Abstract
Circular RNAs (circRNAs) have been reported to regulate the hepatocellular carcinoma (HCC) chemoresistance and tumor progression by regulating gene expression. However, the underlying molecular mechanisms of HCC sorafenib resistance regulated by circRNAs remain unclear. Here, higher expression of circUBE2D2 was directly associated with low survival rate in HCC patients. Functional experiments showed that circUBE2D2 promoted the glycolysis (Warburg effect) and sorafenib resistance in vitro, and knockdown of circUBE2D2 repressed the tumor growth in vivo. Mechanistically, circUBE2D2 was predominantly localized in the cytoplasm and sponged miR-889-3p, which in turn targeted the 3’-UTR of LDHA mRNA. Therefore, circUBE2D2 exerted an oncogenic role through miR-889-3p/LDHA axis. In conclusion, these findings demonstrate that circUBE2D2 accelerated the HCC glycolysis and sorafenib resistance via circUBE2D2/miR-889-3p/LDHA axis, which provides a novel approach for HCC treatment.
Keywords: Hepatocellular carcinoma, circular RNA, sorafenib, glycolysis, LDHA
Introduction
Hepatocellular carcinoma (HCC) is the most common subtype of liver cancer (about 90% occurrence rate), causing the second leading pathogenesis of global cancer-related death [1,2]. Recent advanced in therapeutic methods have been achieved, however, the mortality of HCC is still enormous. The high mortality of HCC frequently caused by metastasis and recurrence, which is be characterized by unclear pathogenesis and molecular mechanism [3,4]. The most essential cause for the HCC tumorigenesis is primarily unclear etiology and partially responsible for the clinical therapeutic strategy [5,6]. Therefore, the main treatment for the HCC should be focused on the in-depth molecular mechanism investigation.
Circular RNAs (circRNAs) are a group of noncoding RNAs with closed covalent loop structure [7,8]. CircRNAs is different from traditional linear mRNAs which are accompanied with 5’ and 3’ terminal structures [9,10]. Benefiting from the closed loop structure, circRNAs are able to resist to the exonucleolytic RNA decay, thereby improving the abundance as compared to parental mRNA. In HCC tumorigenesis, circRNAs regulate the multiple pathophysiological processes. For example, the highly expressed circRNA Cdr1as expression accelerated the proliferation and migration of HCC cells by sponging miR-1270 [11]. High circABCB10 expression is directly associated with HCC patients’ poor survival in clinical investigation through acting as a sponge of miR-670-3p to increase HMG20A level [12]. Hence, these data support the pivotal role of circRNAs in the HCC.
Sorafenib is the first targeted drug for the advanced HCC therapy and the sorafenib resistance is a major obstacle for the treatment [13]. However, sorafenib resistance is widespread for the advanced HCC due to the aerobic glycolysis enhancement. Hence, in-depth study of the molecular mechanisms involved HCC tumorigenesis is significant for the accurate treatment. The current view indicates that sorafenib resistance is closely correlated with glycolysis. In tumor progression, this phenomenon is termed as aerobic glycolysis (Warburg effect) and characterized by enhanced glucose uptake and lactate production. Here, present research focuses on the potential roles of circRNAs in the HCC sorafenib resistance and glycolysis. circUBE2D2 is a novel circRNA generated from exon 4/5/6 (hsa_circ_0006716, 216 bp). These findings demonstrate that circUBE2D2 accelerated the HCC glycolysis and sorafenib resistance, which provides a novel approach for HCC treatment.
Materials and methods
Clinical specimens’ collection
Clinical samples were collected from patients who were diagnosed as pathological HCC from June 2018 to May 2019 at Wuming Hospital. Tissue samples were taken during operation that surgically removed and from liver tumor and paired adjacent normal tissue. None chemotherapy or radiotherapy was administrated prior to surgical excision. The ethical approval was obtained for Ethics Committee of Wuming Hospital (Table 1).
Table 1.
Correlation of circUBE2D2 level with HCC patients’ clinicopathological characteristic
| Total (30 samples) | circUBE2D2 | p value | ||
|---|---|---|---|---|
|
| ||||
| low (n=15) | high (n=15) | |||
| Age (years) | ||||
| ≥60 | 14 | 8 | 6 | 0.426 |
| <60 | 16 | 7 | 9 | |
| Gender | ||||
| Male | 16 | 9 | 7 | 0.682 |
| Female | 14 | 6 | 8 | |
| Cirrhosis | ||||
| Absent | 13 | 8 | 5 | 0.121 |
| Present | 17 | 7 | 10 | |
| TNM | ||||
| I-II | 15 | 7 | 8 | 0.0* |
| III-IV | 15 | 8 | 7 | |
| Tumor size | ||||
| ≥5 cm | 20 | 9 | 11 | 0.001* |
| <5 cm | 10 | 6 | 4 | |
P<0.05 represents statistical difference.
