Abstract
Aims:
Chronic obstructive pulmonary disease (COPD) is a systemic disease. Several long non-coding RNAs (lncRNAs) have been identified to be aberrantly expressed in COPD patients. This study investigated the role of lncRNA cancer susceptibility candidate 2 (CASC2) in COPD, as well as its potential mechanism.
Methods:
Fifty smokers with COPD and another 50 smokers without COPD were recruited. Receiver operating characteristic curve was constructed to assess the diagnostic value of CASC2 in COPD patients. 16HBE cells were treated with cigarette smoke extract (CSE) to establish a cell model. qRT-PCR was used for the measurement of mRNA levels. The cell viability and apoptosis were detected by using Cell Counting Kit-8 and flow cytometry assay. Enzyme-linked immunosorbent assay was performed to detect the levels of proinflammatory cytokines. Luciferase reporter assay was performed for the target gene analysis.
Results:
Serum CASC2 was dramatically decreased in COPD patients compared with smokers without COPD, and was positively associated with FEV1 (forced expiratory volume in one second). Serum CASC2 was overexpressed in severe COPD patients, and had the diagnostic accuracy to distinguish COPD patients from smokers. CASC2 overexpression alleviated CSE-induced apoptosis and inflammation in 16HBE cells. CASC2 functions as a ceRNA of miR-18a-5p. Upregulation of miR-18a-5p reversed the influence of CASC2 on cell apoptosis and inflammation in 16HBE cells. IGF1 was the target gene of miR-18a-5p.
Conclusion:
CASC2 was downregulated in COPD patients and it might be a promising biomarker for the disease diagnosis. Overexpression of CASC2 might inhibit the bronchial epithelial cell apoptosis and inflammation via targeting miR-18a-5p/IGF1 axis.
The reviews of this paper are available via the supplemental material section.
Keywords: bronchial epithelial cell apoptosis, CASC2, diagnosis, inflammatory, miR-18a-5p
Introduction
Chronic obstructive pulmonary disease (COPD), a systemic disease, is closely associated with systemic inflammation and skeletal muscle dysfunction. 1 According to the latest Chinese COPD epidemiological survey, the prevalence of COPD among Chinese over 40 years of age increased from 8.2% in 2008 to 13.7% in 2015. 2 COPD contributes to significant morbidity and mortality worldwide, leading to enormous consequences for societies and economies. 3 Both environmental and genetic factors have been reported to play an important role in the development of COPD. 4 Cigarette smoking is considered to be the most important risk factor for COPD. 5 It is also suggested that genetic and epigenetic changes could contribute to COPD immune response and disease progression. 6 Unfortunately, there is no effective treatment or cure for COPD, thus it is of great significance to explore the etiology and potential pathogenesis of COPD, and new effective therapeutic approaches are warranted.
Long non-coding RNA (lncRNA) is a group of RNA molecules discovered in recent years, with a length greater than 200 nucleotides. lncRNA does not encode proteins, but participates in the regulation of gene expression in the form of RNA at various levels, such as epigenetic regulation, transcriptional regulation, and post-transcriptional regulation, and so on. The aberrant expression of lncRNA has been identified to be involved in respiratory diseases, such as lung cancer, acute respiratory distress syndrome, pulmonary hypertension, pulmonary tuberculosis, and silicosis. 7 Analyzing the relationship of lncRNA with the occurrence and development of COPD may provide new biomarkers and drug targets for the diagnosis and treatment of COPD. lncRNA cancer susceptibility candidate 2 (CASC2) is one of the recently discovered lncRNAs. It has been widely reported to be aberrantly expressed in a variety of cancers. 8 Low expression of CASC2 is identified in diabetic nephropathy rat model. Upregulation of CASC2 can inhibit the release of inflammatory factors and further relieve the disease process. 9 Another study has also reported the low expression level of CASC2 during the development of acute lung injury (ALI). Upregulation of CASC2 can alleviate the lipopolysaccharide (LPS)-induced inflammatory response in the ALI cell model. 10 The findings prompted us to fully explore the role of CASC2 in COPD.
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules that participate in the regulation of various cellular processes, such as cell differentiation, proliferation, apoptosis, and autophagy. 11 With the discovery of the regulatory mechanism of endogenous competitive RNA (ceRNA), miRNA is no longer regarded as an independent element in diseases, and the role of ceRNA regulatory network in various human diseases gradually attracts more and more attention. Many lncRNAs have been reported to have regulatory elements of miRNAs, and can regulate the miRNA expression via sponging miRNA. 12 A recent study has reported the direct binding relationship between CASC2 and miR-18a-5p. CASC2 functions as a sponge of miR-18a-5p in cholangiocarcinoma. 13 The dysregulation of miR-18a-5p has been reported to participate in respiratory diseases. In a study about influenza A, miR-18a-5p is suggested to be involved in the regulation of the pulmonary innate immune response. 14 Another study also reported the upregulation of miR-18a-5p during the lung damage process caused by nanosized SiO2. 15 However, the role and underlying mechanism of miR-18a-5p in COPD remain unknown.
Therefore, the present study aimed to investigate the expression level of lncRNA CASC2 in COPD, and further explore its potential mechanism in bronchial epithelial cell apoptosis and inflammation with the involvement of miR-18a-5p/IGF1. We aimed to provide a reliable experimental basis for further exploring the mechanism of COPD.
Materials and methods
Study subjects
The study was designed with the approval of the ethics committee of Shanghai Pudong New Area Gongli Hospital, and written informed consent was obtained from each participant.
A total of 100 smokers were recruited, in which 50 patients diagnosed with COPD were recruited in the study according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria 16 and another 50 cases had no COPD. All cases had a smoking history of at least 20 pack-years. The GOLD spirometry criterion for the diagnosis of COPD was a post-bronchodilator forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <0.7. Based on the FEV1 impairment, the severity of COPD was defined as follows: GOLD stage I (mild), FEV1 ⩾80% predicted; GOLD stage II (moderate), 50% ⩽FEV1 <80% predicted; GOLD stage III (severe) 30% ⩽FEV1 <50% predicted; GOLD stage IV (very severe), FEV1 <30% predicted. Among 50 COPD patients, three cases were in COPD stage I, 27 were in COPD stage II, 20 were in COPD stage III. Additionally, another 40 healthy individuals who had no history of smoking were also included. All subjects had no other concomitant diseases, including significant cardiac dysfunction, active infection (hepatitis, tuberculosis, etc.), and neurological or psychiatric disorders. Five-milliliter blood samples were collected from each individual. After blood centrifugation, the serum samples were collected and stored at −80°C until analysis.
