Abstract
Objective: This study aimed to explore the therapeutic effect of surgery using platelet-rich plasma combined with a skin flap transplant on open foot fractures with a soft tissue defect. Methods: From February 2017 to March 2020, 72 patients with open foot fractures and soft tissue defects were recruited as the study cohort. The patients who underwent surgery with just a flap transplant were placed in the control group (the CG) (35 cases), and the patients who underwent surgery using platelet-rich plasma combined with a flap transplant were placed in the research group (the RG) (37 cases). The wound volume changes before and after the treatment, the wound healing times, the fracture healing times, and any adverse prognostic reactions were observed. The pre- and post-treatment VAS and SF-36 scores were observed and recorded. Results: The total effective rate in the RG (100.00%) was significantly higher than the total effective rate in the CG (88.57%) (P=0.034). After the treatment, the wound volumes were lower in both groups, and the volume in the RG was smaller than it was in the CG at 3 weeks and 6 weeks after the treatment (P < 0.05). The average wound healing time in the RG (22.40 ± 2.10 days) was significantly lower than it was in the CG (32.20 ± 3.30 days) (P > 0.05). The average fracture healing time in the RG (6.50 ± 2.20 months) was significantly lower than it was in the RG (7.51 ± 2.33 months) (P > 0.05). The total incidence of adverse reactions to the treatment in the RG was 2.70%, and in the CG it was 11.43%. After the treatment, the VAS scores in the RG were significantly lower than they were in the CG (P < 0.05), and the SF-36 scores in the RG were significantly higher than the SF-36 scores in the CG (P < 0.05). Conclusion: Platelet-rich plasma combined with a skin flap transplant can accelerate the healing times of wounds and fractures and lessen the occurrence of adverse reactions during the patients’ treatment.
Keywords: Open fractures of the foot, soft tissue defects, platelet-rich plasma combined with skin flap transplants
Introduction
Fractures are a common worldwide health problem [1]. When patients suffer a fracture, the skin and subcutaneous soft tissue covering the fracture site are sometimes damaged and broken, which exposes the end of the fracture. This phenomenon is called an open fracture [2]. Sometimes, serious injuries may occur in the complete soft tissue capsule, as the wounds can lead to the exposure of the fracture hematoma with the contaminated area and the potentially complex soft tissue components required for the reconstruction, so the treatment of open injuries is more complicated [3]. These fractures are prone to various complications, especially infections, complications that may affect the treatment outcome and increase the incidence of adverse reactions and the cost of treatment. The treatment can not only protect life, limb, and function, but it can also prevent infection [4,5]. As the injury becomes more complex, it involves more complex soft tissue reconstruction, and it may even require an urgent free tissue transfer, which requires close cooperation between orthopedic surgeons and plastic surgeons. Some studies have shown that it is best to complete the whole surgical reconstruction within 48~72 hours [6].
Skin flap transplants are common in wound repair and organ reconstruction [7]. Because the biological process of wound healing is complex, the activity is reduced, the local growth factor quantity is insufficient, or the regulation of many factors is lost, so the wound healing becomes difficult. Therefore, the application of skin flaps in plastic surgery is limited [8]. Among the many factors affecting the repair of tissue defects, it is reported that growth factors and cytokines can promote healing [9], and exogenous cytokines have been used to promote healing clinically. Platelet-rich plasma is an autologous platelet concentrate obtained from fresh whole blood through centrifugation. The high concentration and functional bioactive molecules in PRP are produced for wound healing, such as TGF-β1, TGF-β2, EGF, FGF, PDGF, VEGF, and IL-1. Studies have shown that using platelet-rich plasma is an effective way to repair and reconstruct fractures and bone defects [10,11]. However, there is no research to show whether platelet-rich plasma combined with skin flap transplantation can play a role in open fractures with soft tissue defects. Therefore, we explored the clinical application value of platelet-rich plasma combined with skin flap transplantation for open fractures with soft tissue defects to inform future treatment.
