Abstract
Objective: To study the correlation of serum uric acid (UA), cystatin C (Cys-C) and high-sensitivity C-reactive protein (hs-CRP) with cognitive impairment in lacunar cerebral infarction. Methods: Total 198 patients with lacunar cerebral infarction were selected and divided into 4 groups according to their cognitive function, with 65 cases in the normal group, 72 cases in the mild cognitive impairment group, 38 cases in the moderate cognitive impairment group and 23 cases in the severe cognitive impairment group. The hs-CRP, serum UA, Cys-C and Montreal Cognitive Assessment (MoCA) were measured upon admission. Results: There were statistical differences in hs-CRP, UA and Cys-C among the four groups (all P<0.001). MoCA was negatively correlated with hs-CRP, UA and Cys-C (all P<0.001). Multivariate logistic regression analysis showed that elevated levels of hs-CRP, UA and Cys-C were the influencing factors of cognitive impairment in patients with lacunar cerebral infarction (all P<0.05). Conclusion: The levels of hs-CRP, UA and Cys-C in patients with lacunar cerebral infarction increase with the aggravation of cognitive impairment, and high hs-CRP, UA and Cys-C are independent risk factors of cognitive impairment in patients with lacunar cerebral infarction.
Keywords: Serum uric acid, cystatin C, high-sensitivity C-reactive protein, lacunar cerebral infarction, cognitive impairment
Introduction
Lacunar cerebral infarction is a type of cerebral small vessel disease, and its incidence rate in China is increasing annually [1]. Studies have shown that cognitive impairment is common in patients with lacunar cerebral infarction, and it can progress to vascular dementia without intervention, thus seriously affecting patients’ quality of life [2,3]. Cognitive impairment in patients with lacunar cerebral infarction is often a non-dementia vascular cognitive impairment, and can also be considered as the early stage of vascular dementia [4,5]. At this stage, patients’ cognitive function can further deteriorate and develop into dementia, and it can also be reversed and returned to a normal cognitive state [6,7].
Previous studies have shown that arteriosclerosis is the pathological basis of lacunar cerebral infarction [8]; inflammation and oxidative stress are initiating factors in the occurrence and development of arteriosclerosis [9,10]. High-sensitivity C-reactive protein (hs-CRP) can reflect the inflammatory state of human body, studies have shown that inflammatory markers are correlated to cognitive impairment [11]. Elevated serum uric acid (UA) can promote oxidative stress, increase the release of inflammatory factors in the body, and accelerate the development of vascular dementia in patients with vascular cognitive impairment [12]. Cystatin C (Cys-C) is an indicator reflecting glomerular filtration function. In recent years, more and more studies have indicated that the increase of Cys-C is correlated with the occurrence of cerebrovascular diseases, with a certain predictive effect [13]. The early stage of cognitive impairment can be reversed after treatment, but once cognitive impairment progresses to vascular dementia, it will result in lifelong disability. Therefore, it is of great significance to use all relevant indicators to predict the occurrence of cognitive impairment and make an early diagnosis. The correlation between the above three indicators and cognitive impairment is still controversial [14,15].
Based on this, lacunar cerebral infarction patients with varying degrees of cognitive impairment were included in this study to measure the hs-CRP, UA and Cys-C, so as to provide more clinical evidence for the prevention and treatment of cognitive impairment caused by lacunar cerebral infarction. The reports are as follows.
Materials and methods
Clinical data
A total of 198 patients with lacunar cerebral infarction who were treated in the Neurology Department of Xinhua Hospital of Ily Kazakh Autonomous Prefecture from January 2017 to January 2020 were selected as the research subjects, and they were followed up for 6 months to evaluate their cognitive function. This study was approved by Ethics Committee of Xinhua Hospital of Ily Kazakh Autonomous Prefecture. All patients or their families in this study signed the informed consent forms.