Cigarette smoke extract preparation
As previously described, cigarette smoke extraction was performed. 17 The smoke from 10 research-grade cigarettes (trade name: Da Qianmen, Shanghai, People’s Republic of China) was passed through 25 ml media, and the cigarette smoke extract (CSE) stock solutions were obtained. Then the pH of the solutions was adjusted to 7.0, and an optical density of 0.43 ± 0.02 at 320 nm was defined as 100% CSE. The CSE containing medium at different concentrations (0%, 0.5%, 1%, 2%, and 4%) was gained by diluting with PBS.
Cell culture and transfection
The human bronchial epithelial cell line 16HBE was purchased from the Chinese Academy of Cell Resource Center (Shanghai, China) and cultured in RPMI 1640 medium containing 10% FBS and incubated at a constant temperature incubator at 37°C and 5% CO2.
To regulate the expression level of CASC2 or miR-18a-5p in vitro, plasmid cloning DNA (pcDNA), pcDNA-CASC2 or miR-18a-5p mimic (5′-UAAGGUGCAUCUAGGCAGAUAG-3′), miR-18a-5p inhibitor (5′-CUAUCUGCACUAGAUGCACCUUA-3′), or the negative control (miR-NC; 5′-CAGUACUUUUGUGUAGUACAA-3′) were transfected into 16HBE cells at the logarithmic growth stage of cells. The sequences were purchased from GenePharma (Shanghai, China). The transfection reagent was lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) and the transfection was performed according to the manufacturer’s instructions.
QRT-PCR
Total RNA was isolated and extracted from different groups of cells, cell culture medium, and clinical serum samples by adding TRIzol, chloroform, isopropanol, and 75% ethanol. Single-stranded cDNA was synthesized from 1 μg RNA using a reverse reaction kit (Promega, Madison, WI, USA). Then real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed on a 7500 real-time PCR system (Applied Biosystems, Mannheim, Germany) using an SYBR green I Mater Mix kit (Invitrogen, Carlsbad, CA, USA). U6 and glyceraldehyde phosphate dehydrogenase (GAPDH) were used as the internal reference for CASC2 and miR-18a-5p. Relative expression of genes in 16HBE cells and that release in the culture medium, as well as in the serum samples, was calculated by using 2−ΔΔCt method.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 was applied for cell proliferation analysis. 16HBE cells in the logarithmic growth phase were inoculated in 2 × 103 into each well of the 96-well plate. After incubation for 48 h, 10 μl CCK-8 (Beyotime, Shanghai, China) reagent was added. After incubation for 2 h, optical density was measured at 490 nm using an iMark Microplate Absorbance Reader (Bio-Rad Laboratories, Hercules, CA, USA).
Flow cytometry assay
Cell apoptosis was assessed using a FITC Annexin V Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ, USA). In accord with the apoptosis kit manufacturer’s instructions, the treated cells were collected and centrifuged. The cells were washed with cold PBS, and then stained at room temperature with annexin V and propidium iodide for 10 min in a dark environment. A FACS Calibur flow cytometer (BD, Biosciences) was used to detect the final cell apoptosis rate.
Enzyme-linked immunosorbent assay (ELISA)
The inflammatory responses in the cell model were analyzed by measuring the levels of proinflammatory cytokines in cell culture supernatants. The ELISA kits (Boster Biotechnology Company, Wuhan, China) were used to detect the levels of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α in accordance with the manufacturer’s protocols.
Luciferase reporter assay
A double-luciferase reporter assay kit was purchased from Transgen Biotech (Beijing, China) and used for the target gene verification. In brief, 24 h after transfection, cells were dissociated by 1 × cell lysis buffer, then mixed in 20 μl cell lysate and 100 μl luciferase reaction reagent. Then relative luciferase activity was detected with a microplate reader (Molecular Devices, LLC). Renilla luciferase was used for normalization. Each sample was repeated three times.
Statistical analysis
The data analysis was carried out by SPSS 17.0 and GraphPad Prism 7.0 software. All data were expressed as mean ± standard deviation (SD). Student’s t-test and one-way analysis of variance were used for the comparison of the statistical differences between groups. Pearson’s analysis was used for correlation analysis. Receiver operating characteristic (ROC) curve was constructed to evaluate the diagnostic value of CASC2 in COPD. A p value less than 0.05 was identified as statistically significant.
Results
Characteristics of the study population
In the present study, 40 non-smokers, 50 smokers without COPD, and 50 smokers with COPD were recruited. As shown in Table 1, there was no significant difference in the age, gender, and BMI distribution among the three groups (all p > 0.05). Additionally, the smoking history also showed no significant difference between smoker and COPD groups (p > 0.05). The levels of forced expiratory volume in one second (FEV1) and FEV1/ forced vital capacity (FVC) were significantly different among the three groups (all p < 0.001).
Table 1.
Characteristics of the study population.