Materials and methods
Clinical data
From February 2017 to March 2020, 72 patients with open foot fractures and soft tissue defects were recruited for this experiment, and 35 of the patients were treated using skin flap transplants were enrolled in the control group (CG), with an average age of 33.5 ± 4.8 years. The remaining 37 patients underwent surgery using platelet-rich plasma combined with a flap transplant and were placed in the research group (RG), with an average age of 33.7 ± 4.4 years. This experiment conformed to the requirements of the Medical Ethics Committee.
Inclusion and exclusion criteria
Inclusion criteria: patients who had not taken drugs that would influence the test results, patients who could cooperate with the research, patients aged 30~60 years and who had complete clinical data, and patients who signed the informed consent forms.
Exclusion criteria: those died during the treatment, patients with vital organ injuries, patients suffering from other cardiovascular and cerebrovascular disorders, patients with physical disabilities, patients who were pregnant, patients suffering from autoimmune diseases or from chronic diseases, patients who transferred out, those with contraindications for the operation, and patients with mental diseases, language disorders and diseases that might affect the results of this study.
Preparation of the platelet-rich plasma
The platelet rich plasma preparation package was opened. A 50 ml syringe was applied to extract the anticoagulant. Blood from median cubital vein was collected using a 50 ml syringe and a blood collection needle, then it was injected into the centrifuge tube, placed in a centrifuge, and mixed with the appropriate amount of normal saline for centrifugation. After that, the air pressure well on the left side of the centrifuge tube cover was opened, and the lowest red blood cell layer at a distance of about 3~5 cm from the interface was extracted and discarded. The remaining liquid in the centrifuge tube was fully shaken and placed in a centrifuge for centrifugation again. The thrombin was prepared by extracting the proper amount of normal saline using a 2 ml syringe. After the second centrifugation, about 3/4 of the supernatant was extracted from the bottom to the top with a 20 ml syringe and discarded, and the remaining liquid in the centrifuge tube was platelet-rich plasma. The lower platelet-rich plasma was extracted and stored in a refrigerator at 4°C for immediate use.
Treatment methods
During debridement, any necrotic or poorly vascularized tissues, abnormal secretions, or foreign bodies in the wounds and cavities were thoroughly removed. A good blood supply was ensured to the remaining soft tissues. Fracture reduction: the anatomic reduction of the articular surface was ensured.
The CG underwent a skin flap transplant: The condition was stable, the granulation tissue grew well, and a skin flap repair operation was performed. Flap design: The anterolateral thigh flap was obtained according to the wound size. The connecting line from the anterior superior iliac crest to the outer edge of the patella was obtained as the axis, the superficial point of the musculocutaneous perforator was measured with ultrasonic Doppler in the range of about 5 cm in diameter around the midpoint of the line. The flap was designed with this point as a sign. The place above this point was taken as 1/3 of the flap, below this point as 2/3 of the flap, inside as 1/3 of the flap, and outside as 2/3 of the flap. The line between this point and the middle point of the inguinal is the surface projection of the descending branch of the lateral circumflex femoral artery, that is, the incision of the free vascular pedicle to be dissected. The mean length of the vascular pedicles was 10.1 cm. Surgical methods: First, the inner side of the flap was cut, the descending limb was tested, whether the Doppler audiometric blood vessels run in the same direction was confirmed before the operations, the outer side of the flap was cut from a medial to a lateral free flap to the lateral femoral muscle space, and the flap was separated under the tensor of the fascia lata to the musculocutaneous perforating point of the descending limb. The skin and deep fascia of the vascular pedicle were cut open. The muscular branches were ligated and cut off. If the cutaneous branch was a perforator of the musculocutaneous artery, a small amount of muscle sleeves can be carried. The accompanying veins could not be damaged when freeing the blood vessels, so as not to affect venous return. When the flap had a good blood supply and active bleeding at the edge after separating it, the flap was then ligated and cut off at the root of the descending limb. The anterolateral femoral cutaneous nerves were removed during the process of removing the flap, and the flap was moved to the foot wound. The descending limb and two accompanying veins were anastomosed with the anterior tibial artery and their companions, respectively, and the anterolateral femoral cutaneous nerve was anastomosed with the foot to suture the skin. The thigh donor site was sutured directly or packed with a skin autograft, and the foot was fixed externally with plaster. Postoperative treatment: the routine application of anti-infective drugs was performed after the operation. Seven months after the operation, the skin flaps were reexamined to determine their ability to detect pain, temperature, pressure, and two point discrimination. Matters needing attention during the operation: the debridement and hemostasis should be thorough, otherwise they will easily cause a subcutaneous hematoma and affect the venous return of the skin flap. Before cutting the flap, a Doppler blood flow meter was used to determine the distribution of the cutaneous branches, and they were marked well. Before the operation, we became familiar with the classification and direction of the descending limb, and the flap design should be better looser rather than tighter.
