Abstract
Objective: This research aimed to study the impact and regulatory mechanism of Trem1 in spinal cord ischemia-reperfusion injury (SCIRI). Method: Temporary aortic cross clamp followed by reperfusion was used to establish SCIRI mice model. Mice motion function was estimated by Basso, Beattie, Bresnahan (BBB) score. Spinal cord infract zone was analyzed by HE and TUNEL staining. High throughput sequencing was performed to explore potential target for SCIRI. N2a cells were used to simulate the pathophysiological process of SCIRI in vitro with oxygen-glucose-serum deprivation/restoration (OGSD/R). RT-PCR and Western blot were token to determine mRNA and protein expression levels. Knockdown of Trem1 was performed with siRNA transfection in vitro and shRNA adenovirus injection in vivo. The relationship between Trem1 and SYK was analyzed by immunoprecipitation and immunofluorescence. Result: We observed that neuronal apoptosis of spinal cord was aggravated after SCIRI. Trem1 expression was dramatically upregulated as shown by high throughput sequencing, RT-PCR and Western blot results. Furthermore, Trem1 triggered apoptosis of N2a cells induced by OGSD/R, and knockdown of Trem1 by siRNAs blocked apoptosis via PI3K/AKT and NF-κB signaling pathway by interacting with SYK. In addition, we found that intrathecal injection of adenovirus with Trem1 shRNA could downregulate SYK and inhibit neuron apoptosis caused by SCIRI in vivo. Conclusion: Trem1 interacts with SYK and mediates neuronal apoptosis via the PI3K/AKT and NF-κB signaling pathway. Trem1 may be a therapeutic candidate for patients with SCIRI.
Keywords: Spinal cord ischemia-reperfusion injury, Trem1, apoptosis, SYK, PI3K/AKT, NF-κB
Introduction
Spinal cord ischemia (SCI) brings about the most acute spinal cord injury and calls for high priority in clinical treatment [1]. SCI can lead to quadriplegia, incontinence, respiratory paralysis and even death [2]. It is responsible for the peripheral nervous system complication after aortic and spine surgery, with a reported occurrence ranging from 2.4% to 40% [3]. Efforts to avoid SCI include establishment of temporary shunts or Cardio-Pulmonary Bypass [4], pretreatment with cerebrospinal fluid drainage [5], ischemic preconditioning and pharmacotherapy [6]. Nevertheless, all these methods are unsatisfactory in preventing spinal cord from injury [7].
Reperfusion plays an important role in the main treatment for SCI [8]. However, there is growing evidence demonstrating that spinal cord ischemia-reperfusion injury (SCIRI) can induce the production of free radical, toxin, inflammatory factors, and ER stress enhancement, causing cell apoptosis and severe lower limb neurologic defect [9,10]. Both traumatic and non-traumatic SCIRI can lead to elevated ROS concentration and imbalance of redox homeostasis, deteriorating spinal cord condition [11].
Although there is enough evidence demonstrating that neuronal apoptosis is of great significance for protection and functional recovery of nerve cells after spinal cord ischemia-reperfusion [12,13], excessive apoptosis results in irressible injury. Clearly, the mechanisms of neuronal apoptosis and functional recovery after SCIRI deserve attention [14].
In this study, we analyzed the mRNA profile of C57BL/6J mice in sham and SCIRI mice model. Interestingly, we found a candidate target, the triggering receptor expressed on myeloid cells-1 (Trem1), that mediates neuronal apoptosis via interaction with SYK. We validated Trem1 function in OGSD/R N2a cell model. In addition, spinal cord neuronal apoptosis in SCIRI mice was significantly decreased after Trem1 knockdown with adenovirus. Trem1 may be a potential therapeutic target for SCIRI.
Methods
Animals
Adult male C57BL/6J mice (20-25 g) were purchased from Model Animal Research Center of Nanjing University and raised in separate cages with light to dark cycle (each part 12 h). The animals were allowed ad libitum to food and water. Second Military Medical University Animal Care Committee gave the permission to all experiments. All the procedures were in accordance with the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals. At least six mice were analyzed for each measurement index.
Cell culture and treatments
N2a cells were cultured in DMEM containing 10% FBS. For transfection, N2a cells were transfected with the indicated Trem1 siRNAs (Trem1-si1: GCCAGACUUUGACAGUGAA; Trem1-si2: CCAUGCUACAAGUUCAAAU; Trem1-si3: CCCAGUGACACAACUACAA) using Lipofectamine 3000 (Invitrogen) in accordance with the manufacturer’s instructions. Briefly, N2a cells with 70-80% confluency were transfected with Lipofectamine 3000 and 10 nM siRNAs. 48 h later after transfection, cells were subjected for qPCR, immunoblotting or TUNEL staining.