| Characteristics | Non-smoker (n = 40) | Smoker (n = 50) | COPD (n = 50) | p value |
|---|---|---|---|---|
| Age, years | 59.25 ± 4.00 | 58.64 ± 4.11 | 58.10 ± 4.45 | 0.438 |
| Sex, male/female | 27/13 | 39/11 | 42/8 | 0.177 |
| BMI, kg/m2 | 21.70 ± 1.80 | 21.73 ± 1.70 | 21.28 ± 1.74 | 0.370 |
| FEV1/FVC, % | 87.53 ± 4.20 | 80.78 ± 5.68 | 56.84 ± 11.59 | <0.001 |
| FEV1, % | 92.38 ± 5.12 | 87.68 ± 5.44 | 56.16 ± 15.95 | <0.001 |
| Smoking, pack-years | – | 38.16 ± 11.27 | 41.08 ± 12.40 | 0.221 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Expression changes and clinical values of serum CASC2 in COPD patients
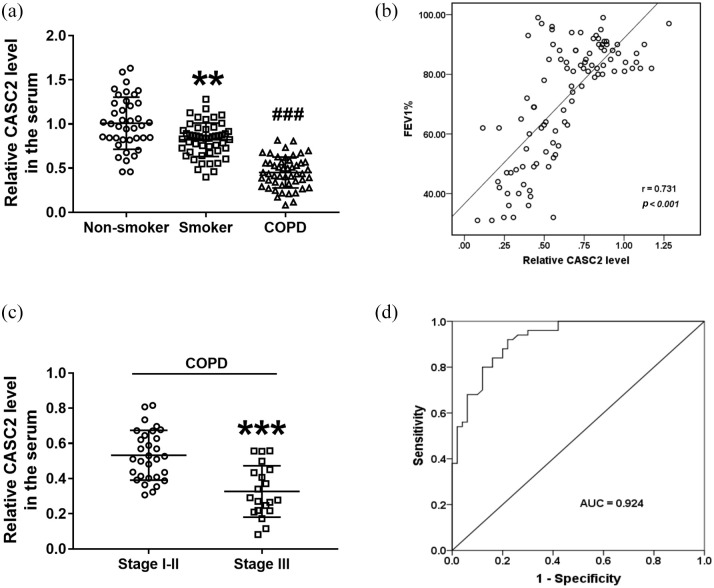
According to the qRT-PCR analysis, it was found that serum CASC2 was low expressed in smokers compared with non-smokers [Figure 1(a); p < 0.05]. Moreover, the serum levels of CASC2 were dramatically decreased in COPD patients compared with smokers without COPD [Figure 1(a); p < 0.001]. Additionally, the correlation analysis results indicated that serum CASC2 was positively associated with FEV1 in all smokers with or without COPD [Figure 1(b); r = 0.731, p < 0.001]. In addition, based on the GOLD stage, the COPD patients were divided into two groups, GOLD stage 1–2 (n = 30), and GOLD stage 3 (n = 20), and CASC2 levels were compared between the two groups. It was found that CASC2 was downregulated significantly in the serum of severe COPD patients compared with the mild and moderate cases [Figure 1(c), p < 0.001]. Moreover, according to the serum CASC2 levels in all smokers with or without COPD, a ROC curve was established. As shown in Figure 1(d), the ROC curve indicated that the area under the curve was 0.924, the sensitivity was 78.0% and the specificity was 92.0% at the cutoff value of 0.680, suggesting that the expression of CASC2 had the ability to distinguish COPD patients from smokers. These results suggested that miR-18a-5p might be involved in the development of COPD for smokers.
Figure 1.
Clinical values of serum CASC2 in COPD patients. (a) Serum CASC2 was low expressed in the smokers compared with non-smokers, and it was dramatically decreased in COPD patients compared with smokers without COPD. **p < 0.01; ###p < 0.001. Differences between groups were compared using one-way analysis of variance. (b) Serum CASC2 was positively associated with FEV1 in all smokers with or without COPD (r = 0.731, p < 0.001). Differences between groups were compared using Student’s t-test. (c) CASC2 was downregulated significantly in the serum of COPD patients in stage I-II compared with that in stage III. ***p < 0.001, compared with stage I–II group. (d) CASC2 had the diagnostic accuracy to distinguish COPD patients from smokers with the AUC of 0.924, the sensitivity of 78.0% and the specificity of 92.0%.
AUC, area under the curve; CASC2, cancer susceptibility candidate 2; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second.
CASC2 overexpression alleviated CSE-induced apoptosis and inflammation in 16HBE cells
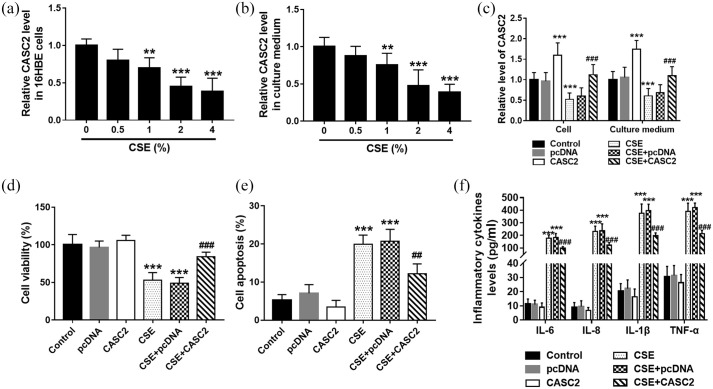
To investigate the role of CASC2 in smoking-triggered COPD, 16HBE cells were treated with CSE to mimic the smoking condition in vitro. 16HBE cells were first treated with CSE at different concentrations. qRT-PCR results indicated that the levels of CASC2 decreased gradually as the CSE concentration increased, and the CASC2 levels were significantly suppressed at 2% and 4% CSE concentration [Figure 2(a)]. But the CASC2 levels showed no significant difference at 2% and 4% CSE concentration. Similar results were also detected in the culture medium [Figure 2(b)]. Therefore, 2% CSE treatment for 24 h was used for the subsequent cell experiments. Then pcDNA-CASC2 was transfected into cells to regulate the level of CASC2. It was found that CASC2 was significantly upregulated by pcDNA-CASC2 transfection in both cell and culture medium [p < 0.001; Figure 2(c)]. The CCK-8 and flow cytometry assay results demonstrated that CASC2 overexpression significantly promoted cell viability, while inhibited CSE-induced cell apoptosis [p < 0.001; Figure 2(d) and (e)]. Then we further investigated the role of CASC2 in inflammatory cytokines release. It was observed that CSE promoted the release of IL-6, IL-8, IL-1β, and TNF-α in 16HBE cells, which was reversed by CASC2 overexpression [p < 0.001; Figure 2(f)]. It was concluded that CASC2 overexpression attenuated CSE-induced apoptosis and inflammation in 16HBE cells.
Figure 2.
CASC2 overexpression alleviated CSE-induced apoptosis and inflammation in 16HBE cells. (a) The levels of CASC2 decreased gradually in 16HBE cells as the CSE concentration increased, and the CASC2 levels were significantly suppressed at 2% and 4% CSE concentration. **p < 0.01; ***p < 0.001. (b) The levels of CASC2 decreased gradually in the cell culture medium as the CSE concentration increased, and the CASC2 levels were significantly suppressed at 2% and 4% CSE concentration. **p < 0.01; ***p < 0.001. (c) CASC2 was significantly upregulated in both cells and cell culture medium by pcDNA-CASC2 transfection. ***p < 0.001, compared with control group; ###p < 0.001 compared with CSE group. (d) and (e) CASC2 overexpression significantly promoted cell viability, while it inhibited CSE-induced cell apoptosis. ***p < 0.001, compared with control group; ###p < 0.001 compared with CSE group. (f) CSE promoted the release of IL-6, IL-8, IL-1β, and TNF-α in 16HBE cells, which was reversed by CASC2 overexpression. ***p < 0.001, compared with the control group; ###p < 0.001 compared with CSE group. Differences between groups were compared using one-way analysis of variance.
