Abstract
Background and aims:
Hyperbaric oxygenation therapy has been used in the treatment of ulcerative colitis in the past few years. However, its efficacy still remains unclear. The aim of the study was to investigate the efficacy of hyperbaric oxygen combination therapy in patients with ulcerative colitis.
Methods:
We conducted a comprehensive study search up to September 2020, from the online databases Embase, PubMed, Cochrane Library, China National Knowledge Infrastructure, WanFang and VIP.
Results:
Thirteen studies comprising 780 patients were included. We found that compared with conventional therapy, hyperbaric oxygen combination therapy was superior in reaching clinical remission [risk ratio (RR)=1.62; 95% confidence interval (CI) 1.42 to 1.84; p < 0.001] and clinical response (RR=1.29; 95% CI 1.21 to 1.38; p < 0.001), with lower disease activity scores [standard mean difference (SMD)= −1.19; 95%CI −1.74 to −0.65; p < 0.001]. An obvious reduction of serum levels of tumor necrosis factor-α (SMD= −1.96; 95%CI −2.50 to −1.41; p < 0.001) and interleukin (IL)-6 (SMD= −2.49; 95% CI −2.84 to −2.15; p < 0.001), and elevation of IL-10 level (SMD=2.40; 95% CI 0.68 to 4.12; p = 0.006) were also observed.
Conclusion:
Hyperbaric oxygen combination therapy was effective in patients with ulcerative colitis, and has potential as a complementary method for its treatment.
Keywords: hyperbaric oxygen, ulcerative colitis, meta-analysis
Introduction
Ulcerative colitis (UC) has emerged as a public health challenge worldwide in the past decade, 1 with the highest prevalence of UC being 505 per 100,000 reported in Norway. 2 The main clinical presentations of UC include bloody mucus in stool, diarrhea, and abdominal pain. While the majority of patients with UC have a mild-to-moderate course, approximately 10−15% of patients suffer from a severe disease course. 3 Therapies include the administration of 5-aminosalicylic acid, corticosteroids, immunosuppressants, and biologics. Some of them have many adverse effects, including infections, malignancy, liver toxicity, myelosuppression et cetera, and some of these are quite expensive.4–6 Although patients can be rescued by surgery, it has a 5% post-operative mortality risk when emergency surgery is performed. 7 Thus, new strategies with fewer adverse effects and lower costs are needed.
Hyperbaric oxygenation therapy (HBOT) is a type of treatment in which people breathe 100% oxygen under a pressure two or three times higher than normal atmospheric pressure at sea level. This therapy increases the oxygen dissolved in blood and causes hyper oxygenation in tissues, which can bring about physiological and biochemical effects. 8 HBOT has been widely used as a treatment in several diseases such as diabetic foot ulcers, radiation tissue injury, and chronic wounds.8–10
Several studies have reported the use of HBOT in the treatment of inflammatory bowel disease; however, most of them are case reports. Whether HBOT has a definitive therapeutic effect in patients with UC remains controversial. Recently, several high-quality randomized controlled trials (RCTs) have been published. This meta-analysis was conducted to examine the efficacy of HBOT in UC based on RCTs and provide evidence for the clinical use of HBOT in UC patients.
Methods
Search strategy and selection criteria
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. 11 We conducted this comprehensive study by searching up to September 2020 the online databases of Embase, Cochrane Library, PubMed, China National Knowledge Infrastructure, WanFang and VIP. Manuscripts written in English and Chinese were included. The following search strategy combining free-text words and MeSH terms was applied in PubMed: (colitis ulcerative[Mesh terms] or Idiopathic Proctocolitis[Title/Abstract] or Ulcerative Colitis[Title/Abstract] or Colitis Gravis[Title/Abstract] or Inflammatory Bowel Disease, Ulcerative Colitis Type[Title/Abstract]) and (Hyperbaric Oxygenation[Mesh terms] or Hyperbaric Oxygenations[Title/Abstract] or Oxygenations, Hyperbaric[Title/Abstract] or Hyperbaric Oxygen Therapy[Title/Abstract] or Hyperbaric Oxygen Therapies[Title/Abstract] or Oxygen Therapies, Hyperbaric[Title/Abstract] or Oxygen Therapy, Hyperbaric[Title/Abstract] or Therapies, Hyperbaric Oxygen[Title/Abstract] or Therapy, Hyperbaric Oxygen[Title/Abstract] or Oxygenation, Hyperbaric[Title/Abstract]). The most recent or most complete study was chosen when several publications reported findings for the same patients. The searching strategy in other databases is introduced in the supplemental material.
Inclusion and exclusion criteria
The following inclusion criteria were used: 1) the study was a randomized controlled trial; 2) study in which patients were diagnosed with UC; 3) study in which hyperbaric oxygenation was used in the intervention group; and 4) at least one outcome was reported in the study.
The following exclusion criteria were used: 1) observational studies, retrospective studies, reviews, case reports, letters, animal trials, and meeting abstracts; 2) studies without full text available or sufficient data for calculation; and 3) studies in which patients were not treated with standard therapy recommended in guidelines, such as Chinese herb intake.
Literature search and selection were conducted by two independent investigators (Pingrun Chen and Yina Li). Any disagreement was resolved by discussion until a consensus was reached.
Data extraction
We extracted the following data: name of the first author, publication year, country, total number of participants, age, sex, treatments, trial duration, clinical outcomes, and adverse events. The primary clinical outcome was clinical remission, and the secondary outcomes included clinical response, disease activity scores, and laboratory test results. Clinical remission or response was identified by the respective article authors (Table 1). Disease activity scores were used to evaluate the severity of UC, and were identified by the article author. Results of laboratory tests included changes in the serum levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-10, and superoxide dismutase (SOD). For the article that reported several outcomes based on different time points, we extracted data at only the longest time point. Adverse effects were also evaluated if mentioned by the authors. Two reviewers (Pingrun Chen and Yina Li) independently completed this period. Cochrane risk of bias tool was used to assess the risk of bias for each study based on six bias domains. 12
Table 1.
