Abstract
Objective: To evaluate the efficacy of Solitaire AB stent mechanical thrombectomy combined with thrombolysis and anticoagulant therapy (AT) in the treatment of cerebral venous sinus thrombosis (CVST) and its effect on neurological function and coagulation indices. Methods: Eighty-two patients with CVST were randomly divided into two groups according to the random number table method. The control group (n=41) were treated with arteriovenous thrombolysis combined with AT, and the observation group were treated with intravascular mechanical thrombectomy plus thrombolysis combined with AT. The effect of the treatment was evaluated 7 days after treatment, and a 6-month follow-up was conductedz after the course of treatment. The clinical efficacy, neurological function (National Institute of Health Stroke Scale (NIHSS), Modified Rankin Scale (mRS)), coagulation function, complications and prognosis were compared between the two groups. Results: The continuous improvement rate of clinical symptoms 7 days after treatment in the observation group (87.80%) was higher than that in the control group (58.54%) (P<0.01); The neurological function scores of both groups after treatment were lower than those before treatment (all P<0.001); The scores of NIHSS and mRS in the observation group were lower than those in the control group 7 days after treatment (all P<0.001). The coagulation indices of fibrinogen (FIB), prothrombin time (PT) and activated partial thromboplastin time (APTT) of 7 days after treatment in the observation group were shorter than those in the control group (all P<0.001), and the D-Dimer (D-D) level in the observation group was higher than that in the control group (P<0.001). The incidences of intracranial hemorrhage, infection, headache, quadriplegia, dizziness and drowsiness in the observation group were lower than those in the control group (all P<0.05). The recanalization rate of venous sinus in the observation group was higher than that in the control group 6 months after treatment (P<0.01). Conclusion: Intravascular mechanical thrombectomy plus thrombolysis combined with AT for patients with CVST is effective, which can effectively improve the coagulation function and promote the recovery of neurological function, with fewer complications and a good prognosis.
Keywords: Cerebral venous sinus thrombosis, mechanical thrombectomy, thrombolysis, anticoagulant therapy, neurological function, coagulation indices
Introduction
Cerebral venous sinus thrombosis (CVST), which can be divided into venous sinus and cerebral venous thrombosis, is a cerebrovascular disease of venous return obstruction caused by a variety of reasons. According to the property of the lesion, CVST can be divided into two types of non-inflammatory and inflammatory lesions, and the latter can be classified as suppurative venous thrombosis, venous sinusitis and thrombophlebitis [1]. Previous studies have shown that CVST mostly occurs in people with wasting diseases, brain trauma, blood disease and heart disease, and its clinical manifestations are systemic symptoms, increased intracranial pressure, cavernous sinus and thrombosis, etc., which affect the healthy life of patients [2]. The clinical manifestations of CVST are complex with high clinical disability rate and fatality rate, and there is no unified standard for its treatment [3,4]. Arterio-venous thrombolysis combined with anticoagulant therapy (AT) is a common treatment intervention in patients with CVST. Although it can improve the symptoms and delay the development of the disease, the long-term prognosis is poor, and the mortality is high [5]. Intravascular mechanical thrombectomy is the treatment to remove the intravascular thrombus tissue with the aid of angiography, which has a wide range of effect, and the blood vessels can be recanalized after the completion of the operation, and the safety is high [6]. Previous study has shown that intravascular mechanical thrombectomy combined with thrombolysis and AT in patients with CVST could reduce clinical mortality and lead to a good prognosis. However, the study did not consider its effects on neurological function and coagulation indices in patients [7]. Therefore, this study evaluated the efficacy of intravascular mechanical thrombectomy and thrombolysis combined with anticoagulant in the treatment of CVST.
Materials and methods
Clinical data
A total of 82 patients with CVST who were treated in our hospital from February 2018 to January 2020 were prospectively selected as the research objects for the study. They were divided into two groups according to the random number table method, the control group (n=41) received intravenous thrombolysis combined with AT, and the observation group (n=41) received intravascular mechanical thrombectomy plus thrombolysis combined with AT. Inclusion criteria: (1) Patients with the disease met the diagnostic criteria of CVST, and who were diagnosed by digital subtraction angiography (DSA) on cerebral blood flow [8]. (2) The patients met the indications for the treatments of thrombolysis, mechanical thrombectomy and AT, and all of them can tolerate the treatments. (3) The patient had complete baseline data and follow-up data. Exclusion criteria: (1) Patients complicated with mental disorders, cognitive abnormalities or autoimmune diseases. (2) Patients with severe hepatic and renal dysfunction, organic diseases or systemic infectious diseases.
