GTC (>6 y a ; ⩾ 2 y b ); LGS (⩾2 y b ) | FS and GTC (⩾ 2 y a / b ); LGS (⩾ 2 y b ) | - | - | Risk of cognitive side effect such as mental slowing and dysphasia |
| Lacosamide | Third generation (2010) | • Voltage-gated sodium channels | FS +/- GS (⩾ 4 y a / b ) | FS (⩾ 4 y) | Retrospective (N = 46) 49 | 48% | – |
| Clobazam | Second generation (1995) | • GABA-A receptor | Epilepsy b | Seizures associated with LGS b (⩾ 2 y) | Retrospective (N = 29) 50 | 69% | Risks from concomitant use with opioids that may result in profound sedation, respiratory depression, coma, and death Risk of physical and/or psychic dependence |
| Brivaracetam | Third generation (2016 adults; 2018 children) | • Synaptic vesicle protein SV2A | FS +/- secondary GS (⩾ 4 y b ) | FS (⩾ 4 y) | Retrospective (N = 44; 3 with TSC) 51 | 43% for the overall population | – |
| Perampanel | Third generation (2012) | • AMPA glutamate receptor | FS ± secondary GS (⩾ 12 y b ); IGE (GTC seizures) (⩾ 12 y b ) | FS ± secondary GS (⩾ 4 y); Primary GTC (⩽12 y b ) | Retrospective (N = 32; 6 with TSC) 52 | TSC 67% at 6 & 12 mo; overall population 44% & 31% at 6 & 12 mo | Risk of serious psychiatric and behavioural reactions including irritability and aggression especially in individuals with cognitive impairment |
Monotherapy.
Adjunctive.
Retention rate at 6 months;
Seizure-freedom or treatment success; Adapted from van der Poest Clement et al 2020, 53 Málaga et al. 2019 54 ; and the respective cited publications and Summary of Product Characteristics (SmPC) and US Highlights of Prescribing Information.
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AS, absence seizures; ES, epileptic spasms; EU, European Union; FS, focal seizures; GABA, gamma aminobutyric acid; GABA-T, GABA-transaminase; GS, generalised seizures; GTC, generaIised tonic clonic; IGE, idiopathic generalised epilepsy; IS, infantile spasms; JME, juvenile myoclonic epilepsy; LGS, Lennox-Gastaut syndrome; MA, marketing authorisation; Mo, month; MOA, mode of action; TS, tonic seizures; TSC, tuberous sclerosis; US, United States; y, year(s).
With regard to studies in TSC patients, VGB was reported to be the most efficacious for IS when prescribed as first treatment, with seizure freedom or ‘treatment success’ occurring in 78% of patients versus 50%–70% for the other ASDs. 46 VGB is also one of only a few treatments with evidence of efficacy in TSC patients from a randomised controlled trial (RCT), albeit in only 22 patients overall; over a 1-month period, all 11 patients (100%) were free of IS in the VGB group as compared with 5/11 (45%) patients treated with hydrocortisone. 41 Other studies, all of which are retrospective observational studies, have reported seizure-freedom rates of 27–89% for IS and 25–46% for other seizures, and ⩾50% response rates of 76–88% for IS and 31–88% for other seizures (Table 1). 53
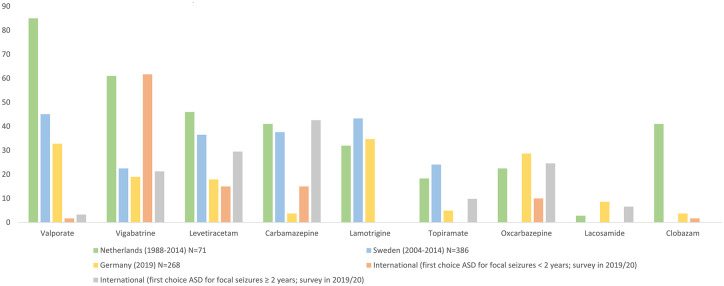
Despite VGB being the recommended first-line therapy, studies in Europe have suggested that it is not always the most commonly prescribed ASD in patients with TSC (Figure 2). Indeed there may be some reluctance to prescribe VGB due to the risk of visual field defects that have been reported to occur in up to a third of patients, which may not be reversible upon discontinuation 55 ; regular vision testing is therefore required although this is not possible in infants and in patients with mental retardation. It should also be noted that VGB may have a relatively low retention rate compared with other ASDs, 56 which may be due to the development of tolerance as a result of its GABAergic mechanism. 57 However, it has been suggested recently that this risk of relapse could be reduced by using a high-dose of VGB, although further studies are warranted to confirm this. 58 Despite these limitations, VGB still remains a pivotal initial treatment option for IS and focal seizure in TSC patients.
Figure 2.
ASD treatment patterns in TSC patients. Data from Overwater et al. 2015 (Netherlands), Welin et al. 2017 (Sweden), Strzelczyk et al. 2021 (Germany) and Słowińska et al. 2020 (International).40,46,59,60
ASD, anti-seizure drugs; TSC, tuberous sclerosis complex.
Preventative treatment with VGB
Highly anticipated results from the EPISTOP trial [ClinicalTrials.gov identifier: NCT02098759] were published in November 2020: this trial, part RCT and part open-label trial depending on the trial site, followed 94 infants with TSC with epileptiform EEG abnormalities who received either conventional VGB treatment (n = 29) (i.e. initiated after the first electrographic or clinical seizure) or preventative VGB (i.e. initiated after EEG epileptiform activity was seen but before seizures had occurred) (n = 25). 61 The median time to onset of seizures was longer in infants who received preventive treatment than in children treated conventionally {Day 614 [95% confidence interval (CI): 474–infinity] versus Day 124 (95% CI): 114–200}. Overall, at 24 months, preventive VGB treatment was associated with reduced risks of clinical seizures [odds ratio (OR)=0.21 (95% CI: 0.04, 0.9); p = 0.032], drug-resistant epilepsy [OR=0.23 (0.06, 0.83); p = 0.025], and IS [OR=0 (0, 0.33); p < 0.001]. In addition, there were no additional safety concerns with preventive treatment. EPISTOP is also designed to identify biomarkers of epileptogenesis and drug-resistant epilepsy, although these analyses are still ongoing.
This study comes off the back of an initial pilot open-label study in 45 infants that showed that the preventative group had a significantly higher proportion of seizure-free patients (93% vs. 35%; p = 0.004), and a lower incidence of drug-resistant epilepsy (7% vs. 42%; p = 0.021). 62 Furthermore, mental retardation was significantly less frequent and severe in the preventative group [14% vs 48%; p = 0.031; mean intelligence quotient (IQ) score 92.3 vs. 68.7; p < 0.05]. Based on this study, consensus guidelines recommend that infants with TSC should be monitored with serial EEG in short intervals that cover wake and sleep phases and are accompanied by video recording. Preventive treatment with VGB in children within 24 months of age should be started if epileptiform activity should occur, with or without clinical manifestations, 17 which is becoming increasingly common in clinical practice. 40 More recently, the 5-year long-term follow up of this study reported that 50% (7/14) of patients in the preventive group remained completely seizure-free compared with only 5% (1/25) in the standard treatment group (p = 0.001). In addition, the median IQ remained higher in the preventive group (94 vs. 46; p < 0.03). 63
Another study, the PREVeNT trial [ClinicalTrials.gov identifier: NCT02849457] has a primary completion date of May 2020. 64 This study is similar to the EPISTOP trial, evaluating preventative VGB treatment in infants <6 months with no history of seizures or IS, although it has the advantage of being a double-blind, placebo-controlled study.