The RG underwent surgery using platelet-rich plasma combined with the skin flap transplant: the patients in the RG were treated with platelet-rich plasma in addition to the surgery described above. The patients were instructed to fast before the operation. Conventional skin preparation was performed. Combined anesthesia was conducted during the operation. The patients were taken in a prone or a supine position, and the wound secretions and necrotic tissues were completely removed after careful debridement, so that the healthy tissues were exposed and the bleeding spots were clearly identified. The wound volume was measured by instilling normal saline, and platelet-rich plasma was injected into the wound to fill it as much as possible. After the plasma solidified and retracted into collagen particles, Vaseline gauze was applied to bind up the wound, and the wound was sealed to prevent the loss of the platelet-rich plasma. Finally, the wound was compressed and bandaged. If the fracture did not heal after the operation, the patient was fixed and braked with plaster support, and the patient’s wound condition was checked in 7~10 days. If the wound was not healed, the platelet-rich plasma was injected again.
Clinical efficacy evaluation
Evaluation criteria for the surgery: Markedly effective: after the repair, the nerves, blood vessels, and tendon tissues all survived well, without any dysfunction, the necrosis and exudation, and the wound area was covered promptly and at the first stage. Effective: after the repair, the nerve, blood vessels, and tendon tissue all survived without any significant dysfunction, and the level of reduction of degree wound area was over 80%. Ineffective: the wound was not covered promptly or effectively, and the blood vessels, nerves, tendons and other tissues were damaged to varying degrees, and there was a certain degree of dysfunction.
Scoring criteria
VAS was used to test the postoperative pain, with a maximum of 10 points. A higher score indicates more serious pain and a worse pain control effect. The patients’ quality of life (QOL) was tested using a questionnaire (SF-36), which involved the physical health (physiological function, role physical, somatic pain, and overall health) and mental health. The total possible score in each dimension was 100 points. A higher score after completing the evaluation indicates a better QOL.
Outcome measures
Main outcome measures: The clinical efficacy of the two groups of patients was observed. The changes in the wound volume before and after the treatment, the wound healing times, the fracture healing times, and any adverse reactions from the treatment were observed.
Secondary outcome measures: the VAS scores and SF-36 scores before and after the treatment were observed.
Statistical analysis
In this study, SPSS 20.0 was used for the statistical analysis, and GraphPad 7 was used for drawing the figures. K-S tests were applied for the distribution analysis of the dose data, in which the normal distribution data were represented as the Means ± SD. Independent sample t tests were applied for the comparisons between groups, paired t tests for the analyses within groups. The count data were represented as the rate (%) and tested using chi-square tests, and were expressed as X2.
Results
Clinical data
There were no significant differences in terms of age, sex, BMI, smoking history, drinking history, exercise habits, marital status, or residence between the RG and the CG (P > 0.05), as shown in Table 1.
Table 1.
