Abstract
Objective: To investigate the combined detection of the autoantibody characteristics in systemic lupus erythematosus (SLE). Methods: 105 SLE patients admitted to our hospital from May 2019 to September 2020 were placed in the SLE examination group (the SE group), 110 patients with rheumatic diseases admitted to our hospital during the same period were placed in the disease control group (the DC group), and 100 healthy people who came to our hospital for physical examinations during the same time period were placed in the healthy control group (the HC group). The SLE patients’ clinical data were recorded. The patients’ antinuclear antibody (ANA), anti-dsDNA, anti-SM, anti-SSA, and anti-rRnp levels were measured. Results: There were no significant differences in the occurrences of fever, alopecia, photosensitivity, interstitial lung disease, Raynaud’s phenomenon, pulmonary arterial hypertension, or osteoporosis. (P > 0.05), but there were higher incidences of arthralgia and butterfly erythema in the female patients (P < 0.05), and higher incidences of renal damage, oral ulcers, nervous system damage, and dysopsia in the male patients (P < 0.05). The positive autoantibody rate in the SE group was significantly higher than it was in the DC and HC groups (P < 0.05). A logistic regression analysis showed that the anti-SM, anti-dsDNA, AnuA, and anti-SSA levels entered the regression equation with statistical differences when P < 0.05. Among the four autoantibodies, the anti-SSA sensitivity was the highest, but its specificity was the lowest, and the specificities of the other three autoantibodies were all more than 97%. The positive rate of anti-dsDNA in its active stage was higher than it was in its inactive stage (P < 0.05). The positive rates of AnuA and anti-SM in their active stages was higher than they were in their inactive stages (P > 0.05). The sensitivities of the combined measurements were higher than the single autoantibody measurements (P < 0.05). Conclusion: Single autoantibody detection has the disadvantage of low sensitivity or specificity. The combined detection of autoantibodies can effectively improve the sensitivity and specificity of SLE detection, so it is worthy of clinical promotion.
Keywords: Systemic lupus erythematosus (SLE), autoantibody characteristics, combined detection of autoantibodies, diagnostic value
Introduction
SLE is a systemic immune disease with a clinically high incidence that mostly affects the female population. It is mainly accompanied by skin injuries, it severely damages patients’ organs, and affects patients’ multiple systems, greatly threatening patients’ physical health and significantly reducing patients’ quality of life [1-3]. At present, the pathogenesis of the disease is still unclear, the clinical symptoms are complex, and the course of the disease is prone to delay and recurrence. It is mainly manifested by humoral immune disorders as well as the involvement of antibodies and multiple systems caused by cellular immune dysfunction and represented by anti-nuclear antibodies [4,5]. Routine examinations, autoantibody detection, and immune system detection, are commonly used in the clinical diagnosis of SLE. Diagnosing early SLE patients and providing effective and timely treatment can effectively improve patients’ prognostic quality and prolong their survival times, but there is a high rate of misdiagnosis in the early stage of SLE, which seriously delays the timing of the treatment [6-8]. Studies have reported that SLE causes damage to multiple organs and systems, so its clinical manifestations are diverse. Patients’ serum testing has revealed that anti-SM, ANA, anti-rRnp, and other antibodies can lead to inflammation, and the combined detection of autoantibodies is more widely applied in the diagnosis of SLE [9-11]. In this study, 105 SLE patients admitted to our hospital from May 2019 to September 2020 were recruited as the study cohort, and the combined detection of the SLE autoantibody characteristics was investigated and analyzed. Now it is reported as follows.
Materials and methods
General information
105 SLE patients admitted to our hospital from May 2019 to September 2020 were placed in the SE group, and they included 32 active patients and 73 inactive patients. Also, 110 patients with rheumatic diseases admitted to our hospital during the same period were placed in the DC group, and they included 47 patients with rheumatoid arthritis, 24 patients with sicca syndrome, 16 patients with mixed connective tissue diseases, 13 patients with polymyositis, and 10 patients with diffuse scleroderma. In addition, 100 healthy people who came to our hospital for physical examinations during the same period were placed in the HC group. The general clinical data such as gender and age were not significantly different (P > 0.05), as shown in Table 1.
Table 1.