CASC2, cancer susceptibility candidate 2; CSE, cigarette smoke extract; pcDNA, plasmid cloning DNA.
CASC2 functions as a ceRNA of miR-18a-5p
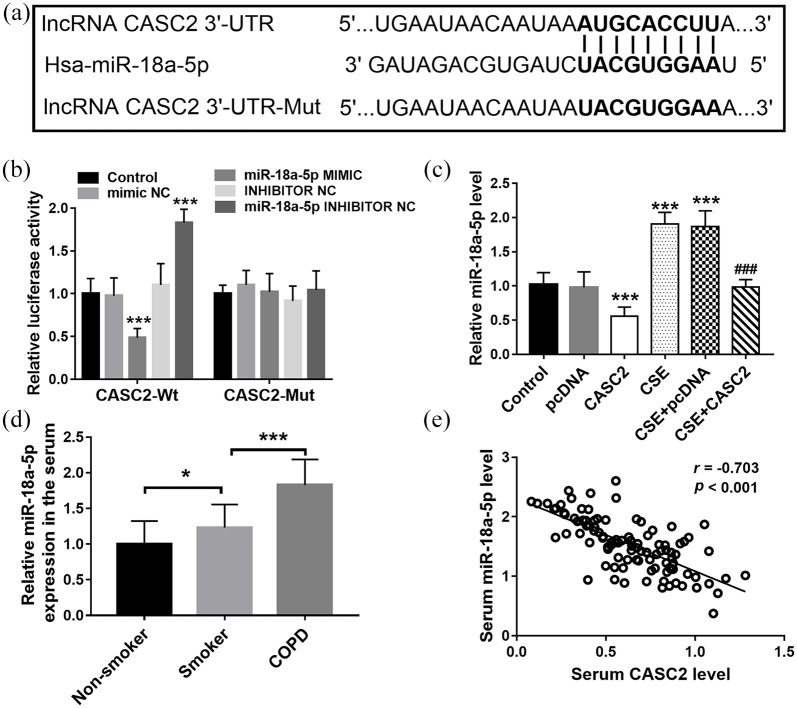
StarBase was used to predict the relationship between CASC2 and miRNAs, and the complementary sequence between miR-18a-5p and CASC2 3′UTR was found [Figure 3(a)]. Then the luciferase reporter assay indicated that miR-18a-5p mimic transfection significantly reduced the luciferase activity of cells co-transfected with CASC2-Wt, whereas miR-18a-5p inhibitor transfection had the opposite effect [Figure 3(b)]. However, the luciferase activity of cells transfected with CASC2-Mut cannot be influenced by the miR-18a-5p expression level [Figure 3(b)].
Figure 3.
CASC2 functions as a ceRNA of miR-18a-5p. (a) The complementary sequence between miR-18a-5p and CASC2 3′-UTR. (b) The luciferase activity of different groups. ***p < 0.001, compared with control group. (c) CSE treatment significantly increased the level of miR-18a-5p, while overexpression of CASC2 led to the reduction of miR-18a-5p expression. ***p < 0.001, compared with control group; ###p < 0.001, compared with CSE group). (d) Serum miR-18a-5p level in COPD patients. *p < 0.05, ***p < 0.001. (e) Serum miR-18a-5p expression showed a negative association with the level of CASC2. Differences between groups were compared using one-way analysis of variance.
CASC2, cancer susceptibility candidate 2; ceRNA, endogenous competitive RNA; COPD, chronic obstructive pulmonary disease; CSE, cigarette smoke extract; lncRNA, long non-coding RNA; Mut, mutant type; NC, negative control; pcDNA, plasmid cloning DNA; Wt, wild type.
The expression of CASC2 was measured in the cell model, and the results indicated that CSE treatment significantly increased the level of miR-18a-5p, while overexpression of CASC2 led to the reduction of miR-18a-5p expression [Figure 3(c)]. Furthermore, increased expression of miR-18a-5p was also detected in the serum of COPD patients compared with the smokers [p < 0.001, Figure 3(d)], and serum miR-18a-5p expression showed a negative association with the level of CASC2 [r = −0.703, p < 0.001; Figure 3(e)]. These data indicate that CASC2 functions as a ceRNA of miR-18a-5p.