Basic clinical data [n (%)]
| RG (n=37) | CG (n=35) | X2 or t | P | |
|---|---|---|---|---|
| Age (years) | 33.7 ± 4.4 | 33.5 ± 4.8 | 0.184 | 0.854 |
| BMI | 22.05 ± 1.24 | 22.02 ± 1.17 | 0.106 | 0.916 |
| Gender | 0.008 | 0.927 | ||
| Male | 12 (32.43) | 11 (31.43) | ||
| Female | 25 (67.57) | 24 (68.57) | ||
| Smoking history | 0.002 | 0.963 | ||
| Yes | 15 (40.56) | 14 (40.00) | ||
| No | 22 (59.46) | 21 (60.00) | ||
| Drinking history | 0.007 | 0.995 | ||
| Yes | 18 (48.65) | 17 (48.57) | ||
| No | 19 (51.35) | 18 (51.43) | ||
| Exercise habit | 0.188 | 0.664 | ||
| Yes | 23 (62.16) | 20 (57.14) | ||
| No | 14 (37.84) | 15 (42.86) | ||
| Marital status | 0.052 | 0.820 | ||
| Married | 31 (83.78) | 30 (85.71) | ||
| Unmarried | 6 (16.22) | 5 (14.29) | ||
| Residence | 0.695 | 0.405 | ||
| Urban | 23 (62.16) | 25 (71.43) | ||
| Rural | 14 (37.84) | 10 (28.57) |
Clinical efficacy of the two groups of patients
The total effective rate in the RG (100.00%) was significantly higher than it was in the CG (88.57%) (P=0.034), as shown in Table 2.
Table 2.
Clinical efficacy [n (%)]
| group | Number of cases | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| RG | 37 | 32 | 5 | 0 | 37 (100.00) |
| CG | 35 | 12 | 19 | 4 | 31 (88.57) |
| X2 | - | - | - | - | 4.477 |
| P | - | - | - | - | 0.034 |
Wound volume changes
There was no significant difference in the wound volumes before the intervention (P > 0.05), but they were reduced after the intervention, and the wound volumes in the RG were smaller than they were in the CG at 3 weeks and 6 weeks after the intervention (P < 0.05), as shown in Figure 1.
Figure 1.
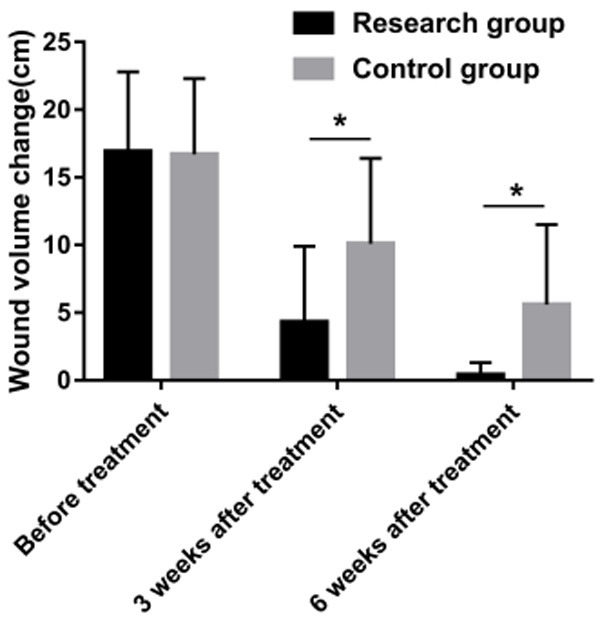
Comparison of the wound volumes. Comparison of the wound volumes in the two groups. Note: * means there were differences between the two groups (P < 0.05).
The wound healing and fracture healing times
The average wound healing time in the RG (22.40 ± 2.10 days) was significantly lower than it was in the CG (32.20 ± 3.30 days) (P > 0.05). The average fracture healing time in the RG (6.50 ± 2.20 months) was lower than it was in the CG (7.51 ± 2.33 months) (P > 0.05), as shown in Figure 2.
Figure 2.