Comparison of the general data among the three groups
| SE group (n=105) | DC group (n=110) | HC group (n=100) | χ2/F | P | |
|---|---|---|---|---|---|
| Gender (male/female) | 21/84 | 26/84 | 23/77 | 0.462 | 0.794 |
| Age (years old) | 41.36±5.28 | 40.85±5.36 | 41.23±5.17 | 0.272 | 0.762 |
| BMI (kg/m2) | 22.73±2.46 | 22.46±2.17 | 22.64±2.28 | 0.384 | 0.682 |
| Education | 0.452 | 0.978 | |||
| Primary education | 31 | 36 | 33 | ||
| Secondary education | 46 | 45 | 40 | ||
| College degree or above | 28 | 29 | 27 |
Inclusion/exclusion criteria
Inclusion criteria
This study was approved by the Medical Ethics Committee of Shandong First Medical University, Shandong Province (approval No. 2019-462). The patients were informed of the whole process and the purpose of the study, and they voluntarily participated in the study and signed the informed consent. The SLE patients all met the ACR classification criteria for SLE [12].
Exclusion criteria
Patients with inflammation, malignant tumors, autoimmune diseases, etc., and a history of connective tissue diseases were excluded from the study.
Methods
5 ml of fasting venous blood from the patients in the three groups was collected in a dry tube and centrifuged at 3000 rpm for 10 min. Thereafter, the sera were separated and stored for testing. The ANA (CAS No: NP766-253) and anti-dsDNA (CAS No: NBP3-07670) antibodies were detected using IIF, anti-SM (CAS No: NBP1-97921SS) and the anti-SSA (CAS No: NB600-101) were detected using ELISA, and the anti-rRnp (CAS No: NB307-670) was detected using Western-blot. The kits were all purchased from the R&D Company (USA), and the detection was carried out in strict accordance with the kit instructions.
Observation indexes
(1) Clinical manifestations. The clinical symptoms of the SLE group were collected and grouped by gender to compare the incidences of the different clinical symptoms. (2) Autoimmune antibody level. The autoantibody levels and the positive expression rates in the different groups were calculated. (3) SLE specific antibodies. Logistic regression analyses were used to determine the SLE-specific antibodies, and the sensitivity and specificity of the single diagnoses and combined diagnoses were calculated.
Statistical analysis
SPSS 19.0 was used to analyze and process the data. The measurement data were expressed as (x̅ ± s) and tested using t-tests. The enumeration data were expressed as [n (%)] and tested using X2 tests. Logistic regression analyses were used to analyze the correlations between the indexes and the results of the diagnoses. A differences was considered statistically significant when P < 0.05.
Results
Comparison of the clinical manifestations in the SLE patients of different genders
The main clinical manifestations of the SLE patients included fever, arthralgia, butterfly erythema, and renal damage, and 73 patients had a fever, accounting for 69.52% (73/105); 57 patients had arthralgia, accounting for 54.29% (57/105); 73 patients had butterfly erythema, accounting for 69.52% (73/105), and 64 patients had renal damage, accounting for 60.95% (64/105). There were no significant differences between the male and female patients in terms of the clinical manifestations such as fever, alopecia, photosensitivity, interstitial lung disease, Raynaud’s phenomenon, pulmonary arterial hypertension, osteoporosis, etc. (P > 0.05). The incidence of arthralgia and butterfly erythema in the female patients was significantly higher than it was in the male patients (P < 0.05). The incidences of renal damage, oral ulcers, nervous system damage, and dysopsia in the male patients was significantly higher than it was in the female patients (P < 0.05), as shown in Table 2.
Table 2.
