Abstract
Introduction
There is an urgent need to develop effective interventional treatments for people with Alzheimer's disease (AD). AD results from a complex multi‐decade interplay of multiple interacting dysfunctional biological systems that have not yet been fully elucidated. Epidemiological studies have linked several modifiable lifestyle factors with increased incidence for AD. Because monotherapies have failed to prevent or ameliorate AD, interventional studies should deploy multiple, targeted interventions that address the dysfunctional systems that give rise to AD.
Methods
This randomized controlled trial (RCT) will examine the efficacy of a 12‐month personalized, multimodal, lifestyle intervention in 60 mild cognitive impairment (MCI) and early stage AD patients (aged 50+, amyloid positivity). Both groups receive data‐driven, lifestyle recommendations designed to target multiple systemic pathways implicated in AD. One group receives these personalized recommendations without coaching. The other group receives personalized recommendations with health coaching, dietary counseling, exercise training, cognitive stimulation, and nutritional supplements. We collect clinical, proteomic, metabolomic, neuroimaging, and genetic data to fuel systems‐biology analyses. We will examine effects on cognition and hippocampal volume. The overarching goal of the study is to longitudinally track biological systems implicated in AD to reveal the dynamics between these systems during the intervention to understand differences in treatment response.
Results
We have developed and implemented a protocol for a personalized, multimodal intervention program for early AD patients. We began enrollment in September 2019; we have enrolled a third of our target (20 of 60) with a 95% retention and 86% compliance rate.
Discussion
This study presents a paradigm shift in designing multimodal, lifestyle interventions to reduce cognitive decline, and how to elucidate the biological systems being targeted. Analytical efforts to explain mechanistic or causal underpinnings of individual trajectories and the interplay between multi‐omic variables will inform the design of future hypotheses and development of effective precision medicine trials.
Keywords: aerobic exercise, Alzheimer's disease, cognitive training, diet, executive function, lifestyle, memory, mild cognitive impairment, multimodal, neuroimaging, nutrition, precision medicine, systems biology, translational research
1. INTRODUCTION
Dementia is one the most important health concerns facing society today. The most common dementia‐related disorder is Alzheimer's disease (AD), the leading cause of disability among individuals older than age 65. 1 Approximately 15 to 20% of people older than 65 years have mild cognitive impairment (MCI). 2 Approximately 15% of individuals with MCI convert to dementia within 1 year, 3 highlighting this prodromal period for intervention. Existing pharmaceutical and non‐pharmaceutical therapies provide modest and short‐term benefits at best. 4 Furthermore, most monotherapies and narrow interventions and are not disease‐modifying therapies; they only treat symptoms of the disease.
A reason for the failure of any of the targeted monotherapeutic approaches is that multiple factors cause AD. By identifying and addressing multiple systemic pathological factors, the actual functional and cognitive improvement in an affected individual could be considerable. Evidence points toward greater effectiveness of multimodal health and lifestyle interventions than single interventions used alone. 5 Effects may be synergistic. Each individual may have a different set of causative factors; therapies must be personalized. The age of personalized medicine enabled by inexpensive testing assays permits comprehensive monitoring of the molecular and physiological subsystems allowing: (1) adjustment of therapies based on which subsystems respond, and (2) understanding the health and disease trajectories in each individual by characterizing time dependencies, dose‐response, and other knowledge‐generating epistemological tests driven by these dense data.
Early detection and intervention are critical. The World Health Organization (WHO) recommends that “proactive management of modifiable risk factors can delay or slow the onset or progression of the ,disease” and stresses the importance of early intervention. 6 Epidemiological studies have linked several modifiable lifestyle factors with risk for late‐life cognitive impairment and AD. 7 , 8 These risk factors can be targeted with multimodal interventions to prevent cognitive decline and co‐morbidities. The most significant results from trials ensue from lifestyle interventions including diet, exercise, cognitive training, and stress reduction. 5 , 9 , 10 Results from multimodal lifestyle prevention trials in asymptomatic older adults show mild but significant effects on cognitive functioning. 11 , 12 , 13 , 14 The largest trials to date have been FINGER, 12 MAPT, 15 and PreDIVA 14 ; the latter two show efficacy only in sub‐populations at increased risk for dementia. These trials have focused on healthy people not yet on the AD spectrum and are aimed to prevent dementia. Additional trials with asymptomatic older adults underway include the US POINTER, 16 SMARRT, 17 and HATICE 18 trials. However, there are no published studies that have examined the efficacy of a personalized, multimodal intervention in individuals with MCI or early AD and confirmed AD neuropathology.
Where previous trials typically target community‐dwelling older adults, and focus on primary risk reduction for dementia, an urgent goal is to intervene in a population at highest risk for progression to dementia: those with AD neuropathology. Astute clinicians have made efforts to define a precision medicine approach to primary risk reduction for cognitive aging, including the Precision Aging model, which has harmonized the risk categories including chronic stress, immune dysfunction, cardiovascular risk, glucose dysregulation, and detailed assessments for cognitive decline in each risk category to develop a custom intervention. 19 , 20 Dr. Dale Bredesen has trailblazed the field of clinical, multi‐component, precision medicine for the treatment of cognitive decline with promising results. 21 However, these approaches have yet to be rigorously tested.