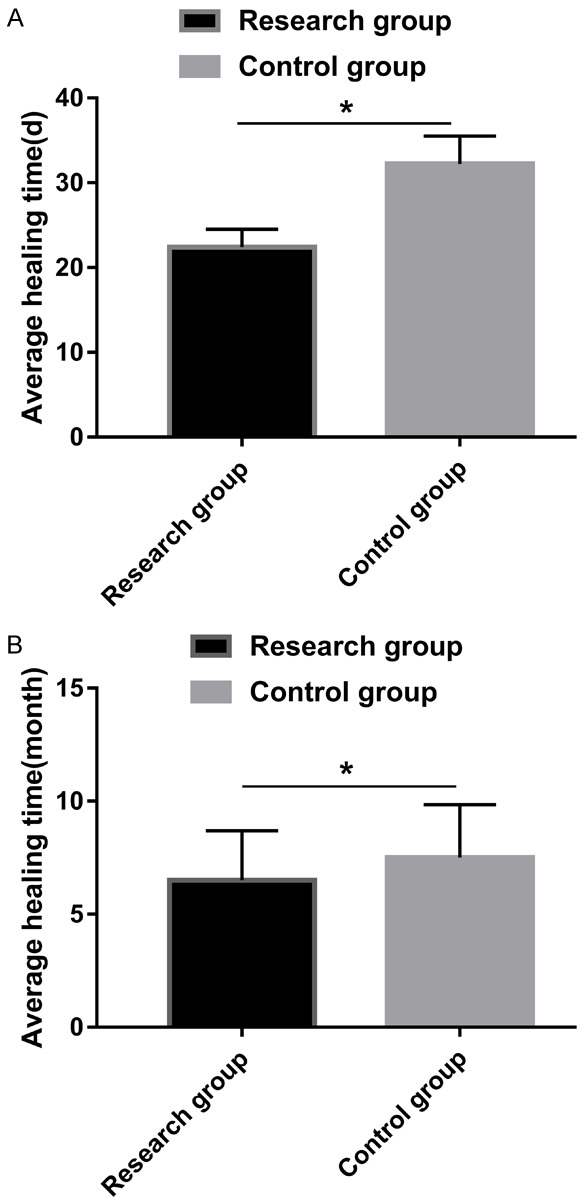
Comparison of patients’ healing times. A: A comparison of the wound healing times between the two groups. B: A comparison of fracture healing times between the two groups. Note: * indicates that there were differences between the two groups (P < 0.05).
Adverse reactions to the surgery
In the RG, there was 1 case of infection for a total incidence rate of 2.70%. In the CG, there were 2 cases of infection, 1 case of important vascular injury and 1 case of ischemic muscle contracture, for a total incidence rate of 11.43%. The incidence of adverse reactions in the RG was significantly lower than it was in the CG (Table 3).
Table 3.
The incidences of adverse reactions [n (%)]
| RG (n=37) | CG (n=35) | |
|---|---|---|
| Infection | 1 (2.70) | 2 (5.71) |
| Osteomyelitis | 0 (0.00) | 0 (0.00) |
| Important vascular injury | 0 (0.00) | 1 (2.86) |
| Peripheral nerve injury | 0 (0.00) | 0 (0.00) |
| Ischemic muscle contracture | 0 (0.00) | 1 (2.86) |
| Fat embolism | 0 (0.00) | 0 (0.00) |
| Total incidence rate (%) | 2.70% | 11.43% |
VAS scores
There was no significant difference in the VAS scores before the intervention (P > 0.05). The SF-36 scores (physiological function, role physical, somatic pain and overall health) of the patients in the RG were significantly higher than the patients in the CG (P < 0.05), as shown in Figure 3.
Figure 3.