Inclusion criteria
(1) Patients met the diagnostic criteria of lacunar cerebral infarction [16]; (2) the clinical data was complete, and patients were able to cooperate; (3) patients were 18-76 years old; (4) patients were awake upon admission and could complete the scale measurements.
Exclusion criteria
(1) Patients were excluded with: non-vascular cognitive impairment or cognitive impairment caused by Alzheimer’s disease; (2) patients who could not cooperate with cognitive function evaluation; (3) patients who were combined with severe cardiopulmonary disease or recent infection; (4) patients who took metabolic drugs affecting hs-CRP, UA and Cys-C within one month of our study; (5) patients with malignant tumors; (6) patients with mental diseases that affect cognition.
Methods and grouping
Methods
Before admission, two tubes of venous blood were extracted, with 5 mL in each tube. Hs-CRP was determined by serum enzyme-linked immunosorbent assay, UA content was measured by automatic biochemical analyzer (Beckman Coulter, USA) and Cys-C was measured by immunoturbidimetry. The kits were all purchased from Shanghai Enzyme Linked Biology Co., Ltd., China.
Grouping
The cognitive function of the patients was measured by Montreal Cognitive Assessment (MoCA), and patients were divided into 4 groups, with 65 cases in the normal group, 72 cases in the mild cognitive impairment group, 38 cases in the moderate cognitive impairment group and 23 cases in the severe cognitive impairment group [17].
Statistical analysis
SPSS 17.0 statistical software was used to analyze the data. Count data was expressed as the number of cases and percentage (n/%). Continuous variables were expressed as mean ± standard deviation (x̅ ± sd). Independent sample t-test was used for the data that conformed to a normal distribution and homogeneity of variances. Pearson’s chi-square test (χ2) was used for the analysis of count data. Logistic regression analysis was used to detect the risk factors of cognitive impairment in lacunar cerebral infarction. Univariate analysis was used for variables with differences. The Ward method was used for variable screening, with inclusion level of 0.05 and exclusion level of 0.1. The risk of decline in MoCA cognitive scoring was expressed by the adjusted odds ratio (OR). P<0.05 was considered statistically significant.
Results
Comparison of general information
There were no differences in gender, age, education, body mass index (BMI), hypertension, carotid atherosclerosis or stenosis, and coronary heart disease among the four groups (all P>0.05). There were statistical differences in diabetes, smoking, drinking, abdominal obesity and hyperhomocysteinemia (all P<0.05). See Table 1.
Table 1.
Comparison of general information among the four groups (n, x̅ ± sd)
| Items | Normal group (n=65) | Mild group (n=72) | Moderate group (n=38) | Severe group (n=23) | χ2/t | P |
|---|---|---|---|---|---|---|
| Gender (Male/Female) | 35/30 | 40/32 | 22/16 | 13/10 | 0.170 | 0.982 |
| Age (year) | 67.4±7.5 | 67.9±7.1 | 68.1±7.6 | 68.2±7.9 | 0.110 | 0.954 |
| Education | 11.2±4.4 | 11.4±4.3 | 11.5±3.9 | 11.4±4.5 | 0.046 | 0.987 |
| BMI (kg/m2) | 20.39±2.03 | 20.45±1.99 | 20.67±2.07 | 20.78±2.08 | 0.311 | 0.817 |
| Combined disease | ||||||
| Diabetes | 34 | 45 | 28 | 19* | 8.881 | 0.031 |
| Hypertension | 35 | 38 | 24 | 14 | 1.437 | 0.697 |
| CHD | 37 | 41 | 23 | 13 | 0.169 | 0.982 |
| Smoking | 42 | 51 | 31 | 20* | 7.803 | 0.049 |
| Drinking | 39 | 41 | 32* | 20* | 13.887 | <0.001 |
| Abdominal obesity | 23 | 34* | 29* | 18* | 22.304 | <0.001 |
| Carotid atherosclerosis or stenosis | 36 | 39 | 26 | 17 | 4.573 | 0.208 |
| Hyperhomocy-steinemia | 47 | 62* | 30* | 23* | 10.166 | 0.017 |
Note: Compared with the normal group;
P<0.05.