Overexpression of miR-18a-5p promoted cell apoptosis and inflammation in 16HBE cells
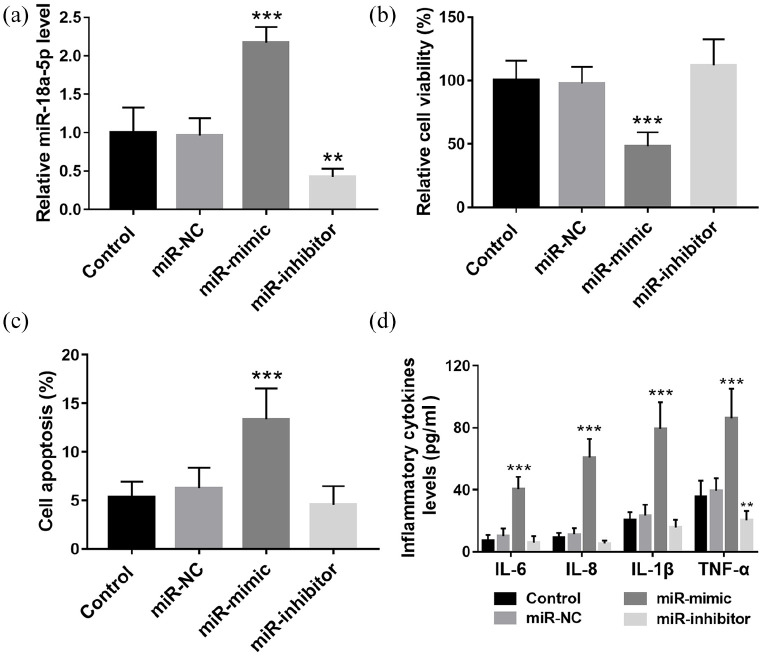
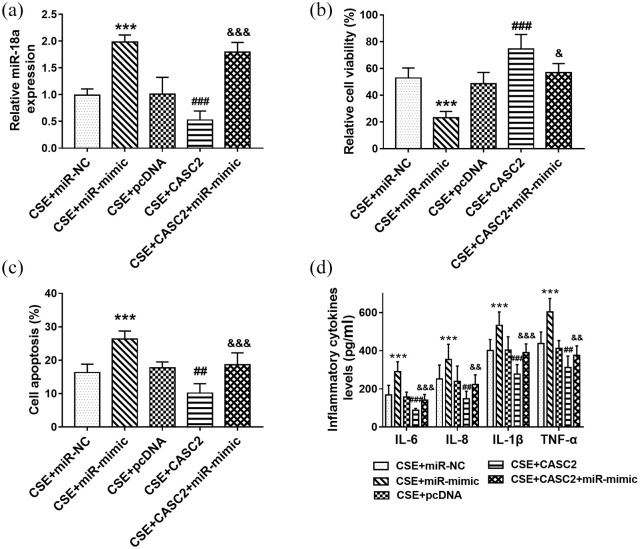
Cell transfection was performed to regulate the level of miR-18a-5p in 16HBE cells. As shown in Figure 4(a), miR-18a-5p mimic transfection significantly elevated the cell miR-18a-5p levels, while miR-18a-5p inhibitor transfection showed the opposite effect (p < 0.01). According to the CCK-8 and flow cytometry assay results, overexpression of miR-18a-5p significantly inhibited cell viability [Figure 4(b); p < 0.001] but promoted cell apoptosis [Figure 4(c); p < 0.01]. MiR-18a-5p downregulation promoted cell viability and inhibited cell apoptosis, but the differences were not significant. Besides, it was found that the levels of IL-6, IL-8, IL-1β, and TNF-α were all increased significantly by miR-18a-5p mimic transfection [Figure 4(d); p < 0.001].
Figure 4.
Overexpression of miR-18a-5p promoted cell apoptosis and inflammation in 16HBE cells. (a) miR-mimic transfection significantly elevated the cell miR-18a-5p levels, while miR-18a-5p inhibitor transfection showed the opposite effect. (b) and (c) Overexpression of miR-18a-5p significantly inhibited cell viability and promoted cell apoptosis. MiR-18a-5p downregulation promoted cell viability and inhibit cell apoptosis, but the differences were not significant. (d) The levels of IL-6, IL-8, IL-1β, and TNF-α were all increased significantly by miR-18a-5p mimic transfection.
**p < 0.01, ***p < 0.001, compared with control group.NC, negative control.
MiR-18a-5p reversed the influence of CASC2 on cell apoptosis and inflammation in 16HBE cells
Then cell transfection was performed to regulate the level miR-18a-5p in vitro. It was observed that miR-18a-5p mimic transfection increased the level of miR-18a-5p remarkably [Figure 5(a)]. According to the CCK-8 results, overexpression of miR-18a-5p aggravated the inhibition of cell viability induced by CSE, and reversed the protective effect of CASC2 on cell viability [Figure 5(b)]. The results of the flow cytometry assay suggested that miR-18a-5p overexpression aggravated CSE-induced cell apoptosis [Figure 5(c)]. Overexpression of CASC2 inhibited cell apoptosis, but the influence was reversed by miR-18a-5p upregulation [Figure 5(c)]. Furthermore, we explored the role of miR-18a-5p in the inflammatory response, and the levels of several inflammatory cytokines were measured. It was found that the levels of IL-6, IL-8, IL-1β, and TNF-α were all increased in CSE treated cells, which was further enhanced by miR-18a-5p overexpression [Figure 5(d)]. Overexpression of CASC2 inhibited the release of inflammatory factors, which was reversed by miR-18a-5p mimic transfection [Figure 5(d)]. The data indicated that overexpression of miR-18a-5p reversed the influence of CASC2 on cell apoptosis and inflammation in 16HBE cells.
Figure 5.
miR-18a-5p reversed the influence of CASC2 on cell apoptosis and inflammatory in 16HBE cells. (a) miR-18a-5p mimic transfection increased the level of miR-18a-5p remarkably. (b) Influence of miR-18a-5p on cell viability. (c) Effect of miR-18a-5p on cell apoptosis. (d) The levels of IL-6, IL-8, IL-1β, and TNF-α in cells after miR-18a-5p upregulated.
***p < 0.001, compared with CSE+miR-NC group.
##p < 0.01, ###p < 0.001, compared with CSE+pcDNA group.
&p < 0.05, &&p < 0.01, &&&p < 0.001, compared with CSE+CASC2 group.
Differences between groups were compared using one-way analysis of variance.
CASC2, cancer susceptibility candidate 2; CSE, cigarette smoke extract; NC, negative control; pcDNA, plasmid cloning DNA.