In our Precision Recommendations for Environmental Variables, Exercise, Nutrition, Training Intervention to Optimize Neurocognition (PREVENTION) pilot randomized controlled trial (RCT) we intervene with multiple modalities to target modifiable risk factors for AD. The program targets six systemic pathways implicated in promoting brain health and/development of AD: inflammation, cardiovascular health, metabolic health, nutritional deficiencies, toxicity, and endocrine functioning. For each study arm we implement a personalized, multimodal intervention, either with or without coaching, to promote positive changes in lifestyle behaviors (exercise, diet, cognitive stimulation, medical management, sleep, stress management, and nutritional supplementation), to help reduce cognitive decline in older adults with MCI or early stage AD with amyloid neuropathology. Our unique systems‐biology approach to data analysis will allow us to elucidate implicated physiological systems impacted by the intervention to more accurately inform future multimodal, precision medicine intervention strategies.
2. METHODS
2.1. Study design
The PREVENTION pilot study is a prospective, 12‐month, two‐arm, RCT (ClinicalTrials.gov NCT04082611) in older adults with early stage AD. Participants are assigned randomly to a personalized, multimodal lifestyle intervention with or without health coaching.
RESEARCH IN CONTEXT
Systematic review: Published studies of randomized controlled trials (RCTs) of lifestyle interventions targeting cognitive health were reviewed, along with epidemiological research, in addition to literature citations of mechanistic studies to extrapolate systemic biological pathways implicated in reducing cognitive decline in Alzheimer's disease (AD).
Interpretation: Our report seeks to inform the AD clinical and research communities of a novel approach to early stage AD intervention, in the form of a data‐driven multimodal lifestyle intervention. To date, preliminary efficacy data show high compliance.
Future directions: With results collected from this longitudinal trial in the next 2 to 3 years we seek to establish an evidence‐based framework for a clinical implementation model of reducing cognitive decline. Our multi‐omic approach applies dense phenotypic with systems‐biology analytical methods to explain mechanistic and causal underpinnings of individual trajectories, and seeks to inform the design of future studies and optimize personalized interventions in patients in the early stages of AD. Our data‐intense trial design enables smaller trials that produce knowledge even for complex interventions.
The primary objectives of PREVENTION are: (1) conduct a pilot feasibility and efficacy study and (2) gather dense data from patients to explore the trajectory of their cognitive health using a systems‐biology approach. To achieve the latter objective, we will analyze longitudinal multi‐omic data. These data include metabolomics, proteomics, genetics, microbiome, behavior metrics, and cognition metrics from each participant assembled as personalized, dense, dynamic data (PD3) 22 clouds. This dense phenotyping approach allows us to create comprehensive data sets on individuals to draw inferences, as opposed to necessitating clinical trials with larger sample sizes (N = 1000 +), which is prohibitive in most cases due to unrealistic recruitment targets of this well‐characterized population and the excessive cost, staffing, and time limitations related to running those trials.
2.2. Participants
The PREVENTION trial examines efficacy in patients early in the disease course in a clinical practice setting. Participants are drawn from the high‐volume Pacific Brain Health Center (PBHC) memory care clinic, which has the benefit of assessing feasibility in a real‐world clinical setting. PBHC is located in Santa Monica, California, with referrals from the surrounding area, including Los Angeles County. PBHC is part of Providence St. Joseph Health system. Sixty (60) participants will be enrolled (30 in each arm). PREVENTION focuses on AD‐type dementia by requiring evidence of amyloid burden as an enrollment criterion, as defined by a positive amyloid positron emission tomography (PET) scan, cerebrospinal fluid (CSF), or blood plasma sample. For PREVENTION, probable AD is defined using the 2018 National Institute on Aging–Alzheimer's Association (NIA‐AA) Research Framework. 24 Our study physicians refer patients for enrollment based on three diagnostic categories: (1) mild AD according to criteria established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA), 25 (2) MCI, according to the Petersen criteria, assessed by neuropsychological assessments, 26 or (3) those with subjective cognitive decline (SCD) as defined by the Subjective Cognitive Decline Initiative working group. 27
Eligibility criteria include: age at least 50‐years‐old, FAST 23 Stage 2‐4 SCD, MCI or early AD no functional impairment, amyloid positivity, English fluency, possess and ability to operate a computer, ability to converse with a coach telephonically, normal or corrected visual and hearing acuity, answers “no” to all items of the PAR‐Q+ 28 or provides physician clearance to participate in a moderately intensive exercise program. Exclusion criteria: non‐AD neurodegenerative disorder (eg, lewy body dementia, frontal‐temporal dementia), existing diagnosis of cerebrovascular disease as the primary cause of cognitive impairment, participant or immediate family members with known AD mutation in the PSEN or APP genes, MMSE below 19 or Clinical Dementia Rating Scale ≥ 2, as evidenced in the patient medical history.