OGSD/R model establishment
N2a cells at logarithmic phase were plated into 12-well plates (1 × 105/well) with complete culture medium. Culture medium was removed completely next day, and the cells were cleaned twice with D-hank’s buffer (pH 7.4). Then, the N2a cells were transferred to apotrophic environment by sugar-free 1640 culture medium without serum. The culture plate was incubated in an anaerobic chamber containing 5% CO2 and 95% N2 at 37°C for 12 h to achieve oxygen-glucose-serum deprivation (OGSD). OGSD and restoration (OGSD/R) were performed with indicated times.
SCIRI mice model
40 male C57BL/6 mice were evenly divided into 2 groups: A Sham group (n=20) and a SCIRI model group (n=20). Mice in sham group underwent thoracic surgery with aortic arch exposed. Spinal cord of mice in SCIRI group were devascularized by cross clamping between the left common carotid artery and the left subclavian artery [15]. The blood supply was restored after 30 min. Basso, Beattie, Bresnahan (BBB) locomotor rating scale was used to assess the motion function of mice. BBB scores ranged from 0-21, representing no hindlimb movement to normal gait movement [16]. After recovering the blood-supply, BBB score was used to detect neural function at 0, 30, 60, 120, 180, 240, 300 and 360 min. After reperfusion with indicated times, the mice were euthanized and their T8-L3 spinal cord segments were isolated.
Intrathecal injection adenovirus with Trem1 shRNA
For in vivo verification test, 24 C57BL/6 mice were divided into 4 groups: a Sham group (n=6, injected with normal saline), a SCIRI group injected with normal saline (n=6), a SCIRI group injected with normal adenovirus (n=6) and a SCIRI group injected with adenovirus containing Trem1 shRNAs (Targeting sequence(F): CCATGCTACAAGTTCAAAT, Loop: CTCGAG, Targeting sequence(R) ATTTGAACTTGTAGCATGG). The titer of adenovirus was 1010 (PFU/ml). All mice were injected intrathecally with injection volume of 20 μl. After injection for 72 h, mice were all anaesthetized and the last three group received ischemia-reperfusion surgery. After reperfusion, neural functions were estimated by BBB score, then all the mice were euthanized and their T8-L3 spinal cord segments were isolated, which were subjected for TUNEL staining and WB.
Hematoxylin-eosin (HE) staining
All the spinal cord tissues were first fixed with 4% PFA. Then the tissues were washed, dehydrated, transparentized and immersed in wax. After successively sliced into 5-μm-thick slices, the paraffin-embedded sections were deparaffinized with xylene, graded ethanol, and mounted on slides for HE staining. Images were captured with an optical microscope.
TUNEL staining
TUNEL FITC Apoptosis Detection Kit (Vazyme) was used for TUNEL staining based on the manufacturer’s instructions. Briefly, the frozen spinal cord slices or N2a cells were fixed in 4% PFA at 4°C overnight. Then the samples were incubated with Proteinase K (20 µg/ml) for 5 min. After incubation with 1 × Equilibration Buffer for 30 min. the samples were treated with FITC-12-dUTP Labeling Mix and Recombinant TdT Enzyme for 1 h. The nucleus was stained with DAPI (2 μg/ml) for 10 min in dark. The TUNEL staining images were photographed with a fluorescence microscope.
Quantitative real-time PCR (qPCR)
Total RNAs were extracted from the treated spinal cord tissues and N2a cells by Trizol reagent (Invitrogen). The ratio of absorbance at 260 nm and 280 nm was detected by spectrophotometer to calculate the RNA concentration. 2 μg total RNA was reversely transcribed by using M-MLV Reverse Transcriptase (Promega) with random hexamer primers to synthesize single-stranded complementary DNA (cDNA). The cDNA product was then amplified by using FastStart Universal SYBR Green Master (Roche) on Applied Biosystems 7900 Real-Time PCR Systems. The qPCR primers were (forward and reverse, respectively) 5’-CCTGTTGTGCTCTTCCATCCTG-3’ and 5’-GGGTTGTAGTTGTGTCACTGG-3’ for Trem1; 5’-CATCACTGCCACCCAGAAGACTG-3’ and 5’-ATGCCAGTGAGCTTCCCGTTCAG-3’ for GAPDH. The relative expression of targeted gene was normalized to GAPDH by using 2-ΔΔCt method.