This study was approved by the hospital Ethics Committee, and the consent form was signed by the patients or their family members.
Methods
Control group: Patients were treated with intravenous sinus thrombolysis. (1) Intravenous sinus thrombolysis, all patients received general anesthesia by tracheal intubation. After the anesthesia took effect, a microcatheter was placed and a total dose of (200-250) × 103 U of urokinase (Techpool Bio-pharma Co., Ltd., specification: 10,000 units, China) was infused at the rate of 1 U per minute. Conventional AT was given within 1 day after the slow injection. (2) AT. AT was the basic treatment for CVST patients. All patients were treated with conventional AT after admission. Heparin sodium (Chengdu Baiyu Pharmaceutical Co., Ltd., specification: 0.6 mL of 6000 AxalU, China) was injected intravenously. After preoperative preparation was improved, the endovascular treatment was carried out within 48 hours. After operation, low molecular heparin was hypodermically injected with a dose of 50-60 IU/kg, twice a day. At the same time, warfarin (Shanghai Sine Pharmaceutical Laboratories Co., Ltd., specification: 5 mg, China) was given at 2.5-6.0 mg/day for 3 consecutive days. After the condition was stable, the dosage of warfarin was adjusted, and INR was controlled to 2.5-3.0. After discharge, warfarin was continued taken orally for 6 months.
Observation group: Intravascular mechanical thrombectomy plus thrombolysis combined with AT were used. The treatment of thrombolysis combined with AT was the same as that of the control group. The methods of intravascular mechanical thrombectomy were as follows: After the patients received general anesthesia by endotracheal intubation, routine disinfection was carried out and hole towels were laid. The approach of left femoral artery was selected for puncture, and 6 F Envoy guiding catheter was inserted. The angiography of cerebral blood flow was visualized and developed quickly and thoroughly. The occlusive site of CVST and collateral circulation around the lesion were determined. The end of the guide tube was placed at the selected blood vessel with the aid of the guide wire. Rebar 18 stent microcatheter (Covidien Company, USA) was placed slightly in front of CVST under the guidance of microwire, and thrombus was removed with Solitaire AB stent (Ev3 Company, USA). At the same time, angiography was performed again to determine the aspiration of CVST and whether it was necessary to remove the thrombus again. The effect of the patients was evaluated 7 days after treatment, and the patients were followed up for 6 months at the end of the course of treatment.
Outcome measurements
The main outcomes
(1) Clinical efficacy. After 7 days treatment, the clinical efficacy of the two groups was evaluated from three aspects: continuous improvement of clinical symptoms (no aggravation of symptoms after treatment, improvement of symptoms), clinical aggravation (poor treatment effect, aggravation of the disease), and no change (no obvious change after treatment). Improvement rate = number of cases of continuous improvement of clinical symptoms/total number of cases × 100% [9]. (2) Neurological function. NIHSS scale (The total score is 42, and the lower the score, the better the effect.) and mRS scale (The total score is 5, and the lower the score, the better the effect.) were used to evaluate the neurological function of patients in both groups before and 7 days after treatment [10,11].
The secondary outcomes
(1) The levels of prothrombin time (PT), partial thromboplastin time (APTT), fibrinogen (FIB) and D-Dimer (D-D) were measured by automatic coagulation analyzer before treatment and 7 days after treatment [12]. (2) Complication: The incidences of intracranial hemorrhage, infection, headache, quadriplegia, dizziness and drowsiness were recorded in the two groups. (3) Prognosis. The venous sinus recanalization rate (complete recanalization, partial recanalization and non-recanalization) was calculated after 6-month follow-up and DSA reexamination in both groups. Venous sinus recanalization rate = (number of complete recanalization + partial recanalization)/total number of cases × 100%.