BMI: body mass index; CHD: coronary heart disease.
Comparison of hs-CRP, UA and Cys-C among the four groups
There were statistical differences in hs-CRP, UA and Cys-C among the four groups (all P<0.001). With the aggravation of the disease, hs-CRP, UA and Cys-C showed an upward trend (all P<0.05). See Table 2.
Table 2.
Comparison of hs-CRP, UA and Cys-C among the four groups
| Items | hs-CRP (mg/L) | UA (μmol/L) | Cys-C (mg/L) |
|---|---|---|---|
| Normal group (n=65) | 3.45±0.73 | 302.32±52.87 | 0.92±0.27 |
| Mild group (n=72) | 5.78±1.14*** | 416.23±48.34*** | 1.19±0.38*** |
| Moderate group (n=38) | 7.23±1.21***,### | 433.64±53.34***,# | 1.39±0.41***,# |
| Severe group (n=23) | 8.76±1.28***,###,&&& | 465.73±54.23***,###,& | 1.62±0.41***,##,& |
| χ2/t | 189.124 | 94.552 | 27.372 |
| P | <0.001 | <0.001 | <0.001 |
Note: Compared with the normal group;
P<0.001.
Compared with the mild group;
P<0.05;
P<0.01;
P<0.001.
Compared with the moderate group;
P<0.05;
P<0.001.
hs-CRP: high-sensitivity C-Reactive Protein; UA: uric acid; Cys-C: Cystatin C.
Correlation of hs-CRP, UA and Cys-C with MoCA
Hs-CRP, UA and Cys-C were negatively correlated with MoCA scores (all P<0.001). See Table 3 and Figures 1, 2 and 3.
Table 3.
Correlation of hs-CRP, UA and Cys-C with MoCA
| MoCA | r | P |
|---|---|---|
| hs-CRP (mg/L) | -0.825 | <0.001 |
| UA (μmol/L) | -0.718 | <0.001 |
| Cys-C (mg/L) | -0.475 | <0.001 |
Note: MoCA: Montreal cognitive assessment; hs-CRP: high-sensitivity C-Reactive Protein; UA: uric acid; Cys-C: cystatin C.
Figure 1.
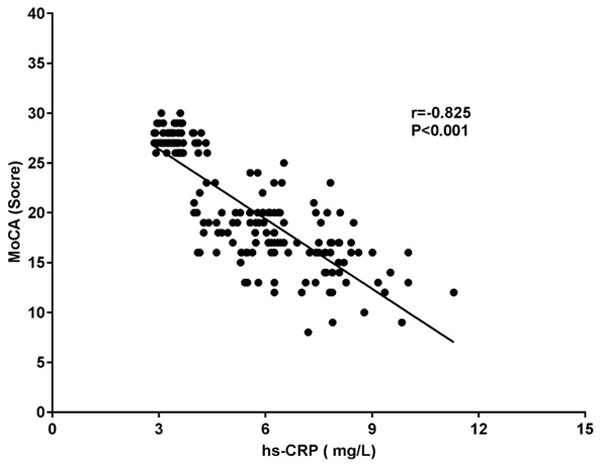
Correlation study between hs-CRP and MoCA. MoCA: Montreal cognitive assessment; hs-CRP: high-sensitivity C-Reactive Protein.
Figure 2.