Cell culture
Normal liver cell lines (Lo-2) and HCC cell lines (SMMC-7721, Huh7, HepG2) were identified from the Chinese Academy of Medical Sciences (Beijing, China). All cell lines were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% FBS (fetal bovine serum, Gibco) in a humidified atmosphere with 5% CO2 at 37°C. For hypoxia culture, cells were cultured under 37°C condition with 5% O2 for 48 h.
Transfection
Small hairpin RNA (shRNA) and complementary DNA (cDNA) plasmids of circUBE2D2 were designed and synthesized by Genomeditech Co., Ltd. (Shanghai, China). Stable transfection cell lines were filtrated using puromycin (2 μg/ml). The miR-889-3p mimics and controls were provided by GeneChem Co., Ltd. (Shanghai, China) and transiently transfected using lipofectamine 2000 (Invitrogen, NY, USA) according to the manufacturer’s instructions. LDHA overexpression (LDHA OE) and controls were designed by GenePharma (Shanghai, China) and transfected using Lipofectamine 2000 (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The sequences were shown in Table S1.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total circRNA or mRNA was separated using TRIzol reagent following the instructions provided by manufacturer (Invitrogen, USA). Reverse transcription was carried out using Prime Script RT reagent kit (Takara, Japan). qRT-PCR was performed by SYBR Prime Script RT-PCR kit (Takara, Japan) or HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the manufacturer’s instruction. All circular RNA, miRNA, mRNA expression levels were calculated using the 2-ΔΔCt method normalized to β-actin expression.
Western blot analysis
Cellular lysates were collected using the lysis buffer (Beyotime Biotechnology, Shanghai, China). The whole proteins were scattered through SDS-PAGE on 10% gel and were transferred to (PVDF) poly vinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked using 5% non-fat milk in PBS-Tween 20 and incubated with primary antibodies (anti-LDHA, ab125683, 1:1000) at 4°C overnight. Subsequently, the PVDF membrane was incubated with secondary antibody rabbit anti-actin (1:2500, Abcam) for 1 h at room temperature. Enhanced chemiluminescence (ECL, Beyotime, Shanghai, China) was used for protein visualization, and the blots were quantified by densitometry (Quantity One software, Bio-Rad).
Actinomycin D and RNase R assay
Total RNA (5 μg) was extracted from HCC cells (HepG2) using RNeasy Mini Kit (Qiagen Inc., Redwood City, CA). RNA was added with 2 mg/ml Actinomycin D or DMSO (Sigma-Aldrich, St. Louis, MO, USA) as the negative control. RNA was incubated with RNase R at 37°C for 10 min. RNase-free water was added to the RNA. Random primer (1 μl) and mRNA primers were used for RT-PCR using RevertAid First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA).
Sorafenib sensitivity assay
The sorafenib sensitivity was detected using CCK-8 assay as previously described [14]. Briefly, HCC cells (1×104 cells/well) were seeded to the 96-well plates. After 24 h incubation, medium was replaced and HCC cells were treated with various doses (0, 0.1, 1, 2, 5, 10, 20 μmol/L) of sorafenib for 24 h. After incubation with 10 μl of CCK-8 kit (Beyotime Institute of Biotechnology, shanghai, China) for 2 h or 4 h. Then, the optical density (OD) value was measured at 450 nm according to the manufacturer’s instructions. The sorafenib sensitivity was determined using the IC50 value (50% decreasing in absorbance normalized to control).
Glucose uptake and lactate production
HCC cells were transfected with sh-circUBE2D2 plasmids or controls for 24 hours. Glucose concentration was detected using Colorimetric Glucose Assay Kit (BioVision, USA) according to the manufacturer’s instructions. Lactate concentration was detected using lactate assay kit (K627, BioVision) according to the manufacturer’s instructions.
Extracellular acidification rate (ECAR) analysis
ECAR was analyzed using the XF96 Bioenergetic Analyzers (Seahorse Bioscience, Agilent Technologies, Santa Clara, CA, USA) as previously described [15]. Subsequently, cells (1×104 cells/well) were seeded into 96-well XF cell culture microplates in selective medium. Cells were administrated with 10 mM glucose, 1 mM oligomycin and 50 mM 2-deoxyglucose (2-DG) was according to the manufacturers’ instructions. ECAR measurement was normalized to total protein content (mpH/min) for ECAR. After 24 h, medium was replaced by XF base medium (pH 7.4).
Subcellular location analysis
The nuclear or cytoplasmic fraction was isolated using PARIS Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The RNAs in nuclear or cytoplasmic fraction were eluted with Elution Solution and detected using RT-PCR. U1 and GAPDH were respectively employed as negative control or positive control for nuclear and cytoplasmic fractions.
Dual-luciferase reporter assay
The sequences of circUBE2D2 and LDHA mRNA 3’-UTR containing miR-889-3p complementary sites were sub-cloned into pGL3 luciferase vector (Promega, Madison, WI, USA), which were respectively named as circUBE2D2-WT or circUBE2D2-Mut, and LDHA-WT or LDHA-Mut. 293T cells were seeded into 96-well plates and co-transfected with pRL-CMV Renilla (6 ng), luciferase reporter (60 ng) and miR-889-3p mimic. After 48 h incubation, the relative luciferase activity was measured based on Firefly/Renilla ration using dual-luciferase reporter assay (Promega, Madison, WI, USA).