Other conventional anti-seizure drugs
Besides VGB, a range of different ASDs are used to treat epilepsy associated with TSC (Figure 2), many of which have been evaluated in retrospective studies in TSC patients, demonstrating ⩾50% responder rates of 37%–69% across studies (Table 1). Of note, brivaracetam (BRV) is a newer ASD approved for adjunctive therapy for focal onset seizures in patients with epilepsy aged ⩾4 years. It is an analogue of levetiracetam (LEV), but is of interest because, in addition to being effective and generally well-tolerated in patients with epileptic encephalopathies, it appears to be associated with a lower incidence of psycho-behavioural adverse events (AEs) compared with LEV.51,65,66
Everolimus
Everolimus (EVE) is an oral protein kinase inhibitor of the mTOR signalling pathway developed over two decades ago. EVE is predominantly known as a cancer treatment; however, due to its mechanism of action as an mTOR inhibitor it also has application as a targeted treatment for TSC patients (Figure 1). 67 In 2017 in the EU and 2018 in the United States (US), EVE gained approval specifically for the treatment of refractory focal-onset seizures associated with TSC, representing an addition to earlier approvals to treat SEGA and renal AMLs.68,69 Another mTOR inhibitor, sirolimus, is indicated for the treatment of patients with LAM, but has so far failed to show significant benefit for reducing seizures in TSC patients. 70
Efficacy for TSC-associated seizures
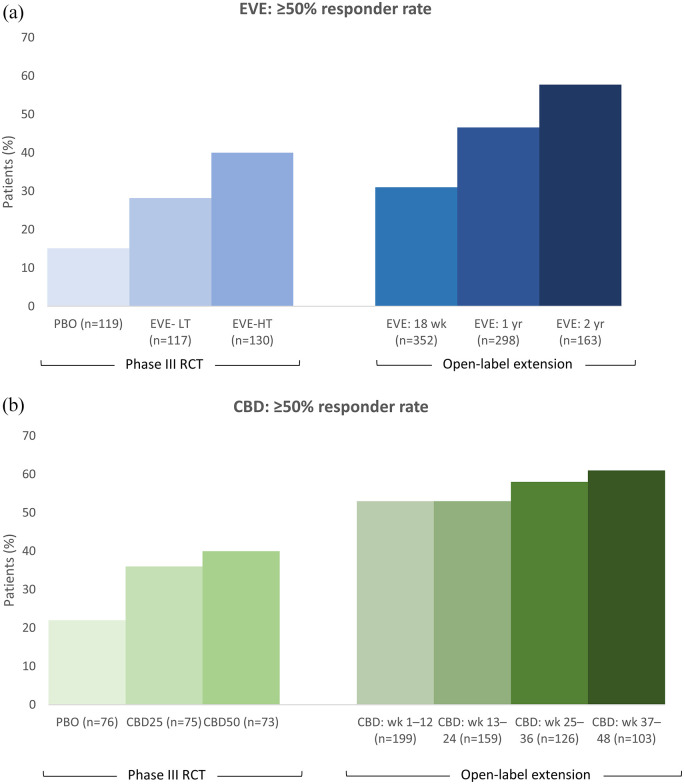
Regarding TSC-associated seizures, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals were based on data from a phase III, double-blind, placebo-controlled RCT (EXIST-3 [ClinicalTrials.gov identifier: NCT01713946]) in which 366 patients were assigned randomly to low-exposure EVE [low trough (LT) range of 3–7 ng/ml; n = 117 (EVE-LT group)], high-exposure EVE [high trough (HT) range of 9–15 ng/ml n = 130 (EVE-HT group)] or placebo (n = 119). 71 The median percentage reduction in seizure frequency was significantly higher in the EVE groups compared with placebo, and significantly higher percentages of patients in the EVE groups achieved 50% or more reductions in seizure frequency (Table 2; Figure 3). In addition, EVE was associated with improvements in the seizure-free rate and the median number of seizure-free days versus placebo. Importantly, EVE has shown efficacy in both younger and older subgroups of children (Supplemental Table S1).72,73
Table 2.
Efficacy of pharmacotherapies from the pivotal RCTs and OLE studies for TSC-associated epilepsy.
| Study design | Patient characteristics | Interventions + comparator | Median baseline monthly TSC-associated seizure frequency | Median percent reduction in TSC-associated seizure; p-value versus PBO | ⩾ 50% responder rate; p value versus PBO | Improvement in overall condition/behaviour/QoL | |
|---|---|---|---|---|---|---|---|
| EVE: EXIST-371,74 | Phase III RCT 18 wks |
• Median (range) age: 10.1 (2.2–56.3) y • Failed >6 prior ASDs: 39% |
EVE-LT (n = 117) | Per week: 8.6 (range: 1.4–192.9) | 29.3% (95% CI 18.8–41.9); p = 0.0028 |
28.2% (95% CI 20.3–37.3); p = 0.0077 | Mean (SD) change in QOLCE total score Responders: 5:8 (11.0); p = 0.016 versus non-responders Non-responders: 1.7 (10.7) Mean (SD) change in QOLIE-AD-48 total score: Responders: 8.2 (12.4); p = 0.155 Non-responders: 2.6 (10.7) Mean (SD) change in QOLIE-31-P total score Responders: 15.2 (15.7); p = 0.021 Non-responders: -0.6 (15.3) |
| EVE-HT (n = 130) | 9.5 (0.3–218.4) | 39.6% (35.0–48.7); p < 0.0001 |
40.0% (31.5–49.0); p < 0.0001 | ||||
| PBO (n = 119) | 10.5 (1.3–231.7) | 14.9% (0.1–21.7) | 15.1% (9.2–22.8) | ||||
| EVE: EXIST-3 OLE 75 | OLE: 48 mo (2 y) | • Median (range) age: 10.03 (2.2–56.3) y | EVE (target exposure, 3–15 ng/mL) (n = 361) | NR | 31.7% (28.5–36.1), 46.7% (40.2–54), 56.9% (50–68.4) at 18 wk, 1 y and 2 y, respectively | 31% (26.2–36.1), 46.6% (40.9–52.5), 57.7% (49.7–65.4) at 18 wk, 1 y and 2 y, respectively |
NR |
| CBD: GWPCARE676 | Phase III RCT 16 wks: 4-wk titration and 12-wk maintenance phase. | • Median (range) age: 11.4 (1.1–56.8) y • Median current ASDs: 3 • Median prior ASDs: 4 |
CBD25 (n = 75) | Per 28 days: 56.0 (IQR: 21.2–101.0) |
48.6% (40.4–55.8); p < 0.00 | 36%; p = 0.07 | Percentage with improvement in overall condition S/CGIC 69%; OR = 2.25; p = 0.0074 |
| CBD50 (n = 73) | 61.0 (36.0–117.0) | 47.5% (39.0–54.8); p = 0.002 | 40%; p = 0.02 | 62%; OR = 1.77; p = 0.0580 | |||
| PBO (n = 76) | 54.1 (26.4–102.0) | 26.5% (14.9–36.5) | 22% | 40% | |||
| CBD: GWPCARE6 OLE 77 | OLE: 48 wks | Median (range) age: 10.8 (1.1–56.8) y | CBD25 with titration up to CBD50 (n = 199) | 56.9 (28.0– 109.0) | 12-wk windows over 48 wks: 54–68% | Wk 1-12: 53% Wk 13–24: 53% Wk 25–36: 58% Wk 37–48: 61% |
S/CGIC: 87% of patients/caregivers at wk 26 P/GIC: 80% of physicians at wk 26 |
ASD, anti-seizure drug; CBD, cannabidiol; CBD25, CBD 25 mg/kg/day; CBD50, CBD 50 mg/kg/day; CI, confidence interval; EVE, everolimus; IQR, interquartile range; HT, high trough (range of 9–15 ng/ml); LT, low trough (range of 3–7 ng/ml); mo, months; NR, not reported; OLE, open-label extension trial; OR, adds ratio; PBO, placebo; P/GIC, Physician Global Impression of Change; QoL, quality of life; QOLCE, Quality of Life in Childhood Epilepsy Questionnaire; QOLIE-AD-48, Quality of Life in Epilepsy Inventory-Adolescents-48; QOLIE-31-P, Patient Weighted Quality of Life in Epilepsy Inventory-Form 31; RCT, randomised controlled trial; S/CGIC, Subject/Caregiver Global Impression of Change; SD, standard deviation; wk, week; y, year(s).
Figure 3.
Responder rates for (a) EVE and (b) CBD from the pivotal RCTs and OLE studies for TSC-associated epilepsy. There are no head-to-head trials of EVE versus CBD, nor any published indirect comparisons. Therefore, any comparisons of the efficacy of the two treatments should be made with caution due to differences in baseline patient demographics and clinical characteristics in the trial populations, and a difference in the placebo effect.
CBD, cannabidiol; CBD25, CBD 25 mg/kg/day; CBD50, CBD 50 mg/kg/day; EVE, everolimus; HT, high trough (range 9–15 ng/ml); LT, low trough (range 3–7 ng/ml); OLE, open-label extension trial; RCT, randomised controlled trial; wk, week; yr, year. The recommended dosage in the OLE trial for EVE was a target trough concentration of 3–15 ng/ml (median dose intensity: 6.76 mg/m2/day [range, 1.1–27.8]) and for CBD it was 25 mg/kg/day with titration up to 50 mg/kg/day (mean modal dose: 27 mg/kg/day).
Of the 366 patients in the core EXIST-3 study, 361 continued in an open-label extension (OLE) to determine the long-term outcomes. 75 Reductions in TSC-associated seizures were sustained with adjunctive EVE, with ⩾50% responder rates increasing over the 2-year period from 31% to 57%, and median percent reductions in seizure frequency increasing from 32% to 57%, although it should be noted that the number of evaluable patients decreased (Figure 3). New responders emerged with a longer EVE treatment duration and 50% of patients experienced persistent responses. The median number of additional seizure-free days (per 28-day period) increased from 2.5 days at week 18, to 4.32 days at 1 year, and 6.15 days at 2 years of EVE treatment, while 15/275 (5%) of patients were seizure-free over the previous 6 months at year 1 and 13/117 (11%) were seizure-free over the previous 6 months at year 2.