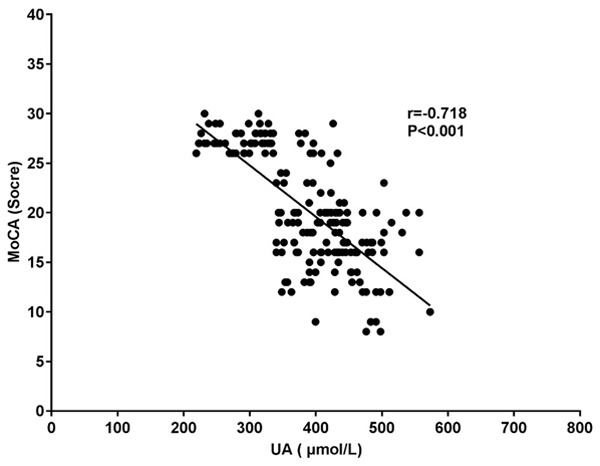
Correlation study between UA and MoCA. MoCA: Montreal cognitive assessment; UA: uric acid.
Figure 3.
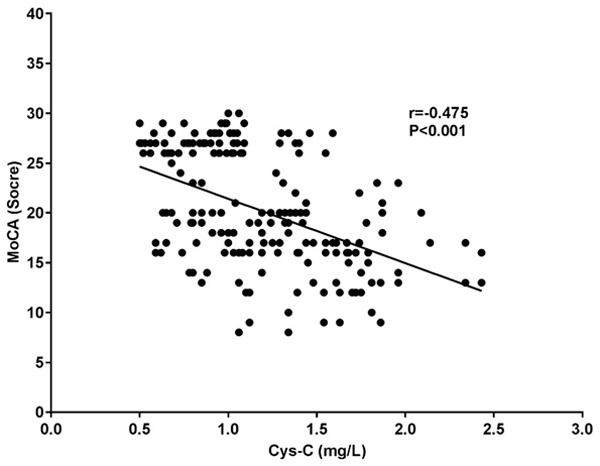
Correlation study between Cys-C and MoCA. MoCA: Montreal cognitive assessment; Cys-C: cystatin C.
Influencing factors of cognitive impairment in patients with lacunar cerebral infarction
Multivariate logistic regression analysis showed that elevated levels of hs-CRP, UA and Cys-C were the influencing factors of cognitive impairment in patients with lacunar cerebral infarction (all P<0.05). See Tables 4 and 5.
Table 4.
Independent variable assignment table of influencing factors of cognitive impairment in patients with lacunar cerebral infarction
| Influencing factors | Independent variable | Assignment |
|---|---|---|
| Diabetes | X1 | Yes =1, no =0 |
| Smoking | X2 | Yes =1, no =0 |
| Drinking | X4 | Yes =1, no =0 |
| Abdominal obesity | X5 | Yes =1, no =0 |
| Hyperhomocysteinemia | X6 | Yes =1, no =0 |
| hs-CRP (mg/L) | X7 | >10 mg/L=1, ≤10 mg/L=0 |
| UA (μmol/L) | X8 | Male ≥420 μmol/L or female ≥360 μmol/L=1, male <420 μmol/L or female <360 μmol/L=0 |
| Cys-C (mg/L) | X9 | >1.09 mg/L=1, ≤1.09 mg/L=0 |
Note: hs-CRP: high-sensitivity C-Reactive Protein; UA: uric acid; Cys-C: Cystatin C.
Table 5.
Influencing factors of cognitive impairment in patients with lacunar cerebral infarction
| Influencing factors | β | SE | Wald value | OR value (95% CI) | P |
|---|---|---|---|---|---|
| hs-CRP (mg/L) | 0.867 | 0.192 | 12.034 | 2.673 (1.167-3.234) | 0.012 |
| UA (μmol/L) | 0.893 | 0.223 | 13.239 | 2.873 (1.233-3.582) | 0.006 |
| Cys-C (mg/L) | 0.728 | 0.148 | 10.762 | 2.089 (1.128-3.122) | 0.017 |
Note: hs-CRP: high-sensitivity C-Reactive Protein; UA: uric acid; Cys-C: Cystatin C; SE: standard error; CI: confidence interval; OR: odds ratio.