Characteristics of the included studies.
| Study | Location | Number | Men (%) | Mean age (years) | Inclusion and exclusion criteria |
|---|---|---|---|---|---|
| Liang 13 | China | 30 | 50 | 37.8 | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Shen 14 | China | 25 | 68 | 34.6 | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Xu 15 | China | 36 | 33.3 | 31 | Inclusion criteria: UC patients; exclusion criteria: NA. The author described that included patients all had abdominal pain, diarrhea. |
| Wang 16 | China | 70 | 52.8 | 32.9 | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Yin 17 | China | 94 | NA | NA | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Nie 18 | China | 138 | NA | NA | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Wang 19 | China | 60 | 33.3 | 31 | Inclusion criteria: UC patients; exclusion criteria: NA. The author described that included patients all had abdominal pain, diarrhea. |
| Zhan and Peng 20 | China | 30 | NA | NA | Inclusion criteria: UC patients; exclusion criteria: NA. |
| Huang and Cao 21 | China | 78 | 52.6 | 39.6 | Inclusion criteria: UC patients, 20−65 years old; exclusion criteria: accompanied by other severe diseases or infectious enteritis, pregnancy, lactation, treated with drugs other than mesalazine one month before study. |
| Dulai et al. 22 | USA | 18 | 50 | 47 (median, intervention group), 31 (median, control group) | Inclusion criteria: UC patients, 18 years or older, moderate to severe UC flare (full Mayo score ⩾6, AND Mayo endoscopic sub-score of 2 or 3), high risk of failing intravenous steroids and needing second-line therapy during hospitalization; exclusion criteria: requiring urgent surgical intervention, HBOT contraindications, intravenous steroids >48 h prior to study |
| Zhong et al. 23 | China | 50 | 56 | 41.7 | Inclusion criteria: UC patients, 18−65 years old, without immunosuppressants or corticosteroids one month prior to study; exclusion criteria: accompanied by other immune disease, other severe diseases, intestinal infection or tumor, allergic to mesalazine, psychiatric disease, pregnancy, lactation |
| Dulai et al. 24 | USA | 11 | 50 (total 20 patients) | 37 (total 20 patients) | Inclusion criteria: UC patients, 18 years or older, moderate to severe UC flare (full Mayo score ⩾6, AND Mayo endoscopic sub-score of 2 or 3), high risk of failing intravenous steroids and needing second-line therapy during hospitalization; exclusion criteria: requiring urgent surgical intervention, contraindication or intolerance to steroid use or any medical condition, HBOT contraindications, intravenous steroids >48 h prior to study |
| Wang and Ma 25 | China | 140 | 47.9 | 40.1 | Inclusion criteria: UC patients; exclusion criteria: accompanied by infectious diarrhea or other severe diseases, treated with corticosteroids, lactation, pregnancy |
HBOT, hyperbaric oxygenation therapy; NA, not accessible; UC, ulcerative colitis.
Statistical analysis
RevMan 5.4.1 and Stata 12.1 software packages were used for the analysis. Discontinuous outcomes including clinical remission and clinical response were characterized by the risk ratio (RR) and 95% confidence interval (CI). Continuous data, including disease activity scores and laboratory test results, were characterized by weighted mean difference (WMD), standardized mean difference (SMD), and 95% CI. Cochrane Q and I2 statistics were calculated to assess the heterogeneity between studies. When significant heterogeneity was observed (p < 0.01 and/or I2>50%), we used a random-effect model; otherwise, the fixed-effect model was applied. We also conducted sensitivity analyses to check the stability of the pooled results. Publication bias was assessed by a funnel plot using the Begg and Egger tests, and significant publication bias was defined as a p-value < 0.05. The trim-and-fill method was applied to estimate the corrected effect size after adjustment for publication bias when it was observed. A two-sided p-value < 0.05 denoted a statistical difference.
Result
Literature search and selection
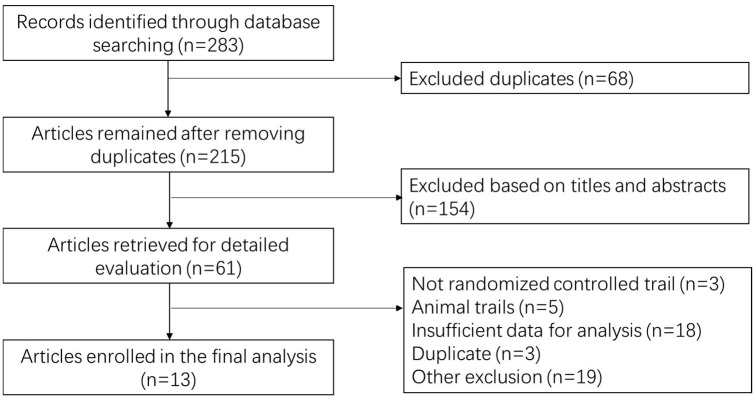
As shown in Figure 1, 283 articles were recorded from databases using the method mentioned above. After removing 68 duplicated records, 215 studies were further screened on the basis of title and abstract. Thirteen studies were finally included for statistical analysis.13–25
Figure 1.
Flow diagram of literature review.