Western blot
Total proteins were extracted and purified from spinal cord tissue or N2a cells using RIPA reagent (1% v/v NP-40, 20 mM Tris-HCL pH 7.4, 5 mM Sodium Pyrophosphate, 5 mM EDTA). The concentration of purified proteins was calculated using BCA Protein Assay Kit (Thermo). For western blot, 30 μg of total protein was separated with 12% SDS-polyacrylamide gel, then the protein was transferred to PVDF membrane (Millipore). The membrane was then subjected to blocking with 5% BSA for 1 h at room temperature, then incubation with primary antibodies against Trem1 (1:1000, Thermo), Caspase-3 (1:2000, CST), Bax (1:2000, Abcam), Bcl2 1:1000, (Abcam), SYK (1:1000, Thermo), pSYK (1:1000, Thermo), pAKT (1:1000, CST), PI3K (1:2000, CST), pP65 (1:1000, CST), TNF-α (1:1000, CST) or GAPDH (1:10000, Sigma) at 4°C overnight. After washing three times with 1X TBST buffer, the membrane was incubated with Peroxidase AffiniPure Goat Anti-Mouse or Goat Anti-Rabbit IgG secondary antibody (Sigma) (1:1000-5000). Finally, the targeted protein in membrane was visualized using an enhanced ECL kit (Thermo) and analyzed using the ImageJ software. The expression level of GAPDH was used as control.
Immunoprecipitation
All operations were performed on ice. The N2a cells were first washed with ice-cold PBS twice, then the cells were lysed with lysis buffer (50 mM Tris-HCl, pH 7.4 with 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100). After centrifugation at 12,000 rpm for 15 min at 4°C, the supernatants were incubated with anti-Trem1 or anti-SYK beads at 4°C for 12 h. Then, the beads were washed with TBS buffer (50 mM Tris-HCl, 150 mM NaCl, pH7.4) for 3 times. Lastly, the beads with antibody conjugated proteins were analyzed by western blot.
Immunofluorescence (IF)
Anti-Trem1 and Anti-SYK antibodies were diluted at 1:300 for immunofluorescence staining. Briefly, N2a cells were plated on slides and fixed with 4% PFA. After fixation, the slides were incubated with Anti-Trem1 or Anti-SYK antibodies at 4°C overnight. Then, fluorescent secondary antibodies (Goat anti-Mouse IgG (H+L), Superclonal Recombinant Secondary Antibody, Alexa Fluor 488 and Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Cyanine3, Thermo) were diluted at 1:1000 and incubated for 1 h. The nucleus was stained with DAPI. Fluorescence microscope was used to photograph.
Statistical analysis
Measurement data were shown as mean ± SD of at least three independent experiments. Comparison between groups was analyzed with two-tailed Student’s t-test. P<0.05 was considered statistically significant.
Results
Apoptosis was increased after SCIRI
To discover new targets for neuroinflammatory injury after SCIRI, a mouse SCIRI model was established. The BBB locomotor rating scale was used to independently assess mice’s hindlimb locomotion by a double-blind method. SCIRI mice got paraplegia in their rear limb after anesthesia recovery and gradually restored motor function after regaining blood supply. And the BBB score of SCIRI mice was markedly decreased after 180 min, due to delayed neuronal damage caused by reperfusion, while all of the mice in the sham group exhibited BBB scores up to 21 after 120 min (Figure 1A).
Figure 1.
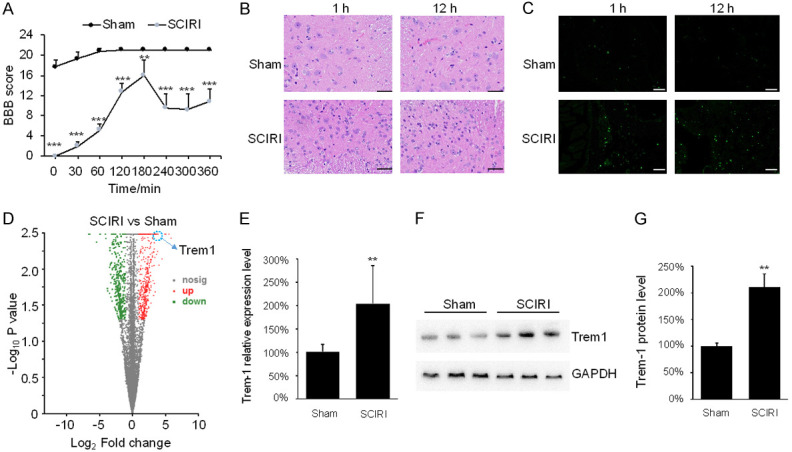
Neuronal apoptosis was increased and the expression level of Trem1 was upregulated after spinal cord ischemia reperfusion injury (SCIRI). (A) BBB score was used to evaluate mice motion after 30, 60 and 120 min of reperfusion in sham mice and SCIRI mice. ***P<0.001, **P<0.01. (B) HE staining was performed on slices from spinal cord specimens. Scare bar: 100 μm. (C) TUNEL staining was performed on slices from spinal cord specimens. Scare bar: 100 μm. (D) The gene expression profile in sham mice and SCIRI mice. (E) The mRNA level of Trem1 in sham mice and SCIRI mice was determined by qPCR. **P<0.01. (F) The protein level of Trem1 in sham mice and SCIRI mice was determined by WB. (G) Quantification of the Trem1 protein level in (F). **P<0.01.