Discussion
Lacunar cerebral infarction is a common type of cerebral small vessel disease, of which the main pathogenesis is arteriosclerosis [18]. Studies have found that patients with lacunar cerebral infarction are prone to cognitive impairment [19,20]. Inflammatory factors not only play an important role in the occurrence and development of lacunar cerebral infarction, but also accelerate the occurrence of cognitive impairment after cerebral infarction in an inflammatory state [11,21]. Some studies have found that the expression of hs-CRP increases in patients with vascular dementia, which may be related to the involvement of hs-CRP in the formation of atherosclerosis [22]. Another study showed that the expression of hs-CRP in patients with non-dementia vascular cognitive impairment increased and was negatively correlated with the MoCA score [23]. However, some studies have found that there is no correlation between the level of hs-CRP and the occurrence of vascular dementia [14]. In this study, it was shown that with the aggravation of cognitive impairment, hs-CRP level presented an increasing trend, which was negatively correlated with MoCA score, and was an independent risk factor for cognitive impairment in patients with lacunar cerebral infarction.
UA has a wide range of physiological functions in the body [24]. The antioxidant properties of UA can reduce the occurrence of oxidative stress and may have a certain neuroprotective effect [25,26]. However, with the oxidative stress response, UA level significantly increased. The anti-oxidation of UA transformed to pro-oxidation due to the high concentration, which can further promote the oxidation of lipoproteins in atherosclerotic plaques and the proliferation of smooth muscle cells, ultimately lead to vascular endothelial damage [27]. Studies have shown that UA in dementia patients with Alzheimer’s disease and Parkinson’s disease is shown to have antioxidant effects, it can protect nerve cells and effectively prevent the occurrence of cognitive impairment. However, high levels of UA in patients with vascular dementia have been found to cause brain white matter atrophy and cognitive decline [28-30]. Studies have indicated that uric acid-lowering therapy may have better clinical benefits for high-risk patients who may develop cognitive impairment [31]. Another study also showed that high levels of UA can promote vascular endothelial damage, produce oxidative stress and promote the release of inflammatory factors [32]. This study showed that with the aggravation of cognitive impairment, UA in patients presented an increasing trend, which was negatively correlated with MoCA score, and was an independent risk factor for cognitive impairment in patients with lacunar cerebral infarction. The result of this study was consistent with the above-mentioned research mechanism.
Cys-C is a reliable indicator of renal function, because it is excreted entirely by the kidneys in the body [33]. In recent years, studies have found that Cys-C level is correlated with the occurrence of cognitive impairment [34]. Studies have reported that there is a positive correlation between the increase of Cys-C and cerebral microvascular hemorrhage in patients with stroke [35]. Cys-C can interfere with the phagocytosis and chemotaxis of granulocytes, promote the release of inflammatory factors, and participate in the occurrence of arteriosclerosis [36]. In elderly patients, increased Cys-C can lead to an increased incidence of cognitive impairment. In patients with renal insufficiency, the higher the Cys-C level, the worse the level of cognition [37]. Another study also found that high levels of Cys-C can induce atherosclerosis, cause damage to vascular walls, promote the release of inflammatory factors and aggravate nerve cell damage [38]. This study showed that with the aggravation of cognitive impairment, Cys-C in patients presented an upward trend, which was negatively correlated with MoCA score. Cys-C was an independent risk factor for cognitive impairment in patients with lacunar cerebral infarction and related to the above research mechanism. In this study, although there were differences in diabetes, smoking, drinking, abdominal obesity, hyperhomocysteinemia and other factors in different groups, they were not independent risk factors leading to cognitive impairment. The result was inconsistent with previous studies and might be related to the small sample size in this study.
The sample size in this study is small, which requires being further expanded in future research. Moreover, the observation time was short, and the follow-up time needs to be further increased to explore the correlation of hs-CRP, UA and Cys-C with cognitive impairment.
To sum up, hs-CRP, UA and Cys-C in patients with lacunar cerebral infarction increase with the aggravation of cognitive impairment, and they are all negatively correlated with MoCA scores. High hs-CRP, UA and Cys-C are independent risk factors of cognitive impairment in patients with lacunar cerebral infarction.