The efficacy of EVE for the treatment of seizures in TSC patients has also been reported in a few single-arm trials and real-world retrospective studies, with ⩾50% responder rates ranging from 27% to 100% across studies of different follow-up periods and different age ranges (including children <2 years and adults ⩾18 years),78–86 together with increases in seizure-free days and seizure freedom rates of 7%-58% (Supplemental Table S2).78,82–85 As with the EXIST-3 study, longitudinal studies, up to 48 months, reported increasing responder rates over time, providing further support that EVE is associated with long-term efficacy for TSC-associated seizures, with the caveat of these studies being smaller, single arm trials (Supplemental Table S2).81,83
TAND outcomes
Health-related QoL (HRQoL) scales were analysed as secondary endpoints in EXIST-3, with patients classified as ‘responders’ having the greatest mean change scores for the majority of domains across a range of HRQoL measures. However, there were low completion rates for the two self-report patient-reported outcome (PRO) measures in the older age groups, which may also have been related to the individuals’ intellectual disability impeding their self-reporting. 74 In a subgroup analysis of Japanese patients in the EXIST-3 study, a positive trend towards an improvement of autism spectrum disorder symptoms, evaluated using the Pervasive Developmental Disorders Autism Society Japan Rating Scale, was observed. 73
In contrast, two RCTs have failed to demonstrate significant differences between EVE and placebo with regard to a wide range of assessments for autism, social and communication skills, IQ, behavioural and emotional problems, sleep quality, QoL, learning and memory, visual motor and fine motor skills, executive functioning, sensory processing and other neuropsychological deficits.87,88 However, the jury is still out on mTOR inhibitors and their potential for improving TAND outcomes: in particular, these two RCTs were conducted in older children (6 years and older) (Supplemental Table S2) whereas, to have an impact, treatment may be needed at earlier stages of brain development. Even in a study of younger children (aged 1.7–13 years), the vast majority already had severe developmental disorder at baseline, and therefore developmental impairment continued to decline, albeit possibly at a slower pace. 78 In this respect, it may not be possible to reverse or significantly delay developmental impairment that is already present, but rather a strategy of prevention before onset may be required (Figure 1). 78
Safety
The most common AEs in the EVE groups included stomatitis, diarrhoea, nasopharyngitis, pyrexia and upper respiratory tract infection, although nasopharyngitis and upper respiratory tract infection were equally common in the placebo group. 71 AEs leading to discontinuations were rare (⩽5%), with stomatitis being the most common reason. In the OLE, the safety profile was generally consistent with that reported in the core study. 75 However, there were two deaths due to pneumonia and septic shock that were suspected to be treatment related. Indeed, EVE has immunosuppressive properties and may therefore predispose patients to infections. Despite this concern, EVE has generally been found to be well tolerated across studies, with the majority of AEs being mild to moderate, including in younger patients.72,82,89
Since the treatment of TSC usually requires polypharmacy, possible interactions with other ASDs and other medications are also important aspects to be taken into consideration. EVE was found to have no effect on pre-dose concentrations of CYP3A4 substrate ASDs such as clonazepam, diazepam, felbamate and zonisamide (ZNS), but has been associated with small (approximately 10%) increases in pre-dose concentrations of the ASDs CBZ, clobazam (CLB) and the CLB metabolite N-desmethylclobazam; these increases may not be clinically significant although dose adjustments for ASDs with a narrow therapeutic index, e.g. CBZ, may be considered.68,69 A clinically significant drug–drug interaction has been reported between EVE and cannabidiol (CBD),90,91 as described in more detail in the CBD section below.
Cannabidiol
Evidence suggests that CBD exerts its anti-convulsive actions through multiple mechanisms including modulation of intracellular calcium and adenosine-mediated signalling, 92 although the understanding of the molecular mechanisms underlying these processes is still in its infancy. Regarding TSC, data from a zebrafish model of the disease showed that CBD was associated with modulation of rpS6, a downstream target of the mTOR pathway, in the brain. In this respect, in vitro and in vivo studies of other disease models including multiple sclerosis, Parkinson’s disease, schizophrenia and cancer have also suggested a role of CBD in modulating the mTOR pathway.93–97 However, CBD appears to have contrasting effects in different environments (i.e. upregulation of the mTOR pathway in some studies and downregulation in others), and more research is needed to further our understanding.93–97 This research is particularly pertinent given that, despite this potential role of CBD in mediating the mTOR pathway, preliminary evidence suggests that CBD treatment at a therapeutic dose for refractory epilepsy does not decrease the volume of SEGAs or AMLs in TSC patients, in contrast with mTOR inhibitors. 98
In addition to its prior indications for Dravet syndrome (DS) and LGS, CBD has recently gained approval by the FDA and the EMA for the treatment of seizures associated with TSC that includes patients 1 year of age and older in the US and 2 years of age and older in the EU.99,100 Of note, CBD is indicated in the EU in conjunction with CLB for DS and LGS, but is licensed without CLB for TSC. 100
Efficacy for TSC-associated seizures
The efficacy and safety of add-on CBD has been evaluated in a phase III RCT (GWPCARE6 [ClinicalTrials.gov identifier: NCT0254476]) conducted in 224 patients with drug-resistant epilepsy associated with TSC, whereby patients were randomised to CBD 25 mg/kg/day (CBD25; n = 75) and 50 mg/kg/day (CBD50; n = 73) or placebo (n = 76) (Table 2). 76 Compared with placebo, CBD was associated with a significantly greater reduction in the percentage change from baseline in the frequency of TSC-associated seizures (Table 2). Higher percentages of patients in the CBD groups achieved a ⩾50% reduction in seizures versus placebo (Table 2; Figure 3). CBD was also associated with a significantly greater reduction in total seizure frequency compared with placebo. In addition, during the 12-week maintenance period, the CBD groups had mean gains of additional seizure-free days over placebo. Furthermore, improvement in overall condition, evaluated using the subject/caregiver global impression of change (S/CGIC), was reported by more patients/caregiver in the CBD groups than with placebo (Table 2). Findings from a post hoc analysis suggested that the onset of the treatment effect occurred early, within the first 2 weeks. 101
Longer-term adjunctive CBD treatment has been evaluated in an OLE to the GWPCARE6 study, involving 199 of the 201 patients who completed the RCT. 77 Reductions in seizures were maintained through 48 weeks, at least 6% of patients remained seizure free during the 12-week windows, and improvement in S/CGIC continued to be reported by a high proportion of proportion of patients/caregivers (Table 2; Figure 3).