Xenograft tumorigenesis
The animal assays were approved by the Wuming Hospital. Male BALB/C nude mice (10 mice, 6-8 weeks) were provided from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Mice were housed under SPF conditions and free access to water/food according to the Guide for the Care and Use of Laboratory Animals. Total 2×106 Huh7 cells were suspended in 100 μL DMEM medium and subcutaneously injected into the same flank of mice. The tumors’ volume was measured every three days after injection using the formula: volume = (length × width2)/2. Three weeks later, mice were sacrificed and tumors were weighed.
Statistical analysis
Results were presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, USA) and SPSS software version 19.0 (SPSS, Inc., Chicago, IL). Comparison between two groups was performed using two-tailed Student’s t-test. Pearson’s correlation analysis was performed for correlation. Overall survival probability analysis for different circUBE2D2 expression was performed using Kaplan-Meier survival curve and log-rank test. P values less than 0.05 were considered as statistical significance.
Results
circUBE2D2 was identified to be upregulated in HCC
In the hypoxia induced HCC cells (HepG2), circRNA microarray analysis was performed to screen the up-regulated or down-regulated circRNA (Figure 1A). Based on the results of high-throughput sequencing, several circRNAs were selected for further examination using qRT-PCR (Figure 1B). Results showed that a novel circRNA, named as circUBE2D2, was significantly up-regulated in the HCC cells (HepG2). For the stability analysis of circUBE2D2, actinomycin D and RNase R treatment were performed. Data showed that circUBE2D2 was stable when treated with actinomycin D (Figure 1C) and RNase R (Figure 1D). In HCC cell lines (SMMC-7721, Huh7, HepG2), circUBE2D2 expression was detected using RT-PCR and data showed that circUBE2D2 expression was significantly up-regulated (Figure 1E). In HCC tissue samples, circUBE2D2 expression was found to be up-regulated as comparing to normal tissue samples (Figure 1F). Kaplan-Meier method was used to calculate the survival curves, showing that higher circUBE2D2 expression indicated the lower survival rate and unfavorable prognosis (Figure 1G). Overall, these findings showed that circUBE2D2 was identified to be up-regulated in HCC.
Figure 1.
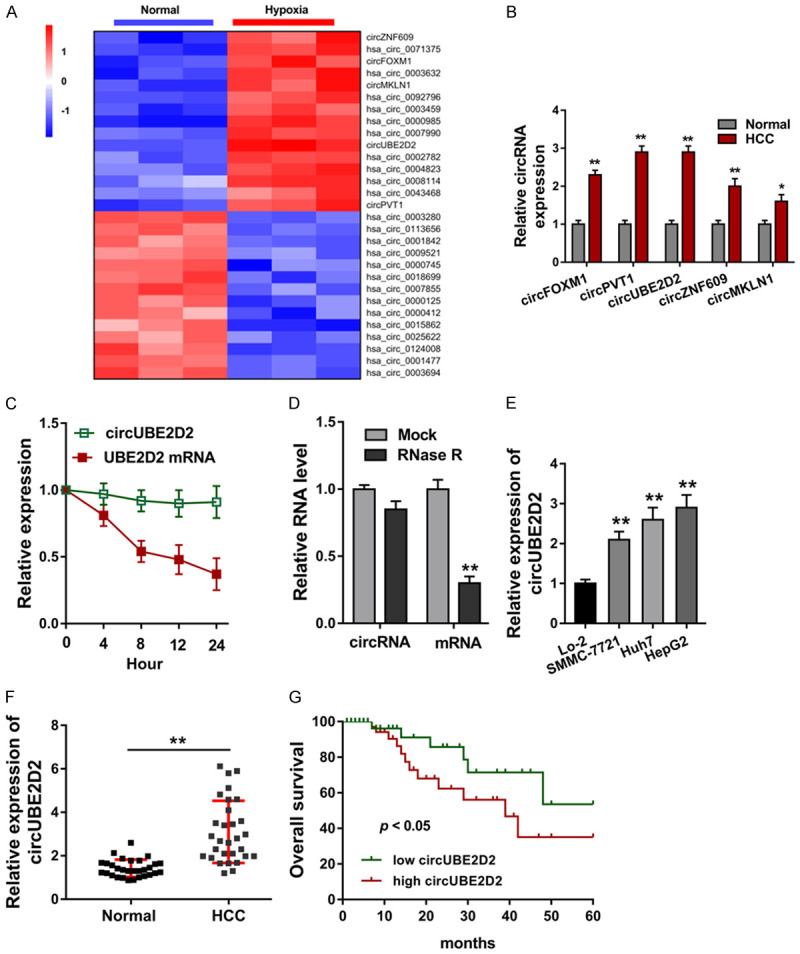
circUBE2D2 was identified to be upregulated in HCC. A. CircRNA microarray analysis was performed to screen the up-regulated or down-regulated circRNA in the hypoxia induced HCC cells (HepG2). B. Several candidate circRNAs (6 circRNAs) were selected for further examination using qRT-PCR. C. Transcription inhibitor (Actinomycin D, Act D) was administrated to measure the stability of circUBE2D2 and UBE2D2 mRNA. D. qRT-PCR was performed to detect the expression of circUBE2D2 and UBE2D2 mRNA with RNase R treatment. E. circUBE2D2 expression was detected using RT-PCR in normal cells (Lo-2) and HCC cell lines (SMMC-7721, Huh7, HepG2). B-E. Experiments were performed in triplicate and were repeated three times. F. In HCC tissue samples, circUBE2D2 expression was detected using RT-PCR as comparing to normal tissue samples (30 samples). G. Kaplan-Meier method was used to calculate the survival curves to showing the survival rate and prognosis of HCC patients (90 cases). **P<0.01 vs. control. *P<0.05 vs. control.
