Abstract
Objective: To investigate the correlation between the serum hepcidin levels and the viral loads in hepatitis C virus (HCV) infected patients. Methods: Sixty HCV-infected patients (the study group) and 50 healthy controls (the control group) were recruited as the study cohort. The liver function and inflammation-related parameters were compared, and the 60 HCV patients were divided into mild (G1-G2), moderate (G3), and severe (G4) groups according to each patient’s inflammatory activity grade (G). The serum iron (SI), ferritin (SF), and transferrin (TRF), hepcidin levels were compared. The relationships between the HCV-RNA, HCV Ag, HCV Ab, albumin (ALB), total bilirubin (TBIL), aminotransferase (ALT), and aspartate aminotransferase (AST) levels and the hepcidin levels was analyzed. The SI, SF, IL-6, ALT, AST, and TBIL levels were significantly higher, and the hepcidin, TRF, and ALB levels were significantly lower in the study group than they were in the control group (P<0.05). The G4 patients’ SI and SF levels were significantly higher than they were in the G3 and the G1-G2 groups (P<0.05). The TRF and hepcidin levels in the G1-G2 group were significantly higher than they were in the G3 and G4 groups (P<0.05). The HCV-RNA, HCV Ag, and HCV Ab levels were negatively correlated with the hepcidin levels (r=-0.7679, r=-0.9062, r=-0.6095, P<0.05), positively correlated with the serum ALB, TBIL, and ALT levels (r=0.9792, r=0.9759, r=0.8236, P<0.05), and not significantly correlated with the AST levels (r=-0.2803, P>0.05). Conclusion: The HCV patients’ serum hepcidin levels showed an abnormal decrease, suggesting that HCV patients may have an iron metabolism disorder, which indicates that there is a possibility of evaluating the HCV patients’ conditions by measuring the hepcidin levels and of improving HCV patients’ prognoses by regulating the iron metabolism.
Keywords: Hepatitis C, HCV, hepcidin, viral load, correlation analysis
Introduction
Hepatitis C is an infectious disease caused by a hepatitis C virus (HCV) infection. HCV is a single-stranded, positive-stranded RNA virus with a spherical shape and a diameter of less than 80 nm, with a shell, nucleobase, and nucleic acid [1]. Studies have shown that there are six genotypes as well as multiple subspecies of HCV worldwide, with the HCV-1b genotype being the most prevalent [2]. HCV’s routes of transmission include blood transmission, sexual transmission, and mother-to-child transmission, with blood transmission and sexual transmission being the most common, and various age groups are generally susceptible to HCV [3,4]. Epidemiology shows that HCV is currently a global epidemic and one of the main causes of liver cancer and cirrhosis. According to the World Health Organization (WHO), the prevalence of global HCV infection is about 3%, about 170 million people are infected with HCV, and the number of new cases of HCV can reach 35,000 per year. Although HCV infection rates vary by region, environment, and medical conditions, HCV infections are still a serious public health problem [5-7].
Current clinical practice indicates that HCV infection leads to hepatocyte injury, which in turn affects a variety of hepatic synthesis and transformation-related metabolic disorders and further accelerates hepatocyte apoptosis and fibrosis. Therefore, the regulation of metabolic disorders is one of the important ways to improve the prognosis of HCV patients [8,9]. Iron is an essential micronutrient for almost all living organisms because it plays critical role in metabolic processes. Research indicates that iron is a component of hemoglobin, myoglobin and many oxidative phosphatases and is widely involved in many metabolic processes. However, some studies have also pointed out that excess iron can also cause damage to the body. Iron in the ionic state can react with oxygen to produce reactive oxygen species (ROS), directly damaging biological membranes and leading to cell death. Hepcidin is a biotin with a negative regulator of iron absorption and recycling and plays an important role in maintaining the body’s iron homeostasis [10]. The liver is a major storehouse of iron and a specific organ for the synthesis of hepcidin, so there is a strong link between iron metabolism disorders and hepatocyte injuries. Non-infectious hepatitis as well as infectious hepatitis can lead to hepatocyte injuries and probably directly affect the body’s iron metabolism. Studies have also found that patients with alcoholic infections, fatty liver disease, and viral infections all have some degree of iron metabolism abnormalities, due to the fact that viral infections may regulate hepcidin’s gene expression [11,12]. The aim of this study was to investigate the correlation between the serum ferritin levels and the viral loads in HCV patients and to preliminarily analyze the effect of ferritin on HCV replication, aiming to provide a clinical basis for the treatment of HCV patients.
