Abstract
Objective: To evaluate the risk factors and clinical outcomes of carbapenem-resistant Klebsiella pneumoniae (K. pneumoniae) (CRKP) infection. Materials and Methods: A case-control study was performed from January 2017 to September 2017. The risk factors and clinical outcomes of CRKP cases (n = 91) were compared with those of the controls infected with carbapenem-susceptible K. pneumoniae (CSKP) (n = 91). Antibiotic susceptibility was determined using Etest while the type of bacteria was identified by Vitek 2. Results: CRKP infection was associated with prior use of carbapenems, β-lactam antibiotics, tigecycline, and hormones; complications with cerebrovascular lesions; chronic obstructive pulmonary disease; as well as prolonged hospitalization. Multivariable analysis showed that the use of carbapenem independently correlates with carbapenem resistance in the multivariable analysis. Carbapenem resistance, mechanical ventilation, tracheotomy, deep vein cannulation, indwelling urinary tract catheter, ICU treatment, and high Acute Physiology, Age, Chronic Health Evaluation II (APACHE II) scores were related to in-hospital mortality. Conclusion: CRKP is a widely spread pathogen associated with high in-hospital mortality. Minimizing the use of antimicrobials, specifically the carbapenems, may be effective to reduce CRKP infection.
Keywords: Nosocomial infections, carbapenem-resistant Klebsiella pneumoniae (CRKP), risk factors, clinical outcomes
Introduction
Carbapenem-resistant enterobacteriaceae (CRE) cause numerous diseases which are spreadable and sometimes untreatable. CRE infection causes high morbidity and mortality [1-3]. Klebsiella pneumoniae (K. pneumoniae) is the most common CRE in human respiratory and intestinal tracts, and a conditional pathogen implicated in nosocomial infection [4]. Data in the China Antimicrobial Surveillance Network (CHINET) showed that the resistance rate of K. pneumoniae to meropenem and imipenem increased to 20.9% and 24.0% (2017) from 3.0% and 2.9% (2005), respectively [5]. As a consequence of extensive use of carbapenems, carbapenem-resistant K. pneumoniae (CRKP) has flourished, which affected the efficiency of antibiotic treatment and prolonged the disease course. In most cases, few therapeutic options exist for eliminating infections due to resistant KPC-producing K. pneumoniae, which led to high mortality [6-8]. The risk factors (RF) and outcomes of CRKP infection [9-12] have been extensively investigated. The RFs may be prolonged hospitalization, invasive procedures, as well as the administration of carbapenems, quinolones, and cephalosporins. In addition, aging, diabetes, and glycopeptide antibiotics have been determined as independent RFs for CRKP infection related death. In this study, we aimed to identify the RFs and the mortality of CRKP infection based on a cohort of patients.
Materials and methods
Patients sourcing
This case-control study was carried out at the First Affiliated Hospital of Nanjing Medical University and got approval from the responsible ethics committee. The informed consent was signed by all the patients. Included were the patients whose K. pneumoniae culture tested positive between January 2017 and September 2017. Each patient whose K. pneumoniae isolate was identified during the study period was considered an eligible subject, regardless his/her repeated positive results. The patients with CRKP were assigned into the CRKP group.
Imipenem-resistant K. pneumoniae isolates and/or K. pneumoniae isolates with meropenem minimum inhibitory concentrations (MICs) ≥2 μg/ml as determined by Vitek 2 system were screened as possible carbapenemase producers. The antimicrobial susceptibility testing was interpreted with Clinical Laboratory Standards Institute (CLSI, 2017).
Totally, 115 CRKP strains were isolated and 91 patients were enrolled. For each case, one control was selected from the patients admitted within the study period with matchable gender, age, etc. All medical records of the patients were analyzed: demographics; medical history and co-morbid conditions; institutions treating the patient before being transferred to our hospital; locations of these institutions; wards; hospital course; treatments administrated before getting infected (such as dialysis, chemotherapy, steroid administration, radiotherapy, hematopoietic stem cell transplantation, surgery, etc.); source of infection; length of stay; ICU treatment; and outcomes. The severity of condition was determined with APACHE II scores.