HE and TUNEL stainings were conducted to confirm the neurological conditions of the spinal cord in SCIRI mice. In the sham group, cells with karyopyknosis were rarely observed in the ventricornu at 1 and 12 h (Figure 1B). Meanwhile, centrally located nucleus in the SCIRI group showed wide distribution and displayed morphological aggregation. Notably, TUNEL-positive cells were rare in the sham group, while FITC-stained positive cells could be seen in the SCIRI spinal cord at 1 h and 12 h after injury (Figure 1C). These data indicate that the apoptosis in spinal cord was increased after SCIRI.
The expression level of Trem1 was upregulated after SCIRI
Then, we performed RNA-seq analysis to determine the mRNA profile of spinal cord. 637 genes were upregulated, and 594 genes were downregulated in the SCIRI mice compared with the sham group (Figure 1D, Supplementary Material). Among these differentially expressed genes, we focused on Trem1, a cell surface receptor that potently induce cascade amplification to inflammatory responses by secretion of pro-inflammatory mediators [17]. To confirm the RNA-seq result, qPCR was performed. There was a substantial increase of the mRNA level of Trem1 after SCIRI (P<0.01) (Figure 1E). Western blotting results further demonstrated that Trem1 was significantly increased after SRICI (Figure 1F, 1G). These data claimed that Trem1 might be potential target for SCIRI.
Oxygen-glucose-serum deprivation/restoration (OGSD/R) cell model construction
To simulate pathophysiological process of SCIRI, we established a stable injury model with OGSD/R in N2a cells. N2a cells were incubated in an anaerobic chamber containing oxygen-free 5% CO2 and 95% N2 at 37°C for 12 h, then returned to the standard culture medium, and incubated for indicated times for recovery under the normoxia culture conditions. The TUNEL-positive cells increased after restoration and the apoptotic cells reached a peak at 1 h after restoration (Figure 2A), indicating that cell apoptosis induced by SCIRI reached the top at 1 h after reperfusion.
Figure 2.
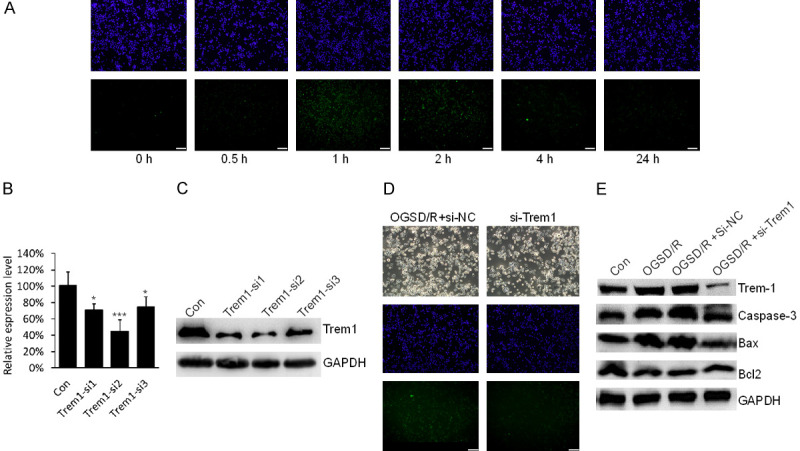
Trem1 triggered apoptosis of N2a cells induced by oxygen-glucose-serum deprivation/restoration (OGSD/R). A. Representative TUNEL-stained N2a cells after oxygen-glucose-serum deprivation/restoration (OGSD/R) with indicated time. Scare bar: 100 μm. B. The mRNA level of Trem-1 in N2a cells after siRNA transfection for 48 h was determined by qPCR. ***P<0.001, *P<0.05. C. The protein level of Trem-1 in N2a cells after siRNA transfection for 48 h was determined by WB. D. TUNEL staining of N2a cells after OGSD/R for 1 h with or without Trem1 siRNA transfection. Scale bar: 100 μm. E. Immunoblotting analysis of Trem1, Caspase-3, Bax and in response to OGD with or without Trem1 siRNA transfection.
Trem1 triggers apoptosis of N2a cells induced by OGSD/R
We next explored the function of Trem1 for SCIRI in OGSD/R N2a cell model. We selected a siRNA to efficiently knock down mRNA and protein level of Trem1. Trem1-si2 showed the best efficiency, so we chose the Trem1-si2 for the following research (Figure 2B, 2C). The apoptotic cells in OGSD/R were decreased when Trem1 was knocked down as determined by TUNEL staining (Figure 2D). In addition, under OGSD/R conditions, the Trem1 protein level was significantly increased, and Caspase-3 and Bax were upregulated, and Bcl2, known as apoptosis inhibitor, was down-regulated (Figure 2E). However, the protein levels of Caspase-3, Bax and Bcl2 were recovered when Trem1 was knocked down in OGSD/R N2a cells (Figure 3C). These data show that Trem1 was required for apoptosis of N2a cells induced by OGSD/R.