circUBE2D2 sensitizes HCC cells sorafenib resistance and glycolysis in vitro
In order to investigate the roles of circUBE2D2 on HCC cells’ biological behavior, the circUBE2D2 knockdown transfection were constructed in HCC cells (HepG2), as well as the overexpression of circUBE2D2 plasmids (Figure 2A). RT-PCR data showed that circUBE2D2 expression was significantly up-regulated or decreased in these transfections (Figure 2A). Sorafenib sensitivity assay showed that circUBE2D2 overexpression sensitized the HCC cells (HepG2) sorafenib resistance, however, circUBE2D2 knockdown transfection reduced the sorafenib resistance (Figure 2B). In other words, circUBE2D2 sensitized the sorafenib resistance of HCC cells. As regarding to the aerobic glycolysis (Warburg effect), glycolytic capacity was detected, including glucose consumption, lactate production and extracellular acidification rate (ECAR). Results illustrated that circUBE2D2 overexpression enhanced the glucose consumption (Figure 2C), lactate production level (Figure 2D) and ECAR quantity (Figure 2E, 2F). Besides, circUBE2D2 knockdown reduced the glucose consumption, lactate production level and ECAR quantity. In conclusion, these findings show that circUBE2D2 sensitizes HCC cells sorafenib resistance and glycolysis in vitro.
Figure 2.
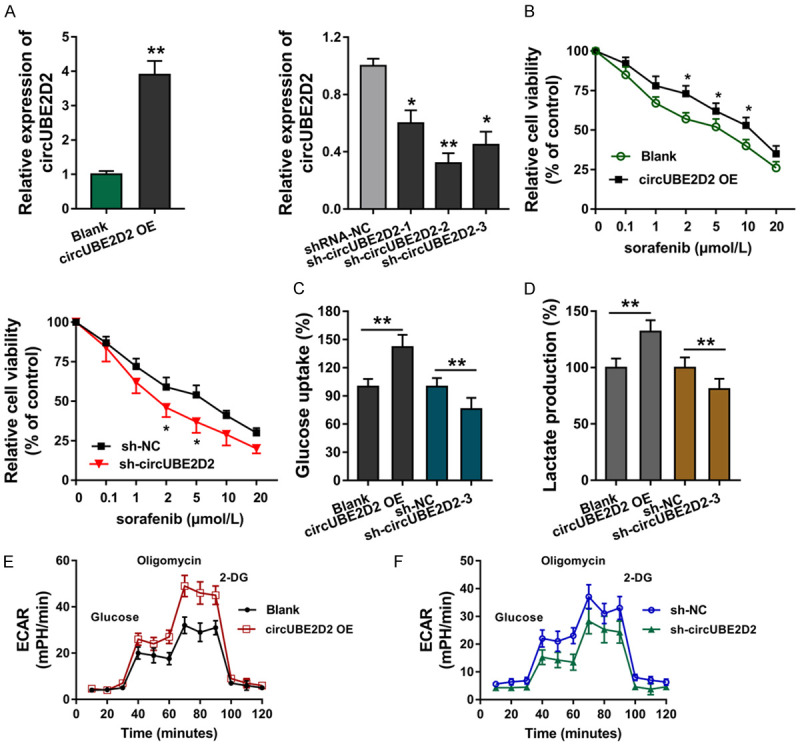
circUBE2D2 sensitizes HCC cells sorafenib resistance and glycolysis in vitro. A. Overexpression of circUBE2D2 plasmids (UBE2D2 overexpression, UBE2D2 OE) and circUBE2D2 knockdown (sh-UBE2D2) were transfected into HCC cells (HepG2) to enhance or silence UBE2D2 expression. Corresponding plasmids acted as controls. RT-PCR detected the circUBE2D2 expression. B. Sorafenib sensitivity analysis using CCK-8 assay showed the relative cellular viability. C. Glucose consumption was detected in HCC cells (HepG2) using glucose assay kit. D. Lactate production was detected using lactate colorimetric assay kit. A-D. Experiments were performed in triplicate and were repeated three times. E, F. Extracellular acidification rate (ECAR) was detected using Seahorse XFe96 analyzer. **P<0.01 vs. control. *P<0.05 vs. control.
