Abstract
Anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) related myositis is a form of immune-mediated necrotizing myopathy (IMNM). Anti-HMGCR autoantibodies target HMGCR, a glycoprotein linked to the endoplasmic reticulum implied in the cholesterol synthesis pathway, and exert a pathogenic effect on skeletal muscle cells. More than 60% of patients affected by HMGCR-related myositis shares statin-exposure in their medical history. Patients commonly experience CK levels elevation, myalgia, muscle weakness and soreness at variable extent, which manifest acutely or sub acutely with a progressively worsening course, in some cases mimicking limb-girdle muscular dystrophies (LGMD) phenotype and treatment is based on an immunosuppressive strategy. Here we present the peculiar case of a previously statins-exposed 72 y.o. asymptomatic man with persistent moderate hyperCKemia and high levels of anti-HMGCR, in which pharmacotherapy has not been initiated yet, while a wait-and-see approach has been adopted instead.
Key words: necrotizing, myopathy, HMGCR, hyperCKemia, statin, antibodies
Introduction
Anti- 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) related myositis is a form of immune-mediated necrotizing myopathy (IMNM) 1, along with anti- signal recognition particle (SRP) myositis. Anti-HMGCR autoantibodies, first identified in 2010, target HMGCR, a glycoprotein linked to the endoplasmic reticulum, implied in the cholesterol synthesis pathway via conversion of HMG-CoA to mevalonic acid; the autoantibodies recognize the intracellular C-terminal end of the enzyme and exert a pathogenic effect on skeletal muscle cells 2. Strikingly, more than 60% of patients affected by HMGCR-related myositis shares statin-exposure in their medical history; moreover, unlike anti-SRP myositis, anti-HMGCRs are not strictly related to malignancy 3. Diagnostic criteria for anti-HMGCR IMNM include elevated serum creatine kinase (CK) levels, presence of anti-HMGCR autoantibodies and proximal muscle weakness mainly in the lower limbs. Muscle biopsy is not required for diagnosis if autoantibodies are detected, nevertheless distinctive histopathological features are the presence of scattered myofibers at various stages of necrosis, fibres regeneration and macrocytes infiltration, while lymphocytic infiltrate is usually absent or poorly represented, and staining for MHC I and C5b-C9 molecules can be positive 4. On the clinical side, patients experience myalgia, muscle weakness and soreness at variable extent, which manifest acutely or sub acutely with a progressively worsening course, in some cases mimicking limb-girdle muscular dystrophies (LGMD) phenotype 5. Treatment is based on an immunosuppressive strategy in which corticosteroids but also methotrexate, rituximab and intravenous immunoglobulins have been safely and effectively employed in many cases 6,7. Here we present the peculiar case of a 72 year old asymptomatic man with persistent hyperCKemia and high levels of anti-HMGCR.
Case presentation
A 72 year old male patient referred to Neuromuscular Unit of Santa Chiara Hospital in Pisa in March 2018, for the detection of persistent moderate hyperCKemia (1000 U/L) on routine blood tests performed since December 2017, without muscular symptoms. The neurologic physical examination was normal. The patient’s medical history included two episodes of intracerebral haemorrage, hypertension, benign prostatic hyperplasia, type 2 diabetes, retinal thrombosis in the right eye. Notably, he also suffered from hypercholesterolemia, for which the patient had been taking Atorvastatin 40 mg per day for 2 years, discontinued soon after hyperCKemia discovery. The patient had been taking also Metformin 500 mg per day and Amlodipine 5 mg per day. No family history for neuromuscular disorders was reported. Subsequent dosages of blood CK levels confirmed an increase of values up to a maximum of 1500 U/L, while, at the six-monthly neurological assessments the patient remains asymptomatic. An electromyography study was made showing a myopathic pattern, with polyphasic, low amplitude MUPs, no spontaneous activity at rest and normal sensory and motor conduction velocities. In November 2019 a muscle biopsy on quadriceps femoris was performed, that showed rare hypo-atrophic, rounded and angled fibres, slight increase of perimysial and endomysial connective tissue and minimal peripheral rimmed positivity at oxidative enzymes staining (Fig. 1). Considering the muscle biopsy features, a genetic testing for LGMDs-associated genes (CAPN, DYSF, ANO5, CAV3, GAA) did not reveal DNA mutations. Myositis-associated autoantibodies including anti-HMGCR were dosed that detected a high anti-HMGCRs title (108.8 cU/ml, normal value < 20 cU/ml). The immunohistochemistry analysis for inflammatory markers was then conducted on the biopsy sample, showing that HLA (Mouse monoclonal anti-Human HLA-ABC Class I Antigen, clone W6/32, MHC I) and MAC staining were negative (Fig. 2). The muscle magnetic resonance imaging (MRI), performed on December 2020, showed a normal muscle trophism with mild fatty infiltration on the proximal part of the right biceps femoris and a minimal oedema on the triceps surae of both legs (Fig. 3). At the last follow-up visit, held in April 2021, the patient was persistently asymptomatic, without signs of muscle weakness but with a persistent hyperCKemia (1900 U/L). In consideration of the absence of symptoms, it was decided to follow the patient periodically to evaluate the clinical and biochemical trend, without any specific treatment.