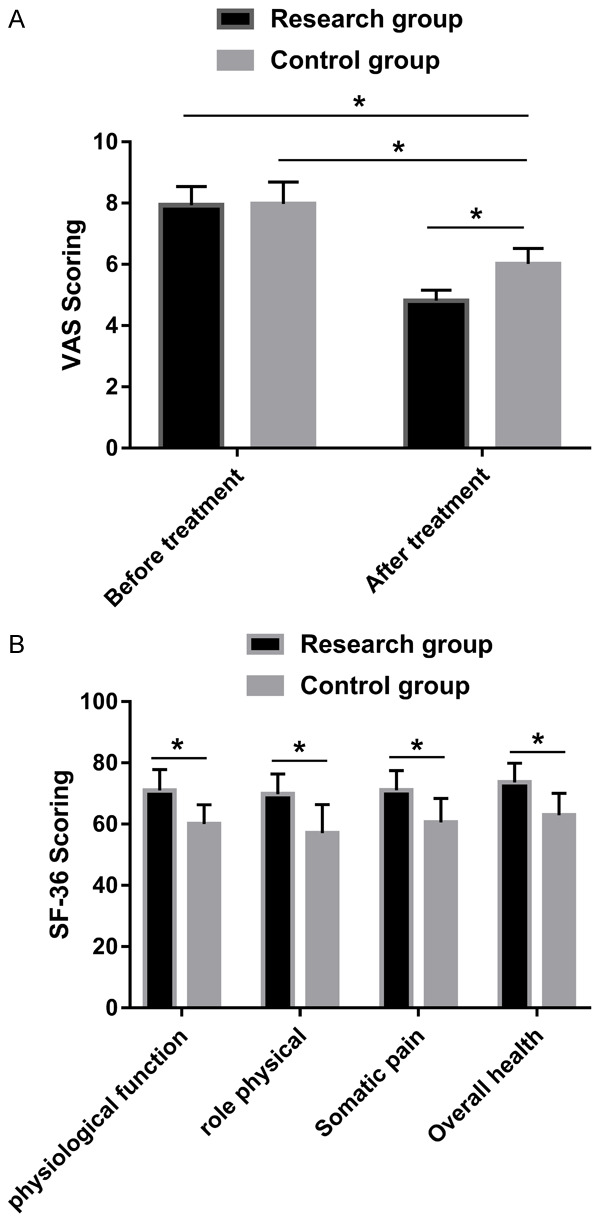
Comparisons of the VAS scores and the SF-36 scores. A: After the treatment, the VAS scores in the RG decreased significantly and were lower than the VAS scores in the CG. B: The SF-36 scores of the patients in the RG were significantly higher than the corresponding scores in the CG. Note: * indicates that there were differences between the two groups (P < 0.05).
Discussion
Among the open fractures encountered in orthopedics and trauma practice, open tibial shaft fractures are relatively common. As the soft tissue around the tibia is very thin, fractures of the tibia often penetrate skin [12]. The principle of treating open fractures is to treat injuries comprehensively, aiming especially to prevent infection. Surgical treatment includes the debridement and irrigation of open wounds and bone and soft tissue reconstruction. A good curative effect depends on early high-quality definite treatment, such as early stable internal fixation and related soft tissue repair. All the elements of the surgical principles are very important and interdependent in order to achieve overall success, but the most critical element seems to be to achieve healthy soft tissue coverage as soon as possible [13-16].
Exogenous cytokines have been used to promote healing clinically. However, due to the limitations of technology, only one growth factor or cytokine is used most frequently, and it can only promote a certain stage of wound healing. Theoretically speaking, the combination of various cytokines in accordance with the biological process of tissue healing can promote tissue healing most effectively [17,18]. In view of the similar biological characteristics of platelet-rich plasma in repairing tissue defects, none of them directly applied PRP to the basal surface of a skin flap, which is the simplest and most convenient method clinically and causes no direct damage to the skin flap, such as a puncture or compression [19-21]. Due to the lack of evidence on the platelet-rich effect, PRP gel was directly applied to the basal part of the skin flap transplant to observe the effect of platelet-rich plasma in promoting the survival of the skin flap, and to explore the possible mechanism of platelet-rich plasma in promoting the survival of the skin flap.
In this study, we first observed the wound volume and found that the wound volume of the RG was smaller than the wound volume of the CG at 3 weeks and 6 weeks after the intervention, and the wound healing time of the RG was significantly shorter than it was in the CG, indicating that platelet-rich plasma combined with skin flap transplantation can effectively improve patients’ wound healing times. We further observed the average healing times of the patients’ fractures and found that the average healing time of the patients in the RG (6.50 ± 2.20 months) was lower than it was in the CG (7.51 ± 2.33 months), suggesting that platelet-rich plasma combined with flap transplantation can improve the healing times of patients’ fractures. We further found that the total incidence of adverse reactions in the RG was 2.70%, but in the CG it was 11.43%. Studies have shown that platelet-rich gel increases the skin flap survival rate. In addition, it reduces the inflammatory reaction in skin flap transplantation, and has a better effect at producing new soft tissue [22]. Maghsoudi and other team members [23] showed that the effectiveness of platelet-rich gel in skin flap transplantation is satisfactory. The possible mechanisms of platelet-rich gel promoting flap survival include platelets, growth factors, immunoreactive factors and fibrin. Platelet enrichment may be a new clinical method for promoting skin flap survival. We observed the VAS and SF-36 scores and found that the VAS scores in the RG were significantly lower than they were in the CG after the intervention, while the SF-36 scores were significantly higher than they were in the CG, which further explains the above results.