Figure 3.
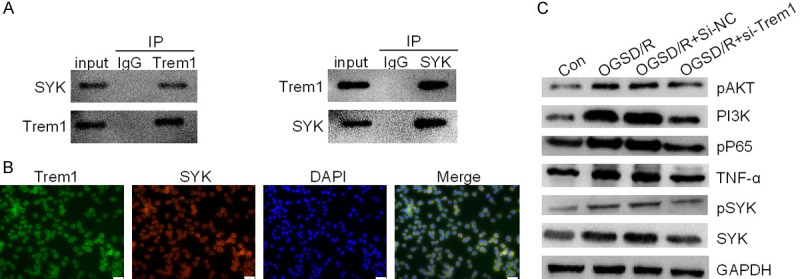
Trem1 activated the PI3K/AKT and NF-κB signaling pathway through interacting with SYK. A. Endogenous Trem1 interacted with exogenous SYK. B. Localization of Trem1 and SYK protein in N2a cells was examined by immunofluorescence assay. Scale bar: 100 μm. C. Protein levels of pAKT, PI3K, p-p65, TNF-α, p-SYK and SYK were detected by immunoblotting after OGSD/R.
Trem1 triggered the PI3K/AKT and NF-κB signaling pathway through interacting with SYK
Trem1, a cell surface receptor, modulates inflammatory responses via coupling with DAP12, then induces phosphorylation of DAP12 on its cytoplasmic immunoreceptor tyrosine-based activation motif and recruitment of the spleen tyrosine kinase (SYK), finally causing inflammatory cytokines and chemokines production [18]. This has been regarded as a launching point of inflammation after ischemic stroke [19]. Here, we found that Trem1 interacted with SYK endogenously with immunoprecipitation analysis (Figure 3A), which was further confirmed by their colocalization in the cell membrane and cytoplasm (Figure 3B). Moreover, the phosphorylation of SYK was significantly increased by OGSD/R in N2a cells (Figure 3C). The phosphorylation of SYK leads to activation of PI3K/AKT pathway and NF-κB pathway [20], therefore, we examined the expression level of PI3K, pAKT, pP65 and TNF-α. Interestingly, we found that OGSD/R treatment increased the expression of PI3K, pAKT, pP65 and TNF-α (Figure 3C). Down-regulation of Trem1 repressed the expression of PI3K, pAKT, pP65 and TNF-α. Therefore, our data suggested that Trem1 triggered the PI3K/AKT and NF-κB signaling pathway through interacting with SYK in OGSD/R N2a cells.
Trem1 mediates spinal cord neuronal apoptosis after SCIRI
To clarify the role of Trem1 in the process of SCIRI in vivo, we constructed Trem1 shRNA adenovirus to knock down the expression level of Trem1 in mice spinal code. After intrathecal injection of adenovirus with Trem1 shRNA for 5 days, mice were operated with SCIRI. Then we assessed the motion function with BBB score. The BBB score of SCIRI mice with Trem1 shRNA injection was significantly increased after 180 min (Figure 4A). Furthermore, knockdown of Trem1 effectively attenuated spinal cord neuronal apoptosis after SCIRI as determined with TUNEL staining (Figure 4B). Consistently, the expression levels of Caspase-3 and Bax were down-regulated, and Bcl2 was up-regulated in Trem1 shRNA group (Figure 4C). In addition, the expression levels of p-SYK, PI3K, pAKT, pP65 and TNF-α were substantially repressed in Trem1 shRNA adenovirus group (Figure 4D). These data demonstrated that Trem1 mediated spinal cord neuronal apoptosis after SCIRI via PI3K/AKT and NF-κB signaling pathway in vivo.
Figure 4.