Safety
The safety profile of the 25 mg/kg/day dose was found to be superior to 50 mg/kg/day; as such the recommended starting dose for TSC patients is 5 mg/kg/day, which can be increased as tolerated to a maintenance dose of 25 mg/kg/day. 99 The most common AEs included diarrhoea, decreased appetite and somnolence. 76 Treatment discontinuations due to an AE were reported in 11% patients in the CBD25 group. Elevated liver transaminases occurred in 9 (12%) patients in the CBD25 group, with the vast majority (81%) of patients with elevations being on concomitant valproate (VPA). A post hoc analysis reported that AEs lasted longer for CBD versus placebo but resolved within the 16-week study in most patients. 101 Furthermore, results from a real-world study have suggested that slow titration of CBD can deliver improved tolerance with comparable efficacy. 102 The AE profile over the long-term in the OLE was similar to that observed previously. 77
With regard to drug–drug interactions, it has been observed that CBD results in increased serum levels of the mTOR inhibitors EVE and sirolimus, requiring reduced mTOR inhibitor dosing to avoid potentially serious AEs.90,91 Also of note is that the risk of transaminase elevations may be increased with concomitant use of VPA and higher doses of CBD, whereby CBD and/or VPA should be reduced or discontinued if clinically significant increases of transaminases occur. 99 In addition, the interaction with CLB, which leads to increased levels of its active metabolite N-desmethylclobazam, may result in increased somnolence and sedation, requiring a dose reduction of CLB. 99 While increases in serum levels of some other ASDs, including TPM, rufinamide (in adults and children), ZNS and eslicarbazepine (in adults) have been reported with increasing doses of CBD, they were within the accepted therapeutic range. 103 Increases in BRV levels in five patients that led to mild AEs in two patients and a reduction of BRV in one patient have also been reported in a small case series. 104
Non-pharmacologic agents
Ketogenic diet and medium-chain triglycerides
The ketogenic diet (KD) is a low carbohydrate, high-fat diet that is designed to mimic the physiological process of fasting whereby the liver produces ketones as an alternative energy source for the brain. Although not fully understood, various mechanisms have been implicated in the role of the KD in treating refractory epilepsy.105,106 In the ‘classic’ KD (a strict diet that can be difficult to adhere to, with very low carbohydrate content and 60–80% of dietary energy provided by long-chain fats) ketone bodies are believed to have a central role.105,106 However, for the more popular, less strict medium-chain triglyceride (MCT) KD, that comprises of the triglycerides heptanoic acid, octanoic acid and decanoic acid, additional key mechanisms of action that are independent of ketones have been reported.105,106 In vivo studies have provided evidence that decanoic acid can directly and selectively inhibits AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors to reduce neuronal excitability, binds PPARγ (peroxisome proliferator-activated receptor gamma) involved in mitochondrial biogenesis and, of particular relevance for TSC, inhibits mTORC1 activity.107–109 Other studies have also demonstrated that the KD can attenuate mTOR signalling pathways,110,111 providing a biological basis for its demonstrated efficacy in controlling seizures in TSC patients. In studies in patients with TSC, the KD is associated with ⩾50% response rates of 68%–83.3%, and seizure-free rates of 33–42% across studies over the short term (3–5 months).112–114 In addition, Youn et al. further demonstrated the long-term efficacy of the KD but only in a proportion of patients, with the number of patients with a >50% response decreasing over time from 58.1% at 6 months to 32.3% at 24 months. 114 Importantly, the KD has also been associated with improvements in cognition and behaviour, 113 although further studies are needed to confirm this observation. Overall, the consensus TSC guidelines from Europe recommend that the KD be considered in early infancy and early childhood when surgery is not an option. 17 The guidelines from the International Ketogenic Diet Study Group from 2018 also propose that the KD be initiated early in the TSC treatment pathway. 115 With this in mind, the KD has been reported to be effective and well tolerated in very young infants with refractory epilepsy, including in infants maintaining a breast milk diet. 116
Vagus nerve stimulation
The consensus TSC guidelines from Europe recommend that vagus nerve stimulation (VNS) – a procedure that involves stimulating the vagus nerve with electrical impulses – be considered in combination with the KD or in cases where the KD is not acceptable. 17 Responder rates (⩾50% reductions in seizures) have been reported in 50%–92% of TSC patients, although these results are from retrospective studies with a small population of patients.117–120 Seizure freedom was rarely reported. VNS may also have a positive impact on level of functioning, 118 adaptive behaviours and QoL, particularly in patients who had the implantation in childhood. 120 Of note, while VNS is used for a wide range of drug-resistant epilepsies, TSC has been associated with a better response to VNS.121,122
Surgery
International, European and United Kingdom (UK) guidelines recommend that epilepsy surgery be considered in medically refractory TSC patients, with early intervention increasing the probability of seizure-freedom.16–18 Patients need to be carefully selected following a risk–benefit assessment requiring extensive pre-surgical evaluations by a team of epilepsy surgery experts (epileptologists, neurosurgeons, neuroradiologists and neuropsychologists). 123 While studies have demonstrated the long-term benefits of epilepsy surgery in a significant proportion of selected TSC patients,124–126 it has traditionally been underutilised due to the potential risk of severe complications including infection and neurocognitive side effects. These complications in the modern era are rare because of the extensive pre-surgical evaluations to determine eligible patients127,128; however, the multifocal nature of epileptogenic tubers and/or their location in deep brain structures excludes many patients. However, novel techniques are being developed, with the potential to expand the number of eligible patients, while reducing the risk of complications.129,130 For example, magnetic resonance imaging (MRI)-guided laser interstitial thermal therapy (MRg-LITT) is a minimally invasive technique that uses heat emitted from a laser device, while the MRI enables real-time accurate monitoring of the thermal ablation process. Initial experiences of using MRg-LITT to identify and treat cortical tubers responsible for clinical seizures in TSC patients have reported seizure freedom in two out of three, 131 and three out of seven TSC patients. 132 Improvements in neuropsychiatric symptoms were also reported. 132 In addition, Hooten et al. reported a novel method that involved a frameless stereotaxy with the aim of expanding MRg-LITT to younger patients, with a successful application of this technique in a 6-month-old infant with TSC. 133
Potential future therapies
There are currently a handful of therapies in various phases of clinical and preclinical development for the treatment of TSC-associated seizures.
Ganaxolone is a positive allosteric modulator of gamma aminobutyric acid A (GABA-A) receptors that is being developed for various rare genetic epilepsy syndromes and treatment of status epilepticus. A phase III trial has recently met its primary endpoint in CDKL5 deficiency disorder, with a significant reduction in median 28-day major motor seizure frequency compared with placebo. 134 The results of its phase II trial in TSC are anticipated to be reported in mid-2021. 135
Soticlestat (TAK-935/OV935) is a highly selective inhibitor of the enzyme cholesterol 24-hydroxylase (CH24H) that is being developed for various developmental and epileptic encephalopathies (DEEs). Its key phase II study, ELEKTRA, met its primary endpoint in reducing seizure frequency in children with DS and LGS. 136 Soticlestat may have application in TSC patients, although it is not clear if trials in this specific population are planned.
Similarly, the clinical development of fenfluramine (FFA) has to date focussed mainly on the treatment of seizures associated with DS and LGS, with approval for the treatment of seizures associated with DS in the US and the EU in mid and late 2020, respectively. 137 A phase II ‘basket’ clinical trial in multiple rare epilepsies including TSC is planned for 2021. 138
Also of interest is a phase I/II clinical trial (STOP2) that started in September 2020 to evaluate the mTOR inhibitor sirolimus for the prevention or delay of seizure onset in TSC infants, although the estimated primary completion is not due for a while yet (September 2022). 139
Basic research is still hugely important for future translational directions in TSC. For example, reductions in neuronal ciliation have recently been implicated in the pathogenesis of TSC, and inhibitors of heat shock protein 90 (Hsp90) were identified as being able to interfere with this action, which could have relevance for future therapeutics. 140
Of course, in light of TSC being a monogenic disease, gene therapy represents the ‘holy grail’ for patients, but with progress to date being confined to animal models, 141 its application is still experimental with many challenges remaining.
Conclusions
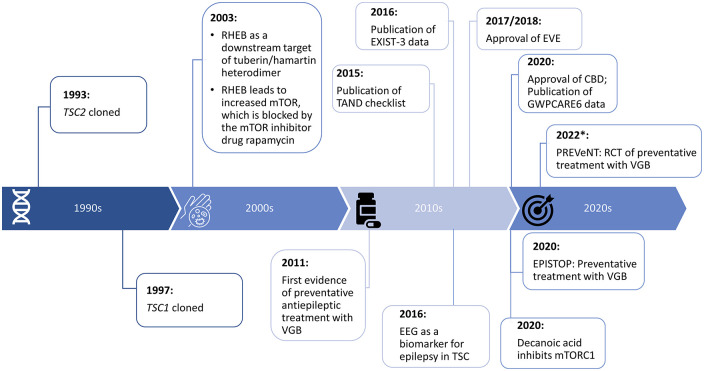
While there has been an awareness surrounding TSC for over 200 years, the genes responsible for this syndrome were not identified until the 1990s (Figure 4) – a breakthrough that furthered the understanding of the mechanisms of the action of the respective proteins. Seminal studies in the early 2000s showed that RHEB was a downstream target of the tuberin/hamartin heterodimer and an upstream regulator of mTOR-mediated signalling, 142 work that continues to be further refined and explored to this day. These discoveries have now been translated into establishing targeted treatments, culminating in the approval of the mTOR inhibitor EVE in 2017/2018. In addition, the newly approved CBD and the already established KD/MCT diet may also have a role in the mTOR pathway, in addition to other putative mechanisms. It is now hoped that the 2020s and beyond will see the benefits of precision treatments consolidated and, in particular, progress investigating their utility for preventing or modifying epileptogenesis may be seen (Figure 4).
Figure 4.
Key milestones towards precision medicine for the amelioration and prevention of TSC-associated epilepsy and TAND. *PREVeNT; Estimated primary completion date of May 2022, and study completion date of December 2022. 64
CBD, cannabidiol; EVE, everolimus; RHEB, Ras homolog enriched in brain; mTOR, mammalian/mechanistic target of rapamycin; RCT, randomised controlled trial; TAND, TSC-associated neuropsychiatric disorders; TSC, tuberous sclerosis complex; VGB, vigabatrin.
The approval of EVE represents an important milestone for the treatment of TSC-associated seizures (Figure 4), especially given the recent data showing its longer-term efficacy, with new responders emerging over time, while 50% of patients experienced sustained responses over 2 years of treatment 75 ; this is in contrast to VGB, which appears to lose efficacy within a year of treatment initiation. 56 In addition, EVE has shown efficacy across a range of TSC manifestations including SEGA, renal AML, skin lesions and cardiac rhabdomyoma, bringing the idea of having a single multi-system treatment for TSC closer. 67 However, the jury is still out on the benefits to TAND outcomes, which may require earlier treatment with EVE.
CBD is also a welcome addition to the treatment armamentarium. Evidence suggests that CBD has multiple cellular targets, 143 and while further studies are required to elucidate the key mechanisms through which it exerts its anti-seizure properties, including its potential role in the mTOR pathway, it has clearly demonstrated efficacy in a proportion of TSC patients with significant reductions in TSC-associated seizure compared with placebo, and ⩾50% responder rates of 53%–61% through 48 weeks of treatment in the OLE (Figure 3, Table 2).