Through the above research, we preliminarily proved the therapeutic value of platelet-rich plasma combined with skin flap transplantation in the treatment of foot open fractures with soft tissue defects. However, there are some limitations to this study. For example, the research participants were relatively homogeneous, and there may be different manifestations among different races. Therefore, a more in-depth experimental analysis will be performed to supplement this.
In conclusion, the application of platelet-rich plasma combined with skin flap transplants in patients with open fractures of the foot and a soft tissue deficiency can improve the healing times of the wounds and fractures and improve the incidence of adverse reactions in the patients’ treatment, so it is worth popularizing in clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Stengel D, Kirschner S, Ekkernkamp A, Bartl C. Evidence-based trauma and orthopedic surgery: 20 years after Sackett. Unfallchirurg. 2016;119:708–714. doi: 10.1007/s00113-016-0209-x. [DOI] [PubMed] [Google Scholar]
- 2.A report by the British Orthopaedic Association/British Association of Plastic Surgeons Working Party on the management of open tibial fractures. September 1997. Br J Plast Surg. 1997;50:570–583. doi: 10.1016/s0007-1226(97)90501-4. [DOI] [PubMed] [Google Scholar]
- 3.Collinge CA, McWilliam-Ross K, Kelly KC, Dombroski D. Substantial improvement in prophylactic antibiotic administration for open fracture patients: results of a performance improvement program. J Orthop Trauma. 2014;28:620–625. doi: 10.1097/BOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 4.Schenker ML, Ahn J, Donegan D, Mehta S, Baldwin KD. The cost of after-hours operative debridement of open tibia fractures. J Orthop Trauma. 2014;28:626–631. doi: 10.1097/BOT.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 5.Schenker ML, Yannascoli S, Baldwin KD, Ahn J, Mehta S. Does timing to operative debridement affect infectious complications in open long-bone fractures? A systematic review. J Bone Joint Surg Am. 2012;94:1057–1064. doi: 10.2106/JBJS.K.00582. [DOI] [PubMed] [Google Scholar]
- 6.Diwan A, Eberlin KR, Smith RM. The principles and practice of open fracture care, 2018. Chin J Traumatol. 2018;21:187–192. doi: 10.1016/j.cjtee.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Wang J, Qiang L, Rui Y, Xue M. Outcomes of using a modified anteromedial thigh perforator flap for repairing the anterolateral thigh free flap donor site: a retrospective clinical review. Medicine (Baltimore) 2018;97:e0491. doi: 10.1097/MD.0000000000010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cam B, Bagdas D, Ozyigit MO, Sagdilek E, Buyukcoskun NI, Ozluk K. Effects of adrenomedullin and glucagon-like peptide on distal flap necrosis and vascularity: the role of receptor systems and nitric oxide. Wounds. 2017;29:163–167. [PubMed] [Google Scholar]
- 9.Janis J, Harrison B. Wound healing: part II. Clinical applications. Plast Reconstr Surg. 2014;133:383e–392e. doi: 10.1097/PRS.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 10.El Backly RM, Zaky SH, Muraglia A, Tonachini L, Brun F, Canciani B, Chiapale D, Santolini F, Cancedda R, Mastrogiacomo M. A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A. 2013;19:152–165. doi: 10.1089/ten.TEA.2012.0357. [DOI] [PubMed] [Google Scholar]
- 11.Rabillard M, Grand JG, Dalibert E, Fellah B, Gauthier O, Niebauer GW. Effects of autologous platelet rich plasma gel and calcium phosphate biomaterials on bone healing in an ulnar ostectomy model in dogs. Vet Comp Orthop Traumatol. 2009;22:460–466. doi: 10.3415/VCOT-09-04-0048. [DOI] [PubMed] [Google Scholar]