Comparison of the clinical manifestations in the SLE patients of different genders
| Clinical manifestations | SE group (n=105) | Male (n=21) | Female (n=84) | χ2 | P |
|---|---|---|---|---|---|
| Fever | 73 (69.52%) | 13 (61.90%) | 60 (71.43%) | 0.719 | 0.698 |
| Arthralgia | 57 (54.29%) | 4 (19.05%) | 53 (63.10%) | 13.1346 | < 0.001 |
| Butterfly erythema | 73 (69.52%) | 5 (23.81%) | 68 (80.95%) | 25.89 | < 0.001 |
| Renal damage | 64 (60.95%) | 18 (85.71%) | 46 (54.76%) | 6.763 | 0.034 |
| Alopecia | 35 (33.33%) | 5 (23.81%) | 30 (35.71%) | 1.071 | 5.585 |
| Oral ulcer | 27 (25.71%) | 10 (47.62%) | 17 (20.24%) | 6.594 | 0.037 |
| Photosensitivity | 26 (24.76%) | 4 (19.05%) | 22 (26.19%) | 0.460 | 0.795 |
| Interstitial lung disease | 13 (12.38%) | 3 (14.29%) | 10 (11.90%) | 0.088 | 0.957 |
| Raynaud’s phenomenon | 28 (26.67%) | 2 (9.52%) | 26 (30.95%) | 3.945 | 0.139 |
| Nervous system damage | 10 (9.52%) | 6 (28.57%) | 4 (4.76%) | 11.05 | 0.004 |
| Pulmonary arterial hypertension | 11 (10.48%) | 3 (14.29%) | 8 (9.52%) | 0.406 | 0.816 |
| Osteoporosis | 5 (4.76%) | 0 (0) | 5 (5.95%) | 1.313 | 0.519 |
| Dystopia | 4 (3.81%) | 3 (14.29%) | 1 (1.19%) | 7.862 | 0.020 |
Comparison of autoantibody detection results among the three groups
The positive rate of autoantibodies in the SE group was significantly higher than it was in the DC and HC groups (all P < 0.05). Additionally, there was a higher detection rate in the patients with SLE and rheumatoid diseases, and a very low detection rate in the normal people, as shown in Table 3.
Table 3.
Comparison of the autoantibody detection results among the three groups
| Detection items | SE group (n=105) | DC group (n=110) | HC group (n=100) | χ2 | P |
|---|---|---|---|---|---|
| ANA | 96 (91.43%) | 67 (60.91%) | 3 (3%) | 165.2459 | < 0.001 |
| Anti-dsDNA | 45 (42.86%) | 3 (2.73%) | 0 (0) | 93.3196 | < 0.001 |
| AnuA | 36 (42.85%) | 3 (2.73%) | 0 (0) | 70.0226 | < 0.001 |
| Anti-SM | 27 (25.71%) | 5 (4.55%) | 0 (0) | 42.9435 | < 0.001 |
| APRA | 11 (10.48%) | 0 (0) | 0 (0) | 22.7961 | < 0.001 |
| Anti-rRNP | 30 (28.57%) | 11 (10%) | 0 (0) | 38.2885 | < 0.001 |
| Anti-SSA | 54 (51.43%) | 25 (22.73%) | 0 (0) | 72.5962 | < 0.001 |
| Anti-SSB | 27 (25.71%) | 8 (7.27%) | 0 (0) | 36.8123 | < 0.001 |
| AHA | 17 (16.19%) | 0 (0) | 0 (0) | 35.9396 | < 0.001 |
Analysis of the nine specific autoantibodies in the sera of the SE group
Using SLE as dependent variable and ANA, anti-dsDNA, AnuA, anti-SM, etc. as covariates, our logistic regression analyses showed that anti-SM, anti-dsDNA, AnuA, and anti-SSA entered the regression equation (P < 0.05), as shown in Table 4.
Table 4.
A logistic regression analysis of the detection results of the 9 specific autoantibodies in the sera of the SE group
| Detection items | B | Wals | P | OR (95% CI) |
|---|---|---|---|---|
| Anti-SM | 1.184 | 6.403 | 0.015 | 3.506 (1.328~9.498) |
| Anti-dsDNA | 1.104 | 6.076 | 0.013 | 1.587 (1.104~2.341) |
| AnuA | 1.105 | 4.814 | 0.024 | 2.941 (1.132~7.832) |
| Anti-SSA | 0.483 | 5.301 | 0.025 | 2.687 (1.165~6.534) |
Analysis of autoantibody detection results of the serum AnuA, anti-dsDNA, anti-SM and anti-SSA in the SE group
Among the four autoantibodies, anti-SSA had the highest sensitivity but the lowest specificity, and the specificity of the other three autoantibodies was all over 97%, as shown in Table 5.
Table 5.
Analysis of the autoantibody detection results of serum AnuA, anti-dsDNA, anti-SM, and anti-SSA in the SE group
| Detection items | Positive (case) | Sensitivity | Specificity |
|---|---|---|---|
| AnuA | 36 | 34.29% | 97.28% |
| Anti-dsDNA | 45 | 42.85% | 97.34% |
| Anti-SM | 27 | 25.71% | 97.32% |
| Anti-SSA | 54 | 51.43% | 67.57% |
Analysis of the serum AnuA, anti-dsDNA, and anti-SM in the active and inactive patients of the SE group
The positive rate of the anti-dsDNA in the active stage was significantly higher than it was in the inactive stage (P < 0.05). The positive rates of AnuA and anti-SM were higher than they were in the inactive stage (P > 0.05), as detailed in Figure 1.