Disclosure of conflict of interest
None.
References
- 1.Jiménez-Balado J, Riba-Llena I, Abril O, Garde E, Penalba A, Ostos E, Maisterra O, Montaner J, Noviembre M, Mundet X, Ventura O, Pizarro J, Delgado P. Cognitive impact of cerebral small vessel disease changes in patients with hypertension. Hypertension. 2019;73:342–349. doi: 10.1161/HYPERTENSIONAHA.118.12090. [DOI] [PubMed] [Google Scholar]
- 2.Balian NR, Alonzo CB, Zurrú MC, Brescacin L, Pigretti SG, Colla Machado PE, Waisman GD, Cristiano E. Clinical predictors of hemorrhagic transformation in non lacunar ischemic stroke. Medicina (B Aires) 2017;77:100–104. [PubMed] [Google Scholar]
- 3.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 2017;131:425–437. doi: 10.1042/CS20160604. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y, Xing Y, Zhu ZD, He Y, Li F, Yang JW, Liu Q, Li FY, Teipel SJ, Zhao G, Jia JP. The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): a randomized controlled trial. Alzheimers Dement. 2019;15:605–614. doi: 10.1016/j.jalz.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Yang JW, Shi GX, Zhang S, Tu JF, Wang LQ, Yan CQ, Lin LL, Liu BZ, Wang J, Sun SF, Yang BF, Wu LY, Tan C, Chen S, Zhang ZJ, Fisher M, Liu CZ. Effectiveness of acupuncture for vascular cognitive impairment no dementia: a randomized controlled trial. Clin Rehabil. 2019;33:642–652. doi: 10.1177/0269215518819050. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Li G, Xu C, Ju C, Qiu X. Training rehabilitation as an effective treatment for patients with vascular cognitive impairment with no dementia. Rehabil Nurs. 2017;42:290–297. doi: 10.1002/rnj.271. [DOI] [PubMed] [Google Scholar]
- 7.Azarpazhooh MR, Hachinski V. Vascular cognitive impairment: a preventable component of dementia. Handb Clin Neurol. 2019;167:377–391. doi: 10.1016/B978-0-12-804766-8.00020-0. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu N, Huang YH, Wei W, Chen F, Zhang WW. Risk factors for silent lacunar infarction in patients with transient ischemic attack. Med Sci Monit. 2016;22:447–453. doi: 10.12659/MSM.895759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun ZQ, Zhao MZ, Bian WW, Ma HF, Sun C. Associations of severity of fatty liver with oxidative stress, SAA, CRP and degree of cerebral arteriosclerosis in cerebral arteriosclerosis patients who have fatty liver. Int J Clin Exp Pathol. 2019;12:3022–3026. [PMC free article] [PubMed] [Google Scholar]
- 10.Sargento-Freitas J, Aday S, Nunes C, Cordeiro M, Gouveia A, Silva F, Machado C, Rodrigues B, Santo GC, Ferreira C, Amorim A, Sousa S, Gomes AC, Castelo-Branco M, Ferreira L, Cunha L. Endothelial progenitor cells enhance blood-brain barrier permeability in subacute stroke. Neurology. 2018;90:e127–e134. doi: 10.1212/WNL.0000000000004801. [DOI] [PubMed] [Google Scholar]
- 11.Narasimhalu K, Lee J, Leong YL, Ma L, De Silva DA, Wong MC, Chang HM, Chen C. Inflammatory markers and their association with post stroke cognitive decline. Int J Stroke. 2015;10:513–518. doi: 10.1111/ijs.12001. [DOI] [PubMed] [Google Scholar]
- 12.Desideri G, Gentile R, Antonosante A, Benedetti E, Grassi D, Cristiano L, Manocchio A, Selli S, Ippoliti R, Ferri C, Borghi C, Giordano A, Cimini A. Uric acid amplifies Aβ amyloid effects involved in the cognitive dysfunction/dementia: evidences from an experimental model in vitro. J Cell Physiol. 2017;232:1069–1078. doi: 10.1002/jcp.25509. [DOI] [PubMed] [Google Scholar]