Figure 1.
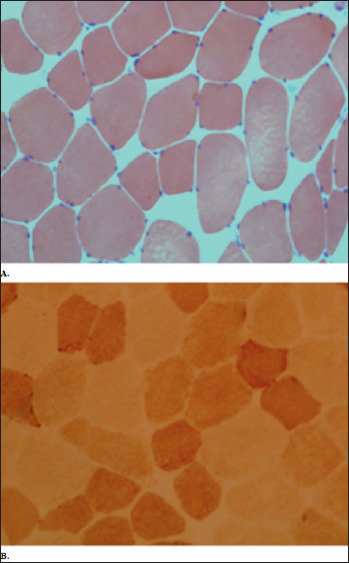
Muscle biopsy. Common staining (A: Haematoxylin&Eosin; B: COX) did not show any specific myopathic sign.
Figure 2.
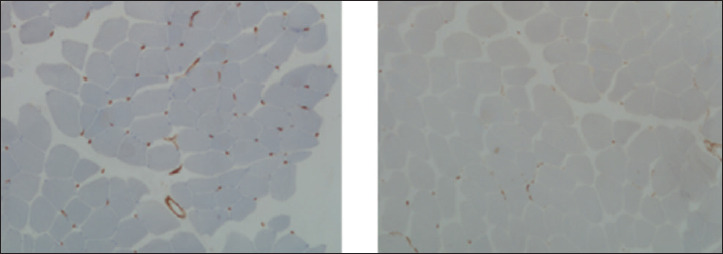
Muscle biopsy. HLA and MAC (C and D) were negative.
Discussion
To our knowledge only few cases of completely and persistently asymptomatic patients diagnosed with anti-HMGCR IMNM have been reported in literature 8. Soares et al. recently described a patient with anti-HMGCR, increasingly high CK levels up to > 6000 U/L in the time of six years and no sign of muscle weakness. He was treated with intravenous immunoglobulins (IVIg) with consequent CK levels decreasing to 2700 U/L.
