Abstract
Objective: To investigate the small intestinal bacterial overgrowth (SIBO) and to evaluate the intestinal barrier function in ulcerative colitis (UC) patients treated with mesalazine and rifaximin. Methods: 96 patients undergoing the methane-hydrogen breath test in our hospital from January 2018 to January 2020 were enrolled in the study group, and 40 healthy persons were enrolled in the control group during this period. The SIBO positive rate of the two groups were collected and compared. Then, the SIBO positive patients were divided into group A and group B. Group A and group B all received mesalazine, and group B received rifaximin plus. The clinical efficacy, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and intestinal barrier function indexes like diamine oxidase (DAO) and D-lactic acid (DLA) were recorded and compared. Results: The study group presented higher SIBO positive rate compared with the control group (56% vs. 25%, P<0.05). After treatment, group B showed better clinical efficacy and lower levels of ESR and CRP than group A (all P<0.05). After treatment, the DAO and D-LA levels of the two groups were decreased, and presented lower levels in group B than group A (all P<0.05). Conclusion: UC patients present a higher positive rate in SIBO. Mesalazine and rifaximin are applied to patients with mild to moderate UC, and their clinical efficacy has been significantly enhanced after the eradication of SIBO.
Keywords: Ulcerative colitis, small intestinal bacterial overgrowth, intestinal barrier function
Introduction
Ulcerative colitis (UC), a non-specific ulcerative colitis, is a chronic inflammatory disease of the rectum and colon, for which, the pathogenesis hasn’t been elucidated [1]. UC is characterized by chronic inflammation and repeated attacks. Thus, UC is challenging to be cured completely, and it usually compiles with clinical symptoms such as fever, bloody diarrhea, abdominal pain, weight loss, etc. As the significant factor to change inflammatory response and immune response, gut flora affects the development of UC. Chronic inflammatory response and immune response disorder are the crucial parts to induce UC [2,3]. The incidence of ulcerative colitis has increased over the years due to the poor lifestyle and tremendous work pressure [4-6].
Small intestinal bacterial overgrowth (SIBO), defined as the increase in external bacteria and resident bacteria in the intestine, leads to food overfermentation, mucosal inflammation, small intestine permeability destruction, malabsorption, and villus damage. SIBO is closely associated with many other diseases such as non-alcoholic fatty liver, irritable bowel syndrome, deep vein thrombosis, diabetes, inflammatory bowel, etc. [7,8]. The imbalance of intestinal symbiotic bacteria and pathogenic bacteria destroys the biological barrier of the intestinal mucosa and poses unavoidable damage to the mechanical and immune barrier [9,10]. The intestinal barrier damage could lead to some conditional pathogenic bacteria in the intestine to translocate and enter the lamina propria to activate the mucosa-related immune system [11-13]. SIBO was detected by the breath test in this study at the advantages of simplicity, low cost, and non-invasiveness. This study aims to investigate SIBO and evaluate the intestinal barrier function in patients with ulcerative colitis.
Materials and methods
General materials
96 patients undergoing the methane-hydrogen breath test in our hospital from January 2018 to January 2020 were enrolled in the study group, and 40 healthy persons were enrolled in the control group during this period. The study group consisted of 28 males and 22 females with a mean age of (42.9±4.3) years old. The control group consisted of 21 males and 19 females with a mean age of (41.7±4.4) years old. No statistical significance was observed in the general materials of the two groups (P > 0.05).
Inclusion and exclusion criteria
Inclusion criteria: 1) Diagnosed with UC [14]; 2) Not taking antibiotics, probiotics etc. recently; 3) With cognitive and communication skills; 4) Approved by the hospital ethics committee, and the written informed consent was provided by the participants.
Exclusion criteria: 1) With the history of colectomy; 2) With sever liver disease, acute infection, other chronic inflammatory diseases; 3) Pregnancy; 4) With diabetes. 5) With long-term PPI and atrophic gastritis.
Methane-hydrogen breathe test
To obtain accurate inspection results, patients must rinse the mouth before taking the substrate orally, then blew it into an airbag. After 30 minutes, the patient took 10 ml lactulose and repeated the above steps every 30 minutes until 8 airbags were collected. In the end, the exhaled air was detected by the Breath Tracker (Quintron, USA).
The following things need to be noted before testing. Patients should take the test on an empty stomach for more than 8 hours and only water throughout the examination. During the examination, intense exercise and smoking were forbidden.