IGF1 is the target gene of miR-18a-5p
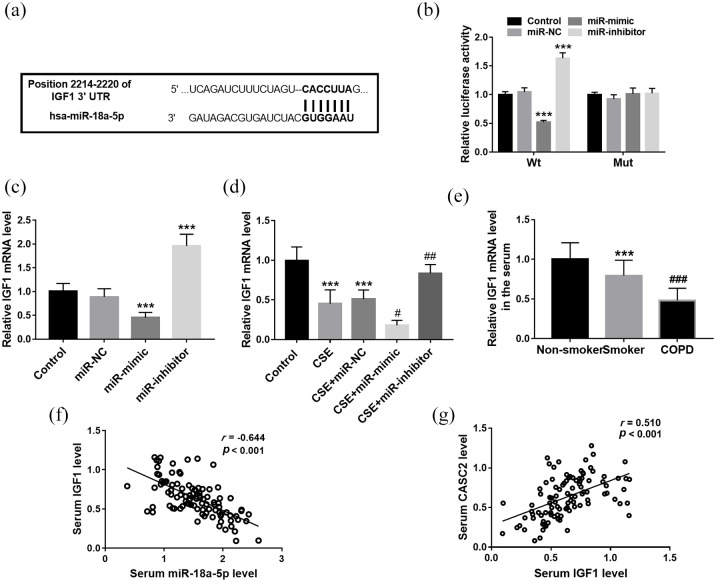
According to the bioinformatics prediction, miR-18a-5p has a putative complementary sequence that was identified in the 3′-UTR of IGF1 [Figure 6(a)]. Then the luciferase reporter assay was performed to confirm whether IGF1 was the target gene of miR-18a-5p. It was found that miR-18a-5p overexpression markedly inhibited the luciferase activity of the Wt 3′-UTR of IGF1, but miR-18a-5p downregulation promoted its luciferase activity [all p < 0.001; Figure 6(b)]. But there was no prominent effect on luciferase activity of IGF1-Mut reporter systems [Figure 6(b)]. In 16HBE cells, IGF1 was downregulated significantly in cells transfected with miR-18a-5p mimic transfection, but was elevated in cells transfected with miR-18a-5p inhibitor [p < 0.001; Figure 6(c)]. Additionally, the mRNA level of IGF1 showed a significant decrease trend in CSE treated cells, but miR-18a-5p mimic transfection significantly reduced the level of IGF1, and miR-18a-5p inhibitor transfection led to the increase of IGF1 [p < 0.001; Figure 6(d)]. Furthermore, the levels of IGF1 were confirmed in the serum of COPD patients. The qRT-PCR results indicated that IGF1 was low expressed in smokers compared with non-smokers; moreover, the smokers with COPD had the highest expression of IGF1 compared with those without COPD, which was consistent with the results observed in CSE treated 16HBE cells [Figure 6(e)]. Serum IGF1 showed negative association with miR-18a-5p level in all smokers [r = −0.644, p < 0.001; Figure 6(f)], but positively correlated with CASC2 levels [r = 0.510, p < 0.001; Figure 6(g)].
Figure 6.
IGF1 is the target gene of miR-18a-5p. (a) According to the bioinformatics prediction, miR-18a-5p has a putative complementary sequence in the 3′-UTR of IGF1. (b) The luciferase activity of cells in different groups. *** p < 0.001. (c) The mRNA level of IGF1 in different cell groups. ***p < 0.001, compared with control group. (d) The mRNA level of IGF1 in different cell groups. ***p < 0.001, compared with control group; #p < 0.05, ##p < 0.01, compared with CSE group. (e) The IGF1 mRNA level in different serum samples. ***p < 0.001, compared with non-smoker group; ###p < 0.01, compared with CSE group. (f) Serum miR-18a-5p showed a negative association with IGF1 level in all smokers. (g) Serum IGF1 showed a negative association with CASC2 level in all smokers. Differences between groups were compared using one-way analysis of variance.
CASC2, cancer susceptibility candidate 2; COPD, chronic obstructive pulmonary disease; CSE, cigarette smoke extract; mRNA, messager RNA; Mut, mutant type; NC, negative control; Wt, wild type.
Discussion
CASC2, a lncRNA, is located on chromosome 10 of the human genome, and plays an important regulatory role in the biological processes of cell proliferation, apoptosis, migration, and invasion. Recent studies have shown that CASC2 can act as an important anticancer factor, and its expression is dysregulated in several human tumors, indicating its important role in the occurrence and development of tumors. In the present study, CASC2 showed low expression in the serum of COPD patients and was positively associated with the level of FEV1. Furthermore, CASC2 was downregulated significantly in the serum of severe COPD patients compared with the mild and moderate cases. Consistently, low expression of CASC2 is identified in a diabetic nephropathy rat model; upregulation of CASC2 can inhibit the inflammatory factor release and further relieve the disease process. 9 Another study also reported the low expression level of CASC2 during the development of ALI, and LPS-induced inflammatory response in the ALI cell model can be suppressed by CASC2 upregulation. 10 These findings supported our results about the potential role of CASC2 in COPD. Furthermore, ROC curve was applied to detect the diagnostic value of serum CASC2 for COPD diagnosis, and serum CASC2 was proved to have the ability to distinguish COPD patients from healthy smokers. The current study focused on the exploration of non-invasive diagnostic markers, so serum samples were collected. The tissue expression level may be critical for COPD phenotype, but lung tissues were not collected in the present study, which should be considered for further exploration.
COPD is a worldwide disease, mainly attributable to the influence of cigarette smoking. 18 There are two main features in COPD: narrowing of the small airways (mainly because of chronic bronchiolitis) and destruction of the alveolar walls (emphysema). The pathological process of COPD has been suggested to be closely related to alveolar epithelial cell apoptosis. 1 In the present study, the human bronchial epithelial cell line 16HBE was recruited for cell experiments, which has been widely used for COPD study in previous researches.19,20 It was found that low expression of CASC2 was detected in both cell and cellular medium of CSE treated 16HBE cells, which was consistent with the results shown in the clinical serum samples. It is known that miRNAs are produced by all cell types, and the same miRNA may be derived from a variety of cell sources, such as endothelial cells, monocytes and macrophages, vascular smooth cells, and platelets, and eventually are secreted in blood. In the present study, the quantitative relations between alterations in intracellular miRNA concentrations and serum miRNA levels are not detected; this would be of interest for further exploration. The cell experiment results suggested that overexpression of CASC2 inhibited CSE-induced apoptosis in 16HBE cells. It should be noted that COPD patients always underwent a chronic inflammatory process, even after smoking cessation. Therefore, inflammation is considered to be one of the major pathological features of COPD, and targeting therapy involving lncRNAs associated with inflammation may be a new and effective means of treating COPD. As previous studies reported, dysregulation of lncRNAs can contribute to the alteration of immune response and homeostatic mechanisms in the lungs of smokers susceptible to COPD, such as MIRI155HG, MEG3, and ANRIL.21–23 Moreover, CASC2 has been widely reported to be involved in the regulation of inflammatory responses and further participate in the disease progression. 10 In the serum of sepsis patients, CASC2 shows a decreasing trend compared with that in healthy controls. 24 CASC2 can inhibit the secretion of inflammatory cytokines in LPS-stimulated human renal tubular epithelial HK‑2 cells. 24 In a study of ALI, CASC2 is reported to attenuate LPS-induced inflammatory response in ALI cell models. 10 Therefore, in the current study, we further explored the role of CASC2 in the inflammatory response, and the levels of several inflammatory cytokines were measured. As expected, it was observed that overexpression of CASC2 inhibited CSE-induced inflammatory response in 16HBE cells, which was consistent with the previous evidence. 10 It is known that COPD encompasses a variety of clinical and pathologic phenotypes ranging from airway inflammation (chronic bronchitis) to destruction of lung tissue (emphysema) and remodeling of the small airways. But in the current study, we focus on just the inflammatory condition of COPD; the association of CASC2 with other phenotypes was not included in the current study. It should be considered for further exploration.