Characteristics of the articles
All included studies were RCTs. A total of 780 patients were included with 397 patients in the intervention group (Tables 1 and 2). The sample sizes ranged from 11 to 140 among these studies. Two studies included patients with moderate to severe flares, while the others included patients without restriction on disease flares. Twelve studies reported the primary and secondary outcomes (clinical response), and only three studies reported disease activity scores (two studies using partial Mayo scores and one study using DAI according to Sutherland and Martin). 26 Results of inflammatory cytokines (TNF-α, IL-6, IL-10, and SOD) were less reported. Only four studies reported changes in TNF-α levels: two reported changes in IL-10 and SOD and two studies reported changes in IL-6 levels. The intervention method was HBOT combined with standard conventional therapy in all studies, and the control method was conventional therapy, except for two studies hosted by Dulai et al.22,24 In these two studies, one study used a sham HBOT therapy combined with conventional therapy as a control protocol. 22 Another study reported by Dulai compared the possible effects of different sessions of HBOT on UC. In the latter, all 20 enrolled patients received HBOT combined with standard therapy, and 11 patients underwent randomization to receive different sessions of HBOT (5-day treatment vs. 3-day treatment). 24 The percentage of males and the age of participants in this study are listed in Table 1, representing all enrolled 20 patients.
Table 2.
Intervention methods, control methods, and outcomes of included trials.
| Study | Intervention group | Outcomes and definition | Outcomes and definitions | Evaluation timepoints | |||
|---|---|---|---|---|---|---|---|
| Style | Sessions of HBOT | Patients enrolled | Style | Patients enrolled | |||
| Liang 13 | HBOT + ST | 24 | 15 (seven mild, eight moderate) | ST | 15 (eight mild, seven moderate) | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, mild inflammation under endoscopy) | Before and after treatment |
| Shen 14 | HBOT + ST | 20−30 | Eight (three mild, four moderate, one severe) | ST | 17 (six mild, 10 moderate, one severe) | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, mild inflammation under endoscopy) | Before and after treatment |
| Xu 15 | HBOT + ST | 36 | 21 | ST | 15 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, mild inflammation under endoscopy), disease activity score (measured using DAI according to Sutherland and Martin 26 ) | Before and after treatment |
| Wang 16 | HBOT + ST | 30 | 36 | ST | 34 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as improvement of clinical symptoms and endoscopic findings) | Before and after treatment |
| Yin 17 | HBOT + ST | 28 | 48 | ST | 46 | Clinical remission (defined as disappearance of clinical symptoms, daily stool frequencies lower than twice, no red or white cells in feces, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, daily stool frequencies lower than four times, fecal red or white cells lower than 10 under high power lens, mild inflammation under endoscopy), results of laboratory test (TNF-α, IL-6) | Before and after treatment |
| Nie 18 | HBOT + ST | 28 | 73 | ST | 65 | Clinical remission (defined as disappearance of clinical symptoms, daily stool frequencies lower than twice, no red or white cells in feces, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, daily stool frequencies lower than four times, fecal red or white cells lower than 10 under high power lens, mild inflammation under endoscopy), expression of cytokines (TNF-α, IL-6) | Before and after treatment |
| Wang 19 | HBOT + ST | 40 | 30 | ST | 30 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as improvement of clinical symptoms and endoscopic findings) | Before and after treatment |
| Zhan and Peng 20 | HBOT + ST | 30 | 15 | ST | 15 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as improvement of clinical symptoms and endoscopic findings) | Before and after treatment |
| Huang and Cao 21 | HBOT + ST | 28 | 40 | ST | 38 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as no abdominal pain, no loose stool with daily frequencies between two to four times, mild inflammation under endoscopy), expression of cytokines (TNF-α, IL-10, SOD) | Before and after treatment |
| Dulai et al. 22 | HBOT + ST | 10 | 10 | Sham HBOT + ST | 8 | Clinical remission (defined as a partial Mayo score of ⩽2 points with no individual sub-score exceeding one point), clinical response (defined as a decrease in partial Mayo score of ⩾2 points with an absolute rectal bleeding sub-score of 0 or 1), disease activity score (measured using partial Mayo score) | Before treatment, and day 3, day 5, day 10 during treatment |
| Zhong et al. 23 | HBOT + ST | 28 | 25 | ST | 25 | Clinical remission (defined as disappearance of clinical symptoms, no positive findings under endoscopy), clinical response (defined as no abdominal pain, no loose stool with daily frequencies between two to four times, mild inflammation under endoscopy), expression of cytokines (TNF-α, IL-10, SOD) | Before and after treatment |
| Dulai et al. 24 | HBOT (five sessions) + ST | 5 | 6 | HBOT (three sessions) + ST | 5 | Disease activity score (measured using partial Mayo score) | Before treatment, and day 3, day 5, day 10 during treatment |
| Wang and Ma 25 | HBOT + ST | 60 | 70 | ST | 70 | Clinical remission (defined as disappearance of clinical symptoms, normal routine stool examination, no positive findings under endoscopy), clinical response (defined as basically disappearance of clinical symptoms, stool white blood cells 0–2 under high power lens, stool red blood cell 0–2 under high power lens, mild inflammation under endoscopy) | Before and after treatment |
DAI, disease activity index; HBOT, hyperbaric oxygenation therapy; SOD, superoxide dismutase; ST, standard therapy.
This meta-analysis included some Chinese articles. Zhong et al. 23 included UC patients who did not take corticosteroids or immunosuppressants one month before the trial and the age of these patients was restricted to between 18 and 65 years of age. They excluded patients who had other comorbidities such as immune disease, endocrine disease, intestinal infection, or tumor. Those who were allergic to treatment drugs or were pregnant were also excluded. Wang and Ma 25 excluded patients who were pregnant, and were treated with corticosteroids or accompanied by infectious diarrhea or other severe diseases. Other Chinese studies did not show clear exclusion criteria. Although these Chinese studies did not describe the exact requirement of disease flares, some studies have provided detailed data about the disease severity of their enrolled patients. For example, Liang 13 included 15 patients (seven patients in the intervention group) with mild disease and 15 patients (eight patients in the intervention group) with moderate disease. Shen 14 included nine patients (three patients in the intervention group) with mild disease, 14 patients (four patients in the intervention group) with moderate disease, and two patients (one patient in the intervention group) with severe disease. Some of the other studies roughly described patients’ symptoms, for example, Xu 15 included patients with loose stools or bloody diarrhea 2−3 times a day, and some patients could reach 10 times a day (Table 1). Most Chinese studies evaluated outcomes including endoscopy before and after the treatment, while Dulai et al. 22 set three time points during the trial (day 3, day 5, day 10) (Table 2).