Materials and methods
General information
60 HCV patients (the study group) and 50 healthy controls (the control group) were enrolled in the study, including 65 male patients and 45 female patients, aged 40-55 years, with an average age of (45.99±2.32) years, an average duration of (2.91±0.21) years, and 20 cases of hypertension and 26 cases of diabetes.
Inclusion criteria: (1) The patients in the study group met the diagnostic criteria for hepatitis C according to the 2015 update of the Guidelines for the Prevention and Treatment of Chronic Hepatitis C [13]. (2) Patients with a clear consciousness and the ability to cooperate with the study. (3) Patients with complete medical records. (4) Patients with good compliance. (5) The study was approved by the hospital ethics committees. (6) The patients or their families signed an informed consent form.
Exclusion criteria: (1) Patients with mental illness. (2) Patients with hepatitis caused by other viruses. (3) Patients with liver cancer. (4) Patients with fatty liver disease or cirrhosis. (5) Patients with autoimmune diseases. (6) Patients with poor compliance.
Elimination criteria: (1) Patients who died during the study, and (2) Patients who asked to withdraw during the study.
Intervention methods
The patients’ fasting venous blood was stored in biochemical tubes with a separating gel, left for 1 h and then centrifuged for 10 min in a low speed centrifuge. The upper layer liquid was collected and stored in a -30°C freezer. The ALB, TBIL, SI, FErr, and Tf levels were measured using a fully automated protein analyzer. The serum ferritin was measured using ELISA, and the IL-6 was measured using a Cobas e601 fully automated biochemical analyzer. The hepatitis C viral loads were measured. The HCV Ag and HCV Ab levels were analyzed using chemiluminescence.
Observation indicators
The differences in the SI, SF, IL-6, ALT, AST, TBIL, hepcidin, TRF, and ALB levels between the two groups were compared. The patients were classified according to their pathological inflammatory activity (G/S staging specification in the Viral Hepatitis Prevention and Control Program [14]) as well as the differences in their SI, SF, TRF, and hepcidin levels. The correlation the between the HCV patients’ hepcidin levels and viral loads was analyzed.
Statistical methods
The data were entered into an EXCEL table, and SPSS 22.0 was used for the data analysis. If the data conformed to a normal distribution, the count data were expressed as [n (%)], and chi-square tests were used to analyze the differences between the groups. The measurement data were expressed as (mean ± standard deviation). T-tests were used for the analyses of the differences between the groups. F tests were used to analyze the differences among multiple groups. The correlation analyses were carried out using Spearman. Independent T tests were used for data that did not conform to a normal distribution. P<0.05 indicates that a difference is statistically significant [15].
Results
Comparison of the differences in the baseline data
There was little difference in the baseline data such as sex, age, mean weight, mean disease duration, or underlying disease (P>0.05), which was comparable (Table 1).
Table 1.
Comparison of the baseline data between the two groups (x̅ ± s)/[n (%)]
| Baseline data | Study group (n=60) | Control group (n=50) | t/X2 | P | |
|---|---|---|---|---|---|
| Gender | Male | 37 | 28 | 0.547 | 0.362 |
| Female | 23 | 22 | |||
| Average age (years) | 45.98±4.33 | 46.01±4.29 | 0.036 | 0.987 | |
| Average weight (kg) | 64.29±3.91 | 64.34±3.89 | 0.066 | 0.917 | |
| Average duration (years) | 2.89±0.28 | 2.93±0.18 | 0.871 | 0.317 | |
| Hypertension | Yes | 10 | 10 | 0.652 | 0.204 |
| No | 50 | 40 | |||
| Diabetes | Yes | 15 | 11 | 0.712 | 0.136 |
| No | 45 | 39 | |||
Comparison of the differences in the liver function and inflammation parameters
The SI, SF, IL-6, ALT, AST, and TBIL levels were significantly higher in the study group patients than in the controls (P<0.05), but the patients in the study group had significantly lower levels of hepcidin, TRF, and ALB than the controls (P<0.05) (Figure 1).