SIBO diagnosis could be established with any one of the following conditions within 120 minutes of oral lactulose. ① Hydrogen concentration > 20 ppm; ② Increase in methane concentration > 12 ppm; ③ Increase in the sum of methane and hydrogen concentration > 15 ppm; ④ Double peak curve throughout the examination.
Treatment methods
All SIBO positive patients in the study group were divided into group A (n=22) and group B (n=28) according the different treatment. All patients received mesalazine (manufacturer: Shanghai Aidefa Pharmaceutical Co., Ltd., national medicine number: H20143164, specification: 0.5 g), 1 g/time, 4 times/d, 6 weeks. Group B received rifaximin (manufacturer: Shenyang Tonglian Pharmaceutical Co., Ltd., national medicine number: H20040052, specification: 0.2 g), 0.2 g/time, 4 times/d, 2 weeks plus.
Clinical efficacy
The clinical efficacy was evaluated by symptom plus microscopic examination or Mayo score alone (See Table 1). The total effective rate = (complete remission + markedly effective + effective)/all cases *100%.
Table 1.
Evaluation criteria for clinical efficacy
| Diagnosis | Complete remission | Markedly effective | Effective | Ineffective |
|---|---|---|---|---|
| Symptom | Disappearance | Significant remission in most | Remission in most | No remission or exacerbation |
| Microscopic examination | Normal | Significant remission | A little remission | No remission |
| Mayo score | Decrease ≥ 95% | Decrease ≥ 70% | Decrease ≥ 30% | Decrease <30% |
Observation indexes
Venous samples were collected on empty stomach at early morning before and after treatment. Automatic biochemical analyzer 7100 (Hitachi, Japan) was adopted to measure CRP by immune scatter turbidity. ESR was detected by VES-matic Cube 200 ESR Analyzer (Sysmex, Japan). DAO and D-LA were detected by Enzyme-linked immunosorbent assay and kits were provided by Shanghai Tongwei Biotechnology Co., Ltd.
Statistical methods
SPSS20.0 was used for processing all the data in this study, and GraphPad Prism 7 (GraphPad Software, San Diego, USA) was adopted for picturing this data. The measurement data was described as (x̅ ± s) and conducted with t-test, and the count data was described as (n %) and conducted with the χ2 test. When P<0.05, the difference was statistically significant.
Results
Comparison of the SIBO positive rate in the control group and study group
The study group presented higher SIBO positive rate compared with the control group (56% vs. 25%, P<0.05). See Table 2.
Table 2.
Comparison of the SIBO positive rate in the control group and study group [n (%)]
| cases | Positive SIBO | Positive rate | |
|---|---|---|---|
| Study group | 89 | 50 | 56.18% |
| Control group | 40 | 10 | 25.00% |
| χ2 | 10.78 | ||
| P | <0.001 |
Comparison of the clinical efficacy of patients in groups A and B
The group B presented greater total effective rate compared with the group a (92.86% vs. 63.64%, P<0.05). See Table 3.
Table 3.
Comparison of the clinical efficacy of patients in groups A and B [n (%)]
| cases | Complete remission | Marked effective | effective | Ineffective | Total effective rate | |
|---|---|---|---|---|---|---|
| group A | 22 | 6 (27.27) | 4 (18.18) | 4 (18.18) | 8 (36.36) | 14 (63.64) |
| group B | 28 | 16 (57.14) | 5 (17.86) | 5 (17.86) | 2 (7.14) | 26 (92.86) |
| χ2 | 6.5747 | |||||
| P | 0.01 |
Comparison of ESR level in groups A and B
Before treatment, there was no statistical difference of ESR between the two groups (P > 0.05). After treatment, the ESR of the two groups both decreased, and group B presented a lower level than group A (all P<0.05). See Figure 1.
Figure 1.