Participants will be 1:1 randomized into either a 12‐month personalized, multimodal intervention with health coaching (MMIC) or personalized, multimodal clinical recommendations only, but not provided coaching or the other active interventions (CR).
2.3. Personalized multimodal intervention program
Participants in both treatment arms will receive personalized, evidence‐based, multimodal lifestyle recommendations for improving their brain health from a study physician. Recommendations, including clinical labs and prescribed lifestyle and nutritional supplement interventions, are reviewed and questions are answered during four quarterly physician visits. For each participant in both study arms, the study physician provides recommendations in the form of a user‐friendly educational resource guide. These include detailed education and guidelines for exercise, diet, cognitive stimulation, and medical management and nutritional supplements and environmental factors, including sleep and stress management (Appendix A).
A personalized program is given to each participant to educate them about and prioritize lifestyle changes. Personalization is informed from their medical history, assessments, clinical blood labs, physical activity levels, body mass index, and apolipoprotein gene (APOE) status (Table 1). They also receive personalized intervention priorities, lab values, and nutritional supplement recommendations (Appendix B).
TABLE 1.
Six systemic pathways to optimize brain health: clinical and discovery laboratory biomarkers
|
Inflammatory markers Stress and inflammation (clinical) Albumin/globulin (A/G) ratio; cortisol, serum; matrix metalloproteinase‐9 (MMP9), serum; interleukin‐6 (IL‐6), serum; high‐sensitivity C‐reactive protein (hs‐CRP), serum; tumor necrosis factor alpha (TNFα), serum. Stress (discovery) Four‐point cortisol (saliva‐at home; assay: ZRT Laboratory, Beaverton, OR). Neurofilament light chain (discovery) Neurofilament light chain (NfL) protein (serum; Quanterix, Billerica, MA). |
|
|
Cardiovascular markers Complete blood count (clinical) White blood cells (WBCs); red blood cells (RBCs); hemoglobin, hematocrit; mean corpuscular volume (MCV); mean corpuscular hemoglobin (MCH); mean corpuscular hemoglobin concentration (MCHC); red cell volume distribution width‐CV (RDW‐CV); platelet count; mean platelet volume (MPV); automated absolute neutrophils; absolute neutrophils; absolute lymphocytes; absolute monocytes; absolute eosinophils; absolute basophils; absolute immature granulocytes; %nRBC; absolute nRBC. Lipids (clinical) Lipid panel: total cholesterol, triglycerides, high‐density lipoprotein (HDL), cholesterol, low‐density lipoprotein (LDL), lipid ratios; LipoFit lipoprotein by NMR: LDL particle number (LDL‐p), small LDL‐p, large very‐low‐density‐lipoprotein (VLDL‐p), HDL‐p (total), large HDL‐p; LDL size, VLDL size, HDL size, total cholesterol, triglycerides, HDL cholesterol, LDL, calculated, EER LipoProfile; omega‐3 and ‐6 fatty acids: eicosapentaenoic acid (EPA); docosahexaenoic acid (DHA); omega‐3 (EPA+DHA) index; omega‐6/omega‐3 ratio; arachidonic acid; EPA/arachidonic acid ratio; lipoprotein (a); oxidized LDL cholesterol; small dense LDL cholesterol. Genetics (clinical) Apolipoprotein E (APOE) genotype; methylenetetrahydrofolate reductase (MTHFR) genotype. Proteomics (discovery) Cardiovascular II; cardiovascular III; inflammation, neurology; and neuro‐exploratory panels collectively assay 1161 unique proteins (plasma; Olink, Uppsala, Sweden). |
|
|
Metabolic markers Comprehensive metabolic panel (clinical) Sodium (Na); potassium (K); chloride (Cl); carbon dioxide (CO2); anion gap; glucose; blood urea nitrogen (BUN); creatinine; calcium; albumin; bilirubin total (calculated); total protein; aspartate aminotransferase (AST) (SGOT); alanine aminotransferase (ALT) (SGPT); alkaline phosphatase (ALP); globulin; A/G ratio; BUN/creatinine ratio. Diabetes risk (clinical) Fasting glucose; hemoglobin A1c (HbA1c); insulin, serum; HOMA‐IR. Metabolomics (discovery) Global Metabolic Profile that surveys ≈1000 metabolites (including amino acids, carbohydrates, lipids, nucleotides, microbiota metabolites, cofactors, and vitamins (plasma; Metabolon, Morrisville, NC). |
|
|
Nutritional deficiencies Vitamins and minerals (clinical) Magnesium, RBC; magnesium, serum; vitamin E, serum; vitamin B12, serum; vitamin B6, serum; homocysteine, serum; vitamin B1 (thiamine), whole blood; vitamin C (ascorbic acid), serum; copper, serum; zinc, serum; glutathione, total; selenium, serum. |
