Abstract
Objective: To study the curative effect of the traditional Chinese medicine (TCM) Shashen Maidong Decoction in treating lung cancer cachexia based on the cancer toxicity theory, and analyze its influence on patients’ serum tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6). Methods: From January 2018 to January 2019, 104 patients with primary lung cancer cachexia diagnosed and treated in our hospital’s oncology department were selected and randomly divided into experimental group and control group, with 52 cases in each group. The control group received routine treatment and nutritional support, while the experimental group received Shashen Maidong Decoction plus. Indexes were compared and analyzed before and after 4 weeks of treatment, including TCM syndrome score, Patient-Generated Subjective Global Assessment (PG-SGA) score, Karnofsky score (KPS), albumin, prealbumin, TNF-α and IL-6 levels. Results: (1) After treatment, both groups’ PG-SGA score, KPS score and TCM syndrome score were better than those before treatment (P < 0.01), and the experimental group’s PG-SGA score, KPS score and TCM syndrome score were higher than those of the control group (P < 0.01). (2) After treatment, both groups’ serum albumin and prealbumin levels were higher than those before treatment (P < 0.05), and the experimental group’s prealbumin level was higher than that in the control group (P < 0.05). (3) After treatment, both groups’ serum levels of TNF-α and IL-6 were lower than those before treatment (P < 0.05), and the experimental group’s levels of TNF-α and IL-6 were lower than those in the control group (P < 0.05). Conclusion: Based on the cancer toxicity theory, the application of Shashen Maidong Decoction in treating lung cancer cachexia has definite therapeutic effects and important clinical values. It can effectively alleviate patients’ symptoms, improve nutritional status, and reduce body’s inflammatory response.
Keywords: Cancer toxicity theory, Shashen maidong decoction, lung cancer cachexia, tumor necrosis factor-A, interleukin-6
Introduction
As is the case worldwide, lung cancer is one of the leading causes of cancer-related deaths, and this is related to the high mortality rate and delayed diagnosis of lung cancer. It is estimated that 50% of primary lung cancer would lead to cachexia, and approximately 20% of patients would die directly from it [1]. The main symptoms are weight loss, fatigue, poor appetite, anemia, depression, etc. Although advancements have been made in early diagnosis and treatment, still majority of cases are diagnosed at a later stage with poor prognosis. Currently, the mainstay for cachexia includes drug therapy, nutrition therapy, and others. However, the key is to treat primary cancer and apply multiple disciplinary therapies on cachexia based on that [2]. Thus, research on treating cancer cachexia with Chinese medicine has been gaining increasing attention. Traditional Chinese Medicine (TCM) believes that [3] cancer toxin is an important player that triggers malignant tumors and metastasis tumors and influences prognosis. On account of that, cancer toxicity theory has positive guiding effects in treating malignant tumors with TCM. Taking etiology, pathogenesis, symptoms and other factors of malignant tumors into consideration, it forms a systematic theory, and claims that cancer toxin, coupled with other factors, generates cancer [4]. This study selected 104 patients with primary lung cancer cachexia, with an attempt to analyze the effects of Shashen Maidong decoction systematically.
Materials and methods
Clinical data
From January 2018 to January 2019, 104 patients with primary lung cancer cachexia diagnosed and treated in our hospital’s oncology department were included. Patients were diagnosed and examined by pathology, cytology, iconography, etc. and met the diagnostic criteria of primary lung cancer [5] and cachexia. Inclusion criteria: (1) patients diagnosed with primary lung cancer cachexia and had been survived for over 3 months, (2) aged over 20 years, (3) patients with KPS score over 70, and TNM staging from III to IV, (4) patients who had not been treated with chemotherapy recently (over a month), (5) patients who knew the research and gave informed consent under conscious condition. Exclusion criteria: (1) patients with hepatic and renal dysfunction and metastatic tumor, (2) patients with severe complicated anemia and cardio-cerebrovascular diseases, (3) patients with blood coagulation disorders, (4) with a history of chemotherapy recently and were allergic to the medicine used in this research, (5) patients who dropped out. This study had obtained approval from our hospital’s research ethics committee. Patients were divided into two groups by random number table method, with 52 cases in each group. The experimental group included 30 males and 22 females, with an average age of 56.21±3.62 years. TNM staging: 18 cases of IIIa staging, 18 cases of IIIb staging, and 16 cases of IV staging. Tumor classification: 44 cases of non-small cell lung cancer (NSCLC) and 8 cases of small cell lung cancer (SCLC). The control group included 28 males and 24 females, with an average age of 57.30±3.41 years. TNM staging: 15 cases of IIIa staging, 20 cases of IIIb staging, and 17 cases of IV staging. Tumor classification: 42 cases of non-small cell lung cancer (NSCLC) and 10 cases of small cell lung cancer (SCLC). The baseline information in the two groups were basically the same (P > 0.05). See Table 1.