Recently, many lncRNAs have been reported to have regulatory elements of miRNAs, and regulate the miRNA expression via sponging miRNAs. 25 A recent study has reported the direct binding relationship between CASC2 and miR-18a-5p; CASC2 functions as a sponge of miR-18a-5p in cholangiocarcinoma. 13 In recent years, several miRNAs have been identified to be aberrantly expressed in COPD patients, and are implicated in the pathogenesis of COPD, such as miR-130a, miR-195-5p, miR-129b.26–28 In the present study, CASC2 functions as a ceRNA of miR-18a-5p in 16HBE cells. And the high expression of miR-18a-5p was detected in the serum of COPD patients. The dysregulation of miR-18a-5p has been reported to participate in several respiratory diseases.14,15 miR-18a-5p can target the glucocorticoid receptor, which is involved in the regulation of inflammatory response and related physiological events. 29 In the current study, the gain and loss function experiments revealed that upregulation of miR-18a-5p aggravated CSE-induced cell apoptosis and inflammatory cytokines release. Moreover, miR-18a-5p overexpression reversed the influence of CASC2 on cell apoptosis and inflammation in 16HBE cells. We concluded that CASC2 might be involved in the development of COPD via sponging miR-18a-5p.
The dysregulation of miRNAs has been widely identified in different human diseases and is involved in the pathogenesis of diseases through binding to the 3′-untranslated region (3′-UTR) of the target gene. 26 Insulin-like growth factor 1 (IGF1) is a well-known anti-apoptotic pro-survival factor, serving a crucial role in maintaining homeostatic processes. 30 In several human disease studies, IGF1 has been identified to be the target gene of miR-18a-5p to participate in disease progression, such as muscle atrophy. 31 Notably, IGF1 has been reported to be low expressed in COPD patients. 32 As the bioinformatics analysis predicted, IGF1 is a candidate target gene of miR-18a-5p, which was confirmed by the luciferase reporter assay. Considering the important role of IGF1 in COPD as previously reported, we guessed that IGF1 might be involved in the regulatory role of miR-18a-5p in COPD. The cell gain and loss function experiments results indicated that overexpression of miR-18a-5p reduced the level of IGF1, while miR-18a-5p downregulation led to the increase of IGF1. Furthermore, a low level of miR-18a-5p was also detected in the serum of COPD patients. Consistently, IGF1 has been reported to be downregulated in COPD patients, and is associated with increased muscle weakness during an acute COPD exacerbation. 33 IGF1 is also known to be a key regulator of muscle mass, and muscle dysfunction is one of the common systemic manifestations of COPD. 34 In addition, IGF1 is also reported to play an important role in the regulation of inflammation in the immune system. 25 The evidence all supported our present results about the potential role of IGF1 in CSE-induced cell apoptosis and inflammation. Furthermore, a negative association of serum IGF1 level with CASC2 level was detected in clinical samples, supporting our conclusion about the involvement of CASC2/miR-18a/IGF1 axis in the disease mechanism. IGF1 can trigger the downstream signaling pathways through binding to its corresponding receptor (IGF1R), of which the most important is the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. 31 The PI3K/Akt pathway has been reported to be involved in the regulation of oxidative stress and inflammatory response, and dysregulation of PI3K/Akt signaling has been observed in both COPD patients and in vitro models.35,36 According to the above findings, we concluded that the CASC2/miR-18a-5p axis in the occurrence of COPD is related to the inhibition of IGF1, which induces the bronchial epithelial cell apoptosis and inflammation, and PI3K/Akt signaling might be involved in the potential mechanism. However, the direct regulatory role between IGF1 and CASC2 was not examined in the current study, and further studies are needed to verify the concrete mechanism.
In conclusion, our findings indicated that CASC2 was downregulated in COPD patients and it might be a promising biomarker for the disease diagnosis. Overexpression of CASC2 might inhibit the bronchial epithelial cell apoptosis and inflammation via targeting miR-18a-5p/IGF1 axis. The present results provide a basis for further study of the molecular mechanisms underlying the development and progression of COPD.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Pudong New Area Health and Family Planning Commission Project (No. PW2020A-19), Shanghai Pudong New Area Gongli Hospital Youth Fund (No. 2018YQNJJ-06) and Shanghai Pudong New Area Gongli Hospital Youth Talent Program (No. GLRq2019-02).
ORCID iD: Lei Zhao  https://orcid.org/0000-0002-2861-9265
https://orcid.org/0000-0002-2861-9265
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Panpan Liu, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Huali Zhang, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Haizhu Zeng, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Yingxia Meng, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Hongchang Gao, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Meilan Zhang, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, Shanghai, PR China.
Lei Zhao, Department of Pulmonary and Critical Care Medicine, Shanghai Pudong New Area Gongli Hospital, 219 Miao-Pu Road, Shanghai 200315, PR China.