Figure 1.
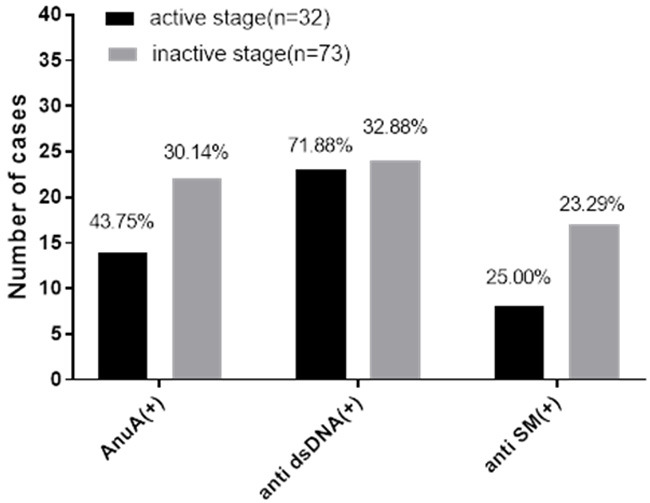
Analysis of the serum AnuA, anti-dsDNA and anti-SM in the active and inactive patients in the SE group. Note: The abscissa indicates AnuA (+), anti-sDNA (+), anti-SM (+), while the ordinate indicates the number of cases. It can be seen from Figure that the positive rates of AnuA, anti-dsDNA, and anti-SM in the active stage were all higher than they were in the inactive stage. The positive rates of the anti-dsDNA were compared among the groups, with significant differences (χ2=13.6845, P < 0.05), and the positive rates of AnuA and anti-SM were compared among the groups, with no significant differences (χ2=1.8299, 0.0360, all P > 0.05).
Combined autoantibody detection results
The sensitivities of the combined autoantibody detections such as AnuA + anti-dsDNA, anti-dsDNA + anti-SM, AnuA + anti-dsDNA, and AnuA + anti-dsDNA + anti-SM were significantly higher than the sensitivity of the single antibody detection (All P < 0.05), and the specificity was higher than 97%, as shown in Tables 5, 6.
Table 6.
Combined autoantibody detection results
| Detection items | Positive (case) | Sensitivity | Specificity |
|---|---|---|---|
| AnuA + Anti-SM | 56 | 53.33% | 97.43% |
| Anti-dsDNA + Anti-SM | 68 | 64.76% | 97.58% |
| AnuA + Anti-dsDNA | 81 | 77.14% | 97.64% |
| AnuA + Anti-dsDNA + Anti-SM | 102 | 97.14% | 98.46% |
Discussion
SLE is a clinically common systemic autoimmune disease involving multiple organs and systems, and it mostly affects the female population. Due to the fact that it is an autoimmune disorder, a variety of autoantibodies are produced against normal cells, which results in inflammation in multiple organs and systems, seriously threatening patients’ physical health [13,14]. At present, the SLE pathogenesis is still unclear, and most studies believe that its pathogenesis is closely related to heredity, the endocrine system, the immune system, etc. With atypical clinical symptoms and the early hidden SLE conditions, once the onset develops rapidly, it will lead to inflammation in multiple organ systems. If not diagnosed and treated early, it will cause serious body damage to the patients and reduce the patients’ quality of life [15-17]. In the early diagnosis of SLE, because of the patients’ atypical clinical manifestations, misdiagnoses can easily occur. Therefore, in the diagnosis of SLE, except for the diagnosis of the clinical symptoms, laboratory antibody detection is also of great significance in the diagnosis of the disease [18]. By improving the diagnostic accuracy, the incidences of misdiagnosis and missed diagnoses are reduced.
Some studies have found that the production of multiple autoantibodies in the sera of SLE patients, such as ANA, is the manifestation of an immune system disorder in the SLE patients. However, besides being present in the SLE patients, ANA is also found in the sera of some patients with connective tissue diseases [19]. Therefore, the ANA detection, which lacks specificity, can be used as a screening tool in the diagnosis of SLE, but it cannot be used as a final diagnostic index. It has been reported in the literature that there are autoantibodies such as anti-SM, AnuA, anti-SSA, anti-dsDNA in ANAs, among which anti-SM and anti-dsDNA have a high specificity in SLE, but they have the disadvantages of low sensitivity [20]. The sensitivity and specificity of an SLE diagnosis can be improved by the combined detection of multiple autoantibodies to reduce the negative impact on the detection population and the disease process, and to decrease the occurrence of misdiagnoses and missed diagnoses.