Figure 1.
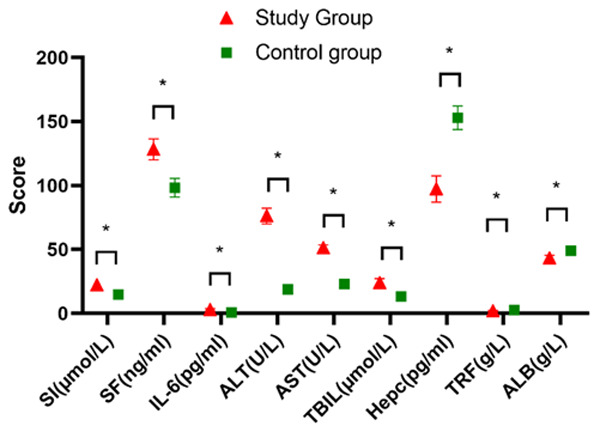
Comparison of the differences in liver function and the inflammation-related indices between the two groups. The SI, SF, IL-6, ALT, AST, and TBIL levels in the study group were significantly higher than they were in the control group (P<0.05), but the Hepc, TRF, and ALB levels in the study group were significantly lower than they were in the control group (P<0.05). * indicates a significant difference between the groups of the same indicator, P<0.05.
Comparison of the hepcidin and iron levels
Among the 60 patients, there were 41 patients in the mild group (G1-G2), 11 patients in the moderate group (G3), and 8 patients in the severe group (G4). The SI and SF levels of the patients in the G4 group were significantly higher than they were in the G3 group, and the G3 group had significantly higher SI and SF levels than the G1-G2 group (P<0.05). At the same time, the TRF and hepcidin levels of the patients in the G4 group were significantly lower than they were in the G3 group, and the G3 group had significantly lower TRF and hepcidin levels than the G1-G2 group (P<0.05) (Figure 2).
Figure 2.
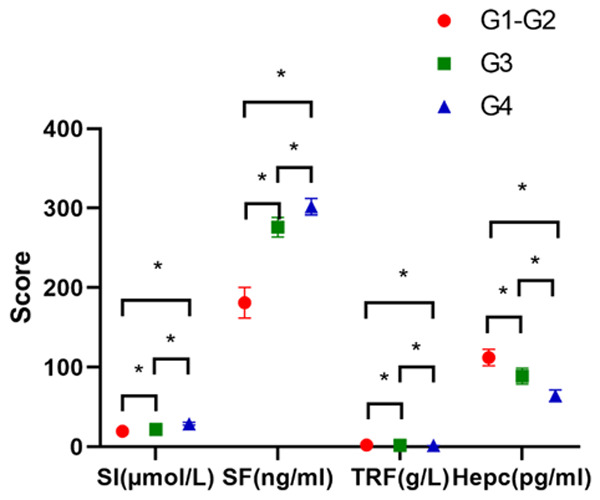
Comparison of the hepcidin and iron levels in the patients with different inflammatory activity. The SI and SF levels in the severe group (G4) were significantly higher than they were in the moderate group (G3), and the SI and SF levels in the G3 group were significantly higher than they were in the mild group (G1-G2) (P<0.05). The TRF and Hepc levels in the G4 group were significantly lower than they were in the G3 group, and the TRF and Hepc levels in the G3 group were significantly lower than they were in the G1-G2 group (P<0.05). * indicates a significant difference between groups of the same indicator, P<0.05.