Table 1.
Comparison of clinical data and information between the two groups
| Indexes | Experimental group (n=52) | Control group (n=52) | t/χ2 | P value |
|---|---|---|---|---|
| Male/Female (n) | 30/22 | 28/24 | 0.16 | 0.69 |
| Age (years) | 56.21±3.62 | 57.30±3.41 | 1.55 | 0.12 |
| TNM staging (n) | - | - | - | - |
| IIIa staging | 18 | 15 | 0.41 | 0.82 |
| IIIb staging | 18 | 20 | ||
| IV staging | 16 | 17 | ||
| Tumor classification | - | - | - | - |
| NSCLC | 44 | 42 | 0.27 | 0.60 |
| SCLC | 8 | 10 |
Methods
The Control group received routine treatments. Medroxyprogesterone (Pfizer Italia S.r.l. SFDA approval number: H20140648) was applied, 250 mg a time, per os, 2 times a day. Nutrition support was provided simultaneously and nutrition supply was provided according to patients’ indications. For patients who cannot eat on his own, tube-fed food and enteral nutrition were provided.
The experimental group received routine treatments and Shashen Maidong decoction at the same time. Prescription composition: Shashen 15 g, Maidong 15 g, Yuzhu 10 g, Tianhua powder 10 g, folium mori 10 g, lentils 10 g, and licorice 6 g. Syndrome differentiation: for patients with phlegm stagnation and dampness resistance, Fuling 15 g, Fabanxia 10 g, and dried tangerine peel 6 g; for patients with heat toxin, Jinqiaomai 15 g, Yuxingcao 15 g, Longkui 10 g, and Zaoxiu 10 g. The medicine was boiled with water, 200 mL decoction was obtained, and the medicine was taken when it’s warm, twice a day, morning and night, and one dose a day. One week is considered a course of treatment, and it was continued for 4 weeks.
Observation indexes
(1) Patient-generated subjective global assessment (PG-SGA) score: PG-SGA score was used to assess overall nutrition condition before and after treatment. PG-SGA score includes body weight change, dietary intake, clinical symptoms, activities and functions, relationship between disease and nutritional needs, metabolic requirement, etc. The total score is 10. 0 to 3 is considered as eutrophic, 4 to 8 is considered as middle or suspected malnutrition, and ≥ 8 is considered as high malnutrition.
(2) Serum albumin and prealbumin levels: the serum albumin and prealbumin levels were tested before and after treatment to record the changes.
(3) Karnofsky (KPS) score: KPS score was used to assess function before and after treatment. KPS score includes patients’ ability to conduct normal activity, daily living activities, etc. The total score is 100, and the higher the score, the better the patients’ function.
(4) TCM syndrome score: based on the Guiding Principles for Clinical Research of New Chinese Medicines (2002 ed), TCM syndrome score was used to assess the syndromes before and after treatment. TCM syndrome score includes 10 dimensions such as coughing, expectoration, chest pain, asthma, lack of energy, sallow complexion, anorexia, etc. The score ranges from 0 to 4 on each dimension, with 0 for no syndrome, 1 for mild syndrome, 2 for moderate syndrome, and 3 for sever syndrome.
(5) Serum TNF-α, IL-6 levels: 3 mL of patients’ peripheral elbow venous blood was drawn respectively before and after treatment, and the serum TNF-α and IL-6 levels were tested by ELISA method.
Assessments on therapeutic effects
Assessment was made based on the TCM syndrome score before and after treatment. Efficacy index = [(score before treatment - score after treatment)/score before treatment] × 100%. Specific criteria: (1) Excellent: cachexia symptoms and other symptoms were significantly alleviated, efficacy index ≥ 70%; (2) Effective: cachexia symptoms and other symptoms were alleviated to some extent, 30% ≤ efficacy index < 70%; (3) Ineffective: cachexia symptoms and other symptoms didn’t change or even worse, efficacy index < 30%. Total effective rate = (Excellent cases + Effective cases)/total cases × 100%.
Statistical analysis
The data were processed by SPSS statistical software 20.0 and the graphs were plotted by GraphPad prism 8.0. The measurement data were represented by (x̅ ± sd) and examined by t test. The enumeration data were represented by % and examined by χ2. P < 0.05 was considered as statistically significant.