Statistical analysis
SPSS18.0 software was used to process the data. The enumeration data were tested by χ2 test and expressed by n (%), and the measurement data were expressed by mean ± standard deviation (x̅ ± sd). All the measurement data were in accordance with normal distribution. The mean values of the two groups were compared with two independent samples t-test. Paired t-test was used to compare the mean before and after intervention in the same group, and repeated measures analysis of variance was used to analyze the repeated data. P<0.05 indicated that the difference was statistically significant.
Results
Comparison of clinical data between the two groups
The clinical data of the two groups were collected. There was no statistical significance in gender, age, course of disease, BMI and clinical manifestations between the observation group and the control group (all P>0.05), and the data were comparable, as shown in Table 1.
Table 1.
Comparison of clinical data between the two groups (n, %)
| Groups | Observation group | Control group | χ2 | P |
|---|---|---|---|---|
| Gender | 1.392 | 0.734 | ||
| Male | 27 (65.85) | 26 (63.41) | ||
| Female | 14 (34.15) | 15 (36.59) | ||
| Age (years) | 43.8±5.8 | 43.7±5.7 | 0.079 | 0.937 |
| Course of disease (Days) | 32.8±4.9 | 32.7±4.9 | 0.185 | 0.854 |
| BMI (kg/m2) | 22.47±1.73 | 22.41±1.69 | 1.683 | 0.335 |
| Clinical manifestations | 1.124 | 0.693 | ||
| Headache with nausea and vomiting | 26 (63.41) | 27 (65.85) | ||
| Epilepsia | 9 (21.95) | 7 (17.07) | ||
| Conscious disturbance | 10 (24.39) | 9 (21.95) | ||
| Visual disturbance | 7 (17.07) | 5 (12.20) |
Note: BMI: body mass index.
Comparison of clinical efficacy in the two groups
After 7 days of treatment, the continuous improvement rate of clinical symptoms in the observation group (87.80%) was higher than that in the control group (58.54%) (P<0.01), as shown in Table 2.
Table 2.
Comparison of clinical efficacy between the two groups (n, %)
| Groups | Continuous improvement of clinical symptoms | Clinical aggravation | No change | Improvement rate |
|---|---|---|---|---|
| Observation group (n=41) | 36 (87.80) | 3 (7.32) | 2 (4.88) | 36 (87.80) |
| Control group (n=41) | 24 (58.54) | 7 (17.07) | 10 (24.39) | 24 (58.54) |
| χ2 | 8.945 | |||
| P | 0.003 |
Comparison of neurological functions in the two groups
The comparison of neurological function between the two groups before treatment was not statistically significant (P>0.05), and the neurological function of both groups was improved 7 days after treatment. The scores of NIHSS and mRS in the observation group were lower than those in the control group 7 days after treatment (all P<0.001), as shown in Table 3 and Figure 1.
Table 3.
Comparison of neurological functions between the two groups (scores, x̅ ± sd)
| Groups | NIHSS | mRS | ||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | 7 days after treatment | Before treatment | 7 days after treatment | |
| Observation group (n=41) | 33.69±4.51 | 7.83±1.59***,### | 3.23±0.34 | 1.15±0.19***,### |
| Control group (n=41) | 34.79±4.94 | 17.47±2.41*** | 3.86±0.48 | 2.68±0.23*** |
Note: Compared with the same group before treatment;
P<0.001.
Compared with the control group 7 days after treatment;
P<0.001.
NIHSS: National Institute of Health Stroke Scale; mRS: Modified Rankin Scale.
Figure 1.
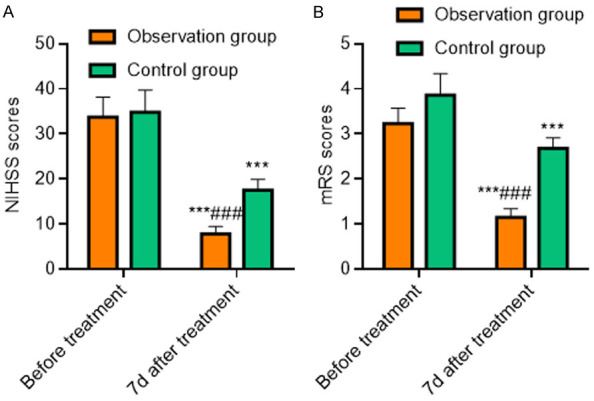
Comparison of neurological function between the two groups. A: NIHSS score; B: mRS score. Compared with the same group before treatment, ***P<0.001; compared with the control group 7 days after treatment, ###P<0.001. NIHSS: National Institute of Health Stroke Scale; mRS: Modified Rankin Scale.