Correlation analysis of the serum hepcidin levels (SHL) and the HCV RNA viral loads in the HCV patients
Our correlation analysis showed a significant negative correlation (r=-0.7679, P<0.001) between the SHLs and the HCV RNA viral loads (Figure 3), with a significant decrease in the HCV RNA levels as the SHLs increased in the HCV patients.
Figure 3.
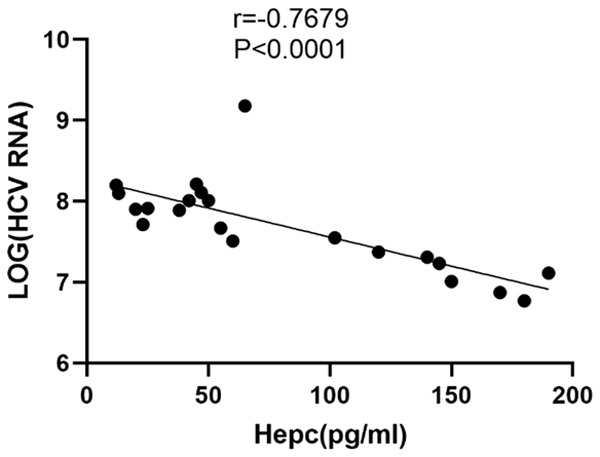
A correlation analysis between the serum ferritin levels and the HCV RNA viral loads in the HCV patients. The correlation analysis showed a significant negative association between the serum ferritin levels and the HCV RNA viral loads in the HCV-infected patients (r=-0.7679, P<0.0001).
Correlation analysis of SHLs in the HCV patients with HCV Ag and HCV Ab
Among the enrolled HCV patients, 21 Ag-positive patients with HCV were randomly analyzed. The SHL of the HCV patients showed a significant negative correlation with their HCV Ag levels (r=-0.9062, P<0.001) (Figure 4A), and it also showed a significant negative correlation with HCV Ab (r=-0.6095, P=0.022) (Figure 4B), indicating that as the SHL of the HCV patients increases, the HCV Ag and HCV Ab levels will gradually decrease.
Figure 4.
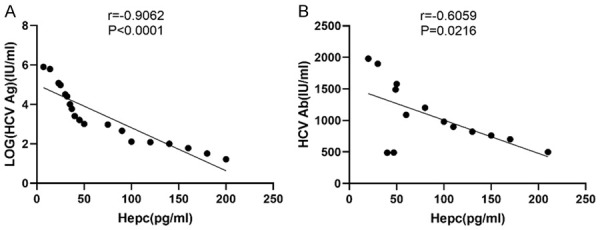
A correlation analysis of the serum hepcidin levels in the HCV-infected patients with their HCV Ag and HCV Ab. The correlation analysis shows a significant negative correlation between the hepcidin levels with the HCV Ag levels (r=-0.9062, P<0.0001) (A) and with HCV Ab (r=-0.6095, P=0.0216) (B).
Correlation analysis of the SHLs in the HCV patients with their ALB and TBIL levels
Sixty HCV patients were included in the study to investigate the correlation between the SHLs and the ALB and TBIL levels, which showed a positive correlation between the SHLs and their ALB levels (r=0.9792, P<0.001) (Figure 5A), and a positive correlation with their TBIL levels (r=0.9759, P<0.001) (Figure 5B), suggesting that as the SHLs increased, the HCV patients’ ALB and TBIL levels also increased significantly.
Figure 5.
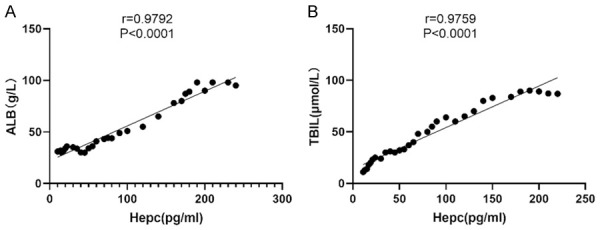
A correlation analysis of the serum ferritin levels in the HCV-infected patients with their ALB and TBIL levels. The serum ferritin levels of the HCV-infected patients were positively correlated with their ALB levels (r=0.9792, P<0.0001) (A) and with their TBIL levels (r=0.9759, P<0.001) (B).