|
|
Toxicity markers Toxins (clinical) Antidiuretic hormone (ADH), plasma; compliment C4a level by radioimmunoassay (RIA); human leukocyte antigen (HLA)‐DR/DQ genotype; leptin, serum; alpha melanocyte stimulating hormone (MSH), plasma; osmolality, plasma; transforming growth factor beta (TGFB1), serum; vascular endothelial growth factor (VEGF), plasma; vasoactive intestinal protein (VIP), plasma. Heavy metals (clinical) Arsenic, whole blood; mercury, whole blood; lead, whole blood; cadmium, whole blood. |
|
|
Endocrine markers Hormones/thyroid (clinical) Total testosterone, serum; free testosterone, serum; estradiol, serum; DHEA‐sulfate, serum; progesterone, serum; pregnenolone, serum; thyroid stimulating hormone (TSH); serum; free T3, serum; free T4, serum; reverse T3, serum. |
|
|
Multiple exploratory systems Gut microbiome (discovery) 16S rRNA sequencing (at‐home stool sample: ≈4400 taxa; DNAgenotek, Ottawa, Canada). Vesicles (discovery) RNA exomes (whole blood; ISB). Whole genome sequencing (discovery) DNA extraction (whole blood; ISB). |
2.3.1. Non‐coached clinical recommendations arm (CR)
Standard of care plus data‐driven, multimodal, and lifestyle clinical recommendations (Appendix B) will be given to participants in the CR arm. These participants will be incentivized to remain in the trial through the same schedule of clinical labs and study physician visits as the MMIC arm. The study physician will support the lifestyle and nutritional supplement recommendations by discussing compliance and providing adjustments as needed. At the end of the trial, participants in the CR arm will have the option to receive health coaching, group exercise, and nutrition counseling for 3 months.
2.3.2. Multimodal intervention with coaching (MMIC) arm
The MMIC arm will also engage with supportive coaching and be provided with the resources to carry out these recommendations. The remote health coach (an RDN) documents and tracks the patient goals. The MMIC arm will receive: 13 one‐on‐one health coaching calls (and email contact as needed) with a health coach (M.R.), seven one‐on‐one diet counseling visits via video or calls with an RDN, 33 live group cognitive‐enhanced, multi‐component (aerobic, strength, and neuromotor training) exercise classes (FitBrain) via video calls led by a CPT, and provided an online neurocognitive training program (BrainHQ from Posit Science). Participants will receive intervention supplies including nutritional supplements, glucose monitors, and ketone monitors free of charge. Follow‐up testing on most outcome measures will occur at around 3, 6, and 12 months, where the midpoint and final assessments have had flexible timing due to coronavirus disease 2019 (COVID‐19).
2.4. Procedures
The Western Institutional Review Board approved this research study on 5/7/2019 (Protocol # 20190583), and informed consent is collected from all participants. Due to COVID‐19, we have transitioned from in‐person consent to remote consent. All participants receive and sign the Experimental Research Subject's Bill of Rights prior to signing the informed consent form. Prior to enrollment in the study, subjects will also complete an authorization of use and disclosure of protected health information and authorization of medical record release.
We monitor each patient for 12 months. We will perform comprehensive, predominately remote assessments and biomarker collection at baseline, and at 3, 6, and at 12 month timepoints to evaluate differences in cognitive trajectory and impact on biomarkers between the two arms (Figure 1). Participants in the CR group may be monitored for up to 18 months if they elect to receive intervention services.
FIGURE 1.
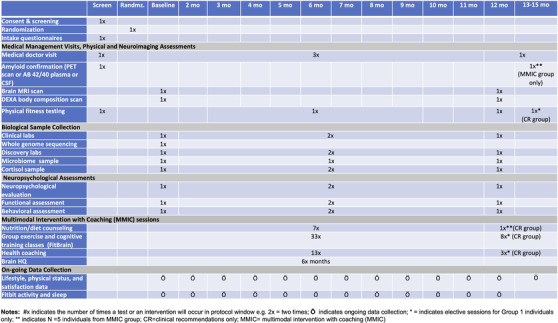
The PREVENTION RCT study timeline
2.4.1. AD pathology confirmation
After consent, but prior to study enrollment, all participants have amyloid status confirmed through PET imaging with Amyvid (Florbetapir F18 Injection), 29 CSF amyloid, or blood plasma AB42/40 ratio. 30
2.4.2. Cognitive tests
A study research staff member trained and supervised by the study's licensed clinical neuropsychologist (S.P.) administers assessments and questionnaires via telehealth. 31 Due to COVID‐19, we have transitioned from in‐person to virtual assessment.