Recorded comorbidities included renal failure, diabetes mellitus, hypertension, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, active cancer, trauma, and history of transplantation. Continuous antibiotic use for ≥72 h during the stay was recorded.
Statistical analysis
With SPSS 20.0 (Chicago, IL, USA) as the analytical tool, dichotomized and categorical data were examined using Fisher’s exact test or chi-squared test. ANOVA or t-test was used for continuous variables. Bivariate analyses were performed to test the corrections between dependent and independent variables. Those indicators with significant differences (P<0.1) were included in the multivariable analysis. The crude and adjusted odds ratios were estimated using Logistic regression analysis. The association of independent variables was expressed as OR with 95% CI. Alpha = 0.05. Graphpad Prism 8 was used to plot the figures.
Results
Basic characteristics of CRKP and control groups
Both groups included 68 males and 23 females, with the average age of (64.0±14) years. There were no differences among cases and controls regarding baseline data such as age, gender, and type of specimens, which were comparable (P>0.05) (Table 1).
Table 1.
Basic characteristics of CRKP and control groups
| Basic information | CRKP group (n = 91) | Control group (n = 91) | P value |
|---|---|---|---|
| Age (x̅ ± s, years old) | 64.0±14 | 64.0 ±14 | 1.00 |
| Male/Female (n/n) | 68/23 | 68/23 | 1.00 |
| Type of specimens (n) | |||
| Sputum | 80 | 80 | 1.00 |
| Mid-stream urine | 4 | 4 | 1.00 |
| Blood | 3 | 3 | 1.00 |
| Alveolar lavage fluid (ALF) | 2 | 2 | 1.00 |
| Discharge | 2 | 2 | 1.00 |
Univariate analysis
Univariable analysis (Table 2) showed that carbapenem-resistant K. pneumoniae infection was related to exposure to some antimicrobials, previous use of hormones, complications with cerebrovascular lesions or COPD, and long length of stay prior to bacterial isolation (P<0.05).
Table 2.
Univariate analysis of risk factors for CRKP infection
| Risk factors | CRKP group (n = 91) | Control group (n = 91) | OR (95% CI) | P value |
|---|---|---|---|---|
| Antibiotic (used prior to bacterial isolation) | ||||
| Carbapenems | 42.00 | 11.00 | 6.23 (2.94-13.24) | <0.0001 |
| Glycopeptide antibiotics | 10.00 | 5.00 | 2.12 (0.70-6.48) | 0.18 |
| β-lactam antibiotics | 21.00 | 10.00 | 2.43 (1.07-5.51) | 0.03 |
| Tigecycline | 10.00 | 2.00 | 5.49 (1.17-25.83) | 0.02 |
| Cephalosporins | 38.00 | 45.00 | 0.73 (0.41-1.32) | 0.30 |
| Quinolones | 9.00 | 6.00 | 1.56 (0.53-4.56) | 0.42 |
| Special treatment | ||||
| Hormones | 12.00 | 4.00 | 3.30 (1.02-10.67) | 0.04 |
| Radiotherapy | 1.00 | 0.00 | 1.01 (0.99-1.03) | 0.32 |
| Chemotherapy | 2.00 | 5.00 | 0.39 (0.07-2.05) | 0.25 |
| Invasive procedures | ||||
| Mechanical ventilation | 52.00 | 43.00 | 1.49 (0.83-2.67) | 0.18 |