In this study, 105 SLE patients admitted to our hospital from May 2019 to September 2020 were recruited as the study cohort, and the combined detection of the SLE autoantibody characteristics were investigated and analyzed. The clinical data of the SLE patients revealed that their main clinical manifestations were fever, arthralgia, butterfly erythema, renal damage, etc. There were no significant differences between the male and female patients in terms of their clinical manifestations such as fever, alopecia, photosensitivity, interstitial lung disease, Raynaud’s phenomenon, pulmonary arterial hypertension, osteoporosis, etc. (P > 0.05). The incidences of arthralgia and butterfly erythema in the female patients were significantly higher than they were in the male patients. The incidences of renal damage, oral ulcers, nervous system damage, and dysopsia in the male patients was significantly higher than it was in the female patients. The study results are consistent with the findings of many other studies [21,22], suggesting that the clinical manifestations of SLE are complex and diverse, and different genders have different clinical symptoms. Among the clinical manifestations of SLE, the common clinical symptoms of the female patients are arthralgia and butterfly erythema, while the male patients more commonly suffer from renal damage, oral ulcers, nervous system damage, dysopsia, etc., and this might have a close relationship with sex hormones. The autoantibody detection showed that the positive rate of autoantibodies in the SE group was significantly higher than it was in the DC and HC groups (All P < 0.05). Additionally, there was a higher detection rate in the patients with SLE and rheumatoid diseases, and a very low detection rate in the normal people, which is consistent with the study of Gorji, et al. [23], suggesting that the autoantibodies such as anti-dsDNA, AnuA, anti-SM, APRA and anti-rRNP have a high detection rate in SLE patients, and have a very low detection rate and a high specificity in other patients with rheumatic diseases and normal people. Our logistic regression analyses demonstrated that anti-SM, anti-dsDNA, AnuA, and anti-SSA entered the regression equation. Among the four autoantibodies, including serum AnuA, anti-dsDNA, anti-SM and anti-SSA, the sensitivity of anti-SSA was the highest, but its specificity was the lowest, and the specificities of the other three autoantibodies were all more than 97%. The study results are consistent with the results of many foreign studies [24,25], indicating that anti-SM, anti-dsDNA, and AnuA have very high specificities, but they have lower sensitivities than the other autoantibodies. By grouping the SLE patients according to their disease processes, it was found that the positive rate of anti-dsDNA in the active stage was significantly higher than it was in the inactive stage, and the positive rates of AnuA and anti-SM were higher than they were in the inactive stage, with no significant differences, which confirms that the expressions of the autoantibodies in the SLE patients are different in the different disease processes. Through the combined detection of anti-SM, anti-dsDNA, and AnuA, we found that the sensitivity of the combined detections of AnuA + anti-dsDNA, anti-dsDNA + anti-SM, AnuA + anti-dsDNA, and AnuA + anti-dsDNA + anti-SM were all significantly higher than the single autoantibody detection, and the specificities were all higher than 97% (All P < 0.05). The sensitivity of the combined detection of the three was the highest, reaching 97.14%, which confirms that the combined detection of autoantibodies can complement each other, effectively improving the sensitivity and specificity of the detection, and reducing the misdiagnosis and missed diagnosis rates. Moreover, an early confirmed diagnosis and early treatment can also beneficially improve the patients’ prognostic quality.
In conclusion, single autoantibody detection has the disadvantage of low sensitivity and specificity. The combined detection of autoantibodies can effectively improve the sensitivity and specificity of SLE detection, significantly improve the detection rate of SLE, effectively distinguish other rheumatic diseases, and reduce missed diagnoses and misdiagnoses in the clinical detection.
Disclosure of conflict of interest
None.