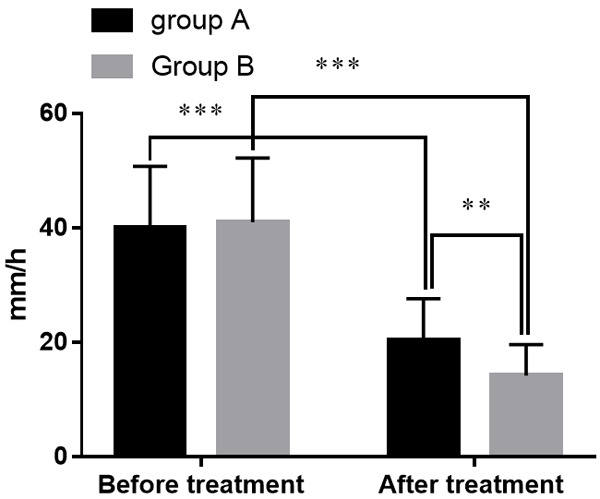
Comparison of ESR levels in groups A and B. Note: The X-axis indicates before and after treatment, and the Y-axis indicates the level of ESR (mm/h). The ESR levels of patients in group A before treatment were (40.02±10.75) mm/h while (20.3±7.3) mm/h after treatment. The ESR levels of patients in group B before treatment were (40.95±11.26) mm/h while (14.2±5.4) mm/h after treatment. **means that a significant difference with P<0.01 and ***means that a significant difference with P<0.001.
Comparison of CRP levels in groups A and B
Before treatment, there was no statistical difference of CRP between the two groups (P > 0.05). After treatment, CRP of the two groups had decreased, and group B presented a lower CRP than group A (all P<0.05). See Figure 2.
Figure 2.
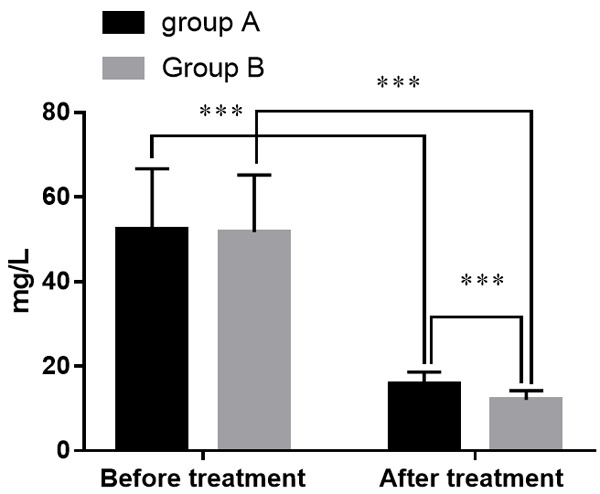
Comparison of CRP levels in groups A and B. Note: The X-axis indicates before and after treatment, and the Y-axis indicates CRP level (mg/l). The CRP levels of patients in group A before treatment were (52.4±14.3) mg/l while (15.8±2.8) mg/l after treatment. The CRP levels of patients in group B before treatment were (51.7±13.5) mg/l while (12.0±2.2) mg/l after treatment. ***means that a significant difference with P<0.001.
Comparison of the serum DAO levels in groups A and B
Before treatment, there was no statistical difference of DAO between the two groups (P > 0.05). After treatment, DAO of the two groups had decreased, lower in group B compared with group A (all P<0.05). See Figure 3.
Figure 3.
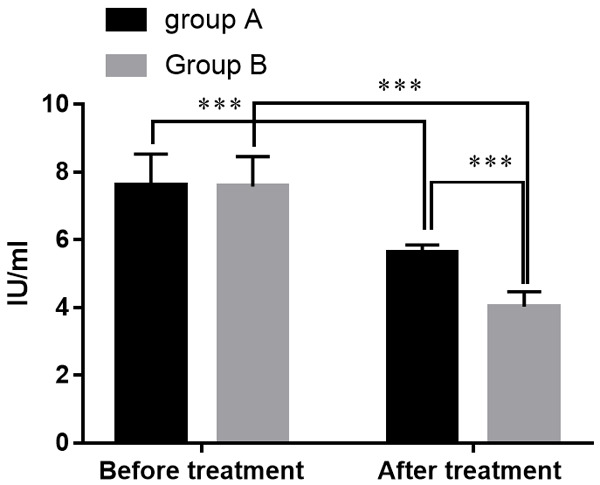
Comparison of serum DAO levels in groups A and B. Note: The X-axis represents before and after treatment, and the Y-axis represents the serum DAO level (IU/ml). The serum DAO levels of patients in group A before treatment were (7.62±0.92) IU/ml while (5.63±0.22) IU/ml after treatment. The serum DAO levels of patients in group B before treatment were (7.58±0.88) IU/ml while (4.03±0.44) IU/ml after treatment. ***means that a significant difference with P<0.001.
Comparison of serum D-LA levels before and after treatment
Before treatment, there was no statistical difference of D-LA between the two groups (P > 0.05). After treatment, D-LA of the two groups had decreased, and group B had a lower level of D-LA (P<0.05). See Figure 4.