- 12.Chai J, Ge J, Zou J. Effect of autologous platelet-rich plasma gel on skin flap survival. Med Sci Monit. 2019;25:1611–1620. doi: 10.12659/MSM.913115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber D, Dulai SK, Bergman J, Buckley R, Beaupre LA. Time to initial operative treatment following open fracture does not impact development of deep infection: a prospective cohort study of 736 subjects. J Orthop Trauma. 2014;28:613–619. doi: 10.1097/BOT.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 14.Gelbard RB, Ferrada P, Yeh DD, Williams BH, Loor M, Yon J, Mentzer C, Khwaja K, Khan MA, Kohli A, Bulger EM, Robinson BRH. Optimal timing of initial debridement for necrotizing soft tissue infection: a practice management guideline from the Eastern association for the surgery of trauma. J Trauma Acute Care Surg. 2018;85:208–214. doi: 10.1097/TA.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 15.Hull PD, Johnson SC, Stephen DJ, Kreder HJ, Jenkinson RJ. Delayed debridement of severe open fractures is associated with a higher rate of deep infection. Bone Joint J. 2014;96-B:379–384. doi: 10.1302/0301-620X.96B3.32380. [DOI] [PubMed] [Google Scholar]
- 16.FLOW Investigators. Bhandari M, Jeray KJ, Petrisor BA, Devereaux PJ, Heels-Ansdell D, Schemitsch EH, Anglen J, Della Rocca GJ, Jones C, Kreder H, Liew S, McKay P, Papp S, Sancheti P, Sprague S, Stone TB, Sun X, Tanner SL, Tornetta P 3rd, Tufescu T, Walter S, Guyatt GH. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med. 2015;373:2629–2641. doi: 10.1056/NEJMoa1508502. [DOI] [PubMed] [Google Scholar]
- 17.Philippeos C, Telerman SB, Oules B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, Watt FM. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol. 2018;138:811–825. doi: 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon YR, Kang EH, Yang CE, Yun IS, Lee WJ, Lew DH. The effect of platelet-rich plasma on composite graft survival. Plast Reconstr Surg. 2014;134:239–246. doi: 10.1097/PRS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Enomoto M, Ukegawa M, Hirai T, Sotome S, Wakabayashi Y, Shinomiya K, Okawa A. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012;129:858–866. doi: 10.1097/PRS.0b013e3182450ac9. [DOI] [PubMed] [Google Scholar]
- 20.Findikcioglu F, Findikcioglu K, Yavuzer R, Lortlar N, Atabay K. Effect of intraoperative platelet-rich plasma and fibrin glue application on skin flap survival. J Craniofac Surg. 2012;23:1513–1517. doi: 10.1097/SCS.0b013e3182597ce6. [DOI] [PubMed] [Google Scholar]
- 21.Karayannopoulou M, Papazoglou LG, Loukopoulos P, Kazakos G, Chantes A, Giannakas N, Savvas I, Psalla D, Kritsepi-Konstantinou M, Dionyssiou D. Locally injected autologous platelet-rich plasma enhanced tissue perfusion and improved survival of long subdermal plexus skin flaps in dogs. Vet Comp Orthop Traumatol. 2014;27:379–386. doi: 10.3415/VCOT-14-02-0030. [DOI] [PubMed] [Google Scholar]
- 22.Mariani E, Canella V, Cattini L, Kon E, Marcacci M, Di Matteo B, Pulsatelli L, Filardo G. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS One. 2016;11:e0156137. doi: 10.1371/journal.pone.0156137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maghsoudi O, Ranjbar R, Mirjalili SH, Fasihi-Ramandi M. Inhibitory activities of platelet-rich and platelet-poor plasma on the growth of pathogenic bacteria. Iran J Pathol. 2017;12:79–87. [PMC free article] [PubMed] [Google Scholar]