It is also of interest that the KD/ MCT diet, with its long-established application in the treatment of retractable epilepsies, may also inhibit mTOR pathway signalling, suggesting that the KD/MCT diet could be a useful early disease-modifying treatment. There is now overwhelming evidence regarding the feasibility and effectiveness of the KD in infants as young as 3 weeks old116,144,145; a recent meta-analysis in infants less than 2 years of age with drug-resistant epilepsy reported ⩾50% responder rates of 59%, while 33% of infants attained seizure freedom. 146 A limitation of the KD, however, especially in older children and adults, is that it is difficult to maintain; the recent evidence that decanoic acid reduces mTORC1 activity in model systems, including astrocytes derived from TSC patients, suggests that a more sustainable diet rich in decanoic acid may be able to produce similar results to the KD, with better compliance. 109
The EPISTOP trial should hopefully pave the way for evaluating targeted treatments that address the underlying pathophysiology of TSC in a preventative capacity, potentially including EVE and CBD. To date, EVE and CBD have been evaluated only in a conventional setting, while it is certainly valid to hypothesise that prophylactic treatment with these treatments, especially EVE, will be even more favourable than VGB due to the targeted mechanism of action, strengthened by data from some animal studies showing the benefits of early treatment with mTOR inhibitors. 147 However, evidence from TSC mouse models has shown that prenatal treatment with mTOR inhibitors may have concerning side-effects including negatively impacting development and neurological symptoms including learning and memory tasks, together with poor birth weight/weight gain.148,149 Given the potential wide-ranging consequences of inhibiting mTOR signalling during foetal development, caution is needed in designing preventative studies using mTOR inhibitors to take into account the safety implications.23,150,151 On the other hand, a case report has described less than optimal outcomes from early treatment with VGB and EVE that may not have been early enough. 152 Indeed, the jury is still out as to whether the results from EPISTOP can be replicated easily in a real-world situation with regard to pin-pointing what the authors called ‘the point of no return’ on the EEG.
Overall, it may also be important to further refine the window of opportunity, as the time between detecting epileptiform EEG activity and the onset of seizures postnatally may be minimal but prenatal treatment with mTOR inhibitors may have safety concerns; identifying additional biomarkers may be useful in this respect. Further research is also needed into elucidating the fundamental molecular mechanisms in TSC, as well as the mechanisms of epileptogenesis, which may translate into identifying new disease-modifying treatments. Overall, due to advances in understanding the molecular genetics and pathophysiology, TSC represents a prototypic clinical disorder for studying epileptogenesis and the impact of precision medicine.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211031100 for Review of the treatment options for epilepsy in tuberous sclerosis complex: towards precision medicine by Susanne Schubert-Bast and Adam Strzelczyk in Therapeutic Advances in Neurological Disorders
Acknowledgments
We would like to thank Amanda Prowse (Lochside Medical Communications Ltd) for providing editorial assistance.
Footnotes
Author contributions: Both authors reviewed the literature, drafted the manuscript, generated the figures, and assume full responsibility for the final publication.
Conflict of interest statement: SS-B reports personal fees from Eisai, Desitin Pharma, GW Pharmaceuticals, LivaNova, UCB Pharma and Zogenix.
AS reports personal fees and grants from Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals, LivaNova, Marinus Pharmaceuticals, Medtronic, UCB Pharma and Zogenix.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing assistance was funded by GW Pharma (Germany) GmbH.
ORCID iD: Adam Strzelczyk  https://orcid.org/0000-0001-6288-9915
https://orcid.org/0000-0001-6288-9915
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Susanne Schubert-Bast, Epilepsy Center Frankfurt Rhine-Main, Center of Neurology and Neurosurgery, Goethe-University Frankfurt, Frankfurt am Main, Germany; Department of Neuropediatrics, Goethe-University Frankfurt, Frankfurt am Main, Germany; LOEWE Center for Personalized and Translational Epilepsy Research (CePTER), Goethe-University Frankfurt, Frankfurt am Main, Germany.
Adam Strzelczyk, Epilepsy Center Frankfurt Rhine-Main, Goethe-University Frankfurt, Schleusenweg 2–16, Frankfurt am Main, 60528, Germany; LOEWE Center for Personalized and Translational Epilepsy Research (CePTER), Goethe-University Frankfurt, Frankfurt am Main, Germany.
References
- 1. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009; 122: 3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 168: 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Napolioni V, Curatolo P. Genetics and molecular biology of tuberous sclerosis complex. Curr Genomics 2008; 9: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jóźwiak S, Kotulska K, Wong M, et al. Modifying genetic epilepsies – results from studies on tuberous sclerosis complex. Neuropharmacology 2020; 166: 107908. [DOI] [PubMed] [Google Scholar]
- 5. Zöllner JP, Franz DN, Hertzberg C, et al. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J Rare Dis 2020; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebrahimi-Fakhari D, Mann LL, Poryo M, et al. Incidence of tuberous sclerosis and age at first diagnosis: new data and emerging trends from a national, prospective surveillance study. Orphanet J Rare Dis 2018; 13: 117–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kingswood JC, d'Augères GB, Belousova E, et al. TuberOus SClerosis registry to increase disease Awareness (TOSCA) - baseline data on 2093 patients. Orphanet J Rare Dis 2017; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kothare SV, Singh K, Chalifoux JR, et al. Severity of manifestations in tuberous sclerosis complex in relation to genotype. Epilepsia 2014; 55: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 9. Peron A, Au KS, Northrup H. Genetics, genomics, and genotype-phenotype correlations of TSC: insights for clinical practice. Am J Med Genet C Semin Med Genet 2018; 178: 281–290. [DOI] [PubMed] [Google Scholar]
- 10. Amin S, Mallick AA, Lux A, et al. Quality of life in patients with tuberous sclerosis complex (TSC). Eur J Paediatr Neurol 2019; 23: 801–807. [DOI] [PubMed] [Google Scholar]
- 11. Jansen AC, Vanclooster S, de Vries PJ, et al. Burden of illness and quality of life in tuberous sclerosis complex: findings from the TOSCA study. Front Neurol 2020; 11: 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tritton T, Bennett B, Brohan E, et al. Health utilities and quality of life in individuals with tuberous sclerosis complex (TSC) who experience epileptic seizures: a web-based survey. Epilepsy Behav 2019; 92: 213–220. [DOI] [PubMed] [Google Scholar]
- 13. Zöllner JP, Conradi N, Sauter M, et al. Quality of life and its predictors in adults with tuberous sclerosis complex (TSC): a multicentre cohort study from Germany. Neurol Res Pract 2021; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grau J, Zöllner JP, Schubert-Bast S, et al. Direct and indirect costs and cost-driving factors of Tuberous sclerosis complex in children, adolescents, and caregivers: a multicenter cohort study. Orphanet J Rare Dis 2021; 16: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zöllner JP, Grau J, Rosenow F, et al. Direct and indirect costs and cost-driving factors in adults with tuberous sclerosis complex: a multicenter cohort study and a review of the literature. Orphanet J Rare Dis 2021; 16: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amin S, Kingswood JC, Bolton PF, et al. The UK guidelines for management and surveillance of Tuberous Sclerosis Complex. QJM 2019; 112: 171–182. [DOI] [PubMed] [Google Scholar]
- 17. Curatolo P, Nabbout R, Lagae L, et al. Management of epilepsy associated with tuberous sclerosis complex: updated clinical recommendations. Eur J Paediatr Neurol 2018; 22: 738–748. [DOI] [PubMed] [Google Scholar]
- 18. Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabbout R, Belousova E, Benedik MP, et al. Epilepsy in tuberous sclerosis complex: findings from the TOSCA study. Epilepsia Open 2019; 4: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 2010; 51: 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pavone P, Polizzi A, Marino SD, et al. West syndrome: a comprehensive review. Neurol Sci 2020; 41: 3547–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curatolo P, Seri S, Verdecchia M, et al. Infantile spasms in tuberous sclerosis complex. Brain Dev 2001; 23: 502–507. [DOI] [PubMed] [Google Scholar]