References
- 1.Vecellio M, Hake VX, Davidson C, Carena MC, Wordsworth BP, Selmi C. The IL-17/IL-23 axis and its genetic contribution to psoriatic arthritis. Front Immunol. 2021;11:596086. doi: 10.3389/fimmu.2020.596086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 3.Sciascia S, Radin M, Yazdany J, Levy RA, Roccatello D, Dall’Era M, Cuadrado MJ. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev. 2017;16:287–293. doi: 10.1016/j.autrev.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–30. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs. 2018;78:355–366. doi: 10.1007/s40265-018-0872-z. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Bae SC, Bass D, Chu M, Egginton S, Gordon D, Roth DA, Zheng J, Tanaka Y. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77:355–363. doi: 10.1136/annrheumdis-2017-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16:440. doi: 10.1007/s11926-014-0440-9. [DOI] [PubMed] [Google Scholar]
- 8.Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G, Hughes G. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 9.Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, Weinstein A, McKay JD, McCune WJ, Petri M, Fettiplace J, Roth DA, Ji B, Heath A. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1125–1134. doi: 10.1002/art.40861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria A, Stohl W, Schwarting A, Okada M, Scheinberg M, van Vollenhoven R, Hammer AE, Groark J, Bass D, Fox NL, Roth D, Gordon D. Efficacy and safety of subcutaneous belimumab in anti-double-stranded DNA-positive, hypocomplementemic patients with systemic lupus erythematosus. Arthritis Rheumatol. 2018;70:1256–1264. doi: 10.1002/art.40511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones A, Muller P, Dore CJ, Ikeji F, Caverly E, Chowdhury K, Isenberg DA, Gordon C, Ehrenstein MR. Belimumab after B cell depletion therapy in patients with systemic lupus erythematosus (BEAT Lupus) protocol: a prospective multicentre, double-blind, randomised, placebo-controlled, 52-week phase II clinical trial. BMJ Open. 2019;9:e032569. doi: 10.1136/bmjopen-2019-032569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Vollenhoven RF, Navarra SV, Levy RA, Thomas M, Heath A, Lustine T, Adamkovic A, Fettiplace J, Wang ML, Ji B, Roth D. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology. 2020;59:281–291. doi: 10.1093/rheumatology/kez279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, Kleoudis C, Groark J, Bass D, Fox NL, Roth D, Gordon D. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69:1016–1027. doi: 10.1002/art.40049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, Roth DA, Gordon D. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase III parent study in the United States. Arthritis Rheumatol. 2018;70:868–877. doi: 10.1002/art.40439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, Amoura Z, Yu X, Mok CC, Santiago MB, Saxena A, Green Y, Ji B, Kleoudis C, Burriss SW, Barnett C, Roth DA. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 16.Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, Enghard P, Richter U, Biesen R, Schneider U, Knebel F, Burmester G, Radbruch A, Mei HE, Mashreghi MF, Hiepe F, Alexander T. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med. 2020;383:1149–1155. doi: 10.1056/NEJMoa2023325. [DOI] [PubMed] [Google Scholar]
- 17.Samy E, Wax S, Huard B, Hess H, Schneider P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int Rev Immunol. 2017;36:3–19. doi: 10.1080/08830185.2016.1276903. [DOI] [PubMed] [Google Scholar]
- 18.Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, Bajema IM, Scherer HU, Toes REM, Huizinga TJW, Rabelink TJ, van Kooten C, Teng YKO. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. 2018;91:45–54. doi: 10.1016/j.jaut.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford) 2017;56:i3–i13. doi: 10.1093/rheumatology/kew401. [DOI] [PubMed] [Google Scholar]
- 20.Carreira PL, Isenberg DA. Recent developments in biologic therapies for the treatment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2019;58:382–387. doi: 10.1093/rheumatology/key064. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Arztebl Int. 2015;112:423–32. doi: 10.3238/arztebl.2015.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise LM, Stohl W. The safety of belimumab for the treatment of systemic lupus erythematosus. Expert Opin Drug Saf. 2019;18:1133–1144. doi: 10.1080/14740338.2019.1685978. [DOI] [PubMed] [Google Scholar]
- 23.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22:1286–94. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 24.Azoicai T, Antoniu S, Caruntu ID, Azoicai D, Antohe I, Gavrilovici C. Belimumab and antipneumococcal vaccination in patients with systemic lupus erythematosus. Expert Rev Clin Immunol. 2018;14:175–177. doi: 10.1080/1744666X.2018.1429269. [DOI] [PubMed] [Google Scholar]
- 25.Basta F, Fasola F, Triantafyllias K, Schwarting A. Systemic lupus erythematosus (SLE) therapy: the old and the new. Rheumatol Ther. 2020;7:433–446. doi: 10.1007/s40744-020-00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