Comparison of coagulation function in the two groups
The comparison of coagulation function between the two groups before treatment was not statistically significant (P>0.05); The FIB levels, PT and APTT in both groups after 7 days of treatment was shorter than those before treatment (all P<0.001), and the level of D-D was higher than that before treatment (all P<0.001). Seven days after treatment, the time of FIB, PT and APTT in the observation group were shorter than those in the control group (all P<0.001), and the level of D-D in the observation group was higher than that in the control group (P<0.001), as shown in Table 4 and Figure 2.
Table 4.
Comparison of coagulation function between the two groups (x̅ ± sd)
| Groups | Observation group | Control group |
|---|---|---|
| FIB (g/L) | ||
| Before treatment | 4.85±1.21 | 4.78±1.24 |
| 7 days after treatment | 3.37±1.10***,### | 4.26±1.08*** |
| D-D (mg/L) | ||
| Before treatment | 2.88±0.21 | 2.77±0.26 |
| 7 days after treatment | 3.77±0.45***,### | 3.15±0.28*** |
| PT (s) | ||
| Before treatment | 14.08±1.52 | 13.82±1.23 |
| 7 days after treatment | 10.54±1.25***,### | 13.24±1.08*** |
| APTT (s) | ||
| Before treatment | 27.93±8.71 | 28.43±8.56 |
| 7 days after treatment | 24.16±3.75***,### | 26.36±3.48*** |
Note: Compared with the same group before treatment;
P<0.001.
Compared with the control group 7 days after treatment;
P<0.001.
FIB: fibrinogen; D-D: D-Dimer; PT: prothrombin time; APTT: activated partial thromboplastin time.
Figure 2.
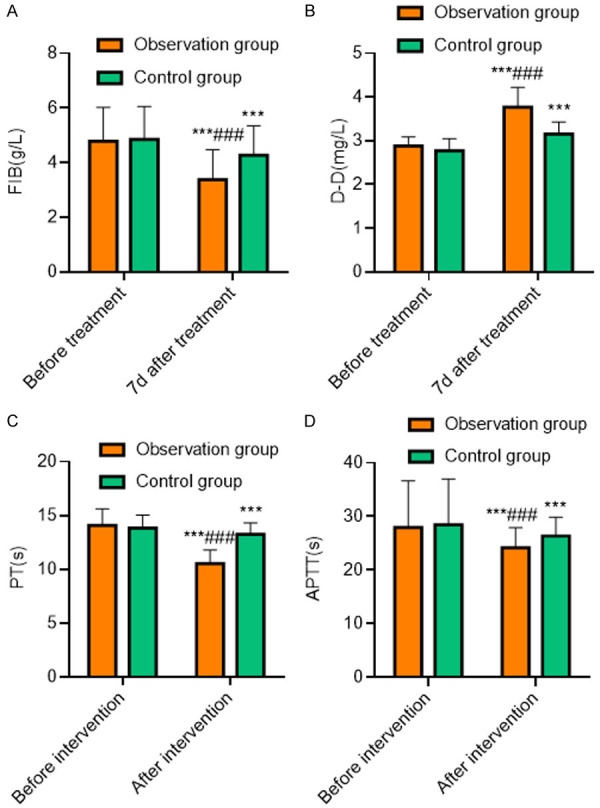
Comparison of coagulation function between the two groups. A: FIB; B: D-D; C: PT; D: APTT. Compared with the same group before treatment, ***P<0.001; compared with the control group 7 days after treatment, ###P<0.001. FIB: fibrinogen; D-D: D-Dimer; PT: prothrombin time; APTT: activated partial thromboplastin time.