Correlation analysis of the SHLs in the HCV patients with their ALT and AST levels
The correlation between the SHL and the ALT, AST levels in the HCV patients was investigated, and the results showed that the SHLs in the HCV patients showed a significant positive correlation with ALT (r=0.8236, P<0.001) (Figure 6A), and there was no significant correlation with AST (r=-0.2803, P=0.2185) (Figure 6B), suggesting that the increase in the SHLs in the HCV patients would be accompanied by an increase in the ALT levels, but no significant changes in the AST levels were observed.
Figure 6.
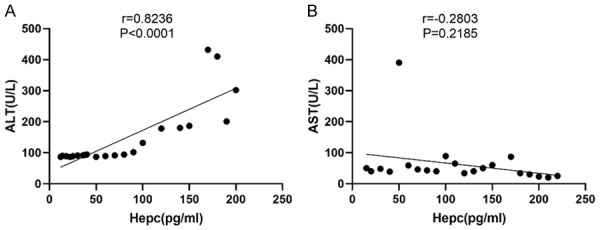
A correlation analysis of the serum hepcidin levels in the HCV-infected patients with their ALT and AST levels. The serum hepcidin levels in the HCV-infected patients showed a significant positive correlation with their ALT levels (r=0.8236, P<0.0001) (A) but no correlation with their AST levels (r=-0.2803, P=0.2185) (B).
Discussion
Statistics show that China still has one of the highest rates of HCV infection, and HCV infections are the main cause of end-stage liver disease, cirrhosis-related liver failure, and liver-cancer related deaths. A 2014 epidemiology report shows that China’s new cases of liver cancer and related deaths ranks the highest worldwide. Hepatitis C is still a serious public health issue, causing a serious burden on society, but it also poses an significant threat to the health of the population. The pathogenesis of hepatitis C is still unclear, and its treatment options are still relatively few. In recent years, there have also been a number of scholars committed to improving the clinical symptoms caused by HCV infection through the regulation of individual metabolism, and they achieved certain results [16,17]. A study of 89 patients with HCV infection showed that the metabolism of sugars, fats, amino acids, and trace elements in patients has a certain change, and the reason may be that the liver is widely involved in the metabolism and biotransformation of the above substances, and HCV infection causes significant damage to the liver cells [18]. Another study found that the iron metabolism in the body is closely related to the liver cell damage, and iron overload aggravates the peroxidation process, thus accelerating the inflammatory process of the body, and inducing liver cell necrosis and liver fibrosis [19].
Hepcidin is a key regulator of the entry of iron into the circulation. It has strong in vitro antimicrobial activity, so it is called a “defensive peptide”. Several experiments have found that hepcidin is a negative regulator of iron absorption and recycling, and it is the only biotin with a negative feedback of iron metabolism found so far. Hepcidin actually plays an essential role in maintaining the body’s iron homeostasis. When the body’s serum iron level is abnormally elevated, the hepcidin mRNA expression is increased and when the body is in an iron-deficient state, and the expression of hepcidin mRNA is significantly reduced, thus achieving a dynamic balance in maintaining the serum iron level [20].