circUBE2D2 targets the miR-889-3p
In HCC cell (HepG2), subcellular fraction analysis found that circUBE2D2 was principally located in the cytoplasm, rather than nuclear (Figure 3A). Online bioinformatics tools (CircNet, CircInteractome, TargetScan) indicated that miR-889-3p were collectively identified (Figure 3B). CircUBE2D2 containing miR-889-3p complementary sites were constructed, which were named as circUBE2D2-WT or circUBE2D2-Mut. Luciferase reporter assay showed that circUBE2D2 was closely connected with miR-889-3p mimics (Figure 3C). RT-PCR showed that miR-889-3p expression was decreased in HCC cells transfected with circUBE2D2 overexpression plasmids, and increased in HCC cells transfected with circUBE2D2 knockdown (Figure 3D). Pearson correlation analysis of circUBE2D2 and miR-889-3p was detected in HCC tissues cohort, suggesting the negative relationship of circUBE2D2 and miR-889-3p (Figure 3E). In conclusion, these data showed that circUBE2D2 targets the miR-889-3p, acting as miRNAs sponge.
Figure 3.
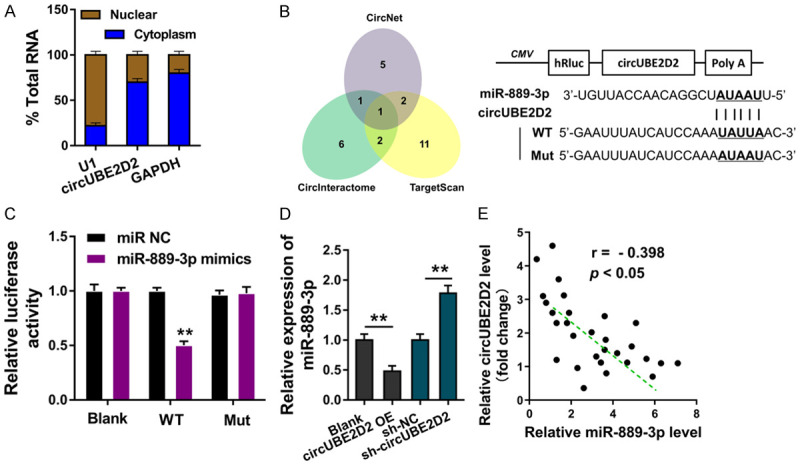
circUBE2D2 targets the miR-889-3p. A. Subcellular fraction analysis showed the circUBE2D2 enrichment in the cytoplasm or nuclear. Experiments were performed in triplicate and were repeated three times. B. Online bioinformatics tools (CircNet, CircInteractome, TargetScan) indicated the collective miRNAs. CircUBE2D2 containing miR-889-3p complementary sites were constructed, which were named as circUBE2D2-WT or circUBE2D2-Mut. C. Luciferase reporter assay showed the luciferase activity of co-transfection of circUBE2D2 and miR-889-3p mimics. D. RT-PCR showed the miR-889-3p expression in HCC cells transfected with circUBE2D2 overexpression plasmids or circUBE2D2 knockdown. C, D. Experiments were performed in triplicate and were repeated three times. E. Pearson correlation analysis showed the relationship of circUBE2D2 and miR-889-3p in HCC tissues cohort (30 samples). **P<0.01 vs. control. *P<0.05 vs. control.
miR-889-3p targets the LDHA mRNA 3’-UTR
Furthermore, the downstream of miR-889-3p was investigated using bioinformatics tools (miRanda, miRBase, TargetScan) indicated that LDHA may function as the target protein of miR-889-3p (Figure 4A). LDHA mRNA 3’-UTR containing miR-889-3p complementary sites was constructed, which were named as LDHA-WT or LDHA-Mut (Figure 4B). Luciferase reporter assay showed that LDHA mRNA was closely connected with miR-889-3p mimics (Figure 4C). RT-PCR showed that LDHA mRNA expression was decreased in HCC cells transfected with miR-889-3p mimics, and recovered when transfected with circUBE2D2 overexpression (Figure 4D). Pearson correlation analysis of LDHA and circUBE2D2 was detected in HCC tissues cohort, suggesting the negative relationship of circUBE2D2 and LDHA (Figure 4E). Overall, these data suggest that miR-889-3p targets the LDHA mRNA 3’-UTR.
Figure 4.