Results
Comparison of the PG-SGA score before and after treatment
Before treatment, the PG-SGA scores of the control group and the experimental group were 6.72±2.05 and 6.93±2.28, respectively (P > 0.05); after treatment, they were 3.92±1.05 and 2.05±0.64, lower than those before treatment, and lower in the experimental group than the control group (all P < 0.01). See Figure 1.
Figure 1.
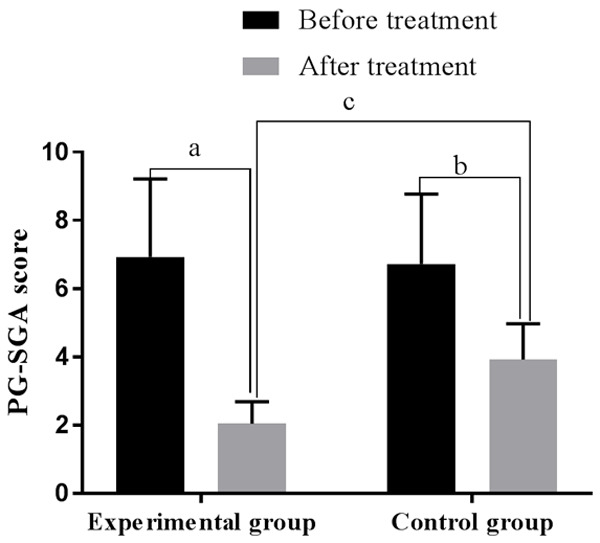
Comparison of the PG-SGA score before and after treatment. Note: The horizontal axis stands for groups and the vertical axis stands for PG-SGA score. The experimental group’s PG-SGA score before treatment was 6.93±2.28, and after treatment was 2.05±0.64 (aP < 0.001). The control group’s PG-SGA score before treatment was 6.72±2.05, and after treatment was 3.92±1.05 (bP < 0.001). Two groups were different after treatment (cP < 0.001).
Comparison of the serum albumin and prealbumin levels before and after treatment
Before treatment, the albumin levels of the control group and the experimental group were 23.09±4.03 and 22.83±4.87, and the prealbumin levels were 136.58±56.88 and 140.21±50.01, respectively (all P > 0.05); after treatment, the albumin levels were 24.61±3.48 and 25.17±4.07, the prealbumin levels were 157.25±23.90 and 171.56±42.05, respectively, greater than those before treatment, and higher for prealbumin in the experimental group than the control group (all P < 0.01). See Table 2.
Table 2.
Comparison of the serum albumin and prealbumin levels before and after treatment (x̅ ± sd, g/L)
| Groups | Cases | Serum albumin | Prealbumin | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | ||
| Experimental group | 52 | 22.83±4.87 | 25.17±4.07a | 140.21±50.01 | 171.56±42.05b |
| Control group | 52 | 23.09±4.03 | 24.61±3.48a | 136.58±56.88 | 157.25±23.90b |
| T value | 0.30 | 0.75 | 0.35 | 2.13 | |
| P value | 0.78 | 0.45 | 0.73 | 0.04 | |
Note: Compared with the same group before treatment;
P < 0.05;
P < 0.05.
Comparison of the KPS before and after treatment
Before treatment, the KPS levels of the control group and the experimental group were 55.09±5.63 and 53.28±5.57, respectively (P > 0.05); after treatment, they were 74.18±10.02 and 62.80±7.16, respectively, higher than those before treatment, and higher in the experimental group than the control group (all P < 0.01). See Figure 2.
Figure 2.
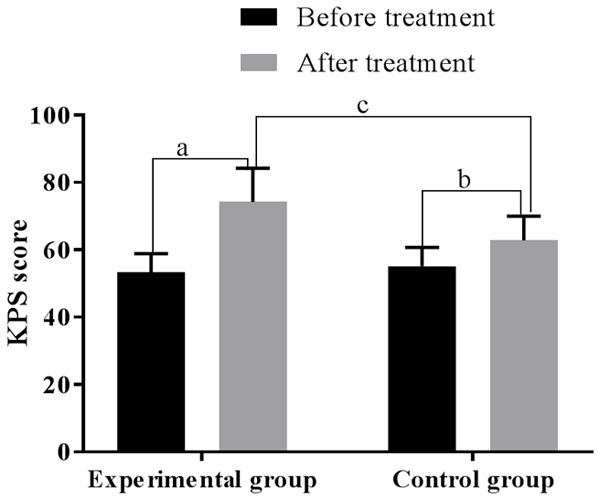
Comparison of the KPS score before and after treatment. Note: The horizontal axis stands for groups and the vertical axis stands for KPS score. The experimental group’s KPS score before treatment was 53.28±5.57, and after treatment was 74.18±10.02 (aP < 0.001). The control group’s KPS score before treatment was 55.09±5.63, and after treatment was 62.80±7.16 (bP < 0.001). Two groups were different after treatment (cP < 0.001).