Neurocognitive assessments are conducted at baseline, ≈3 and 6 months and at 12 months. Our primary outcome measure of episodic memory is the RAVLT (NIH Toolbox, 32 Supplemental). Global cognitive ability is assessed with the Montreal Cognitive Assessment,* Wechsler Memory Scale 4th edition (WMS‐IV) Visual Reproduction I & II Test,* Oral Trail‐Making Test B,* WMS‐IV Visual Paired Associates I & II Recall and Recognition Tests,** and ecological memory is assessed with the Rivermead Story Delay sub‐test.** Receptive vocabulary, working memory, and processing speed are assessed with tests from the NIH Toolbox: Picture Vocabulary,*** List Sorting Working Memory,* Oral Symbol Digits,* Picture Sequence Test,* and Oral Reading Recognition Test. Self‐reported cognitive assessments include: PROMIS 2.0 Cognitive Functions and Cognitive Functional Abilities. To calculate the cognitive composite relevant to AD, selected test score results will be transformed into a z‐score. 33 , 34 *Included in the cognitive composite score. **Collected at Baseline and 12 months. ***Collected at Baseline only.
2.4.3. Clinical and patient‐reported assessments
Demographic, health, and other information regarding patients’ risk status for the development of AD are collected at study baseline to assist in guiding clinical recommendations. Patient‐oriented outcome assessments administered at baseline, and 3, 6, and 12 months include: (1) Perceived Stress Scale‐4 (PSS‐4), 35 (2) PROMIS‐2 36 Cognitive Functions, ‐2 Cognitive Functional Abilities Profiles, and ‐29 Profile v2.1, and (3) Functional Activities Questionnaire (FAQ). At baseline and 12 months we collect: (1) FAST staging, (2) MESA 10‐year Coronary Heart Disease Risk Score, 37 (3) International Physical Activity Questionnaire (IPAQ), 38 and (4) Food Frequency Questionnaire (FFQ). 39
2.4.4. Clinical and discovery biomarker collection
Table 1 details clinical tests that can be ordered for the study by the study physician to inform their treatment plan or to include for dense data discovery purposes. Clinical data are collected at baseline, twice throughout the trial (at ≈3 and 6 months), and at 12 months, and they are analyzed locally at the Providence Saint John's Health Center Pathology Laboratory or sent out to QUEST or ARUP laboratories. Plasma, serum, and whole blood samples are also bio‐banked at our research institute.
2.4.5. Biometric and fitness data
All participants undergo a DEXA scan to explore body composition. A fitness assessment by a CPT is at baseline and 6 and 12 months, including a Short Physical Performance Battery (SPPB) of lower extremities 40 and an NIH 2‐minute walk test of cardiovascular endurance. 41
2.4.6. Continuous activity monitoring
Physical activity, heart rate, and sleep data are collected on a nearly continuous basis by a FitBit Charge 3 tracker during the 12‐month intervention.
2.4.7. Magnetic resonance imaging (MRI)
An MRI scan is conducted on a GE 3T at baseline and at 12 months to examine changes in our primary outcome measure of hippocampal volume. MRI data are processed using the Neuroreader 42 neuroimaging software to extract normalized region brain volumes. Neuoreader is an FDA‐cleared brain volumetric software package that has validated that hippocampal volume remains the best single volumetric predictor of conversion from MCI to AD. 43
Additional exploratory outcome scans for future analyses include: 15‐direction DTI, resting‐state functional MRI, and 3D ASL.
2.5. Statistical analyses
2.5.1. Primary outcomes
We will measure and analyze ease of recruitment and the retention rate. We expect to have low attrition (≤30%) for both arms and a high adherence to the multimodal lifestyle recommendation‐coached intervention, with a target of ≥70% adherence to be an effective intervention. 39 In response to the COVID‐19 pandemic we developed a predominately remote trial delivery. Remote delivery should lead to increased protocol adherence. Adherence will be calculated as the ratio of completed to assigned sessions. We have systematically considered retention and behavioral engagement strategies. 44 , 45 , 46 To determine adherence to coached intervention we track the attendance to: health coaching calls, FitBrain group exercise sessions, dietary counseling calls, usage of computerized cognitive training, and consumption of supplements (as determined by the study physician). Compliance reports from CRA interviews will generate subjective compliance scores to each of the seven primary intervention components: exercise, diet, cognitive stimulation, social interaction, supplements, sleep, and stress management. The ability to assess individual adherence to study sessions and compliance to the primary interventions will allow investigation of the relative contribution of each to overall effect. Activity and sleep data will be aggregated using a custom FitBit data aggregation platform. These will be objective measures of compliance, will assist with health coaching, and will help determine if patients increase their physical activity (METS/minutes) and sleep habits (FitBit Sleep Score). The study physician also generates a measure of subjective compliance from the patient, corroborated by their care partner, using the Clinician Rating Scale every 3 months. 47 Subjective compliance is rated on a scale from 1 to 7 (7 the highest compliance).