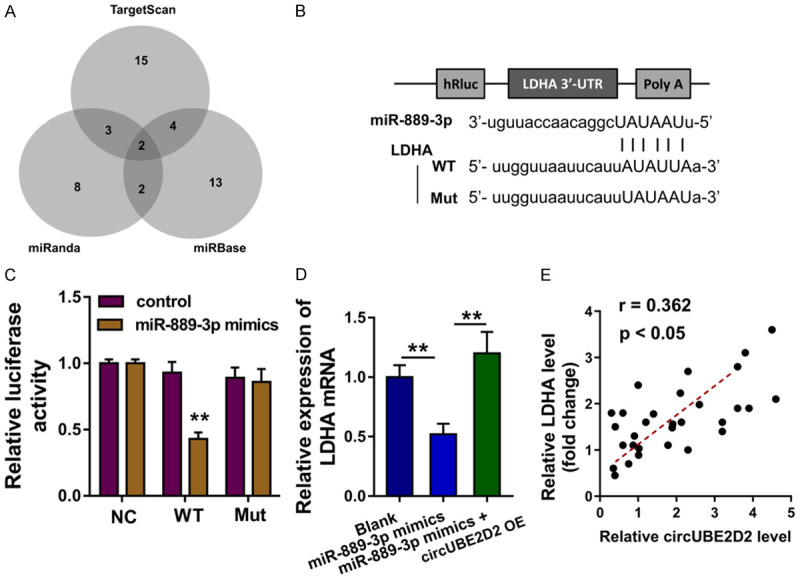
miR-889-3p targets the LDHA mRNA 3’-UTR. A. Bioinformatics tools (miRanda, miRBase, TargetScan) indicated that LDHA may function as the target protein of miR-889-3p. B. LDHA mRNA 3’-UTR containing miR-889-3p complementary sites was constructed, which were named as LDHA-WT or LDHA-Mut. C. Luciferase reporter assay showed the closely connection within miR-889-3p and LDHA mRNA. D. RT-PCR showed the LDHA mRNA expression in HCC cells (HepG) transfected with miR-889-3p mimics and circUBE2D2 overexpression. C, D. Experiments were performed in triplicate and were repeated three times. E. Pearson correlation analysis of LDHA and circUBE2D2 was detected in HCC tissues cohort (30 samples). **P<0.01 vs. control.
circUBE2D2/miR-889-3p/LDHA axis regulates the HCC sorafenib resistance and glycolysis
In previous research, our findings confirmed that circUBE2D2 could target the miR-889-3p/LDHA axis. In further investigation, rescue assays were performed to detect the cooperative roles of circUBE2D2 miR-889-3p/LDHA axis. Sorafenib sensitivity assay showed that miR-889-3p mimics transfection significantly repressed the HCC cells (HepG2) sorafenib sensibility, and circUBE2D2 overexpression co-transfection recovered the sorafenib sensitivity (Figure 5A). Besides, LDHA overexpression activated the sorafenib sensibility and circUBE2D2 knockdown co-transfection inhibited the sorafenib sensibility. Glycolytic capacity analysis found that miR-889-3p mimics transfection repressed the glucose uptake (Figure 5B) and lactate production (Figure 5C), and circUBE2D2 overexpression co-transfection recovered the glucose uptake and lactate production. Moreover, LDHA overexpression up-regulated the glucose uptake and lactate production, while circUBE2D2 knockdown co-transfection inhibited them. In vivo xenograft assay showed that circUBE2D2 knockdown remarkedly repressed the tumor weight (Figure 5D) and volume (Figure 5E). Overall, these findings supports that circUBE2D2/miR-889-3p/LDHA axis regulates the HCC sorafenib resistance and glycolysis.
Figure 5.
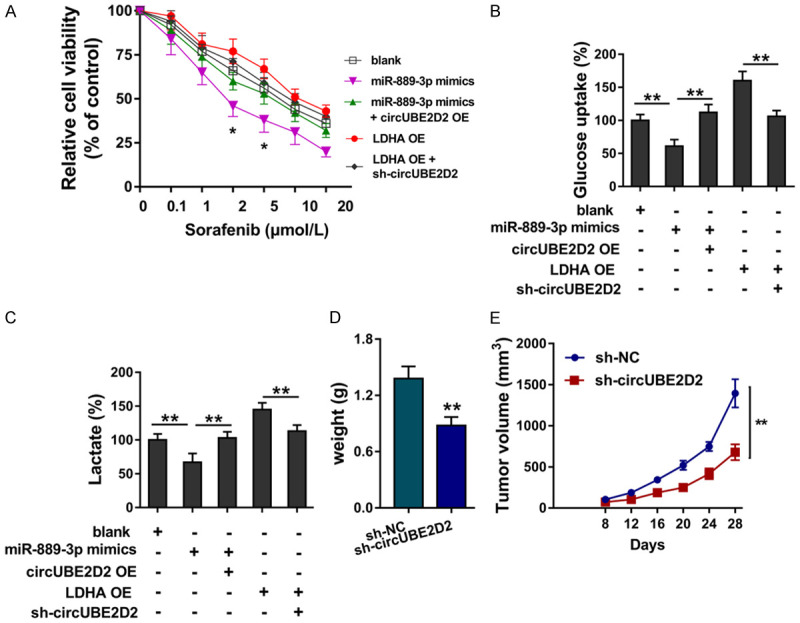
circUBE2D2/miR-889-3p/LDHA axis regulates the HCC sorafenib resistance and glycolysis. (A) Sorafenib sensitivity assay was performed using HCC cells (HepG2). HepG2 cells were transfected with miR-889-3p mimics and/or circUBE2D2 overexpression (circUBE2D2 OE), LDHA overexpression (LDHA OE) and/or circUBE2D2 knockdown (sh-circUBE2D2). (B) Glucose consumption was detected in HCC cells (HepG2) using glucose assay kit. (C) Lactate production was detected using lactate colorimetric assay kit. (A-C) Experiments were performed in triplicate and were repeated three times. (D) Tumor weight and (E) volume were detected using in vivo mice xenograft assay (10 mice). **P<0.01 vs. control. *P<0.05 vs. control.