Comparison of the TCM syndrome score before and after treatment
Before treatment, the TCM syndrome scores of the control group and the experimental group were 20.74±6.08 and 21.22±5.30 (P > 0.05); after treatment, they were 10.34±3.42 and 6.13±3.25, lower than those before treatment, and lower in the experimental group than the control group (all P < 0.01). See Figure 3.
Figure 3.
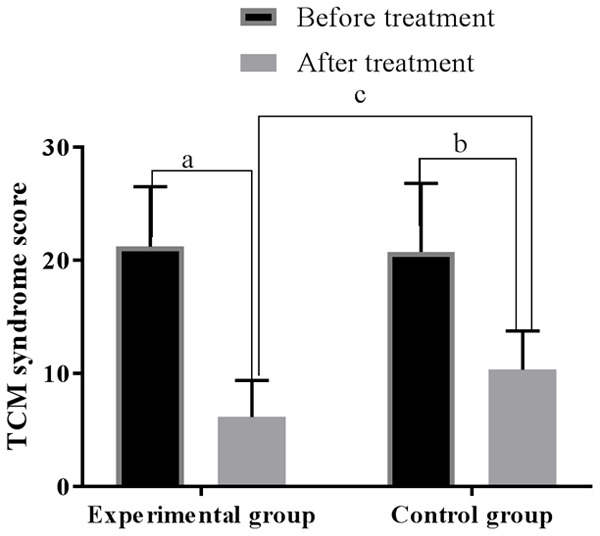
Comparison of the TCM syndrome score before and after treatment. Note: The horizontal axis stands for groups and the vertical axis stands for TCM syndrome score. The experimental group’s TCM syndrome score before treatment was 21.22±5.30, and after treatment was 6.13±3.25 (aP < 0.001). The control group’s TCM syndrome score before treatment was 20.74±6.08, and after treatment was 10.34±3.42 (bP < 0.001). Two groups were different after treatment (cP < 0.001).
Comparisons of two groups’ total effective rate
The total effective rate of the experimental group was higher than that of the control group (92.31% vs. 71.15%, P < 0.05). See Table 3.
Table 3.
Comparisons of the total effective rate [n (%)]
| Groups | Cases | Excellent | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| Experimental group | 52 | 28 (53.85) | 20 (38.46) | 4 (7.69) | 48 (92.31) |
| Control group | 52 | 17 (32.69) | 20 (38.46) | 15 (28.85) | 37 (71.15) |
| χ2 value | 7.792 | ||||
| P value | 0.005 |
Comparison of the TNF-α and IL-6 levels before and after treatment
Before treatment, the TNF-α levels of the control group and the experimental group were 120.32±17.68 and 120.51±18.26, respectively, and the IL-6 levels were 138.47±19.28 and 139.20±20.32, respectively (all P > 0.05); after treatment, the TNF-α levels were 91.16±12.20 and 63.06±13.45, the IL-6 levels were 97.36±14.18 and 70.12±10.25, lower than those before treatment, and lower in the experimental group than the control (all P < 0.01). See Table 4.
Table 4.
Comparison of the TNF-α and IL-6T levels before and after treatment (x̅ ± sd)
| Groups (n) | TNF-α (ng/L) | IL-6 (ng/L) | ||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Experimental group (n=52) | 120.51±18.26 | 63.06±13.45a | 139.20±20.32 | 70.12±10.25a |
| Control group (n=52) | 120.32±17.68 | 91.16±12.20b | 138.47±19.28 | 97.36±14.18b |
| T value | 1.06 | 7.12 | 0.14 | 13.02 |
| P value | 0.06 | < 0.001 | 0.07 | 0.01 |
Note: Compared with the same group before treatment;
P < 0.05;
P < 0.05.