Meta-analysis findings
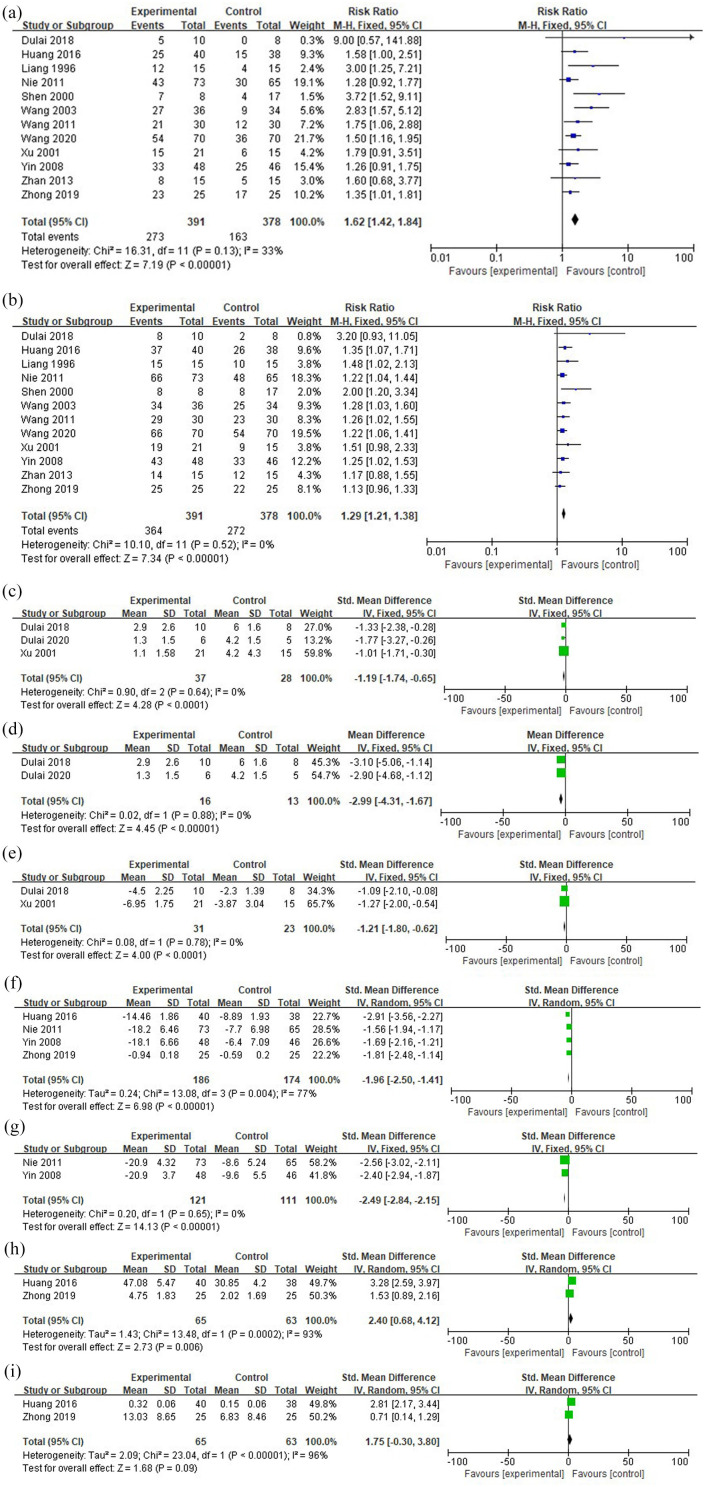
The intervention groups were superior to the control groups in the induction of clinical remission (RR=1.62; 95% CI 1.42 to 1.84; p < 0.001; I2=33%) and clinical response (RR=1.29; 95% CI 1.21 to 1.38; p < 0.001; I2=0%). Furthermore, the intervention groups had significantly lower disease activity scores than the control groups (SMD= −1.19; 95% CI −1.74 to −0.65; p < 0.001; I2=0%) (Figure 2). Comparing two studies reporting partial Mayo scores, intervention groups also had lower scores than the control groups (WMD= −2.99; 95% CI −4.31 to −1.67; p < 0.00001; I2=0%). Changes in disease activity scores before and after the treatment were also evaluated, and these indicated that disease activity scores of the intervention groups dropped more significantly than those of the control groups (SMD= −1.21; 95% CI= −1.80 to −0.62; p < 0.001; I2=0%). Comparing the level of cytokines, HBOT combination therapy significantly decreased the levels of serum TNF-α (SMD= −1.96; 95% CI −2.50 to −1.41; p < 0.001; I2=77%) and IL-6 (SMD= −2.49; 95% CI −2.84 to −2.15; p < 0.001; I2=0%), and increased the levels of serum IL-10 (SMD=2.40; 95% CI 0.68 to 4.12; p = 0.006; I2=93%). However, the changes in SOD were not significant (SMD=1.75; 95% CI −0.30 to 3.80; p = 0.09; I2=96%) (Figure 2).
Figure 2.