Discussion
Sorafenib is a molecular targeted drug used in the treatment of advanced HCC. It can inhibit the proliferation of tumor cells and avoid tumor growth and recurrence. This multi-target drug could extend the HCC patient’s life, however, the clinical effect is not as good as we expected. The main reason for the dilemma is the sorafenib resistance. Thus, the solution to the problem is to eliminate the resistance from molecular level.
In this research, we focused on the potential role of circRNA circUBE2D2 in the HCC sorafenib resistance. Firstly, in the hypoxia treated HCC cells, we screened the dysregulated circRNAs using the circRNA microarray analysis. Based on the results of high-throughput sequencing, several circRNAs were selected for further examination using qRT-PCR. Results showed that a novel circRNA, named as circUBE2D2, was significantly up-regulated in the HCC cells (HepG2). For the stability analysis of circUBE2D2, actinomycin D and RNase R treatment were performed. Data showed that circUBE2D2 was stable when treated with actinomycin D and RNase R. Moreover, the abnormal circUBE2D2 overexpression was closely correlated with the unfavorable prognosis. Thus, we identified this novel circRNA, which might be oncogenic factor in the HCC.
Given that sorafenib resistance was reported to be connected with aerobic glycolysis [16,17], the hypoxia-related novel circUBE2D2 might be concerned with the sorafenib resistance. In cellular research, we performed the gain and loss of functional assays to detect the sorafenib resistance IC50 and glycolytic capacity. Results illustrated that circUBE2D2 overexpression enhanced the glucose consumption, lactate production level and ECAR quantity. Besides, circUBE2D2 knockdown reduced the glucose consumption, lactate production level and ECAR quantity. Thus, our findings confirmed the conclusion that circUBE2D2 accelerated the sorafenib resistance and aerobic glycolysis.
The role of ncRNA in the HCC sorafenib resistance has been wildly reported. circRNA expression profile was detected in sorafenib-resistant HCC cells and the differentially expressed circRNAs play critical roles in HCC sorafenib resistance [18]. For example, high lncRNA HOTAIR expression was found to be increased in the sorafenib resistance, and HOTAIR knockdown increased the sensitivity of sorafenib by up-regulating miR-217 [19]. Another example, lncRNA H19 expression was negatively related to HCC cells’ sorafenib sensitivity [20]. LncRNA NEAT1 expression was significantly associated with sorafenib chemoresistance patterns and HCC prognosis, demonstrating the regulation of miR-149-5p/AKT1 axis [21]. Overall, we could conclude the critical role of ncRNA in the HCC sorafenib resistance.
CircRNA is a critical group RNA in the human transcriptome [22]. In HCC tumorigenesis, numerous circRNAs were found to participate the pathophysiological process. For example, circRNA circSLC3A2 is predominantly localized in the cytoplasm and exhibits an oncogenic role by sponging miR-490-3p and promoting PPM1F protein expression, which indicates a positive correlation with poor survival for HCC patients [23]. CircRNA circFBXO11 is associated with poor prognosis of HCC patients and its overexpression promotes the proliferation and oxaliplatin resistance through targeting miR-605, thereby targeting FOXO3/ABCB1 axis [24]. Overall, these results demonstrate that circRNAs function as critical oncogenic elements in HCC.