- 23. Davis PE, Filip-Dhima R, Sideridis G, et al. Presentation and diagnosis of tuberous sclerosis complex in infants. Pediatrics 2017; 140: e20164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Słowińska M, Jóźwiak S, Peron A, et al. Early diagnosis of tuberous sclerosis complex: a race against time. How to make the diagnosis before seizures? Orphanet J Rare Dis 2018; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strzelczyk A, Schubert-Bast S. Expanding the treatment landscape for Lennox-Gastaut syndrome: current and future strategies. CNS Drugs 2021; 35(1): 61–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Vries PJ, Belousova E, Benedik MP, et al. TSC-associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis 2018; 13: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Vries PJ, Whittemore VH, Leclezio L, et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol 2015; 52: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prabowo AS, Anink JJ, Lammens M, et al. Fetal brain lesions in tuberous sclerosis complex: TORC1 activation and inflammation. Brain Pathol 2013; 23: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Talos DM, Sun H, Zhou X, et al. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One 2012; 7: e35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Specchio N, Pietrafusa N, Trivisano M, et al. Autism and epilepsy in patients with tuberous sclerosis complex. Front Neurol 2020; 11: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curatolo P, Napolioni V, Moavero R. Autism spectrum disorders in tuberous sclerosis: pathogenetic pathways and implications for treatment. J Child Neurol 2010; 25: 873–880. [DOI] [PubMed] [Google Scholar]
- 32. Capal JK, Bernardino-Cuesta B, Horn PS, et al. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav 2017; 70: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jansen FE, Vincken KL, Algra A, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 2008; 70: 916–923. [DOI] [PubMed] [Google Scholar]
- 34. Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology 2007; 68: 62. [DOI] [PubMed] [Google Scholar]
- 35. Zimmer TS, Broekaart DWM, Gruber V-E, et al. Tuberous sclerosis complex as disease model for investigating mTOR-related gliopathy during epileptogenesis. Front Neurol 2020; 11: 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Major P, Rakowski S, Simon MV, et al. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia 2009; 50: 147–154. [DOI] [PubMed] [Google Scholar]
- 37. De Ridder J, Lavanga M, Verhelle B, et al. Prediction of neurodevelopment in infants with tuberous sclerosis complex using early EEG characteristics. Front Neurol 2020; 11: 582891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Domańska-Pakieła D, Kaczorowska M, Jurkiewicz E, et al. EEG abnormalities preceding the epilepsy onset in tuberous sclerosis complex patients - a prospective study of 5 patients. Eur J Paediatr Neurol 2014; 18: 458–468. [DOI] [PubMed] [Google Scholar]
- 39. Wu JY, Peters JM, Goyal M, et al. Clinical electroencephalographic biomarker for impending epilepsy in asymptomatic tuberous sclerosis complex infants. Pediatr Neurol 2016; 54: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Słowińska M, Kotulska K, Szymańska S, et al. Approach to preventive epilepsy treatment in tuberous sclerosis complex and current clinical practice in 23 countries. Pediatr Neurol 2021; 115: 21–27. [DOI] [PubMed] [Google Scholar]
- 41. Chiron C, Dumas C, Jambaqué I, et al. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res 1997; 26: 389–395. [DOI] [PubMed] [Google Scholar]
- 42. Camposano SE, Major P, Halpern E, et al. Vigabatrin in the treatment of childhood epilepsy: a retrospective chart review of efficacy and safety profile. Epilepsia 2008; 49: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 43. Pellock JM, Faught E, Foroozan R, et al. Which children receive vigabatrin? Characteristics of pediatric patients enrolled in the mandatory FDA registry. Epilepsy Behav 2016; 60: 174–180. [DOI] [PubMed] [Google Scholar]
- 44. Friedman D, Bogner M, Parker-Menzer K, et al. Vigabatrin for partial-onset seizure treatment in patients with tuberous sclerosis complex. Epilepsy Behav 2013; 27: 118–120. [DOI] [PubMed] [Google Scholar]
- 45. van der Poest Clement EA, Sahin M, Peters JM. Vigabatrin for epileptic spasms and tonic seizures in tuberous sclerosis complex. J Child Neurol 2018; 33: 519–524. [DOI] [PubMed] [Google Scholar]
- 46. Overwater IE, Bindels-de Heus K, Rietman AB, et al. Epilepsy in children with tuberous sclerosis complex: chance of remission and response to antiepileptic drugs. Epilepsia 2015; 56: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 47. Franz DN, Tudor C, Leonard J, et al. Lamotrigine therapy of epilepsy in tuberous sclerosis. Epilepsia 2001; 42: 935–940. [DOI] [PubMed] [Google Scholar]
- 48. Collins JJ, Tudor C, Leonard JM, et al. Levetiracetam as adjunctive antiepileptic therapy for patients with tuberous sclerosis complex: a retrospective open-label trial. J Child Neurol 2006; 21: 53–57. [DOI] [PubMed] [Google Scholar]
- 49. Geffrey AL, Belt OD, Paolini JL, et al. Lacosamide use in the treatment of refractory epilepsy in tuberous sclerosis complex. Epilepsy Res 2015; 112: 72–75. [DOI] [PubMed] [Google Scholar]
- 50. Jennesson M, van Eeghen AM, Caruso PA, et al. Clobazam therapy of refractory epilepsy in tuberous sclerosis complex. Epilepsy Res 2013; 104: 269–274. [DOI] [PubMed] [Google Scholar]
- 51. Willems LM, Bertsche A, Bösebeck F, et al. Efficacy, retention, and tolerability of brivaracetam in patients with epileptic encephalopathies: a multicenter cohort study from Germany. Front Neurol 2018; 9: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chang FM, Fan PC, Weng WC, et al. The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure 2020; 75: 82–86. [DOI] [PubMed] [Google Scholar]
- 53. van der Poest, Clement E, Jansen FE, Braun KPJ, et al. Update on drug management of refractory epilepsy in tuberous sclerosis complex. Paediatr Drugs 2020; 22: 73–84. [DOI] [PubMed] [Google Scholar]
- 54. Málaga I, Sánchez-Carpintero R, Roldán S, et al. New anti-epileptic drugs in paediatrics. Anales de Pediatría (English Edition) 2019; 91: 415.e411. [DOI] [PubMed] [Google Scholar]
- 55. SABRIL®. Summary of product characteristics, https://www.medicines.org.uk/emc/product/4279/smpc#gref (accessed January 2021).
- 56. Kluger G, Berz K, Holthausen H. The long-term use of vigabatrin and lamotrigine in patients with severe childhood onset epilepsy. Eur J Paediatr Neurol 2001; 5: 37–40. [DOI] [PubMed] [Google Scholar]
- 57. Neal MJ, Shah MA. Development of tolerance to the effects of vigabatrin (gamma-vinyl-GABA) on GABA release from rat cerebral cortex, spinal cord and retina. Br J Pharmacol 1990; 100: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hussain SA, Schmid E, Peters JM, et al. High vigabatrin dosage is associated with lower risk of infantile spasms relapse among children with tuberous sclerosis complex. Epilepsy Res 2018; 148: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Welin KO, Carlqvist P, Svensson A, et al. Epilepsy in tuberous sclerosis patients in Sweden - Healthcare utilization, treatment, morbidity, and mortality using national register data. Seizure 2017; 53: 4–9. [DOI] [PubMed] [Google Scholar]
- 60. Strzelczyk A, Grau J, Bast T, et al. Prescription patterns of antiseizure drugs in tuberous sclerosis complex (TSC)-associated epilepsy: a multicenter cohort study from Germany and review of the literature. Expert Rev Clin Pharmacol 2021; 14: 749–760. [DOI] [PubMed] [Google Scholar]
- 61. Kotulska K, Kwiatkowski DJ, Curatolo P, et al. Prevention of epilepsy in infants with tuberous sclerosis complex in the EPISTOP trial. Ann Neurol 2021; 89(2): 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jóźwiak S, Kotulska K, Domańska-Pakieła D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol 2011; 15: 424–431. [DOI] [PubMed] [Google Scholar]
- 63. Jóźwiak S, Słowińska M, Borkowska J, et al. Preventive antiepileptic treatment in tuberous sclerosis complex: a long-term, prospective trial. Pediatr Neurol 2019; 101: 18–25. [DOI] [PubMed] [Google Scholar]
- 64. ClinicalTrials.gov. NCT02849457: preventing epilepsy using vigabatrin in infants with tuberous sclerosis complex, https://clinicaltrials.gov/ct2/show/NCT02849457 (accessed December 2020).
- 65. Nissenkorn A, Tzadok M, Bar-Yosef O, et al. Treatment with brivaracetam in children - The experience of a pediatric epilepsy center. Epilepsy Behav 2019; 101: 106541. [DOI] [PubMed] [Google Scholar]
- 66. Villanueva V, López-González FJ, Mauri JA, et al. BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurol Scand 2019; 139: 360–368. [DOI] [PubMed] [Google Scholar]
- 67. Lechuga L, Franz DN. Everolimus as adjunctive therapy for tuberous sclerosis complex-associated partial-onset seizures. Expert Rev Neurother 2019; 19: 913–925. [DOI] [PubMed] [Google Scholar]
- 68. VOTUBIA®. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/votubia-epar-product-information_en.pdf (accessed November 2020).
- 69. AFINITOR® /AFINITOR DISPERZ®. Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022334s040,203985s013lbl.pdf (accessed November 2020).