| Tracheotomy | 31.00 | 22.00 | 1.62 (0.849-3.09) | 0.14 |
| CVC | 70.00 | 58.00 | 1.90 (0.99-3.63) | 0.05 |
| Surgery | 52.00 | 58.00 | 0.76 (0.42-1.38) | 0.36 |
| Nasogastric intubation | 45.00 | 38.00 | 1.36 (0.76-2.45) | 0.30 |
| Urinary catheterization | 69.00 | 66.00 | 1.19 (0.61-2.31) | 0.61 |
| Implants | 13.00 | 10.00 | 1.35 (0.56-3.26) | 0.50 |
| Dialysis | 13.00 | 5.00 | 2.87 (0.98-8.41) | 0.05 |
| General information | ||||
| Mean length of hospitalization prior to bacterial isolation | 19.60 | 11.70 | <0.0001 | |
| ICU treatment | 61.00 | 56.00 | 1.27 (0.69-2.33) | 0.44 |
| Mean APACHE II scores | 12.60 | 12.40 | 0.23 | |
| Basic disease conditions | ||||
| Cardiovascular diseases | 52.00 | 52.00 | 1.0 (0.56-1.80) | 1.00 |
| Cerebrovascular diseases | 37.00 | 23.00 | 2.03 (1.08-3.81) | 0.03 |
| COPD | 10.00 | 2.00 | 5.50 (1.17-25.83) | 0.02 |
| Malignant tumors | 12.00 | 21.00 | 0.51 (0.23-1.10) | 0.08 |
| Renal failure | 13.00 | 7.00 | 2 (0.76-5.27) | 0.16 |
| Diabetes | 11.00 | 16.00 | 0.645 (0.28-1.28) | 0.30 |
| History of transplantation | 3.00 | 0.00 | 1.03 (1.0-1.07) | 0.08 |
| Trauma | 12.00 | 10.00 | 1.23 (0.50-3.01) | 0.65 |
Multivariate analysis
We also screened out the independent RFs for CRKP infection using logistic multiple regression analysis. The variables with P<0.1 in the univariate analysis were further processed with the multivariate analysis. The results showed that use of β-lactam antibiotics (OR: 2.09, 95% CI: 1.38-3.15) prior to bacterial isolation was an independent RF for CRKP infection (Table 3).
Table 3.
Independent risk factors for CRKP infection
| Risk factors | OR value | 95% CI | P value |
|---|---|---|---|
| β-lactam antibiotics | 2.09 | 1.38-3.15 | <0.0001 |
Mortality-associated RFs in patients in the two groups
The RFs showing close association with K. pneumoniae-induced mortality included carbapenem resistance (OR: 2.23, 95% CI: 1.03-4.81), mechanical ventilation (OR: 5.932, 95% CI: 2.33-15.14), tracheotomy (OR: 2.504, 95% CI: 1.17-5.37), deep vein cannulation (OR: 19.17, 95% CI: 2.55-144.06), and urinary catheterization (OR: 3.204, 95% CI: 1.07-9.63). ICU treatment (OR: 2.607, 95% CI: 1.07-6.36) and high APACHE II scores were also associated with in-hospital mortality (Table 4, P<0.05) (Figure 1).
Table 4.
Analysis of mortality risk factors in K. pneumoniae infected patients
| Risk factors | Number of deaths | Number of survived patients | OR value (95% CI) | P value |
|---|---|---|---|---|
| Carbapenem resistance | 23 | 68 | 2.23 (1.03-4.81) | 0.039 |
| Mechanical ventilation | 29 | 66 | 5.932 (2.33-15.14) | 0.0001 |
| Tracheotomy | 16 | 37 | 2.504 (1.17-5.37) | 0.016 |
| CVC | 34 | 94 | 19.17 (2.55-144.06) | <0.0001 |
| Urinary catheterization | 31 | 104 | 3.204 (1.07-9.63) | 0.03 |
| ICU | 28 | 89 | 2.607 (1.07-6.36) | 0.031 |
| APACHEII scores | 16.65 | 11.48 | <0.0001 |
Figure 1.