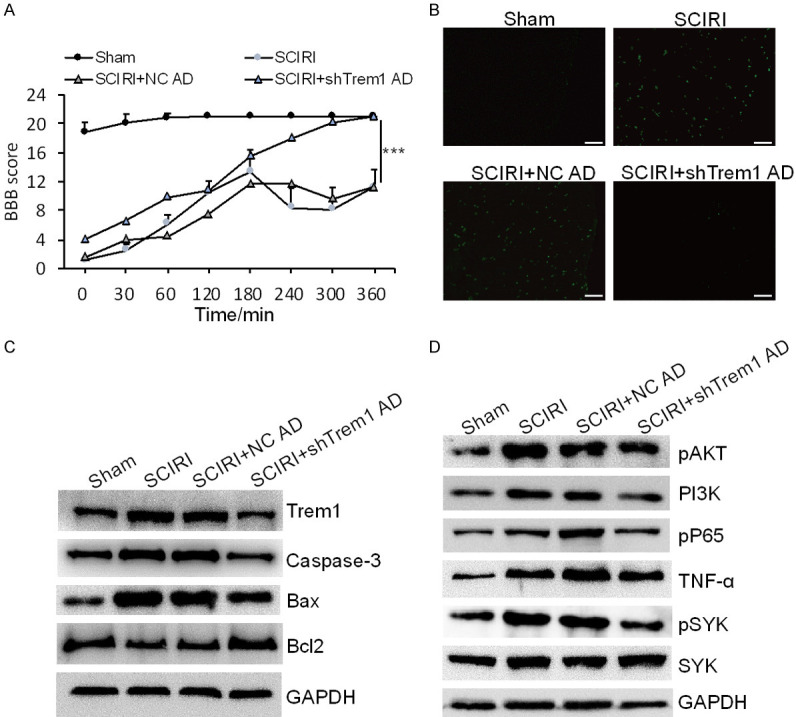
Knockdown of Trem1 ameliorated spinal cord neuronal apoptosis after SCIRI. A. BBB score evaluated mice motion after 30, 60 and 120 min of reperfusion in sham mice and SCIRI mice with or without Trem1 shRNA adenovirus. ***P<0.001. B. TUNEL staining was performed on slices from spinal cord specimens. Scale bar: 100 μm. C. Immunoblotting analysis of Trem1, Caspase-3, Bax and Bcl2 in sham mice and SCIRI mice with or without Trem1 shRNA adenovirus. D. Immunoblotting analysis of pAKT, PI3K, p-p65, TNF-α, p-SYK and SYK in sham mice and SCIRI mice with or without Trem1 shRNA adenovirus.
Discussion
Neuroinflammation and cellular apoptosis caused by SCIRI result in neurological dysfunction [21]. We found that spinal cord neuronal apoptosis was significantly increased after SCIRI. The expression of Trem1 was significantly increased in SCIRI spinal cord and OGSD/R N2a cell model. Moreover, Trem1 activated the PI3K/AKT and NF-κB signaling pathway via interacting with SYK. In addition, knockdown of Trem1 ameliorated spinal cord neuronal apoptosis after SCIRI via PI3K/AKT and NF-κB signaling pathway in vivo.
SCIRI often leads to irreversible neurological impairments, which may be associated with apoptosis induced by inflammation [22]. Proinflammatory cytokines induced by SCIRI could lead to neuronal apoptosis and even death [23]. Furthermore, complex pathological alterations were observed in reperfusion injury [24], confirming that early intervention in secondary damage makes huge difference to impede the aggressive apoptosis process [25].
Trem1 is a surface activating receptor expressed on neutrophils, monocytes, and macrophages, playing an essential role in amplifying inflammatory responses through regulation of pro-inflammatory mediators [17,26]. It has been reported that Trem1 was found on hepatocellular carcinoma cells. Therefore, it might work as a prognostic factor for cancer prevention [27]. Moreover, inhibition of Trem1 alleviates early brain injury after subarachnoid hemorrhage via downregulation of the signal pathway of p38MAPK/MMP-9 and conservation of ZO-1 [28]. Here we found that Trem1 was a key target for spinal cord neuronal apoptosis and inhibition of Trem1 may exert positive effect for therapies of SCIRI. Previous studies have shown that inhibition of Trem1 by shRNA in macrophages suppresses cancer cell invasion in vitro [29]. Moreover, disrupting Trem1 by the synthetic peptide blocker markedly downregulated the lipopolysaccharide, which caused upregulation of proinflammatory factors through inducible nitric oxide synthase (iNOS), cyclooxygenase-2, and NF-κB [30], and had a cancer type independent, therapeutically beneficial antitumor activity in pancreatic cancer [31].
We observed that knockdown of Trem1 ameliorated motion function and spinal cord neuronal apoptosis after SCIRI in vivo. Trem1 inhibition, via synthetic soluble TREM-1 protein mimickers, plays a role in protecting from inflammatory disorders. Meanwhile, this mimicry does not affect the normal antibacterial response [32]. Identifying specific TREM-1 ligands, which possess good biosafety, is essential to explore new avenues to SCIRI.
In conclusion, Trem1 mediates neuronal apoptosis in SCIRI mice and OGSD/R N2a cell model. Importantly, repression of PI3K/Akt and NF-κB signaling pathway via SYK could be crucial for the effects of Trem1 inhibition. Overall, Trem1 is likely to act as therapeutic candidate for patients with SCIRI.
Acknowledgements
Supported by grants from the general project of National Natural Science Fund of China: No. 81873764.
Disclosure of conflict of interest
None.