Discussion
Cachexia accounts for major proportion of lung cancer-related death, and it involves multiple systems and organs and results in bleak prognosis. To date, the exact mechanism of cachexia remains unclear, yet several factors that are involved are documented. (1) patients with malignant tumors have poor appetite due to the changes of uptake central mechanism, (2) anemia reduces metabolic supply, (3) sarcolysis and lipolysis affects metabolism, (4) the metabolism mediums produced by the body are involved. Once the cachexia occurs, the effects of radical operation, radiotherapy and chemotherapy will all be compromised. The common treatment is palliative nutrition support therapy, but the effects and prognosis are somber [6]. In recent years, along with the advanced clinical research on TCM, it has played an increasingly positive role in treating tumors and their complications.
Lung cancer is believed to be lung accumulation of toxin in TCM. Ancient Chinese doctors had certain knowledge on lung cancer, they mostly described it as tumor, cancer, abdominal mass, accumulation, etc. Zhongzi Li from the Ming Dynasty wrote, ‘accumulation occurs due to the lack of Qi and toxin thus take its place’. He pointed out that malignant tumors were generated because our body’s Qi was not strong enough to fight toxin [7]. Zhongying Zhou, a famous practitioner of Chinese medicine, proposed the cancer toxicity theory, considering that malignant tumors are highly toxic and cause the deficiency of Qi. As is known, cancer toxin is the key factor of forming malignant tumors; pathological factors like deficiency, phlegm, blood stagnation, and dampness are prone to binding with cancer, which form the complex pathogenesis of malignant tumors. On one hand, cancer toxin is the etiology of malignant tumors. On the other hand, it is a production of pathology, and interplay caused by external and internal factors. External causes include six pathogenic factors, which reflect on fleshy exterior, block the flow of Qi, result in body fluid deficiency and poor metabolism, and further accumulate into blocks. Internal causes include pathological factors like depression, improper diet, and chronic illness, which affects liver regulation and spleen transportation and causes deficiency, phlegm, blood stagnation, dampness and heat combined together to form toxin and hence triggers the disease. Therefore, cancer toxin is the etiology of lung cancer, which is caused by other pathological factors as well. Thus, cancer toxin is a concern in treating malignant tumors [8]. Cachexia is a common symptom for patients with late stage lung cancer. There is no corresponding term in TCM. According to some descriptions of symptoms resemble to cachexia in TCM literature and combine with cachexia’s clinical symptoms, it is considered as a kind of ‘consumptive disease’ in TCM. For example, there are related descriptions in Suwen, like dried-up appearances and obvious pulse, which are all the symptoms of cachexia with bleak prognosis. The pathological symptoms are consumptive, Qi deficiency, spleen and stomach deficiency, essence and blood asthenia, weak body, insufficient blood, and so on. Chinese medicine considers that, consumptive diseases are due to endowment weakness, over-stress, and improper diet. Patients with consumptive diseases often lack Qi and blood in internal organs, causing the deficiency of Yin and Yang, generating malnutrition and body consumption. Along with the pathological changes, phlegm and blood stagnation occur. Deficiency syndrome becomes excess syndrome, and the two combine together and accumulate, resulting in blood gas stasis. It constantly consumes patients’ Qi, worsens the conditions, and then leads to excess syndrome and deficiency syndrome. It was recorded in ancient literature that this disease occurs mainly at viscera, spleen, and kidney. Asthenic diseases of spleen and kidney are the root of cancer cachexia [9].
Shashen Maidong decoction uses Shashen and Maidong as dominant composition, aiming at clearing heat and moistening dryness. Yuzhu and Tianhua powders serve as adjuvants; they can strengthen the function of moistening lung and stomach. Raw lentils and licorice serve as adjuvant as well, they can replenish Qi and stomach, replenish spleen and benefit lung. Folium mori can dredge the lung collaterals and remove heat from the lung to relieve cough. Licorice coordinates the property of herbs to make prescription. With the medicines combined together, the effects of phlegm reduction, blood stasis dissipation, heat-clearing, detoxicating, Qi replenishment and spleen invigoration take place [10]. According to modern pharmacological research, Shashen is rich in polysaccharide and glucoside, which help strengthening the function of immunity and antibiosis. It was reported that the effective constituent of Shashen can participate in the process of molecular recognition and cell adhesion, and it can also regulate body defense ability [11]. Animal experiments prove that Shashen polysaccharide can improve rats’ peripheral blood lymphocyte level and regulate immune function [11]. Maidong polysaccharide can improve body’s immune function and promote the production of antibody and addiment. Also, prior trials show that Shashen Maidong decoction has the function of inflammatory response, protecting gastric mucosa and boosting immune system [12].