Comparison of the incidence of complications in the two groups
The incidences of intracranial hemorrhage, infection, headache, quadriplegia, dizziness and drowsiness in the observation group were lower than those in the control group (P<0.05), as shown in Table 5.
Table 5.
Comparison of complications between the two groups (n, %)
| Groups | Observation group | Control group | χ2 | P |
|---|---|---|---|---|
| n | 41 | 41 | ||
| Intracranial hemorrhage | 0 (0.00) | 1 (2.44) | ||
| Infection | 1 (2.44) | 2 (4.88) | ||
| Headache | 0 (0.00) | 3 (7.32) | ||
| Quadriplegia | 1 (2.44) | 3 (7.32) | ||
| Dizziness and drowsiness | 0 (0.00) | 0 (0.00) | ||
| Incidences | 2 (4.88) | 9 (21.95) | 5.145 | 0.023 |
Comparison of prognosis in the two groups
Six months after treatment, the recanalization rate of venous sinus in the observation group was higher than that in the control group (P<0.01), as shown in Table 6.
Table 6.
Comparison of prognosis between the two groups (n, %)
| Groups | Complete ecanalization | Partial recanalization | Non-recanalization | Recanalization rate |
|---|---|---|---|---|
| Observation group (n=41) | 29 (70.73) | 9 (21.95) | 3 (7.32) | 38 (92.68) |
| Control group (n=41) | 21 (51.22) | 7 (17.07) | 13 (31.71) | 28 (68.29) |
| χ2 | 7.765 | |||
| P | 0.005 |
Discussion
Thrombus is a small mass of blood flow formed on the surface of the exfoliated or repaired part of the inner surface of the blood vessel of the cardiovascular system. The conventional treatment methods are mainly thrombolysis combined with AT. Although it can improve the symptoms, but the long-term prognosis is poor, the recanalization rate is low, and the incidence of complications is increased. Therefore, to actively seek for new treatment methods is of great significance to improve the symptoms of patients. In recent years, intravascular mechanical thrombectomy plus thrombolysis combined with AT has been applied in patients with CVST, and the effect is ideal [13].
After 7 days of treatment, the continuous improvement rate of clinical symptoms in the observation group (87.80%) was higher than that in the control group (58.54%). The neurological function of both groups was improved after treatment. The scores of NIHSS and mRS in the observation group were lower than those in the control group 7 days after treatment, suggesting that intravascular mechanical thrombectomy plus thrombolysis combined with AT for CVST patients can achieve a good therapeutic effect, which is helpful to reduce the injury of neurological function and benefit the recovery of patients. Domestic scholars gave intravascular mechanical thrombectomy plus thrombolysis combined with AT to patients with CVST [14]. The results show that the patients can achieve a higher improvement rate after treatment. AT is the main treatment for patients with CVST. Oral warfarin after intravenous drip of heparin sodium can inhibit the synthesis of coagulation factors involved in vitamin K in the liver, and play a good anticoagulant effect [15,16]. Previous studies have shown that the use of heparin sodium and warfarin can promote carboxylase in liver mitochondria to convert glutamic acid of the above coagulation factors into γ-carboxyglutamic acid, and it combine with calcium ions to play an anticoagulant effect [17]. However, most of the patients with CVST are seriously ill, so it is difficult to get a good effect with a single AT, and there are great limitations during the treatment of patients [18,19]. Thrombolytic therapy is also a common treatment in patients with CVST, which can help to reduce venous pressure and bleeding risk by reopening occluded venous sinuses in a short time [20,21]. In this study, the time of FIB, PT and APTT after 7 days of in the observation group were shorter than those in the control group. The level of D-D in the observation group was higher than that in the control group, suggesting that intravascular mechanical thrombectomy plus thrombolysis combined with anticoagulation is helpful to improve coagulation and reduce the incidence of rebleeding in patients with CVST. Previous scholars have targeted 10 CVST patients, 1 patient died 48 hours after thrombolytic therapy, and most of the patients had a good prognosis. Mechanical thrombectomy is also a common treatment in patients with CVST. Mechanical extrusion and extraction of venous sinus thrombus are carried out by using saccule, Merci thrombectomy device and Solitaire thrombectomy device, which can promote thrombus decomposition and fragmentation [22,23]. Clinically, the use of intravascular mechanical thrombectomy plus thrombolysis combined with anticoagulation in patients with CVST can gain the advantages of different treatment methods, with high safety and good prognosis [24,25]. In this study, the incidence of intracranial hemorrhage, infection, headache, quadriplegia, dizziness and drowsiness in the observation group was lower than that in the control group, and the recanalization rate of venous sinus in the observation group was higher than that in the control group 6 months after treatment, suggesting that intravascular mechanical thrombectomy plus thrombolysis combined with anticoagulation therapy for CVST can achieve a good prognosis.