- 13.Chang AX, Wu SX, Yang QH, Kang ZX, Li YF. Effects of high-dose atorvastatin on prevention of contrast-induced nephropathy after cerebrovascular intervention. Int J Clin Exp Med. 2019;12:10494–10501. [Google Scholar]
- 14.Liu YT, Chen HJ, Zhao K, He WL, Lin SS, He JC. High levels of plasma fibrinogen are related to poststroke cognitive impairment. Brain Behav. 2019;9:e01391. doi: 10.1002/brb3.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JY, Lan TY, Tang GJ, Tang CH, Chen TJ, Lin HY. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis Res Ther. 2015;17:139. doi: 10.1186/s13075-015-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinese Society of Neurology and Neurology Cerebrovascular Disease Study Group of the Chinese Medical Association. Guidelines for diagnosis and treatment of acute ischemic stroke in China 2014. Chin J Neurol. 2015;48:246–257. [Google Scholar]
- 17.Writing Group of Guidelines for Diagnosis and Treatment of Dementia and Cognitive Impairment in China and Professional Committee of Cognitive Disorders, Neurologist Branch, Chinese Medical Association. 2018 Chinese guidelines for the diagnosis and treatment of dementia and cognitive impairment (V): diagnosis and treatment of mild cognitive impairment. Chin J Med. 2018;98:1294–1301. [Google Scholar]
- 18.Xu X, Gao YY, Liu RY, Qian L, Chen Y, Wang XY, Xu Y. Progression of white matter hyperintensities contributes to lacunar infarction. Aging Dis. 2018;9:444–452. doi: 10.14336/AD.2017.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang C, Li MC, Zhang XN, Liu H, Mao ZF, Sun HG. Association between post-stroke depression in lacunar stroke patients and rehospitalization. Int J Clin Exp Med. 2018;11:4126–4131. [Google Scholar]
- 20.Zhou YN, Gao HY, Zhao FF, Liang YC, Gao Y, Liu XH, Wang T, Wang ZG, Wu QJ. The study on analysis of risk factors for severity of white matter lesions and its correlation with cerebral microbleeds in the elderly with lacunar infarction. Medicine (Baltimore) 2020;99:e18865. doi: 10.1097/MD.0000000000018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen LL, Yang QH, Ding R, Liu D, Chen ZJ. Carotid thickness and atherosclerotic plaque stability, serum inflammation, serum MMP-2 and MMP-9 were associated with acute cerebral infarction. Exp Ther Med. 2018;16:5253–5257. doi: 10.3892/etm.2018.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PJ, Mabe S, Sherwood A, Babyak MA, Doraiswamy PM, Welsh-Bohmer KA, Kraus W, Burke J, Hinderliter A, Blumenthal JA. Association between insulin resistance, plasma leptin, and neurocognition in vascular cognitive impairment. J Alzheimers Dis. 2019;71:921–929. doi: 10.3233/JAD-190569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An XL, Li CL. Analysis of risk factors for vascular cognitive impairment in patients with cerebral infarction. Cell Biochem Biophys. 2015;71:673–677. doi: 10.1007/s12013-014-0246-4. [DOI] [PubMed] [Google Scholar]
- 24.Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 25.Pasalic D, Marinkovic N, Feher-Turkovic L. Uric acid as one of the important factors in multifactorial disorders--facts and controversies. Biochem Med (Zagreb) 2012;22:63–75. doi: 10.11613/bm.2012.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Méndez-Hernández E, Salas-Pacheco J, Ruano-Calderón L, Téllez-Valencia A, Cisneros-Martínez J, Barraza-Salas M, Arias-Carrión O. Lower uric Acid linked with cognitive dysfunction in the elderly. CNS Neurol Disord Drug Targets. 2015;14:564–566. doi: 10.2174/1871527314666150430161659. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Kawaguchi A, Tomiyama H, Ishizu T, Matsumoto C, Higashi Y, Takase B, Suzuki T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Takemoto Y, Hano T, Sata M, Ishibashi Y, Maemura K, Ohya Y, Furukawa T, Ito H, Yamashina A, Node K. Cross-sectional and longitudinal associations between serum uric acid and endothelial function in subjects with treated hypertension. Int J Cardiol. 2018;272:308–313. doi: 10.1016/j.ijcard.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Ye BS, Lee WW, Ham JH, Lee JJ, Lee PH, Sohn YH. Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer’s disease biomarker related cognitive decline? Eur J Neurol. 2016;23:948–957. doi: 10.1111/ene.12969. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, O’Reilly ÉJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. 2016;86:520–526. doi: 10.1212/WNL.0000000000002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaaren BF, Vernooij MW, Dehghan A, Vrooman HA, de Boer R, Hofman A, Witteman JC, Niessen WJ, Breteler MM, van der Lugt A, Ikram MA. The relation of uric acid to brain atrophy and cognition: the Rotterdam Scan Study. Neuroepidemiology. 2013;41:29–34. doi: 10.1159/000346606. [DOI] [PubMed] [Google Scholar]
- 31.Latourte A, Soumaré A, Bardin T, Perez-Ruiz F, Debette S, Richette P. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis. 2018;77:328–335. doi: 10.1136/annrheumdis-2016-210767. [DOI] [PubMed] [Google Scholar]
- 32.Crosta F, Occhiuzzi U, Passalacqua G, Occhiuzzi E, Cimini A, Grassi D, Ferri C, Marini C, Borghi C, Desideri G. Association between the serum uric acid levels and lacunar infarcts in the elderly. J Mol Neurosci. 2018;65:385–390. doi: 10.1007/s12031-018-1096-0. [DOI] [PubMed] [Google Scholar]
- 33.Bang JY, Kim SO, Kim SG, Song JG, Hwang GS. Cystatin-C is associated with partial recovery of kidney function and progression to chronic kidney disease in living kidney donors: observational study. Medicine (Baltimore) 2017;96:e6037. doi: 10.1097/MD.0000000000006037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Wei YK, Zhu J. Early markers of kidney dysfunction and cognitive impairment among older adults. J Neurol Sci. 2017;375:209–214. doi: 10.1016/j.jns.2017.01.071. [DOI] [PubMed] [Google Scholar]
- 35.Zhao AM, Qiu WR, Mao LJ, Ren JG, Xu L, Yao MJ, Bilinksi K, Chang D, Liu JX. The efficacy and safety of Jiedu Tongluo granules for treating post-stroke depression with qi deficiency and blood stasis syndrome: study protocol for a randomized controlled trial. Trials. 2018;19:275. doi: 10.1186/s13063-018-2633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadidi NN, Huna Wagner RL, Lindquist R. Nonpharmacological treatments for post-stroke depression: an integrative review of the literature. Res Gerontol Nurs. 2017;10:182–195. doi: 10.3928/19404921-20170524-02. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Kurella-Tamura M, Ackerson L, Hoang TD, Anderson AH, Duckworth M, Go AS, Krousel-Wood M, Kusek JW, Lash JP, Ojo A, Robinson N, Sehgal AR, Sondheimer JH, Steigerwalt S, Townsend RR. Higher levels of cystatin C are associated with worse cognitive function in older adults with chronic kidney disease: the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2014;62:1623–1629. doi: 10.1111/jgs.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Sun L. Cystatin C in cerebrovascular disorders. Curr Neurovasc Res. 2017;14:406–414. doi: 10.2174/1567202614666171116102504. [DOI] [PubMed] [Google Scholar]