The results of this study show that after the experimental group was treated with Shashen Maidong decoction in addition to the routine treatment, both groups’ PG-SGA score, serum albumin level, prealbumin level, KPS score and TCM syndrome score were better than those before treatment (P < 0.05), and the experimental group was superior to the control group (P < 0.05). This proves that Shashen Maidong decoction can effectively alleviate the cachexia syndromes, improve patients’ function condition and nutritional condition.
In lung cancer cachexia, significant increase of pro-inflammatory factor activity and inflammatory response occur, resulting in the cancerous lesions break down, metabolism reinforcement, and synthesis reduction. IL-6 is an inflammatory medium synthesized and released by lymphocytes and alveolar macrophages. TNF-α is an important factor that triggers the inflammatory response, causes inflammatory cell exudation, and generates and aggravates inflammatory response. According to clinical research [13], Shashen Maidong decoction can effectively regulate immunoglobulin (Sig) A and IL-6 levels, protect patients’ respiratory system, reduce pneumonia rats inflammatory cell infiltration, and lower serum IL-6 and TNF-α levels. The authors found that there were no significant differences between the two groups in serum TNF-α and IL-6 levels (P > 0.05) before treatment. After treatment, the experimental group’s serum TNF-α and IL-6 levels were lower than those of the control group (P < 0.05). The results strongly supported the above mentioned finding, and it proves that Shashen Maidong decoction can effectively lower body’s inflammatory cytokines levels.
We thus recommend that cancer toxicity theory is beneficial to clinical treatment of malignant tumor. In view of this theory, the application of Shashen Maidong Decoction on treating lung cancer cachexia has meaningful outcome.
Disclosure of conflict of interest
None.
References
- 1.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Soff G. Thrombosis and hemostasis in cancer. Scope of the problem and overview. Cancer Treat Res. 2019;179:1–9. doi: 10.1007/978-3-030-20315-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, Canin B, Cohen HJ, Holmes HM, Hopkins JO, Janelsins MC, Khorana AA, Klepin HD, Lichtman SM, Mustian KM, Tew WP, Hurria A. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018;36:2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng S, Lin Z, Wang Y, Wang Z, Li P, Zheng Y. Psoriasis therapy by Chinese medicine and modern agents. Chin Med. 2018;13:16. doi: 10.1186/s13020-018-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26:72–80. doi: 10.1007/s11655-019-3064-0. [DOI] [PubMed] [Google Scholar]
- 6.Curry JL, Torres-Cabala CA, Kim KB, Tetzlaff MT, Duvic M, Tsai KY, Hong DS, Prieto VG. Dermatologic toxicities to targeted cancer therapy: shared clinical and histologic adverse skin reactions. Int J Dermatol. 2014;53:376–84. doi: 10.1111/ijd.12205. [DOI] [PubMed] [Google Scholar]
- 7.Pak VN. α-fetoprotein-binding toxins and teratogens against cancer. Ther Deliv. 2019;10:1–3. doi: 10.4155/tde-2018-0068. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, McClees SF, Afaq F. Pomegranate for prevention and treatment of cancer: an update. Molecules. 2017;22:177. doi: 10.3390/molecules22010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sang T, Qiu W, Li W, Zhou H, Chen H, Zhou H. The relationship between prevention and treatment of colorectal cancer and cancerous toxin pathogenesis theory basing on gut microbiota. Evid Based Complement Alternat Med. 2020;2020:7162545. doi: 10.1155/2020/7162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien SM, Klampatsa A, Thompson JC, Martinez MC, Hwang WT, Rao AS, Standalick JE, Kim S, Cantu E, Litzky LA, Singhal S, Eruslanov EB, Moon EK, Albelda SM. Function of human tumor-infiltrating lymphocytes in early-stage non-small cell lung cancer. Cancer Immunol Res. 2019;7:896–909. doi: 10.1158/2326-6066.CIR-18-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Zhou Y. Shashen-maidong decoction-mediated IFN-γ and IL-4 on the regulation of Th1/Th2 imbalance in RP rats. Biomed Res Int. 2019;2019:6012473. doi: 10.1155/2019/6012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng P, Li J, Chen Y, Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog Mol Biol Transl Sci. 2019;163:423–444. doi: 10.1016/bs.pmbts.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui X, Zhang M, Han X, Zhang R, Chen L, Liu Y, Xiang Y, Xie T. Combination of traditional Chinese medicine and epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of non-small cell lung cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20683. doi: 10.1097/MD.0000000000020683. [DOI] [PMC free article] [PubMed] [Google Scholar]