Figure 4.
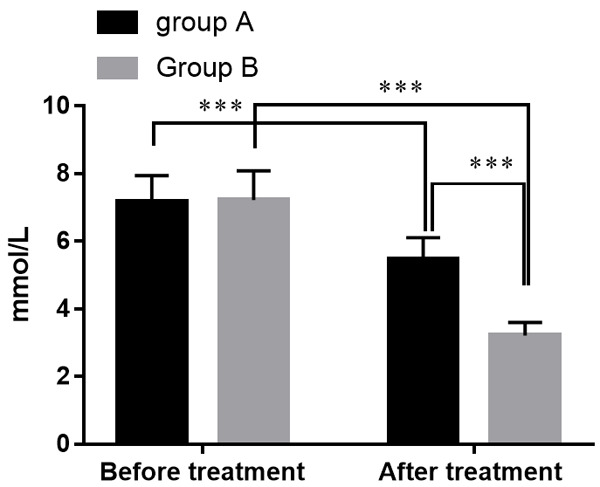
Comparison of serum D-LA levels in groups A and B. Note: The X-axis represents before and after treatment, and the Y-axis represents the serum D-LA value (in mmol/L). The serum D-LA levels of patients in group A were (7.18±0.77) mmol/L before treatment while (5.48±0.63) mmol/L after treatment. The serum D-LA levels of patients in group B were (7.22±0.87) mmol/L before treatment while (3.22±0.38) mmol/L after treatment. ***means that a significant difference with P<0.001.
Discussion
UC usually exists in the colon and occasionally extends to the terminal ileum. Previous studies have demonstrated the importance of SIBO in patients with UC who present a higher positive rate in SIBO than healthy persons [15]. A large volume of published studies have found that the abnormal manifestation of the small intestine function in patients with UC and animal models is decreased in intestinal fluid, absorption of D-xylose, amino acids, and fat [16,17]. There is a significant decrease in absorption of intestinal fluid and electrolytes in UC patients. Even in the remission period, the intestinal secretion capacity of patients with UC is different from a healthy person. Their proximal colonic mucosa is more sensitive to cAMP-dependent secretion but insensitive to Ca2+-dependent secretion. Genetic factors increase the intestinal permeability of patients with UC, even in remission. One study by Axel Dignass et al. [18] carried out a lactulose hydrogen breath test and a wireless dynamic capsule (a general gastrointestinal motility detection system that detects pressure, pH, and temperature) on patients, proving that the delay of orofecal transit time was prone to SIBO. IL-1β increase in the circular colic muscles may result in motility disorders of the colon in patients with UC due to hydrogen peroxide production.
It is now well established from a variety of studies that intestinal flora changes are one of the essential mechanisms inducing UC [19]. It has been confirmed that functions of normal intestinal flora are mostly manifested to inhibit the growth of pathogenic bacteria, strengthen the role of the epithelial barrier, and regulate inflammation through the Toll-like receptor pathway [20,21]. Previous studies have shown a decrease in Firmicutes and Bacteroides in patients with UC but an increase in Proteobacteria and Actinomycetes. It has also been reported that the relationships between fecal microbiota and UC. Panagiotis Kourkoulis [22] et al. performed fecal microbiota transplantation on 35 UC patients, resulting in 31% ineffective and 69% remission. It has proved that a variety of probiotics maintained intestinal homeostasis by blocking the harm effects of bacteria, increased the integrity of the epithelial barrier, promoted innate immunity, and balanced inflammatory factors, thereby maintaining the remission of UC and preventing recurrence. Intestinal mucosal barrier function plays the role of the normal intestinal environment, and the dysfunction would lead to inflammatory bowel disease. D-LA, as the fermentation of intestinal bacteri, mainly exists in a highly active endoenzyme of the intestinal villus. When the intestinal mucosa function is damaged, D-LA and DAO enter the systemic circulation through blood, so D-LA and DAO was the important indexes to measure the intestinal barrier function.