The results of the present study showed that the SI, SF, IL-6, ALT, AST and TBIL levels in the HCV-infected individuals were significantly higher than they were in the control group, while the hepcidin, TRF and ALB levels were significantly lower than they were in the control group, with significant differences between the groups (P<0.05), indicating that, on the one hand, HCV infection can damage liver function, but on the other hand, it does affect the body’s iron metabolism. Some scholars have carried out controlled studies on patients with hepatitis B, patients with hepatitis C, and healthy individuals and analyzed the differences in the routine liver function indicators, and the results showed that hepatitis B/C patients had significant abnormalities in their liver function indices such as ALT, AST, and TBIL as well as their iron metabolism-related indices such as TRF, total iron binding capacity (TIBC), and ferritin saturation (TS), and the differences between the hepatitis B and hepatitis C patient groups were also pronounced. For example, the mean level of TRF was 2.5±0.56 g/L in patients with hepatitis B and 2.17±0.48 g/L in patients with hepatitis C [21,22]. We concluded that the liver is one of the main organs where iron is stored and transformed. Under normal circumstances, iron is stored in hepatocytes and macrophages, which are released for metabolism when the body is deficient in iron, and hepcidin plays the role of regulating the iron homeostasis. The SI and S represent the level of free iron in the body. We found that HCV infection significantly affects liver function and leads to abnormal liver metabolism, and this is one reason for the decrease in the SHLs in HCV patients. In addition, retrospective analysis results have shown that HCV structural proteins are involved in the regulation of signaling pathways related to hepcidin expression and the down-regulation of hepcidin expression, thus affecting iron metabolism, which is consistent with the results of this study [23].
This study also analyzed the correlation between the serum hepcidin levels and the viral loads in HCV patients. HCV patients were divided into three groups according to each patient’s degree of inflammation. A controlled study of 98 patients with hepatitis C and 80 healthy controls showed that the TRF and TIBC levels of the hepatitis C patients were negatively correlated with age. The SI, SF and TS levels were positively correlated with the ALT, AST and TBIL levels. The SF and TS levels were negatively correlated with the ALB level, and the TRF and TIBC levels were negatively correlated with the ALT, AST and TBIL levels. This shows that there is a significant correlation between the serum iron metabolism indices and the inflammatory activity of the liver immune cells. Patients with hepatitis C have a significant iron overload compared with healthy individuals, and the reason may be that HCV virus infection leads to liver cell damage and large amounts of iron ions enter the blood [24]. We concluded that hepcidin is an important substance that can maintain the stability of the extracellular iron levels and the total iron levels, so the liver’ health status will have a significant impact on the iron metabolism. HCV viral load is the most direct indicator of hepatitis C. An elevated viral load often indicates that the HCV virus is highly active. HCV Ag and HCV Ab are important indicators for determining the infectivity of hepatitis C and determining the indication for treatment and viral resistance. As mentioned above, the hepcidin level reflects the liver function. Therefore, the higher the HCV-RNA, HCV Ag, and HCV Ab levels the lower the hepcidin levels in patients with hepatitis C [25]. ALB, ALT, and TBIL are all clinical indicators that reflect the hepatic synthesis and conversion. HCV infection exacerbates the symptoms of iron overload, so as the inflammatory activity of the liver increases, there is a significant increase in the hepcidin level [26].
In summary, the abnormal decrease in serum hepcidin in HCV-infected patients suggests that iron metabolism may be dysregulated, suggesting the possibility of evaluating the patients’ condition by measuring the hepcidin levels in the HCV patients and improving the prognosis of HCV patients by regulating their iron metabolism. The shortcoming of this study is its lack of experiments exploring the specific mechanism of hepcidin and HCV replication, which will be investigated in the future to improve the prognosis of HCV patients.
Acknowledgements
This work was supported by the scientific research funds of the Hebei Provincial Health Department (No. 20130226).
Disclosure of conflict of interest
None.