Forest plots. (a) Clinical remission. (b) Clinical response. (c) Disease activity scores. (d) Partial Mayo scores. (e) Changes in disease activity scores. (f) Changes in serum TNF-α level. (g) Changes in serum IL-6 level. (h) Changes in serum IL-10 level. (i) Changes in serum superoxide dismutase level.
CI, confidence interval; IV, inverse variance; M-H, Mantel-Haenszel; Std., standardized.
Risk of bias
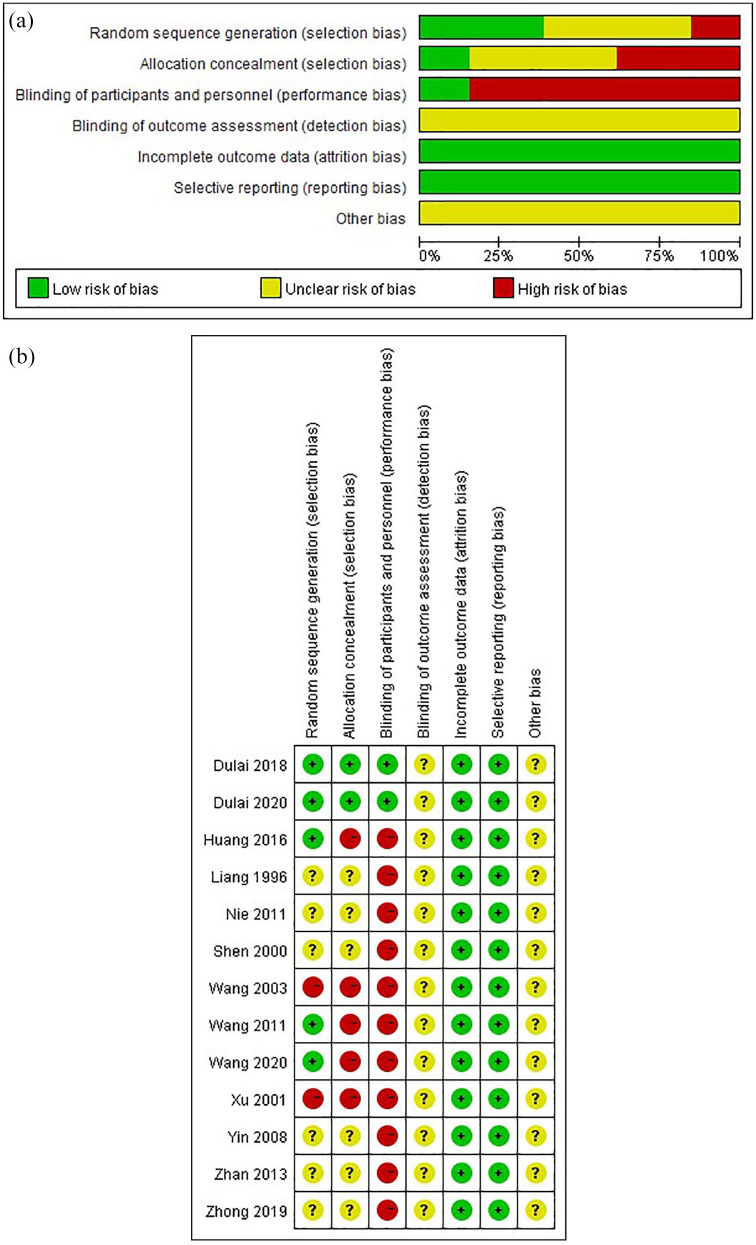
All included studies underwent an evaluation of the risk of bias. The summary of the risk of bias is presented in Figure 3.
Figure 3.
Risk bias of included studies using Cochrane risk of bias tool.
Publication bias and sensitivity analysis
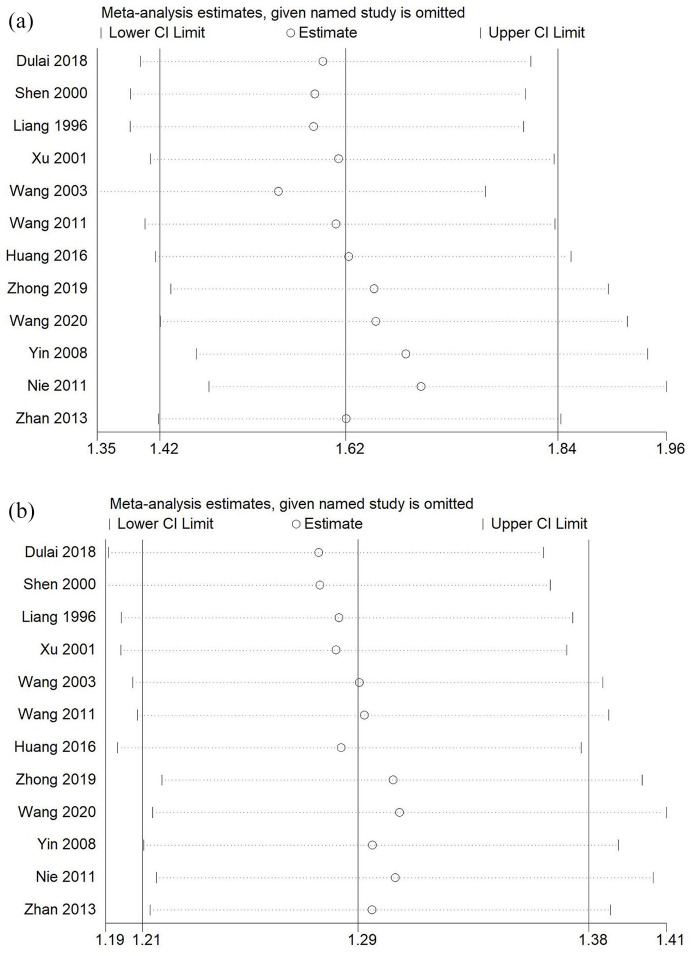
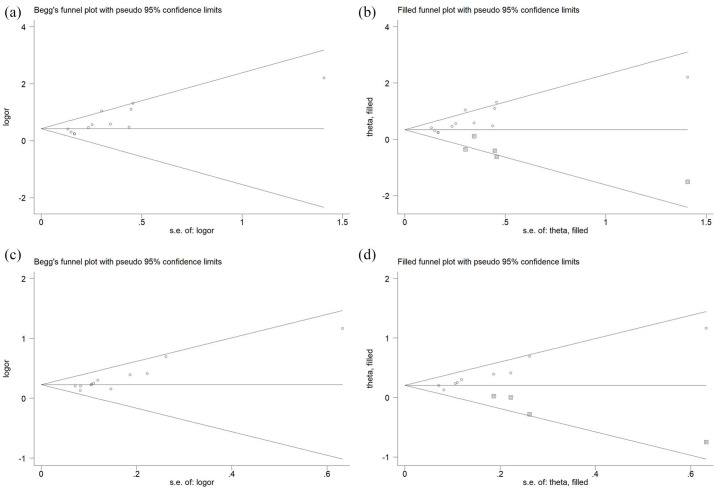
We also conducted a sensitivity analysis for clinical remission and response. We omitted each study in sequence to determine whether doing so had significant influence on the outcomes. No relative change was observed after the removal of each study (Figure 4). Publication bias was found based on the Begg and Egger tests (clinical remission: p = 0.002; clinical response: p < 0.001). Further analysis using trim-and-fill was conducted. Adjusted pooled estimates still indicated the superiority of HBOT combination therapy in reaching clinical response (adjusted RR=1.226; 95% CI 1.152 to 1.305; p < 0.001) and clinical remission (adjusted RR=1.411; 95% CI 1.253 to 1.590; p < 0.001) (Figure 5).
Figure 4.
Sensitivity analysis. (a) Analysis for clinical remission. (b) Analysis for clinical response.
Figure 5.
Publication bias and trim-and-fill method. (a) Publication bias for clinical remission. (b) Trim-and-fill method for clinical remission. (c) Publication bias for clinical response. (d) Trim-and-fill method for clinical response.
CI, confidence interval.
Adverse effect