- 70. Overwater IE, Rietman AB, Bindels-de Heus K, et al. Sirolimus for epilepsy in children with tuberous sclerosis complex: a randomized controlled trial. Neurology 2016; 87: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 71. French JA, Lawson JA, Yapici Z, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016; 388: 2153–2163. [DOI] [PubMed] [Google Scholar]
- 72. Curatolo P, Franz DN, Lawson JA, et al. Adjunctive everolimus for children and adolescents with treatment-refractory seizures associated with tuberous sclerosis complex: post-hoc analysis of the phase 3 EXIST-3 trial. Lancet Child Adolesc Health 2018; 2: 495–504. [DOI] [PubMed] [Google Scholar]
- 73. Mizuguchi M, Ikeda H, Kagitani-Shimono K, et al. Everolimus for epilepsy and autism spectrum disorder in tuberous sclerosis complex: EXIST-3 substudy in Japan. Brain Dev 2019; 41: 1–10. [DOI] [PubMed] [Google Scholar]
- 74. de Vries PJ, Franz DN, Curatolo P, et al. Measuring health-related quality of life in tuberous sclerosis complex – psychometric evaluation of three instruments in individuals with refractory epilepsy. Front Pharmacol 2018; 9: 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Franz DN, Lawson JA, Yapici Z, et al. Everolimus for treatment-refractory seizures in TSC: extension of a randomized controlled trial. Neurol Clin Pract 2018; 8: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thiele EA, Bebin EM, Bhathal H, et al. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: a placebo-controlled randomized clinical trial. JAMA Neurol 2020: e204607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thiele E, Bebin EM, Filloux F, et al. Long-term safety and efficacy of cannabidiol (CBD) for the treatment of seizures in patients with tuberous sclerosis complex (TSC) in an open-label extension (OLE) trial (GWPCARE6). Neurology 2020; 94: 677. [Google Scholar]
- 78. Kadish NE, Riedel C, Stephani U, et al. Developmental outcomes in children/adolescents and one adult with tuberous sclerosis complex (TSC) and refractory epilepsy treated with everolimus. Epilepsy Behav 2020; 111: 107182. [DOI] [PubMed] [Google Scholar]
- 79. Kilincaslan A, Kok BE, Tekturk P, et al. Beneficial effects of everolimus on autism and attention-deficit/hyperactivity disorder symptoms in a group of patients with tuberous sclerosis complex. J Child Adolesc Psychopharmacol 2017; 27: 383–388. [DOI] [PubMed] [Google Scholar]
- 80. Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol 2013; 74: 679–687. [DOI] [PubMed] [Google Scholar]
- 81. Krueger DA, Wilfong AA, Mays M, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology 2016; 87: 2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saffari A, Brösse I, Wiemer-Kruel A, et al. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age - a multicenter retrospective study. Orphanet J Rare Dis 2019; 14: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Samueli S, Abraham K, Dressler A, et al. Efficacy and safety of everolimus in children with TSC - associated epilepsy - pilot data from an open single-center prospective study. Orphanet J Rare Dis 2016; 11: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Svarrer EMM, Fischer CM, Frederiksen MG, et al. Everolimus as adjunctive treatment in tuberous sclerosis complex-associated epilepsy in children. Dan Med J 2019; 66: A5582. [PubMed] [Google Scholar]
- 85. Wiegand G, May TW, Ostertag P, et al. Everolimus in tuberous sclerosis patients with intractable epilepsy: a treatment option? Eur J Paediatr Neurol 2013; 17: 631–638. [DOI] [PubMed] [Google Scholar]
- 86. Stockinger J, Strzelczyk A, Nemecek A, et al. Everolimus in adult tuberous sclerosis complex patients with epilepsy: too late for success? A retrospective study. Epilepsia 2021; 62(3): 785–794. [DOI] [PubMed] [Google Scholar]
- 87. Krueger DA, Sadhwani A, Byars AW, et al. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann Clin Transl Neurol 2017; 4: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Overwater IE, Rietman AB, Mous SE, et al. A randomized controlled trial with everolimus for IQ and autism in tuberous sclerosis complex. Neurology 2019; 93: e200–e209. [DOI] [PubMed] [Google Scholar]
- 89. Willems LM, Rosenow F, Schubert-Bast S, et al. Efficacy, retention and tolerability of everolimus in patients with tuberous sclerosis complex: A survey-based study on patients’ perspectives. CNS Drugs 2021; DOI: 10.1007/s40263-021-00839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ebrahimi-Fakhari D, Agricola KD, Tudor C, et al. Cannabidiol elevates mechanistic target of rapamycin inhibitor levels in patients with tuberous sclerosis complex. Pediatr Neurol 2020; 105: 59–61. [DOI] [PubMed] [Google Scholar]
- 91. Wiemer-Kruel A, Stiller B, Bast T. Cannabidiol interacts significantly with everolimus-report of a patient with tuberous sclerosis complex. Neuropediatrics 2019; 50: 400–403. [DOI] [PubMed] [Google Scholar]
- 92. Nichol K, Stott C, Jones N, et al. The proposed multimodal mechanism of action of cannabidiol (CBD) in epilepsy: modulation of intracellular calcium and adenosine-mediated signaling (P5.5-007). Neurology 2019; 92: P5.5-007. [Google Scholar]
- 93. Giacoppo S, Pollastro F, Grassi G, et al. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017; 116: 77–84. [DOI] [PubMed] [Google Scholar]
- 94. Renard J, Loureiro M, Rosen LG, et al. Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J Neurosci 2016; 36: 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kalenderoglou N, Macpherson T, Wright KL. Cannabidiol reduces leukemic cell size – but is it important? Front Pharmacol 2017; 8: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther 2011; 10: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 97. Gugliandolo A, Pollastro F, Bramanti P, et al. Cannabidiol exerts protective effects in an in vitro model of Parkinson's disease activating AKT/mTOR pathway. Fitoterapia 2020; 143: 104553. [DOI] [PubMed] [Google Scholar]
- 98. Barnett JR, Grinspoon RA, Harisinghani M, et al. The efficacy of cannabidiol on renal angiomyolipoma and subependymal giant cell tumor volume in tuberous sclerosis complex. J Clin Neurosci 2020; 77: 85–88. [DOI] [PubMed] [Google Scholar]
- 99. EPIDIOLEX®. Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210365s005s006s007lbl.pdf (accessed November 2020).
- 100. Epidyolex. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/epidyolex-epar-product-information_en.pdf (accessed June 2021 2021).
- 101. Wu J, Cock H, Devinsky O, et al. Time to onset of cannabidiol (CBD) treatment effect and resolution of adverse events (AEs) in the tuberous sclerosis complex (TSC) phase 3 randomized controlled trial (GWPCARE6). Neurology 2020; 94: 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. D’Onofrio G, Kuchenbuch M, Hachon-Le Camus C, et al. Slow titration of cannabidiol add-on in drug-resistant epilepsies can improve safety with maintained efficacy in an open-label study. Front Neurol 2020; 11: 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chang BS. Cannabidiol and serum antiepileptic drug levels: the ABCs of CBD with AEDs. Epilepsy Curr 2018; 18: 33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Klotz KA, Hirsch M, Heers M, et al. Effects of cannabidiol on brivaracetam plasma levels. Epilepsia 2019; 60: e74–e77. [DOI] [PubMed] [Google Scholar]
- 105. Augustin K, Khabbush A, Williams S, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol 2018; 17: 84–93. [DOI] [PubMed] [Google Scholar]
- 106. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 2017; 30: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chang P, Augustin K, Boddum K, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016; 139: 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Simeone TA, Matthews SA, Samson KK, et al. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol 2017; 287: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Warren EC, Dooves S, Lugarà E, et al. Decanoic acid inhibits mTORC1 activity independent of glucose and insulin signaling. Proc Natl Acad Sci USA 2020; 117: 23617–23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McDaniel SS, Rensing NR, Thio LL, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011; 52: e7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Singh A, Mettler T, Oh H, et al. The ketogenic diet attenuates both hyperactivity in mTOR pathway and astrogliosis through regulation of AMPK signaling in the epileptic brain. FASEB J 2018; 32: 805–811. [Google Scholar]
- 112. Kossoff EH, Thiele EA, Pfeifer HH, et al. Tuberous sclerosis complex and the ketogenic diet. Epilepsia 2005; 46: 1684–1686. [DOI] [PubMed] [Google Scholar]