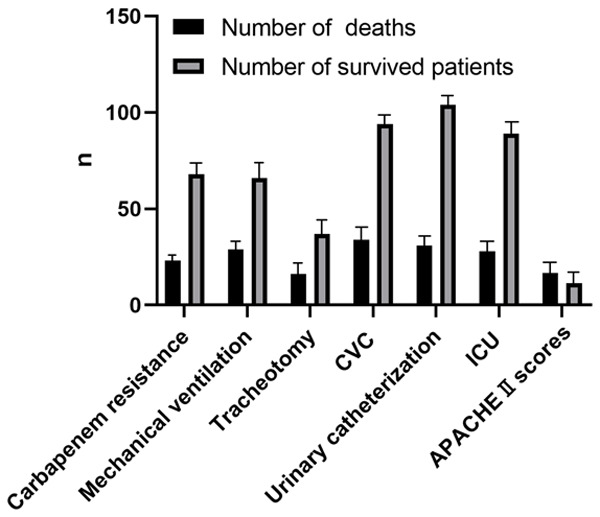
Analysis of mortality risk factors in K. pneumoniae infected patients.
Discussion
Resistant gram-negative K. pneumoniae causes various disorders, such as meningitis, pneumonia, wound and blood infection [13]. K. pneumoniae is an opportunistic pathogen that can survive in the hospital over a long period of time, and pose on patients more pathological, psychological and economic burdens. Extended-spectrum β-lactamase (ESBL) enzymes produced by the bacteria can raise the host’s resistance to antibiotic agents. ESBL-producing K. pneumoniae has been isolated in the hospitals around the world for years and rampant use of carbapenem increases its resistantance [14-18]. Since CRKP strain was first isolated in our hospital in 2005, many cases of CRKP infection have emerged in the ICU and other departments. Therefore, clarifying the RFs for CRKP infection has become an urgent need for such field.
In this study, we demonstrated that CRKP infection was associated with severity of illness, prolonged hospitalization, complications with cerebrovascular lesions and COPD, prior use of carbapenems, β-lactam antibiotics, tigecycline and hormones. Prior use of carbapenems is an independent RF. We also found that CRKP infection increased in-hospital mortality. The use of antibiotics showed causative association with CRKP infection. Antibiotics, such as carbapenem and β-lactamase inhibitors, were mainly administered for critically ill patients, thus increasing the patient’s susceptibility to infection by multidrug-resistant organisms [19-21].
Our study showed that prior carbapenems use is an independent RF for CRKP infection, which is consistent with the results reported previously [22]. KPC-producing K. pneumoniae is an emerging pathogen associated with in-hospital mortality, which were usually treated with antimicrobial agents. Our research findings highlight the importance of minimizing unnecessary and inappropriate use of antimicrobial drugs, especially carbapenems. Restricting carbapenems can reduce drug selection pressure and antimicrobial resistance. Early identification of pathogens and less antibiotic therapy are crucial for preventing CRKP infection. Susceptible populations include the patients suffering from severe cerebrovascular diseases, COPD patients having a long history of hospitalizations and use of antibiotics, or those needing hormones to enhance their immune system. Antibiotic resistance, mechanical ventilation, tracheotomy, deep vein cannulation, urinary catheterization, ICU treatment, and high APACHE II scores are associated with high mortality in patients. Therefore, it is also necessary to reduce invasive procedures to avoid CRKP infection.
Our findings showed that KPC-producing K. pneumoniae strains were the main reason causing in-hospital infection and could raise in-hospital mortality. Early detection and effective measures should be taken to restrict the spread of carbapenemase-producing organisms.
Disclosure of conflict of interest
None.