We are seeking to determine the efficacy of the personalized multimodal lifestyle intervention in early stage AD patients to reduce (or reverse) cognitive decline and elucidate individual differences in disease progression. We believe that the coached intervention will promote greater compliance with the recommendations, producing greater improvements in cognitive functioning and brain volumetrics compared participants those in the CR arm. We will employ a linear mixed model to characterize the change over time of our primary neuropsychological outcome measure (RAVLT learning score) and neural biomarker outcome (hippocampal volume) and our secondary outcome measures (composite cognitive score and BUN). We will also conduct exploratory analyses on the neuroimaging data sets (DTI, resting state functional MRI, and ASL) to assess effects of compliance on brain structure and function.
2.5.2. Systems‐biology personal, dense, dynamic data analysis
We will conduct analyses of molecular and physiological subsystems that drive and respond to healthy and disease states to create understanding of health and disease trajectories in each individual. We will perform time‐dependency, dose‐response, and other knowledge‐generating analyses. Thousands of inter‐omic correlations will be computed using personal, dense, dynamic data clouds to identify associations that could be followed up with perturbation experiments. We will partition the correlations into data communities, which place biomarkers in context within biological networks. We will also apply multiple machine‐learning techniques for classification. These analyses are facilitated by contextual and validation data in our aggregated database of all prior studies from the Institute for Systems Biology (ISB) and coupled with larger databases such as ADNI and AMP‐AD. This enables demonstration of consistency of newly acquired data with past knowledge and data. The ISB‐led Pioneer 100 Wellness Project study demonstrated our longitudinal, multi‐omic data analytical approach. 22 Analysis of data from individuals on a trajectory of early stage AD will permit exploration between measured variables and models of causation that can further advance the knowledge of and research into the pathologies that contribute to brain degeneration. Random assignment to intervention arms increases the diversity of interventions to increase the epistemological value of systems analyses. We will also curate outlier values to identify perturbed biological networks. Functional analysis of these outliers can—even on an individual basis—inform elements of a patient's biology that have been pushed into abnormal states that reflect pathologies.
Our dense data and research design will help facilitate identification of individual components of the therapy that were or were not effective. Most of the components of our intervention have already been shown to be individually effective, so it is arguably most important at this point in time to evaluate the ensemble. By combining these interventions, we are testing synergistic effects that may not be detectable by trials that test only one intervention at a time. Fully combinatorial strategies are infeasible because the number of trial arms required grows as 2n with the number of interventions. Therefore, we believe future multimodal trials should include dense data collection and systems analyses.
3. RESULTS
The PREVENTION study enrollment began in September 2019. We have consented 37 patients, of which 3 are currently in the screening phase awaiting amyloid results, 12 did not meet eligibility criteria, 2 declined enrollment post‐screening, and 20 enrolled and randomized into the trial (10 CR, 10 MMIC). To date we have enrolled 1 SCD (FAST Stage 2), 15 MCI (FAST Stage 3), and 4 early AD patients (FAST Stage 4). One patient has completed the study. We have a retention of 95% (one patient from the MMIC group has withdrawn from the study due to COVID‐19‐related concerns regarding personal health and safety). Average age is 69 years (range: 52 to 80); 53% are female.
Adherence has been high in the coached arm (Table 2). Adherence to dietary counseling is 86%. Adherence to the FitBrain group exercise sessions is 80%. Self‐reported enjoyment and engagement in those sessions has been very high and no adverse events during FitBrain were reported. Adherence to the telephone‐delivered health coaching is 75%. All participants in the coached arm have utilized the BrainHQ platform. Average program usage is 2 hours per month (range: 0.2 to 5 hours per month). Participants in MMCI average 6 of a possible 7 subjective compliance and are interested and engaged in the program and require little prompting to remain compliant. This initial adherence and compliance data demonstrate feasibility, including engagement and adoption, of this personalized, multimodal lifestyle intervention program in early stage AD patients.
TABLE 2.
Interim compliance with the PREVENTION personalized, multimodal lifestyle intervention (N = 20). Mean (standard deviation)
| Overall compliance with multimodal lifestyle intervention | Score |
|---|---|
| Clinician rating scale (1‐7) | 6.0 (1.1) |
| Proportion of compliance | 86% (16%) |
| Compliance with intervention components | |
|---|---|
| Supplements | 92.5% (9%) |
| Social activity | 89.2% (9%) |
| Stress management | 88.3% (10%) |
| Exercise | 85% (25.4%) |
| Sleep | 84.2 (22.3%) |
| Cognitive stimulation/learn new things | 83.3% (25%) |
| Diet | 82.5% (24.5%) |
4. DISCUSSION
Although no disease‐modifying pharmacological therapies exist for early stage AD, preventative lifestyle modification strategies and interventions have been posited to be the most promising means to slow AD‐related cognitive decline. 48 Furthermore, the COVID‐19 pandemic has exacerbated barriers that prevent individuals from fully engaging in healthy lifestyle behaviors to ameliorate and remediate cognitive decline. To promote sustainable behavioral changes through lifestyle modification, it is critical that preventative interventions are personalized, allowing for individual variability, personal preferences, and variations in access. 5 Health coaching can encourage, inspire, and empower patients to reach their maximum potential. 49
The PREVENTION trial is designed to evaluate the efficacy of a personalized, predominantly remote‐based, multimodal intervention for the treatment of early stage cognitive decline due to AD neuropathology. In this trial we are also systematically identifying biomarkers across all physiological systems for the earliest possible detection of the AD transition in individuals as well as biomarkers for assessing and improving the AD in response to multimodal systems therapeutic approaches. The PREVENTION trial design should enable not just paradigm shifts in the conduct of clinical trials, but also innovations in therapy, including a focus on multimodal therapies that are delivered through coaching interventions.