In this study, patients with UC were treated with mesalazine and rifaximin. Rifaximin improves the clinical efficacy of mesalazine on UC after the eradication of SIBO. The group B presented greater total effective rate than group A. In accordance with the present results, previous studies [23,24] have demonstrated that rifaximin helped alleviate the clinical symptoms of patients with UC, like the results in this study. In addition, mesalazine could decrease ESR and CRP levels. After treatment, there was a decrease in the ESR and CRP levels, and group B presented a significantly lower level (P<0.05), indicating eradication of SIBO beneficial to alleviate the condition. The adjustment of serum DAO and D-LA indexes facilitated the improvement of intestinal mucosal barrier function. Patients with UC presented abnormally high expression levels of serum D-LA and DAO, positively correlated with the disease severity [25]. The results showed a significant lower serum DAO and D-LA in group A (P<0.05), providing a stronger evidence. It is an observational study with a small number of participants and short follow-up. A randomized controlled study with a large sample is needed to confirm this conclusion further.
To sum up, patients with UC present a higher positive rate in SIBO in comparison with healthy persons. Mesalazine and rifaximin are applied to patients with mild to moderate UC, and their clinical efficacy has been significantly improved after the eradication of SIBO, indicating that this treatment works on patients with UC and significantly enhances the intestinal mucosal barrier function.
Disclosure of conflict of interest
None.
References
- 1.Li S, Wu B, Fu W, Reddivari L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int J Mol Sci. 2019;20:2588. doi: 10.3390/ijms20102588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrukh A, Mayberry JF. Surveillance for colorectal cancer and chemoprevention in ulcerative and Crohn’s colitis: the need for clinical strategies to increase effectiveness. JGH Open. 2019;3:370–373. doi: 10.1002/jgh3.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinisch W, Bressler B, Curtis R, Parikh A, Yang H, Rosario M, Røseth A, Danese S, Feagan B, Sands BE, Ginsburg P, Dassopoulos T, Lewis J, Xu J, Wyant T. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a post hoc analysis of GEMINI 1. Inflamm Bowel Dis. 2019;25:803–810. doi: 10.1093/ibd/izy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz Karadag F, Arslan F, Caskurlu H, Cag Y, Vahaboglu H. Efficacy of antiviral treatment in cytomegalovirus detected ulcerative colitis: meta-analysis of available data. Scand J Gastroenterol. 2019;54:1346–1352. doi: 10.1080/00365521.2019.1688860. [DOI] [PubMed] [Google Scholar]
- 5.Binabaj MM, Asgharzadeh F, Avan A, Rahmani F, Soleimani A, Parizadeh MR, Ferns GA, Ryzhikov M, Khazaei M, Hassanian SM. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. J Cell Physiol. 2019;234:11654–11661. doi: 10.1002/jcp.27823. [DOI] [PubMed] [Google Scholar]
- 6.Bugda Gwilt K, Olliffe N, Hoffing RA, Miller GM. Trace amine associated receptor 1 (TAAR1) expression and modulation of inflammatory cytokine production in mouse bone marrow-derived macrophages: a novel mechanism for inflammation in ulcerative colitis. Immunopharmacol Immunotoxicol. 2019;41:577–585. doi: 10.1080/08923973.2019.1672178. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen B, Haastrup P, Wehberg S, Kjeldsen J, Waldorff FB. Predictors of health-related quality of life in patients with moderate to severely active ulcerative colitis receiving biological therapy. Scand J Gastroenterol. 2020;55:656–663. doi: 10.1080/00365521.2020.1768282. [DOI] [PubMed] [Google Scholar]
- 8.Hagelund LM, Elkjær Stallknecht S, Jensen HH. Quality of life and patient preferences among Danish patients with ulcerative colitis - results from a survey study. Curr Med Res Opin. 2020;36:771–779. doi: 10.1080/03007995.2020.1716704. [DOI] [PubMed] [Google Scholar]
- 9.Rumer KK, Dehghan MS, Sceats LA, Trickey AW, Morris AM, Kin C. Use of biological medications does not increase postoperative complications among patients with ulcerative colitis undergoing colectomy: a retrospective cohort analysis of privately insured patients. Dis Colon Rectum. 2020;63:1524–1533. doi: 10.1097/DCR.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amouzadeh-Ghadikolai O, Reicht G, Quehenberger F, Robier C. Basophilia of the peripheral blood in patients with ulcerative colitis. Scand J Gastroenterol. 2020;55:248–250. doi: 10.1080/00365521.2019.1710247. [DOI] [PubMed] [Google Scholar]