References
- 1.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen M, Eschenauer GA, Bryan M, O’Neil K, Furuya EY, Della-Latta P, Kubin CJ. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 2010;67:180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Quinn JP. The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids, and transposons. Clin Infect Dis. 2009;49:1739–1741. doi: 10.1086/648078. [DOI] [PubMed] [Google Scholar]
- 4.Correa L, Martino MD, Siqueira I, Pasternak J, Gales AC, Silva CV, Camargo TZ, Scherer PF, Marra AR. A hospital-based matched case-control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect Dis. 2013;13:80. doi: 10.1186/1471-2334-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, Zhang XJ, Zhang CX, Ji P, Xie Y, Kang M, Wang CQ, Wang AM, Xu YH, Shen JL, Sun ZY, Chen ZJ, Ni YX, Sun JY, Chu YZ, Tian SF, Hu ZD, Li J, Yu YS, Lin J, Shan B, Du Y, Han Y, Guo S, Wei LH, Wu L, Zhang H, Kong J, Hu YJ, Ai XM, Zhuo C, Su DH, Yang Q, Jia B, Huang W. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother. 2017;61:e02349-16. doi: 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papagiannitsis CC, Malli E, Florou Z, Sarrou S, Hrabak J, Mantzarlis K, Zakynthinos E, Petinaki E. Emergence of sequence type 11 Klebsiella pneumoniae coproducing NDM-1 and VIM-1 metallo-β-lactamases in a Greek hospital. Diagn Microbiol Infect Dis. 2017;87:295–297. doi: 10.1016/j.diagmicrobio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Han JH, Goldstein EJ, Wise J, Bilker WB, Tolomeo P, Lautenbach E. Epidemiology of carbapenem-resistant Klebsiella pneumoniae in a network of long-term acute care hospitals. Clin Infect Dis. 2017;64:839–844. doi: 10.1093/cid/ciw856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills JP, Talati NJ, Alby K, Han JH. The epidemiology of carbapenem-resistant klebsiella pneumoniae colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol. 2016;37:55–60. doi: 10.1017/ice.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akturk H, Sutcu M, Somer A, Aydın D, Cihan R, Ozdemir A, Coban A, Ince Z, Citak A, Salman N. Carbapenem-resistant klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: risk factors for progression to infection. Braz J Infect Dis. 2016;20:134–140. doi: 10.1016/j.bjid.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. A retrospective study of risk factors for carbapenem-resistant Klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10:208–213. doi: 10.3855/jidc.6697. [DOI] [PubMed] [Google Scholar]
- 12.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotgiu G, Are BM, Pesapane L, Palmieri A, Muresu N, Cossu A, Dettori M, Azara A, Mura II, Cocuzza C, Aliberti S, Piana A. Nosocomial transmission of carbapenem-resistant Klebsiella pneumoniae in an Italian university hospital: a molecular epidemiological study. J Hosp Infect. 2018;99:413–418. doi: 10.1016/j.jhin.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Hawkey PM. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008;14(Suppl 1):159–165. doi: 10.1111/j.1469-0691.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 15.van Duin D, Doi Y. The global epidemiology of carbapenemase-producing enterobacteriaceae. Virulence. 2017;8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, Xie L, Yang C, Ma X, Li H, Li W, Zhang X, Liao K, Man S, Wang S, Wen H, Li B, Guo Z, Tian J, Pei F, Liu L, Zhang L, Zou C, Hu T, Cai J, Yang H, Huang J, Jia X, Huang W, Cao B, Wang H. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62:e01882–17. doi: 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martirosov DM, Lodise TP. Emerging trends in epidemiology and management of infections caused by carbapenem-resistant Enterobacteriaceae. Diagn Microbiol Infect Dis. 2016;85:266–275. doi: 10.1016/j.diagmicrobio.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogan C, Kaye KS, Chopra T, Hayakawa K, Pogue JM, Lephart PR, Bheemreddy S, Lazarovitch T, Zaidenstein R, Perez F, Bonomo RA, Marchaim D. Outcomes of carbapenem-resistant enterobacteriaceae isolation: matched analysis. Am J Infect Control. 2014;42:612–620. doi: 10.1016/j.ajic.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Gagliotti C, Giordani S, Ciccarese V, Barozzi A, Giovinazzi A, Pietrantonio AM, Moro ML, Pinelli G, Sarti M. Risk factors for colonization with carbapenemase-producing klebsiella pneumoniae in hospital: a matched case-control study. Am J Infect Control. 2014;42:1006–1008. doi: 10.1016/j.ajic.2014.05.028. [DOI] [PubMed] [Google Scholar]