Regarding safety, no serious adverse effects were reported in the included studies. One patient enrolled in the study hosted by Dulai et al. 22 developed headache during HBOT treatment. However, it was later proved to be related to the use of mesalamine. Liang 13 reported that some patients felt discomfort in their ears, which could be relieved by swallowing. No patient developed claustrophobia or psychological intolerance, vision changes, seizures, or other evidence of oxygen toxicity.
Meta-analysis with applicable data
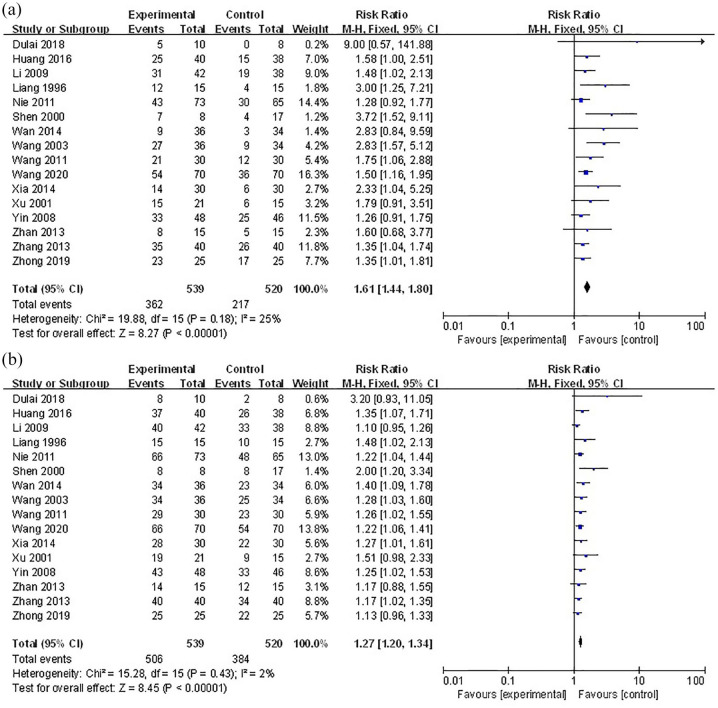
We conducted another meta-analysis based on all applicable data from the RCTs we searched, including those that had been excluded. Another four studies were enrolled in this meta-analysis, and the results showed that patients who underwent HBOT combination therapy performed better in reaching clinical remission (RR=1.61; 95% CI 1.44 to 1.80; p < 0.001; I2=25%) and clinical response (RR=1.27; 95% CI 1.20 to 1.34; p < 0.001; I2=2%) (Figure 6).
Figure 6.
Meta-analysis with all applicable data. (a) Forest plot for clinical remission. (b) Forest plot for clinical response.
The reasons for excluding these four studies varied. For example, Xia 27 described the superiority of HBOT combined with Shenlingbaishu powder compared with sulfasalazine, with a response rate of 93.3% in the intervention group and 73.3% in the control group. We excluded this research because Chinese herbs are not included in conventional drugs. Li and Zhu 28 enrolled 80 patients with UC, and evaluated outcomes every week during the therapy, but did not clearly explain the definitions of outcomes.
Discussion
Our results demonstrated that, compared with conventional therapy, hyperbaric oxygen therapy combined with standard treatment was more effective in achieving clinical remission and response, with lower disease activity scores, and significantly reduced serum levels of TNF-α and IL-6 and elevated IL-10 levels.