However, there are also some shortcomings in this study, such as a short time span and a small number of cases. Thus, it needs to be further studied and discussed.
In summary, intravascular mechanical thrombectomy combined with thrombolysis combined and AT in patients with CVST is effective, which can effectively improve the coagulation function and promote the recovery of neurological function, with fewer complications and a good prognosis.
Disclosure of conflict of interest
None.
References
- 1.Shang K, Li H, Luo X. Cerebral venous sinus thrombosis due to hyperhomocysteinemia with cystathionine-β-synthase (CBS) gene mutation: a case report. Medicine (Baltimore) 2019;98:e14349. doi: 10.1097/MD.0000000000014349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Q, Chai X, Xiao C, Cao X. A case report of oral contraceptive misuse induced cerebral venous sinus thrombosis and dural arteriovenous fistula. Medicine (Baltimore) 2019;98:e16440. doi: 10.1097/MD.0000000000016440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsanos AH, Tsivgoulis G. Is intravenous thrombolysis still necessary in patients who undergo mechanical thrombectomy? Curr Opin Neurol. 2019;32:3–12. doi: 10.1097/WCO.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 4.Dawit S, Das DM, Acierno MD, O’Carroll CB. Teaching neuroImages: congenital variant misdiagnosed as cerebral venous sinus thrombosis: clinical pitfall. Neurology. 2019;92:e2064–e2065. doi: 10.1212/WNL.0000000000007372. [DOI] [PubMed] [Google Scholar]
- 5.Smith E, Kumar V. BET 1: does a normal D-dimer rule out cerebral venous sinus thrombosis (CVST)? Emerg Med J. 2018;35:396–397. doi: 10.1136/emermed-2018-207777.1. [DOI] [PubMed] [Google Scholar]
- 6.An H, Fan CQ, Duan JG, Ren Y, Dong K, Zhang Q, Ji XM, Huang XQ. Severe hyperhomocysteinemia with two novel mutations of c.154T>C and c.457G>A in cystathionine beta-synthase gene. Chin Med J (Engl) 2018;131:2368–2370. doi: 10.4103/0366-6999.241801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung V, Sastry A, Srivastava S, Wilcock D, Parrott A, Nayak S. Mechanical thrombectomy in acute ischaemic stroke: a review of the different techniques. Clin Radiol. 2018;73:428–438. doi: 10.1016/j.crad.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Vere DW, Sykes CH, Armitage P. Venous thrombosis during dextrose infusion. Lancet. 1960;2:627–630. doi: 10.1016/s0140-6736(60)91697-4. [DOI] [PubMed] [Google Scholar]
- 9.Xian Z, Chen Y, Chen L, Lu Q, Huang G, Qin Q, Zeng J, Liang Z. A clinical research on the potential pathogenesis of somatic cancer related cerebral venous sinus thrombosis. Medicine (Baltimore) 2019;98:e15134. doi: 10.1097/MD.0000000000015134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deskur A, Zawada I, Błogowski W, Starzyńska T. Cerebral venous sinus thrombosis in a young patient with ulcerative colitis: a case report. Medicine (Baltimore) 2019;98:e17428. doi: 10.1097/MD.0000000000017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayanna MB, Shantha GPS, Giudici M. Efficacy and safety of mechanical thrombectomy in the treatment of acute ischemic anterior circulation stroke: a systematic review and meta-analysis. J Am Coll Cardiol. 2018;71:A2049. [Google Scholar]