We expect completion of the PREVENTION trial within the next 2 years. From this trial we seek to develop an evidence‐based framework for a clinical implementation model of reducing cognitive decline. Our multi‐omic, systems‐biology analytical efforts to explain mechanistic or causal underpinnings of individual trajectories, seeks to inform the design of future hypotheses and to improve and better personalize the intervention in patients in the early stage of AD.
CONFLICT OF INTEREST
None.
Supporting information
Appendix A
Appendix B
ACKNOWLEDGMENTS
We would like to thank the patients and families that participated in this research, without which this research would not be possible. This study has benefited from the clinical infrastructure of the Pacific Brain Health Center (PBHC), specifically Mihae Kim, NP, Claudia Wong, NP, and Kyrsten Cardenas, and all of the PBHC clinical staff members. We are also grateful to the clinical research infrastructure and leadership provided by the Providence Saint John's Cancer Institute, specifically, Dr. Neil Martin, Dr. Santosh Kesari, Brian Anderson, and Elena Berezhnikh. We are grateful to the excellent integrative, nutritional counseling from Elizabeth Baron Cole & Associates, including registered dietitian nutritionists: Elizabeth Baron Cole, Evette Richardson, and Jordan Stachel. This work was supported by Providence St. Joseph Health, Seattle, WA [Alzheimer's Translational Pillar (ATP)]; Saint John's Health Center Foundation; Pacific Neuroscience Institute Foundation, including the generous support of the Singleton and McLoughlin families; and the National Institutes of Health [U01AG046139, RF1AG057443, U01AG061359, R01AG062514, R21AG061494].
McEwen SC, Merrill DA, Bramen J, et al. A systems‐biology clinical trial of a personalized multimodal lifestyle intervention for early Alzheimer's disease. Alzheimer's Dement. 2021;7:e12191. 10.1002/trc2.12191
Trial registration: ClinicalTrials.gov: NCT0408261. Registered on September 9, 2019.
REFERENCES
- 1. World Health Organization . Dementia Fact Sheets. https://www.who.int/news‐room/fact‐sheets/detail/dementia Published 2020. Accessed March 11, 2020.
- 2. Alzheimer's Association . 2019 Alzheimer's disease facts and figures. Alzheimer's Dement. 2019;15(3):321‐387. 10.1016/j.jalz.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 3. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment report of the guideline development, dissemination, and implementation. Neurology. 2018;90(3):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cummings JL, Morstorf T, Zhong K, Alzheimer's disease drug‐development pipeline: few candidates, frequent failures. Alzheimer's Res Ther. 2014;6(4):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bott NT, Hall A, Madero EN, et al. Face‐to‐face and digital multidomain lifestyle interventions to enhance cognitive reserve and reduce risk of Alzheimer's disease and related dementias: a review of completed and prospective studies. Nutrients. 2019;11(9):2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Risk Reduction of Cognitive Decline and Dementia WHO Guidelines. Geneva; 2019. [PubMed] [Google Scholar]
- 7. Edwards GA, Gamez N, Escobedo G, Calderon O, Moreno‐Gonzalez I, Modifiable risk factors for Alzheimer's disease. Front Aging Neurosci. 2019;11(JUN):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131(5):645‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population‐based data. Lancet Neurol. 2014;13(8):788‐794. [DOI] [PubMed] [Google Scholar]
- 11. Mavros Y, Gates N, Wilson GC, et al. Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: outcomes of the study of mental and resistance training. J Am Geriatr Soc. 2017;65(3):550‐559. [DOI] [PubMed] [Google Scholar]
- 12. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255‐2263. [DOI] [PubMed] [Google Scholar]
- 13. Rebok GW, Ball K, Guey LT, et al. Ten‐year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Charante EPM, Richard E, Eurelings LS, et al. Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet. 2016;388(10046):797‐805. [DOI] [PubMed] [Google Scholar]
- 15. Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16(5):377‐389. [DOI] [PubMed] [Google Scholar]
- 16. Alzheimer's Association . U.S. POINTER | Alzheimer's Association. https://alz.org/us‐pointer/overview.asp Accessed January 18, 2021.