References
- 1.Pullen LC. Hepatitis C infection may be a blessing for patients in need of a transplant. Am J Transplant. 2018;18:2373–2374. doi: 10.1111/ajt.15091. [DOI] [PubMed] [Google Scholar]
- 2.Sharafi H, Alavian SM. Hepatitis C resistance to NS5A inhibitors: is it going to be a problem? World J Hepatol. 2018;10:543–548. doi: 10.4254/wjh.v10.i9.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelaziz AO, Shousha HI, Said EM, Soliman ZA, Shehata AA, Nabil MM, Abdelmaksoud AH, Elbaz TM, Abdelsalam FM. Evaluation of liver steatosis, measured by controlled attenuation parameter, in patients with hepatitis C-induced advanced liver fibrosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2018;30:1384–1388. doi: 10.1097/MEG.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 4.Bivigou-Mboumba B, Amougou-Atsama M, Zoa-Assoumou S, M’Boyis Kamdem H, Nzengui-Nzengui GF, Ndojyi-Mbiguino A, Njouom R, François-Souquière S. Hepatitis B infection among HIV infected individuals in Gabon: occult hepatitis B enhances HBV DNA prevalence. PLoS One. 2018;13:e0190592. doi: 10.1371/journal.pone.0190592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhoute X, Penaranda G, Raoul JL, Sellier F, Castellani P, Oules V, Perrier H, Lefolgoc G, Pol B, Campanile M, Bayle O, Beaurain P, Monnet O, Bourlière M. Hepatocellular carcinoma recurrence in hepatitis C virus-related cirrhosis treated with direct-acting antivirals: a case-control study. Eur J Gastroenterol Hepatol. 2018;30:368–375. doi: 10.1097/MEG.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 6.Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952–953. doi: 10.1056/NEJMe1801662. [DOI] [PubMed] [Google Scholar]
- 7.Scott LJ. Ledipasvir/sofosbuvir: a review in chronic hepatitis C. Drugs. 2018;78:245–256. doi: 10.1007/s40265-018-0864-z. [DOI] [PubMed] [Google Scholar]
- 8.Liang TJ, Ward JW. Hepatitis C in injection-drug users - a hidden danger of the opioid epidemic. N Engl J Med. 2018;378:1169–1171. doi: 10.1056/NEJMp1716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Nam JY, Lee JH, Lee HA, Chang Y, Lee HY, Cho H, Lee DH, Cho YY, Cho EJ, Yu SJ, Lee JM, Kim YJ, Yoon JH. Intensity of surveillance for hepatocellular carcinoma determines survival in patients at risk in a hepatitis B-endemic area. Aliment Pharmacol Ther. 2018;47:1490–1501. doi: 10.1111/apt.14623. [DOI] [PubMed] [Google Scholar]
- 10.Tiittala P, Ristola M, Liitsola K, Ollgren J, Koponen P, Surcel HM, Hiltunen-Back E, Davidkin I, Kivelä P. Missed hepatitis B/C or syphilis diagnosis among Kurdish, Russian, and Somali origin migrants in Finland: linking a population-based survey to the national infectious disease register. BMC Infect Dis. 2018;18:137. doi: 10.1186/s12879-018-3041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermann C, Wendeler D, Nienhaus A. Hepatitis C in healthcare personnel: secondary data analysis of therapies with direct-acting antiviral agents. J Occup Med Toxicol. 2018;13:16. doi: 10.1186/s12995-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, Wang X, Zhang B, Zhang Q, Chen L, Nie M, Liu Y, Meng B, Huang H, Jiang W, Zeng Y, Li W, Wu K, Hou Y, Wiman KG, Li Z, Zhang H, Peng R, Zhu S, Pan-Hammarström Q. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood. 2018;131:2670–2681. doi: 10.1182/blood-2017-11-817601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bil JP, Schrooders PA, Prins M, Kouw PM, Klomp JH, Scholing M, Huijbregts LP, Sonder GJ, Waegemaekers TC, de Vries HJ, Meijer W, Zuure FR, Tostmann A. Integrating hepatitis B, hepatitis C and HIV screening into tuberculosis entry screening for migrants in the Netherlands, 2013 to 2015. Euro Surveill. 2018;23:17-00491. doi: 10.2807/1560-7917.ES.2018.23.11.17-00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciupe SM. Modeling the dynamics of hepatitis B infection, immunity, and drug therapy. Immunol Rev. 2018;285:38–54. doi: 10.1111/imr.12686. [DOI] [PubMed] [Google Scholar]