References
- 1. Tzouvelekis A, Laurent G, Bouros D. Stem cell therapy in chronic obstructive pulmonary disease. Seeking the Prometheus effect. Curr Drug Targets 2013; 14: 246–252. [DOI] [PubMed] [Google Scholar]
- 2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018; 391: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 3. Chen PK, Hsiao YH, Pan SW, et al. Independent factors associate with hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease requiring intensive care unit admission: Focusing on the eosinophil-to-neutrophil ratio. PLoS One 2019; 14: e0218932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Ortega VE, Ampleford EJ, et al. Genome-wide association study of lung function and clinical implication in heavy smokers. BMC Med Genet 2018; 19: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De la, Garza MM, Cumpian AM, Daliri S, et al. COPD-type lung inflammation promotes K-ras mutant lung cancer through epithelial HIF-1alpha mediated tumor angiogenesis and proliferation. Oncotarget 2018; 9: 32972–32983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapellos TS, Bassler K, Aschenbrenner AC, et al. Dysregulated functions of lung macrophage populations in COPD. J Immunol Res 2018; 2018: 2349045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X, Chen D, Yu L. The value of circulating long non-coding RNA maternally expressed gene 3 as a predictor of higher acute respiratory distress syndrome risk and 28-day mortality in sepsis patients. J Clin Lab Anal 2020; 34: e23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palmieri G, Paliogiannis P, Sini MC, et al. Long non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol 2017; 111: 31–38. [DOI] [PubMed] [Google Scholar]
- 9. Min XQ, Xie Y. LncRNA CASC2 alleviates the progression of diabetic nephropathy by regulating the miR-144/SOCS2 signalling axis. Kidney Blood Press Res 2020; 45: 837–849. [DOI] [PubMed] [Google Scholar]
- 10. Li X, Mo J, Li J, et al. lncRNA CASC2 inhibits lipopolysaccharide-induced acute lung injury via miR-27b/TAB2 axis. Mol Med Rep 2020; 22: 5181–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Liu X, Wu Y, et al. MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget 2017; 8: 32370–32379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Liu Z, Yao B, et al. Long non-coding RNA CASC2 suppresses epithelial-mesenchymal transition of hepatocellular carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer 2017; 16: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng L, Liu YH, Nie S, et al. LncRNA CASC2 inhibits cell proliferation, metastasis and EMT through miR-18a/SOCS5 axis in cholangiocarcinoma. Eur Rev Med Pharmacol Sci 2020; 24: 8367–8376. [DOI] [PubMed] [Google Scholar]
- 14. Brogaard L, Larsen LE, Heegaard PMH, et al. IFN-lambda and microRNAs are important modulators of the pulmonary innate immune response against influenza A (H1N2) infection in pigs. PLoS One 2018; 13: e0194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang H, Zhang Y, Li W, et al. Altered microRNA expression profiles in lung damage induced by nanosized SiO2. Bioengineered 2017; 8: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 17. Moscovis SM, Gordon AE, Al Madani OM, et al. Virus infections and sudden death in infancy: the role of interferon-gamma. Front Immunol 2015; 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu W, Li R, Sun Y. Increased IFN-gamma-producing Th17/Th1 cells and their association with lung function and current smoking status in patients with chronic obstructive pulmonary disease. BMC Pulm Med 2019; 19: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ouyang Y, Liu X, Li H, et al. RNA Sequencing analyses reveal the potential mechanism of pulmonary injury induced by gallium arsenide particles in human bronchial epithelioid cells. Sci Rep 2020; 10: 8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu T, Shi C, Li H, et al. Curcumin suppresses cigarette smoke extract-induced oxidative stress through PPARgamma/NF-κB pathway in human bronchial epithelial cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2018; 38: 1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li N, Liu Y, Cai J. LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomed Pharmacother 2019; 117: 109015. [DOI] [PubMed] [Google Scholar]
- 22. Bi H, Wang G, Li Z, et al. Long Noncoding RNA (lncRNA) Maternally Expressed Gene 3 (MEG3) participates in chronic obstructive pulmonary disease through regulating human pulmonary microvascular endothelial cell apoptosis. Med Sci Monit 2020; 26: e920793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ge J, Geng S, Jiang H. Long noncoding RNAs antisense noncoding RNA in the INK4 locus (ANRIL) correlates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in patients with chronic obstructive pulmonary disease. J Clin Lab Anal 2019; 33: e22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Wei J, Shang F, et al. Long noncoding RNA CASC2 ameliorates sepsis induced acute kidney injury by regulating the miR155 and NF-κB pathway. Int J Mol Med 2020; 45: 1554–1562. [DOI] [PubMed] [Google Scholar]
- 25. Miao WJ, Yuan DJ, Zhang GZ, et al. lncRNA CASC2/miR18a5p axis regulates the malignant potential of nasopharyngeal carcinoma by targeting RBBP8. Oncol Rep 2019; 41: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 26. Li S, Jiang L, Yang Y, et al. MiR-195-5p inhibits the development of chronic obstructive pulmonary disease via targeting siglec1. Hum Exp Toxicol 2020; 39: 1333–1344. [DOI] [PubMed] [Google Scholar]
- 27. Wu Y, Guan S, Ge Y, et al. Cigarette smoke promotes chronic obstructive pulmonary disease (COPD) through the miR-130a/Wnt1 axis. Toxicol In Vitro 2020; 65: 104770. [DOI] [PubMed] [Google Scholar]
- 28. Chi Y, Di Q, Han G, et al. Mir-29b mediates the regulation of Nrf2 on airway epithelial remodeling and Th1/Th2 differentiation in COPD rats. Saudi J Biol Sci 2019; 26: 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Y, Zhao L, Yan Y, et al. PBMCs to stress-associated miR-18a-5p and miR-22-3p ratios as new indicators of metabolic syndrome. Biomed Res Int 2020; 2020: 8159342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castilla-Cortazar I, Aguirre GA, Femat-Roldan G, et al. Is insulin-like growth factor-1 involved in Parkinson’s disease development? J Transl Med 2020; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu C, Wang M, Chen M, et al. miR-18a induces myotubes atrophy by down-regulating IgfI. Int J Biochem Cell Biol 2017; 90: 145–154. [DOI] [PubMed] [Google Scholar]
- 32. Coskun F, Ege E, Uzaslan E, et al. Evaluation of thyroid hormone levels and somatomedin-C (IGF-1) in patients with Chronic Obstructive Pulmonary Disease (COPD) and relation with the severity of the disease. Tuberk Toraks 2009; 57: 369–375. [PubMed] [Google Scholar]
- 33. Crul T, Spruit MA, Gayan-Ramirez G, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest 2007; 37: 897–904. [DOI] [PubMed] [Google Scholar]
- 34. Roberston MJ, Raghunathan S, Potaman VN, et al. CRISPR-Cas9-induced IGF1 gene activation as a tool for enhancing muscle differentiation via multiple isoform expression. FASEB J 2020; 34: 555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun X, Chen L, He Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab 2019; 20: 301–304. [DOI] [PubMed] [Google Scholar]
- 36. Wang Z, Li R, Zhong R. Extracellular matrix promotes proliferation, migration and adhesion of airway smooth muscle cells in a rat model of chronic obstructive pulmonary disease via upregulation of the PI3K/AKT signaling pathway. Mol Med Rep 2018; 18: 3143–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211028072 for LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis by Panpan Liu, Huali Zhang, Haizhu Zeng, Yingxia Meng, Hongchang Gao, Meilan Zhang and Lei Zhao in Therapeutic Advances in Respiratory Disease