- 17. Yaffe K, Barnes DE, Rosenberg D, et al. Systematic multi‐domain alzheimer's risk reduction trial (SMARRT): study protocol. J Alzheimer's Dis. 2019;70(s1):S207‐S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbera M, Mangialasche F, Jongstra S, et al. Designing an internet‐based multidomain intervention for the prevention of cardiovascular disease and cognitive impairment in older adults: the HATICE trial. J Alzheimer's Dis. 2018;62(2):649‐663. [DOI] [PubMed] [Google Scholar]
- 19. Hodes JF, Oakley CI, O'Keefe JH, et al. Alzheimer's “Prevention” vs. “Risk Reduction”: transcending Semantics for Clinical Practice. Front Neurol. 2019;9(JAN):1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryan L, Hay M, Huentelman MJ, et al. Precision aging: applying precision medicine to the field of cognitive aging. Front Aging Neurosci. 2019;11(JUN):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bredesen DE, Sharlin K, Jenkins D, et al. Reversal of cognitive decline: 100 patients. J Alzheimer's Dis Park. 2018;08(05):1‐6. [Google Scholar]
- 22. Price ND, Magis AT, Earls JC, et al. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat Biotechnol. 2017;35(8):747‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer's disease: reliability, validity, and ordinality. Int Psychogeriatrics. 1992;4(3):55‐69. [DOI] [PubMed] [Google Scholar]
- 24. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer's Dement. 2017;13(3):296‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warburton DER, Jamnik VK, Bredin SSD, Gledhill N. The physical activity readiness questionnaire for everyone (PAR‐Q+). Heal Fit J Canada. 2011;4(2):3‐17. [Google Scholar]
- 29. Rosenberg PB, Wong DF, Edell SL, et al. Cognition and amyloid load in Alzheimer disease imaged with florbetapir F 18 (AV‐45) positron emission tomography. Am J Geriatr Psychiatry. 2013;21(3):272‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma β‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):E1647‐E1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta‐analysis. Neuropsychol Rev. 2017;27(2):174‐186. [DOI] [PubMed] [Google Scholar]
- 32. Weintraub S, Dikmen SS, Heaton RK, et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014;20(6):567‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langbaum JB, Hendrix SB, Ayutyanont N, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer's disease. Alzheimer's Dement. 2014;10(6):666‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isaacson RS, Hristov H, Saif N, et al, Individualized clinical management of patients at risk for Alzheimer's dementia. Alzheimer's Dement. 2019;15(12):1588‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385‐396. [PubMed] [Google Scholar]
- 36. Cella D, Riley W, Stone A, et al. The patient‐reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol. 2010;63(11):1179‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McClelland RL, Jorgensen NW, Budoff M, et al. 10‐Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors derivation in the MESA (Multi‐Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) STUDY And the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755‐762. [DOI] [PubMed] [Google Scholar]
- 39. Barclay AW, Flood VM, Brand‐Miller JC, Mitchell P. Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi‐quantitative food‐frequency questionnaire. Public Health Nutr. 2008;11(6):573‐580. [DOI] [PubMed] [Google Scholar]
- 40. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. Journals Gerontol. 1994;49(2):M85‐94. [DOI] [PubMed] [Google Scholar]
- 41. Bohannon RW, Wang YC, Gershon RC. Two‐minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys Med Rehabil. 2015;96(3):472‐477. [DOI] [PubMed] [Google Scholar]
- 42. Ahdidan J, Raji CA, DeYoe EA, et al. Quantitative neuroimaging software for clinical assessment of hippocampal volumes on MR imaging. J Alzheimer's Dis. 2015;49(3):723‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanpitukpongse TP, Mazurowski MA, Ikhena J, Petrella JR. Predictive utility of marketed volumetric software tools in subjects at risk for Alzheimer disease: do regions outside the hippocampus matter?. Am J Neuroradiol. 2017;38(3):546‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith DL. Patient nonadherence in clinical trials: could there be a link to postmarketing patient safety?. Drug Inf J. 2012;46(1):27‐34. [Google Scholar]
- 45. Robiner WN. Enhancing adherence in clinical research. Contemp Clin Trials. 2005;26(1):59‐77. [DOI] [PubMed] [Google Scholar]
- 46. Matsui D. Strategies to measure and improve patient adherence in clinical trials. Pharmaceut Med. 2009;23(5‐6):289‐297. [Google Scholar]
- 47. Kemp R, Hayward P, Applewhaite G, Everitt B, David A. Compliance therapy in psychotic patients: randomised controlled trial. Br Med J. 1996;312(7027):345‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merrill DA, Small GW. Prevention in psychiatry: effects of healthy lifestyle on cognition. Psychiatr Clin North Am. 2011;34(1):249‐261. [DOI] [PubMed] [Google Scholar]
- 49. Hayes E, Kalmakis KA. From the sidelines: coaching as a nurse practitioner strategy for improving health outcomes. J Am Acad Nurse Pr. 2007;19(11):555‐562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A
Appendix B