- 15.DeBose-Scarlett A, Balise R, Kwon D, Vadaparampil S, Chen SX, Schiff ER, Ayala GP, Thomas E. Obstacles to successful treatment of hepatitis C in uninsured patients from a minority population. J Transl Med. 2018;16:178. doi: 10.1186/s12967-018-1555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berchtold L, Zanetta G, Dahan K, Mihout F, Peltier J, Guerrot D, Brochériou I, Ronco P, Debiec H. Efficacy and safety of rituximab in hepatitis B virus-associated PLA2R-positive membranous nephropathy. Kidney Int Rep. 2018;3:486–491. doi: 10.1016/j.ekir.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingani M, Akita T, Ouoba S, Sanou AM, Sugiyama A, Tarnagda Z, Ohisa M, Tinto H, Mishiro S, Tanaka J. High prevalence of hepatitis B infections in Burkina Faso (1996-2017): a systematic review with meta-analysis of epidemiological studies. BMC Public Health. 2018;18:551. doi: 10.1186/s12889-018-5432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danane J, Meskaf A, Allali K. Optimal control of a delayed hepatitis B viral infection model with HBV DNA-containing capsids and CTL immune response. Optim Control Appl Methods. 2018;39:1262. [Google Scholar]
- 19.Saito H, Umemura T, Joshita S, Yamazaki T, Fujimori N, Kimura T, Komatsu M, Matsumoto A, Tanaka E, Ota M. KIR2DL2 combined with HLA-C1 confers risk of hepatitis C virus-related hepatocellular carcinoma in younger patients. Oncotarget. 2018;9:19650–19661. doi: 10.18632/oncotarget.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alidjinou EK, Michel C, Canva V, Ajana F, Hober D, Bocket L. Very slow decline of hepatitis B virus surface antigen and core related antigen in chronic hepatitis B patients successfully treated with nucleos(t)ide analogues. J Med Virol. 2018;90:989–993. doi: 10.1002/jmv.25028. [DOI] [PubMed] [Google Scholar]
- 21.Pang Q, Zhou L, Qu K, Cui RX, Jin H, Liu HC. Validation of inflammation-based prognostic models in patients with hepatitis B-associated hepatocellular carcinoma: a retrospective observational study. Eur J Gastroenterol Hepatol. 2018;30:60–70. doi: 10.1097/MEG.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 22.Gadhachanda VR, Eastman KJ, Wang Q, Phadke AS, Patel D, Yang W, Marlor CW, Deshpande M, Huang M, Wiles JA. Ferrocene-based inhibitors of hepatitis C virus replication that target NS5A with low picomolar in vitro antiviral activity. Bioorg Med Chem Lett. 2018;28:3463–3471. doi: 10.1016/j.bmcl.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Angelidakis G, Sturgis E, Economides MP, Ying J, Torres H. Impact of chronic hepatitis C virus infection in patients with non-oropharyngeal cancers. J. Clin. Oncol. 2018;36:e18027. doi: 10.1016/j.oraloncology.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Liang Y, Cao X, Ding Q, Zhao Y, He Z, Zhong J. Hepatitis C virus NS4B induces the degradation of TRIF to inhibit TLR3-mediated interferon signaling pathway. PLoS Pathog. 2018;14:e1007075. doi: 10.1371/journal.ppat.1007075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura K, Que L, Shimadu M, Koura M, Ishihara Y, Wakae K, Nakamura T, Watashi K, Wakita T, Muramatsu M. Flap endonuclease 1 is involved in cccDNA formation in the hepatitis B virus. PLoS Pathog. 2018;14:e1007124. doi: 10.1371/journal.ppat.1007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JF, Xiong HL, Cao JL, Wang SJ, Guo XR, Lin BY, Zhang Y, Zhao JH, Wang YB, Zhang TY, Yuan Q, Zhang J, Xia NS. A cell-penetrating whole molecule antibody targeting intracellular HBx suppresses hepatitis B virus via TRIM21-dependent pathway. Theranostics. 2018;8:549–562. doi: 10.7150/thno.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]