- 11.Kotani S, Fukuba N, Kawashima K, Mishima Y, Sonoyama H, Okimoto E, Tada Y, Oka A, Tamagawa Y, Oshima N, Mishiro T, Tobita H, Shibagaki K, Moriyama I, Ishimura N, Kushiyama Y, Fujishiro H, Ishihara S. Prevalence of functional dyspepsia-like symptoms in ulcerative colitis patients in clinical remission and overlap with irritable bowel syndrome-like symptoms. Scand J Gastroenterol. 2020;55:560–564. doi: 10.1080/00365521.2020.1761998. [DOI] [PubMed] [Google Scholar]
- 12.Magdziak A, Szlak J, Mróz A, Wieszczy P, Zagórowicz E. A stool test in patients with active ulcerative colitis helps exclude cytomegalovirus disease. Scand J Gastroenterol. 2020;55:664–670. doi: 10.1080/00365521.2020.1771760. [DOI] [PubMed] [Google Scholar]
- 13.Pettersson N, Kragsbjerg F, Hamrin A, Bergman S, Forsblad-d’Elia H, Karling P. Increased chronic pain in patients with ulcerative colitis is mostly associated to increased disease activity. A cross-sectional case-control study. Scand J Gastroenterol. 2020;55:1193–1199. doi: 10.1080/00365521.2020.1820567. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018;1:CD011450. doi: 10.1002/14651858.CD011450.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier KA, Dwyer PA, Vessey JA, Shea J, Pratt P. The efficacy of antihistamines in preventing reactions to infliximab in patients with crohn disease/ulcerative colitis: a review of the evidence. Gastroenterol Nurs. 2020;43:345–349. doi: 10.1097/SGA.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47:162–175. doi: 10.1111/apt.14422. [DOI] [PubMed] [Google Scholar]
- 17.Pachler FR, Bisgaard T, Mark-Christensen A, Toft G, Laurberg S. Impact on fertility after failure of restorative proctocolectomy in men and women with ulcerative colitis: a 17-year cohort study. Dis Colon Rectum. 2020;63:816–822. doi: 10.1097/DCR.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 18.Dignass A, Waller J, Cappelleri JC, Modesto I, Kisser A, Dietz L, DiBonaventura M, Wood R, May M, Libutzki B, Bargo D. Living with ulcerative colitis in Germany: a retrospective analysis of dose escalation, concomitant treatment use and healthcare costs. J Med Econ. 2020;23:415–427. doi: 10.1080/13696998.2019.1707210. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Xiang L, Li L. Therapeutic efficacy of the combination therapy of corticosteroids and 5-aminosalicylic acid for treatment of pyoderma gangrenosum with ulcerative colitis. Indian J Dermatol. 2020;65:38–41. doi: 10.4103/ijd.IJD_505_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Carvello M, Lightner AL, Spinelli A, Kotze PG. Up-to-date surgery for ulcerative colitis in the era of biologics. Expert Opin Biol Ther. 2020;20:391–398. doi: 10.1080/14712598.2020.1718098. [DOI] [PubMed] [Google Scholar]
- 21.Lavryk OA, Stocchi L, Shawki S, Aiello A, Church JM, Steele SR, Hull TL. Redo IPAA after a failed pouch in patients with crohn’s disease: is it worth trying? Dis Colon Rectum. 2020;63:823–830. doi: 10.1097/DCR.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 22.Kourkoulis P, Michalopoulos G, Katifelis H, Giannopoulou I, Lazaris AC, Papaconstantinou I, Karamanolis G, Gazouli M. Leucine-rich alpha-2 glycoprotein 1, high mobility group box 1, matrix metalloproteinase 3 and annexin A1 as biomarkers of ulcerative colitis endoscopic and histological activity. Eur J Gastroenterol Hepatol. 2020;32:1106–1115. doi: 10.1097/MEG.0000000000001783. [DOI] [PubMed] [Google Scholar]
- 23.Walter E, Hausberger SC, Groß E, Siebert U. Health-related quality of life, work productivity and costs related to patients with inflammatory bowel disease in Austria. J Med Econ. 2020;23:1061–1071. doi: 10.1080/13696998.2020.1801187. [DOI] [PubMed] [Google Scholar]
- 24.Chhibba T, Ma C. Is there room for immunomodulators in ulcerative colitis? Expert Opin Biol Ther. 2020;20:379–390. doi: 10.1080/14712598.2020.1708896. [DOI] [PubMed] [Google Scholar]
- 25.Song WB, Lv YH, Zhang ZS, Li YN, Xiao LP, Yu XP, Wang YY, Ji HL, Ma L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J Gastroenterol. 2009;15:3916–9. doi: 10.3748/wjg.15.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]