Abbreviations
- OGSD/R
Oxygen-glucose-serum deprivation/restoration
- SCIRI
Spinal cord ischemia-reperfusion injury
Supporting Information
References
- 1.Craig A, Tran Y, Middleton J. Psychological morbidity and spinal cord injury: a systematic review. Spinal Cord. 2009;47:108–114. doi: 10.1038/sc.2008.115. [DOI] [PubMed] [Google Scholar]
- 2.Parent S, Mac-Thiong JM, Roy-Beaudry M, Sosa JF, Labelle H. Spinal cord injury in the pediatric population: a systematic review of the literature. J Neurotrauma. 2011;28:1515–1524. doi: 10.1089/neu.2009.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coselli JS, LeMaire SA, Conklin LD, Koksoy C, Schmittling ZC. Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2002;73:1107–1115. doi: 10.1016/s0003-4975(02)03370-2. discussion 1115-1106. [DOI] [PubMed] [Google Scholar]
- 4.Flores J, Shiiya N, Kunihara T, Matsuzaki K, Yasuda K. Risk of spinal cord injury after operations of recurrent aneurysms of the descending aorta. Ann Thorac Surg. 2005;79:1245–1249. doi: 10.1016/j.athoracsur.2004.09.064. discussion 1249. [DOI] [PubMed] [Google Scholar]
- 5.Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, Gorelik S, Slobogean GP, Umedaly H, Giffin M, Nikolakis MA, Street J, Boyd MC, Paquette S, Fisher CG, Dvorak MF. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine. 2009;10:181–193. doi: 10.3171/2008.10.SPINE08217. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitani K, Kawaguchi M, Kawamata M, Kakinohana M, Kato S, Hasuwa K, Yamakage M, Yoshikawa Y, Nishiwaki K, Hasegawa K, Inagaki Y, Funaki K, Matsumoto M, Ishida K, Yamashita A, Seo K, Kakumoto S, Tsubaki K, Tanaka S, Ishida T, Uchino H, Kakinuma T, Yamada Y, Mori Y, Izumi S, Shimizu J, Furuichi Y, Kin N, Uezono S, Kida K, Nishimura K, Nakai M, Ohnishi Y. Cerebrospinal fluid drainage to prevent postoperative spinal cord injury in thoracic aortic repair. J Anesth. 2021;35:43–50. doi: 10.1007/s00540-020-02857-w. [DOI] [PubMed] [Google Scholar]
- 7.Fakhoury M. Spinal cord injury: overview of experimental approaches used to restore locomotor activity. Rev Neurosci. 2015;26:397–405. doi: 10.1515/revneuro-2015-0001. [DOI] [PubMed] [Google Scholar]
- 8.Gökce EC, Kahveci R, Gökce A, Cemil B, Aksoy N, Sargon MF, Kısa Ü, Erdoğan B, Güvenç Y, Alagöz F, Kahveci O. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine. 2016;24:949–959. doi: 10.3171/2015.10.SPINE15612. [DOI] [PubMed] [Google Scholar]
- 9.Talavera MM, Nuthakki S, Cui H, Jin Y, Liu Y, Nelin LD. Immunostimulated arginase II expression in intestinal epithelial cells reduces nitric oxide production and apoptosis. Front Cell Dev Biol. 2017;5:15. doi: 10.3389/fcell.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao L, Zhang Z, Xu W, Li T, Ying G, Qin B, Li J, Zheng J, Zhao T, Yan F, Zhu Y, Chen G. Natrium benzoate alleviates neuronal apoptosis via the DJ-1-related anti-oxidative stress pathway involving akt phosphorylation in a rat model of traumatic spinal cord injury. Front Mol Neurosci. 2019;12:42. doi: 10.3389/fnmol.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaki AM, El-Tanbouly DM, Abdelsalam RM, Zaki HF. Plumbagin ameliorates hepatic ischemia-reperfusion injury in rats: role of high mobility group box 1 in inflammation, oxidative stress and apoptosis. Biomed Pharmacother. 2018;106:785–793. doi: 10.1016/j.biopha.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Qi L, Jiang-Hua M, Ge-Liang H, Qing C, Ya-Ming L. MiR-34a inhibits spinal cord injury and blocks spinal cord neuron apoptosis by activating phatidylinositol 3-kinase (PI3K)/AKT pathway through targeting CD47. Curr Neurovasc Res. 2019;16:373–381. doi: 10.2174/1567202616666190906102343. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Xue P, Fu L, Zhang J, Jiang J, Guo X, Bao G, Xu G, Sun Y, Chen J, Cui Z. HAX1 is associated with neuronal apoptosis and astrocyte proliferation after spinal cord injury. Tissue Cell. 2018;54:1–9. doi: 10.1016/j.tice.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Su P, Pan Z, Liu D, Niu Y, Zhu W, Yao P, Song Y, Sun Y. Combination therapy with hyperbaric oxygen and erythropoietin inhibits neuronal apoptosis and improves recovery in rats with spinal cord injury. Phys Ther. 2019;99:1679–1689. doi: 10.1093/ptj/pzz125. [DOI] [PubMed] [Google Scholar]