- 113. Park S, Lee EJ, Eom S, et al. Ketogenic diet for the management of epilepsy associated with tuberous sclerosis complex in children. J Epilepsy Res 2017; 7: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Youn SE, Park S, Kim SH, et al. Long-term outcomes of ketogenic diet in patients with tuberous sclerosis complex-derived epilepsy. Epilepsy Res 2020; 164: 106348. [DOI] [PubMed] [Google Scholar]
- 115. Kossoff EH, Zupec-Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018; 3: 175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dressler A, Trimmel-Schwahofer P. The ketogenic diet for infants: how long can you go? Epilepsy Res 2020; 164: 106339. [DOI] [PubMed] [Google Scholar]
- 117. Elliott RE, Carlson C, Kalhorn SP, et al. Refractory epilepsy in tuberous sclerosis: vagus nerve stimulation with or without subsequent resective surgery. Epilepsy Behav 2009; 16: 454–460. [DOI] [PubMed] [Google Scholar]
- 118. Major P, Thiele EA. Vagus nerve stimulation for intractable epilepsy in tuberous sclerosis complex. Epilepsy Behav 2008; 13: 357–360. [DOI] [PubMed] [Google Scholar]
- 119. Parain D, Penniello MJ, Berquen P, et al. Vagal nerve stimulation in tuberous sclerosis complex patients. Pediatr Neurol 2001; 25: 213–216. [DOI] [PubMed] [Google Scholar]
- 120. Zamponi N, Petrelli C, Passamonti C, et al. Vagus nerve stimulation for refractory epilepsy in tuberous sclerosis. Pediatr Neurol 2010; 43: 29–34. [DOI] [PubMed] [Google Scholar]
- 121. Colicchio G, Montano N, Fuggetta F, et al. Vagus nerve stimulation in drug-resistant epilepsies. Analysis of potential prognostic factors in a cohort of patients with long-term follow-up. Acta Neurochir (Wien) 2012; 154: 2237–2240. [DOI] [PubMed] [Google Scholar]
- 122. Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 2011; 115: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 123. Rosenow F, Bast T, Czech T, et al. Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the Austrian, German, and Swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment. Epilepsia 2016; 57: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 124. Jansen FE, van Huffelen AC, Algra A, et al. Epilepsy surgery in tuberous sclerosis: a systematic review. Epilepsia 2007; 48: 1477–1484. [DOI] [PubMed] [Google Scholar]
- 125. Liang S, Zhang J, Yang Z, et al. Long-term outcomes of epilepsy surgery in tuberous sclerosis complex. J Neurol 2017; 264: 1146–1154. [DOI] [PubMed] [Google Scholar]
- 126. Liu S, Yu T, Guan Y, et al. Resective epilepsy surgery in tuberous sclerosis complex: a nationwide multicentre retrospective study from China. Brain 2020; 143: 570–581. [DOI] [PubMed] [Google Scholar]
- 127. Bjellvi J, Flink R, Rydenhag B, et al. Complications of epilepsy surgery in Sweden 1996-2010: a prospective, population-based study. J Neurosurg 2015; 122: 519–525. [DOI] [PubMed] [Google Scholar]
- 128. d’Orio P, Rizzi M, Mariani V, et al. Surgery in patients with childhood-onset epilepsy: analysis of complications and predictive risk factors for a severely complicated course. J Neurol Neurosurg Psychiatry 2019; 90: 84–89. [DOI] [PubMed] [Google Scholar]
- 129. Treiber JM, Curry DJ, Weiner HL, et al. Epilepsy surgery in tuberous sclerosis complex (TSC): emerging techniques and redefinition of treatment goals. Childs Nerv Syst 2020; 36: 2519–2525. [DOI] [PubMed] [Google Scholar]
- 130. Youngerman BE, Save AV, McKhann GM. Magnetic resonance imaging-guided laser interstitial thermal therapy for epilepsy: systematic review of technique, indications, and outcomes. Neurosurgery 2020; 86: E366–E382. [DOI] [PubMed] [Google Scholar]
- 131. Stellon MA, Cobourn K, Whitehead MT, et al. “Laser and the Tuber”: thermal dynamic and volumetric factors influencing seizure outcomes in pediatric subjects with tuberous sclerosis undergoing stereoencephalography-directed laser ablation of tubers. Childs Nerv Syst 2019; 35: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 132. Tovar-Spinoza Z, Ziechmann R, Zyck S. Single and staged laser interstitial thermal therapy ablation for cortical tubers causing refractory epilepsy in pediatric patients. Neurosurg Focus 2018; 45: E9. [DOI] [PubMed] [Google Scholar]
- 133. Hooten KG, Werner K, Mikati MA, et al. MRI-guided laser interstitial thermal therapy in an infant with tuberous sclerosis: technical case report. J Neurosurg Pediatr 2018; 23: 92–97. [DOI] [PubMed] [Google Scholar]
- 134. Marinus Pharmaceuticals. CDKL5 deficiency disorder (CDD) phase 3 topline data results, https://ir.marinuspharma.com/events-and-presentations/event-details/2020/CDKL5-Deficiency-Disorder-CDD-Phase-3-Topline-Data-Results/default.aspx (2020, accessed December 2020).
- 135. ClinicalTrials.gov. NCT04285346: adjunctive ganaxolone treatment (Part A) in TSC followed by long-term treatment (Part B) (TSC), https://clinicaltrials.gov/ct2/show/NCT04285346 (accessed December 2020).
- 136. Takeda. Phase 2 ELEKTRA study of soticlestat (TAK-935/OV935) meets primary endpoint reducing seizure frequency in children with Dravet syndrome or Lennox-Gastaut syndrome, http://www.takeda.com/newsroom/newsreleases/2020/phase-2-elektra-study-of-soticlestat-tak-935ov935-meets-primary-endpoint-reducing-seizure-frequency-in-children-with-dravet-syndrome-or-lennox-gastaut-syndrome/ (2020, accessed August 2020).
- 137. Strzelczyk A, Schubert-Bast S. Therapeutic advances in Dravet syndrome: a targeted literature review. Expert Rev Neurother 2020: 20(10): 1065–1079. [DOI] [PubMed] [Google Scholar]
- 138. Zogenix. Investor update 2019: exploring new potential indications for FINTEPLA®, https://zogenixinc.gcs-web.com/static-files/dafef97d-0dff-49c0-bb33-18bf54b04b61 (2019, accessed December 2020).
- 139. ClinicalTrials.gov. NCT04595513: stopping tsc onset and progression 2: epilepsy prevention in TSC infants (STOP2), https://clinicaltrials.gov/ct2/show/NCT04595513?term=prevention&cond=Tuberous+Sclerosis&draw=2&rank=1 (accessed January 2021).
- 140. Di Nardo A, Lenoël I, Winden KD, et al. Phenotypic screen with TSC-deficient neurons reveals heat-shock machinery as a druggable pathway for mTORC1 and reduced cilia. Cell Rep 2020; 31(12): 107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Prabhakar S, Cheah PS, Zhang X, et al. Long-term therapeutic efficacy of intravenous AAV-mediated hamartin replacement in mouse model of tuberous sclerosis type 1. Mol Ther Methods Clin Dev 2019; 15: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci 2003; 28: 573–576. [DOI] [PubMed] [Google Scholar]
- 143. Friedman D, French JA, Maccarrone M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol 2019; 18: 504–512. [DOI] [PubMed] [Google Scholar]
- 144. Kim SH, Shaw A, Blackford R, et al. The ketogenic diet in children 3 years of age or younger: a 10-year single-center experience. Sci Rep 2019; 9: 8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dressler A, Trimmel-Schwahofer P, Reithofer E, et al. The ketogenic diet in infants—advantages of early use. Epilepsy Res 2015; 116: 53–58. [DOI] [PubMed] [Google Scholar]
- 146. Lyons L, Schoeler NE, Langan D, et al. Use of ketogenic diet therapy in infants with epilepsy: a systematic review and meta-analysis. Epilepsia 2020; 61: 1261–1281. [DOI] [PubMed] [Google Scholar]
- 147. Schubert-Bast S, Rosenow F, Klein KM, et al. The role of mTOR inhibitors in preventing epileptogenesis in patients with TSC: current evidence and future perspectives. Epilepsy Behav 2019; 91: 94–98. [DOI] [PubMed] [Google Scholar]
- 148. Anderl S, Freeland M, Kwiatkowski DJ, et al. Therapeutic value of prenatal rapamycin treatment in a mouse brain model of tuberous sclerosis complex. Hum Mol Genet 2011; 20: 4597–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Way SW, Rozas NS, Wu HC, et al. The differential effects of prenatal and/or postnatal rapamycin on neurodevelopmental defects and cognition in a neuroglial mouse model of tuberous sclerosis complex. Hum Mol Genet 2012; 21: 3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Curatolo P, Aronica E, Jansen A, et al. Early onset epileptic encephalopathy or genetically determined encephalopathy with early onset epilepsy? Lessons learned from TSC. Eur J Paediatr Neurol 2016; 20: 203–211. [DOI] [PubMed] [Google Scholar]
- 151. Curatolo P, Bjørnvold M, Dill PE, et al. The role of mTOR inhibitors in the treatment of patients with tuberous sclerosis complex: evidence-based and expert opinions. Drugs 2016; 76: 551–565. [DOI] [PubMed] [Google Scholar]
- 152. Moavero R, Marciano S, Graziola F, et al. Combined targeted treatment in early onset epilepsy associated with tuberous sclerosis. Epilepsy Behav Case Rep 2016; 5: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211031100 for Review of the treatment options for epilepsy in tuberous sclerosis complex: towards precision medicine by Susanne Schubert-Bast and Adam Strzelczyk in Therapeutic Advances in Neurological Disorders