No significant heterogeneity was detected among clinical remission, clinical response, disease activity scores, or changes in the serum level of IL-6. Significant heterogeneity was observed when comparing the changes in TNF-α and IL-10 levels. Sensitivity analysis was performed, and no obvious changes in our estimates were found, which indicated the robustness of our results. Publication bias was found in clinical remission and response, but it was proved to have no influence by trim-and-fill method.
Although many case reports have demonstrated the effectiveness of HBOT, it remains controversial whether HBOT is suitable for UC. Pagoldh et al. 29 reported no superiority of HBOT combination therapy compared with standard treatment, which is inconsistent with our results. There may be several reasons to explain the different conclusions. First, although it was a RCT, this study was open-label, without blinding and allocation concealment, which may result in a high risk of bias. Second, only four patients completed the HBOT protocol, indicating that the effectiveness may not be estimated correctly owing to the small sample size. We excluded this study because of insufficient data for analysis. On the contrary, the studies included in our analysis that reported clinical remission and response enrolled more patients and had more detailed data. To avoid placebo effect, Dulai et al. 22 even used a sham control protocol in which patients breathed room air (21% oxygen) under a pressure of 1.2 ATA. This high-quality study demonstrated that HBOT is well tolerated and effective for UC patients.
In this study, we included articles in Chinese. Meta-analysis serves as a statistical method, which can combine the results from different studies but of a similar topic. It was based on fully incorporating relevant researches. Since we have a good understanding of only English and Chinese, the languages of the studies are restricted to English and Chinese. And unexpectedly, we found more trials published in Chinese than in English. We selected the searching results according to our inclusion and exclusion criteria, which were established at the beginning. All the included studies must have at least one outcome, and they must have available full texts and the patients treated with standard therapy according to the guidelines. The definitions of the outcomes should be listed in the article, and the exact sessions of hyperbaric oxygen therapy are also needed. And based on these strategies, we did our research. We have to admit that some of the Chinese researches provided little data on the selection of patients, especially those which were published several years ago. And some data which may have a great influence on the result were also less reported, such as disease flares. This is one of the limitations of our research.
HBOT has been widely used to treat chronic wounds and diabetic foot ulcers. Several possible mechanisms may explain the effects of HBOT. It promotes wound healing by increasing oxygen delivery to the hypoxic tissues. Dhamodharan et al. 30 reported that HBOT can induce angiogenesis in the tissue, which was demonstrated by the significantly increased expression of angiogenesis markers such as EGF, VEGF, PDGF, FGF-2, and CXCL10. Other studies also demonstrated that HBOT can suppress the expression of pro-inflammatory cytokines including IL-1, IL-6, and TNF-α, and stimulate the expression of anti-inflammatory cytokines such as IL-10.31−34 Our results were consistent with these findings.
To the best of our knowledge, this is the first meta-analysis to evaluate the validity of HBOT in the treatment of UC. We demonstrated a superior effect of HBOT combination therapy in the treatment of UC. There was no significant heterogeneity between the studies for clinical remission and response. Our study has several limitations. First, the sample size was small in most of the included studies, and the number of enrolled studies for meta-analysis may not be large enough for sufficiently evaluating the validity of HBOT combination therapy. In addition, only 2–4 studies reported the laboratory results, and thus the cogency of the results may be limited. We do think high-quality RCTs are under urgent demand. Second, some of the studies had high or unclear risk of randomization and blinding. Third, publication bias of clinical remission was observed, which may indicate that some studies remained hypothetically unpublished. Although publication bias was detected, it did not influence our results after the trim-and-fill method. Fourth, the baseline and control strategies differed between studies. The study hosted by Dulai et al. 22 enrolled UC patients with moderate-to-severe flares and used a sham control method. However, other researchers used traditional drugs such as mesalazine as a control method. Some studies enrolled patients without restrictions on disease severity. And still, some questions need to be answered. For example, it remains unclear whether different sessions of HBOT may show different treatment effects and how many sessions of HBOT should be recommended for the treatment of UC with respect to disease severity. Therefore, more high-quality RCTs are required.
Conclusion
In the present study, we demonstrated that HBOT combined with standard therapy improved outcomes in UC patients, including clinical remission, clinical response, disease severity scores, and laboratory test results, compared with standard therapy alone. In conclusion, HBOT could serve as a complementary treatment in patients with UC.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_17562848211023394 for Systematic review with meta-analysis: effectiveness of hyperbaric oxygenation therapy for ulcerative colitis by Pingrun Chen, Yina Li, Xian Zhang and Yan Zhang in Therapeutic Advances in Gastroenterology
Acknowledgments
We thank Dr. Wang Yan (Department of Thoracic Surgery, West China Hospital, Sichuan University) for guidance of data interpretation.