- 15.Bell MT, Puskas F, Smith PD, Agoston VA, Fullerton DA, Meng X, Weyant MJ, Reece TB. Attenuation of spinal cord ischemia-reperfusion injury by specific α-2a receptor activation with dexmedetomidine. J Vasc Surg. 2012;56:1398–1402. doi: 10.1016/j.jvs.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Scheff SW, Saucier DA, Cain ME. A statistical method for analyzing rating scale data: the BBB locomotor score. J Neurotrauma. 2002;19:1251–1260. doi: 10.1089/08977150260338038. [DOI] [PubMed] [Google Scholar]
- 17.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki Y, Nakano Y, Mishiro K, Takagi T, Tsuruma K, Nakamura M, Yoshimura S, Shimazawa M, Hara H. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep. 2013;3:3177. doi: 10.1038/srep03177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Chen F, Fang B, Zhang Z, Dong Y, Tong X, Ma H. MiR-128-3p alleviates spinal cord ischemia/reperfusion injury associated neuroinflammation and cellular apoptosis via SP1 suppression in rat. Front Neurosci. 2020;14:609613. doi: 10.3389/fnins.2020.609613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu J, Sun H, Zhang Y, Xu W, Wang C, Fang Y, Zhao J. Neuroprotective effects of luteolin against spinal cord ischemia-reperfusion injury by attenuation of oxidative stress, inflammation, and apoptosis. J Med Food. 2018;21:13–20. doi: 10.1089/jmf.2017.4021. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Yao Y, He R, Meng Y, Li N, Zhang D, Xu J, Chen O, Cui J, Bian J, Zhang Y, Chen G, Deng X. Methane ameliorates spinal cord ischemia-reperfusion injury in rats: antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic Biol Med. 2017;103:69–86. doi: 10.1016/j.freeradbiomed.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Xie L, Wang Z, Li C, Yang K, Liang Y. Protective effect of nicotinamide adenine dinucleotide (NAD(+)) against spinal cord ischemia-reperfusion injury via reducing oxidative stress-induced neuronal apoptosis. J Clin Neurosci. 2017;36:114–119. doi: 10.1016/j.jocn.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Sun H, Wei H, Dong M, Zhang Y, Xu W, Fang Y, Zhao J. Astaxanthin alleviates spinal cord ischemia-reperfusion injury via activation of PI3K/Akt/GSK-3β pathway in rats. J Orthop Surg Res. 2020;15:275. doi: 10.1186/s13018-020-01790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boufenzer A, Lemarié J, Simon T, Derive M, Bouazza Y, Tran N, Maskali F, Groubatch F, Bonnin P, Bastien C, Bruneval P, Marie PY, Cohen R, Danchin N, Silvestre JS, Ait-Oufella H, Gibot S. TREM-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ Res. 2015;116:1772–1782. doi: 10.1161/CIRCRESAHA.116.305628. [DOI] [PubMed] [Google Scholar]
- 27.Duan M, Wang ZC, Wang XY, Shi JY, Yang LX, Ding ZB, Gao Q, Zhou J, Fan J. TREM-1, an inflammatory modulator, is expressed in hepatocellular carcinoma cells and significantly promotes tumor progression. Ann Surg Oncol. 2015;22:3121–3129. doi: 10.1245/s10434-014-4191-7. [DOI] [PubMed] [Google Scholar]
- 28.Sun XG, Duan H, Jing G, Wang G, Hou Y, Zhang M. Inhibition of TREM-1 attenuates early brain injury after subarachnoid hemorrhage via downregulation of p38MAPK/MMP-9 and preservation of ZO-1. Neuroscience. 2019;406:369–375. doi: 10.1016/j.neuroscience.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Sigalov AB. A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock. Int Immunopharmacol. 2014;21:208–219. doi: 10.1016/j.intimp.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng CW, Chen NF, Sung CS, Kuo HM, Yang SN, Chen CL, Hung HC, Chen BH, Wen ZH, Chen WF. Therapeutic effect of modulating TREM-1 via anti-inflammation and autophagy in Parkinson’s disease. Front Neurosci. 2019;13:769. doi: 10.3389/fnins.2019.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen ZT, Sigalov AB. Novel TREM-1 inhibitors attenuate tumor growth and prolong survival in experimental pancreatic cancer. Mol Pharm. 2017;14:4572–4582. doi: 10.1021/acs.molpharmaceut.7b00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81–95. doi: 10.1016/j.pharmthera.2017.02.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