Anti-HMGCRs tend to be a very specific finding of inflammatory process, as it was not found in patients with other related diseases other than myositis 5. In our case, statins may be the cause of a cytotoxic myopathy frequently observed, while the other medications that the patient was taking (Meformin and Amplodipine) to our knowledge are not associated to hyperCKemia, neither alone nor in combination. The peculiarity of the case here presented is that CK values are slightly, but steadily increased through the last three years of clinical monitoring. According to the scientific literature, statin withdrawal is effective in the remission of myositis only in rare, mild cases, and many patients require immunosuppressive treatment 5. Notably, in our case muscle biopsy did not show any necrotic or inflammatory features but only minor and not specific myopathic changes. Even muscle MRI also did not show any specific pattern of inflammation or extensive involvement, but only minor and non-specific aspects that could be age-related and non-pathologic. Furthermore, the case we describe highlights the opportunity and importance of including the anti-HMGCR test in cases of hyperCKemia and previous exposure to statins, even when the clinical examination is unremarkable over time. The pathogenetic role of anti-HMGCRs was described by Bergua et al. in 2018, in an in vivo study that examined the effects of IMNM patients’ IgGs in mice 9. They observed muscle damage and complement activation, thus demonstrating the direct effect of antibodies on tissues and providing the basis for hypothesizing plasma exchange as an effective treatment in severe forms. Given the pathogenic role of autoantibodies, we believe that in these cases it could be useful to timely monitor their serum levels and compare them to CK levels. In milder or asymptomatic stable cases, like the one we describe, in our opinion, the timing and extent of the treatment should be questioned, because none of the therapeutic options currently in use does not carry potential side effects or risks, especially in current times of pandemic, when immunosuppressive therapy should be a considered choice. Consequently, in our case, we chose to proceed with clinical and laboratory follow-up instead of starting with drug administration, although we are aware that a broader longitudinal follow-up is necessary to establish more solid management indications, to identify early signs of clinical deterioration and initiate therapy promptly. In our view this case highlights the opportunity of testing anti-HMGCR antibodies in patients statin-exposed showing high CK levels, even when muscle weakness or myalgia are not present, and inflammation and necrosis are not evident on biopsy or muscle MRI, as anti-HMGCRs are now widely and easily detectable in many laboratories and correct diagnosis leads to proper follow-up and potential treatment when needed.
Figures and tables
Acknowledgement
Not applicable.
Footnotes
Ethical consideration
The manuscript in part or in full has not been submitted or published anywhere. The manuscript has been at the EAN 2021 conference and is not under copyright.
Funding
The study did not receive any funding.
Conflict of interest
No potential conflict of interest was reported by the authors.
Author contributions
FT wrote the paper.
GA processed and analyzed muscle tissue involved in the study.
LC processed muscle tissue involved in the study and provided genetic analysis.
GS drafted the work and revised it.
GR approved the work for publication.
References
- 1.Crisan E, Patil VK. Neuromuscular complications of statin therapy. Curr Neurol Neurosci Rep 2020;20:47. https://doi.org/10.1007/s11910-020-01064-0 10.1007/s11910-020-01064-0 [DOI] [PubMed] [Google Scholar]
- 2.Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol 2018;14:215-224. https://doi.org/10.1080/1744666X.2018.1440206 10.1080/1744666X.2018.1440206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713-721. https://doi.org/10.1002/art.30156 10.1002/art.30156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allenbach Y, Benveniste O, Stenzel W, et al. Nat Rev Rheumatol 2020;16:689-701. https://doi.org/10.1038/s41584-020-00515-9 10.1038/s41584-020-00515-9 [DOI] [PubMed] [Google Scholar]
- 5.Mohassel Payam, Landon-Cardinal Océane, Foley A. Reghan, et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol Neuroimmunol Neuroinflamm 2019;6:e523. https://doi.org/10.1212/NXI.0000000000000523 10.1212/NXI.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treppo E, Infantino M, Benucci M, et al. Efficacy and safety of high-dose immunoglobulin-based regimen in statin-associated autoimmune myopathy: a multi-center and multi-disciplinary retrospective study. J Clin Med 2020;9:3454. https://doi.org/10.3390/jcm9113454 10.3390/jcm9113454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer A, Troyanov Y, Drouin J, et al. Statin-induced anti-HMGCR myopathy: successful therapeutic strategies for corticosteroid-free remission in 55 patients. Arthritis Res Ther 2020;22:5. https://doi.org/10.1186/s13075-019-2093-6 10.1186/s13075-019-2093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares IFZ, Comprido VF, Hsu BRRHS, et al. Immune-mediated necrotising myopathy in asymptomatic patients with high creatine kinase. BMJ Case Rep 2020;13:e235457. https://doi.org/10.1136/bcr-2020-235457 10.1136/bcr-2020-235457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergua C, Chiavelli H, Allenbach Y, et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann Rheum Dis 2019;78:131-139. https://doi.org/10.1136/annrheumdis-2018-213518 10.1136/annrheumdis-2018-213518 [DOI] [PubMed] [Google Scholar]