- 12.Sun J, He Z, Nan G. Cerebral venous sinus thrombosis presenting with multifocal intracerebral hemorrhage and subarachnoid hemorrhage: a case report. Medicine (Baltimore) 2018;97:e13476. doi: 10.1097/MD.0000000000013476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang ACO, Tsang FCP, Lee R, Leung GKK, Lui WM. Response to letter regarding “Combined aspiration thrombectomy and continuous intrasinus thrombolysis for cerebral venous sinus thrombosis: technical note and case series”. Neuroradiology. 2019;61:847–848. doi: 10.1007/s00234-019-02239-4. [DOI] [PubMed] [Google Scholar]
- 14.Soni P, Koech H, Silva D, Das P, Sindwani R, Dobri G, Recinos PF. Cerebral venous sinus thrombosis after transsphenoidal resection: a rare complication of cushing disease-associated hypercoagulability. World Neurosurg. 2020;134:86–89. doi: 10.1016/j.wneu.2019.10.077. [DOI] [PubMed] [Google Scholar]
- 15.Chen WB, Wang XL. Cerebral venous sinus thrombosis as the first manifestation of JAK2(V617F)-positive essential thrombocythemia. Chin Med J (Engl) 2018;131:748–750. doi: 10.4103/0366-6999.226903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Tang Z, Zhu W, Xu S. Clinical reasoning: a teenager with persistent headache. Neurology. 2019;92:e1526–e1531. doi: 10.1212/WNL.0000000000007184. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Xu W, Li T, Chen J, Shao A, Yan F, Chen G. Stem cell therapy: a promising therapeutic method for intracerebral hemorrhage. Cell Transplant. 2018;27:1809–1824. doi: 10.1177/0963689718773363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alrohimi A, Jassal R. Headache in guillain-barré syndrome: diagnostic and management implications. Can J Neurol Sci. 2018;45:240–242. doi: 10.1017/cjn.2017.247. [DOI] [PubMed] [Google Scholar]
- 19.Chiasakul T, De Jesus E, Tong J, Chen Y, Crowther M, Garcia D, Chai-Adisaksopha C, Messé SR, Cuker A. Inherited thrombophilia and the risk of arterial ischemic stroke: a systematic review and meta-analysis. Blood. 2018;132(Suppl 1):2518. doi: 10.1161/JAHA.119.012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cioni S, Casseri T, Vallone IM, Gennari P, Romano DG, Leonini S, Acampa M, Tassi R, Martini G, Bracco S. Letter regarding “combined aspiration thrombectomy and continuous intrasinus thrombolysis for cerebral venous sinus thrombosis: technical note and case series”. Neuroradiology. 2019;61:845–846. doi: 10.1007/s00234-019-02232-x. [DOI] [PubMed] [Google Scholar]
- 21.Shao X, Wang Q, Shen J, Liu J, Chen S, Jiang X. Treatment of traumatic depressed compound skull fractures. J Craniofac Surg. 2019;30:2239–2244. doi: 10.1097/SCS.0000000000005982. [DOI] [PubMed] [Google Scholar]
- 22.Bevan R, Patel C, Bhatti I, Te Water Naude J, Gibbon F, Leach P. Surgical management of raised intracranial pressure secondary to otogenic infection and venous sinus thrombosis. Child Nerv Syst. 2020;36:349–351. doi: 10.1007/s00381-019-04353-3. [DOI] [PubMed] [Google Scholar]
- 23.Afridi FA, Gomes J, Mysore SA, Ahmed R, Kushnir AA. The effect of prolonged antenatal IVIG treatment on the development of gestational alloimmune liver disease in the neonate. Blood. 2020;136:13–14. [Google Scholar]
- 24.Kristoffersen ES, Harper CE, Vetvik KG, Zarnovicky S, Hansen JM, Faiz KW. Incidence and mortality of cerebral venous thrombosis in a norwegian population. Stroke. 2020;51:3023–3029. doi: 10.1161/STROKEAHA.120.030800. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Ren M, Meng R, Wang F, Ji X. Dural arteriovenous fistula formation complicated cerebral venous sinus stenosis after venous sinus stenting. World Neurosurg. 2018;120:400–402. doi: 10.1016/j.wneu.2018.08.230. [DOI] [PubMed] [Google Scholar]