In conclusion, we identified the novel hypoxia-related circRNA circUBE2D2 in HCC, which is up-regulated in tissue and cells and positively correlates with HCC patients’ poor prognosis. Results demonstrated that circUBE2D2 promotes the glycolysis and sorafenib resistance by targeting the miR-889-3p/LDHA axis. This finding may provide an effective promising target for therapy in HCC.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kumari R, Sahu MK, Tripathy A, Uthansingh K, Behera M. Hepatocellular carcinoma treatment: hurdles, advances and prospects. Hepat Oncol. 2018;5:HEP08. doi: 10.2217/hep-2018-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miguet M, Adam JP, Blanc JF, Lapuyade B, Bernard P, Buscail E, Neau-Cransac M, Vendrely V, Laurent C, Chiche L. Multidisciplinary meetings specific to hepatocellular carcinoma: How to proceed? J Visc Surg. 2019;156:217–227. doi: 10.1016/j.jviscsurg.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Hiraoka A, Kumada T, Michitaka K, Kudo M. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. 2019;8:312–325. doi: 10.1159/000494844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy CM, McCarthy M, O’Donoghue K. Recurrent hepatocellular carcinoma in pregnancy: a case report and literature review. Obstet Med. 2019;12:202–204. doi: 10.1177/1753495X18784074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu SK, Chawla YK, Dhiman RK, Singh V, Duseja A, Taneja S, Kalra N, Gorsi U. Rupture of hepatocellular carcinoma: a review of literature. J Clin Exp Hepatol. 2019;9:245–256. doi: 10.1016/j.jceh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanakis N, Kehagias E, Matthaiou N, Samonakis D, Tsetis D. Transcatheter arterial chemoembolization combined with radiofrequency or microwave ablation for hepatocellular carcinoma: a review. Hepat Oncol. 2018;5:HEP07. doi: 10.2217/hep-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach DH, Lee SK, Sood AK. Circular RNAs in cancer. Mol Ther Nucleic Acids. 2019;16:118–129. doi: 10.1016/j.omtn.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Yu F, Li P, Wang K. Emerging function and clinical significance of exosomal circRNAs in cancer. Mol Ther Nucleic Acids. 2020;21:367–383. doi: 10.1016/j.omtn.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Qi X, Liu L, Hu X, Liu J, Yang J, Yang J, Lu L, Zhang Z, Ma S, Li H, Yun X, Sun T, Wang Y, Wang Z, Liu Z, Zhao W. Emerging epigenetic regulation of circular RNAs in human cancer. Mol Ther Nucleic Acids. 2019;16:589–596. doi: 10.1016/j.omtn.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Yu F, Chen X, Li P, Wang K. The underlying mechanisms of noncoding RNAs in the chemoresistance of hepatocellular carcinoma. Mol Ther Nucleic Acids. 2020;21:13–27. doi: 10.1016/j.omtn.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y, Lv X, Yin W, Zhou L, Hu Y, Zhou A, Qi F. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY) 2019;11:8182–8203. doi: 10.18632/aging.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Cai L, Lei X, Wang D. Circular RNA ABCB10 promotes hepatocellular carcinoma progression by increasing HMG20A expression by sponging miR-670-3p. Cancer Cell Int. 2019;19:338. doi: 10.1186/s12935-019-1055-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhang K, Wang T, Zhou H, Feng B, Chen Y, Zhi Y, Wang R. A novel aurora-a inhibitor (MLN8237) synergistically enhances the antitumor activity of sorafenib in hepatocellular carcinoma. Mol Ther Nucleic Acids. 2018;13:176–188. doi: 10.1016/j.omtn.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu Y, Ma F, Huang W, Fang S, Li M, Wei T, Guo L. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16:5. doi: 10.1186/s12943-016-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Zuo X, Zhang Y, Han G, Zhang L, Wu J, Wang X. MiR-3662 suppresses hepatocellular carcinoma growth through inhibition of HIF-1α-mediated Warburg effect. Cell Death Dis. 2018;9:549. doi: 10.1038/s41419-018-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu A, Ji Q, Ma Y, Tai Y, Wang Y, Shen C, Liu Y, Wang T, Han J, Zhao C, Ma L, Liu W. Activator of thyroid and retinoid receptor increases sorafenib resistance in hepatocellular carcinoma by facilitating the Warburg effect. Cancer Sci. 2020;111:2028–2040. doi: 10.1111/cas.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu MY, Tang YP, Liu JJ, Liang R, Luo XL. Global transcriptomic study of circRNAs expression profile in sorafenib resistant hepatocellular carcinoma cells. J Cancer. 2020;11:2993–3001. doi: 10.7150/jca.39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X, Zhang W, Ye Y, Li H, Cheng L, Zhang M, Zheng S. LncRNA HOTAIR contributes to sorafenib resistance through suppressing miR-217 in hepatic carcinoma. Biomed Res Int. 2020;2020:9515071. doi: 10.1155/2020/9515071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Liu Y, Li Z, Li H, Li X, Yan L, Mao J, Shen J, Chen W, Xue F. Long non-coding RNA H19 is involved in sorafenib resistance in hepatocellular carcinoma by upregulating miR-675. Oncol Rep. 2020;44:165–173. doi: 10.3892/or.2020.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu Y, Tang G, Wu X, Wu C. LncRNA NEAT1 modulates sorafenib resistance in hepatocellular carcinoma through regulating the miR-149-5p/AKT1 axis. Saudi J Gastroenterol. 2020;26:194–203. doi: 10.4103/sjg.SJG_4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Li P, Song Y, Ge YX, Meng XM, Huang C, Li J, Xu T. Progress and prospects of circular RNAs in Hepatocellular carcinoma: novel insights into their function. J Cell Physiol. 2018;233:4408–4422. doi: 10.1002/jcp.26154. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Chen W, Jin M, Hou L, Chen X, Zhang R, Zhang J. CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Mol Cancer. 2018;17:165. doi: 10.1186/s12943-018-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Qin X, Wu R, Wan L, Zhang L, Liu R. Circular RNA circFBXO11 modulates hepatocellular carcinoma progress and oxaliplatin resistance through miR-605/FOXO3/ABCB1 axis. J Cell Mol Med. 2020;24:5152–5161. doi: 10.1111/jcmm.15162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