Footnotes
Author contributions: Pingrun Chen − study design, literature search, data collection, data interpretation, figures, writing. Yina Li − study design, literature search, data collection, data interpretation, figures, writing. Xian Zhang − study design, literature search, data collection, data interpretation, figures, writing. Yan Zhang – study design, data interpretation, figures, writing. Guarantor of the article: Yan Zhang.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Pingrun Chen  https://orcid.org/0000-0002-5915-2530
https://orcid.org/0000-0002-5915-2530
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Pingrun Chen, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Yina Li, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Xian Zhang, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
Yan Zhang, Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
References
- 1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 3. Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018; 16: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am 2014; 43: 525–541. [DOI] [PubMed] [Google Scholar]
- 5. Dulai PS, Siegel CA, Colombel JF, et al. Systematic review: monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD. Gut 2014; 63: 1843–1853. [DOI] [PubMed] [Google Scholar]
- 6. Dulai PS, Thompson KD, Blunt HB, et al. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol 2014; 12: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Al-Darmaki A, Frolkis AD, et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology 2015; 149: 928–937. [DOI] [PubMed] [Google Scholar]
- 8. Löndahl M, Fagher K, Katzman P. What is the role of hyperbaric oxygen in the management of diabetic foot disease? Curr Diab Rep 2011; 11: 285–293. [DOI] [PubMed] [Google Scholar]
- 9. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev 2016; 4: Cd005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tejada S, Batle JM, Ferrer MD, et al. Therapeutic effects of hyperbaric oxygen in the process of wound healing. Curr Pharm Des 2019; 25: 1682–1693. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang GH. Efficacy of hyperbaric oxygen on ulcerative colitis. Zhong Guo Shi Yong Nei Ke Za Zhi 1996; 16: 619. [Google Scholar]
- 14. Shen XF. Hyperbaric oxygen therapy of ulcerative colitis. Jiao Tong Yi Xue 2000; 14: 363. [Google Scholar]
- 15. Xu J. Efficacy evaluation on 21 cases of ulcerative colitis treated with hyperbaric oxygen. Zhong Hua Hang Hai Yi Xue Yu Gao Qi Ya Yi Xue Za Zhi 2001; 8: 173–174. [Google Scholar]
- 16. Wang L. Clinical application of hyperbaric oxygen in the treatment of ulcerative colitis. Zhong Guo Chang Kuang Yi Xue 2003; 16: 476–477. [Google Scholar]
- 17. Yin WB. Influence of hyperbaric oxygen on TNF-α and IL-6 in patients with ulcerative colitis. Yi Xue Xin Xi 2008; 21: 661–663. [Google Scholar]
- 18. Nie YL. Influence of hyperbaric oxygen on TNF-α and IL-6 in patients with ulcerative colitis. Zhong Guo Zhong Yi Yao Zi Xun 2011; 3: 12–13. [Google Scholar]
- 19. Wang XQ. Efficacy evaluation of hyperbaric oxygen combined with sulfasalazine in the treatment of ulcerative colitis. Yi Xue Xin Xi 2011; 24: 119. [Google Scholar]
- 20. Zhan YH, Peng NN. Clinical application of hyperbaric oxygen in the treatment of ulcerative colitis. Yi Xue Xin Xi 2013; 26: 427. [Google Scholar]
- 21. Huang K, Cao QX. Effect of hyperbaric oxygen combined with mesalazine in the treatment of ulcerative colitis. Zhong Hua Wu Li Yi Xue Yu Kang Fu Za Zhi 2016; 38: 379–380. [Google Scholar]
- 22. Dulai PS, Buckey JC, Raffals LE, et al. Hyperbaric oxygen therapy is well tolerated and effective for ulcerative colitis patients hospitalized for moderate–severe flares: a phase 2A pilot multi-center, randomized, double-blind, sham-controlled trial. Am J Gastroenterol 2018; 113: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 23. Zhong LD, Zhong C, Chen HB, et al. Study on the application of mesalazine sustained-release granules combined with hyperbaric oxygen therapy in patients with ulcerative colitis. Zhong Guo Yi Xue Chuang Xin 2019; 16: 44–47. [Google Scholar]
- 24. Dulai PS, Raffals LE, Hudesman D, et al. A phase 2B randomised trial of hyperbaric oxygen therapy for ulcerative colitis patients hospitalised for moderate to severe flares. Aliment Pharmacol Therap 2020; 52(6): 955–963. [DOI] [PubMed] [Google Scholar]
- 25. Wang LL, Ma FQ. Hyperbaric oxygen combined with mesalazine in the treatment of 70 cases of ulcerative colitis. Zhong Hua Hang Hai Yi Xue Yu Gao Qi Ya Yi Xue Za Zhi 2020; 27: 112–114. [Google Scholar]
- 26. Sutherland LR, Martin F. 5-Aminosalicylic acid enemas in treatment of distal ulcerative colitis and proctitis in Canada. Dig Dis Sci 1987; 32: 64s–66s. [DOI] [PubMed] [Google Scholar]
- 27. Xia X. Effect of Shenlingbaishu powder combined with hyperbaric oxygen on ulcerative colitis. Zhong Hua Hang Hai Yi Xue Yu Gao Qi Ya Yi Xue Za Zhi 2014; 21: 130–131. [Google Scholar]
- 28. Li YH, Zhu JF. Clinical application of hyperbaric oxygen in the treatment of ulcerative proctitis. Zhong Guo Xian Dai Pu Tong Wai Ke Jin Zhan 2009; 12: 161,184. [Google Scholar]
- 29. Pagoldh M, Hultgren E, Arnell P, et al. Hyperbaric oxygen therapy does not improve the effects of standardized treatment in a severe attack of ulcerative colitis: a prospective randomized study. Scand J Gastroenterol 2013; 48: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 30. Dhamodharan U, Karan A, Sireesh D, et al. Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Rad Biol Med 2019; 138: 53–62. [DOI] [PubMed] [Google Scholar]
- 31. Karadurmus N, Sahin M, Tasci C, et al. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol Pol 2010; 61: 275–279. [PubMed] [Google Scholar]
- 32. Bosco G, Vezzani G, Mrakic Sposta S, et al. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J Enzyme Inhib Med Chem 2018; 33: 1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kudchodkar B, Jones H, Simecka J, et al. Hyperbaric oxygen treatment attenuates the pro-inflammatory and immune responses in apolipoprotein E knockout mice. Clin Immunol 2008; 128: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li F, Fang L, Huang S, et al. Hyperbaric oxygenation therapy alleviates chronic constrictive injury-induced neuropathic pain and reduces tumor necrosis factor-alpha production. Anesth Analg 2011; 113: 626–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_17562848211023394 for Systematic review with meta-analysis: effectiveness of hyperbaric oxygenation therapy for ulcerative colitis by Pingrun Chen, Yina Li, Xian Zhang and Yan Zhang in Therapeutic Advances in Gastroenterology