Abstract
Objective: To evaluate the effect of combination therapy of meridian acupuncture and massage on motor development in children with spastic cerebral palsy (SCP). Methods: A total of 113 children with SCP in our hospital were allocated into research group (63 cases, treated with meridian acupuncture plus massage) and control group (50 cases, treated with routine rehabilitation measures). Clinical efficacy and alterations of inflammatory factors were observed. Peabody Developmental Motor Scale (PDMS) and gross motor function measure (GMFM-88; sitting, standing, walking) were employed for the assessment of motor ability. Changes in muscle tension were monitored with the Ashworth scale (AS), and modified Barthel index (MBI) and Gesell’s Developmental Schedule (GDS) were used to evaluate children’s daily activities, language, fine motor skills, and adaptability. Finally, the development of children in the two groups was monitored. Results: The research group had higher total effective rate than the control group (P=0.018). After treatment, the levels of interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) in the research group were lower than those in the control group (P<0.05); the PDMS and AS scores were reduced in both groups, and the reduction was greater in research group (P<0.05); GFMF-88, Barthel and GDS scores increased in both groups, especially in the research group; children in research group were better developed than those in control group (P<0.05). Conclusion: Meridian acupuncture plus massage contributes to a significant improvement of motor development in children with SCP.
Keywords: Meridian acupuncture plus massage, spastic cerebral palsy in children, efficacy, motor development
Introduction
Cerebral palsy (CP), a permanent non-progressive developmental disorder, may result in non-progressive brain injury during fetal or infant development [1]. It is classified according to the degree of motor disturbance and the site of involvement, and ataxia, athetosis, spasm and hypotonia are the most common types [2], accounting for 60-70% of children with CP [3]. CP is characterized by abnormal muscle tension and motor skills induced by the damage to the developing brain [4], thereby leading to muscle weakness, spasm and motor disorders, as well as loss of muscular balance and coordination, ultimately restricting daily activities [5]. Children with spastic cerebral palsy (SCP) are prone to abnormal posture and joint deformity due to long-term limb spasm [6], threatening their growth and quality of life. Although several risk factors, including placental abruption, intrauterine infection, parents’ bad habits, and heredity, have been reported clinically [7], the heterogeneity of SCP makes the pathogenesis unclear and leads to the lack of effective prevention measures [8].
At present, the diagnosis of CP is based on the clinical manifestations including motor patterns. Western medicine, such as drugs for promoting neuron recovery, is usually used, followed by motor rehabilitation training, so as to alleviate limb dyskinesia [9]. Surgical treatments are available in special conditions, but their clinical efficacy is limited and the therapeutic effect remains disappointing [10]. Traditional Chinese medicine (TCM), a discipline that studies human physiology, and pathology, as well as diagnosis and prevention of diseases, has attracted considerable attention in the treatment of CP [11]. There is evidence that acupuncture, massage and other TCM methods can effectively promote the dredging of meridians and relieve spasm [12]. As pointed out by Lee et al., meridian acupuncture upregulates the positive expression of nerve growth factor [13]. Also, Ghafoor et al. reported that acupuncture therapy is convenient and efficient, and its combination with massage is able to enhance nerve recovery [14]. Therefore, we wonder whether this combination therapy may also have a favorable effect for children with SCP. To verify this, we investigated the efficacy of meridian acupuncture plus massage and its influence on the development of children with SCP. The present study aims to summarize superior treatments and provide reference and guidance for improving treatment effect, and in addition, enhance the quality of life and reduce the economic burden of the family and the society.
General data
A total 113 children with SCP treated in Guiyang Maternal and Child Health Hospital from August, 2017 to September, 2019 were allocated into research group (63 cases, treated with meridian acupuncture plus massage) and control group (50 cases, treated with routine rehabilitation measures). The experiment was approved by the ethics committee of our hospital and all participants signed the informed consent.
Inclusion and exclusion criteria
The children included were diagnosed with SCP by our doctors with reference to standards of Chinese guidelines for rehabilitation of cerebral palsy (2015) [15], laboratory and imaging tests, and were subsequently treated in our hospital. Family members of the children aged from 1 to 5 years were informed of this study and agreed to cooperate. Children with other congenital diseases, other developmental abnormalities, incomplete clinical data and other types of cerebral palsy were excluded.
Treatment procedures
Control group: The patients were given routine rehabilitation measures. After a series of examinations, they were given baclofen tablets (2.5 mg/time, 4 times/d) and intravenous injection of ganglioside (20 mg/time) containing glucose injection at 0.05 g/mL or sodium chloride injection. Targeted treatments such as transcutaneous electrical nerve stimulation or neuromuscular stimulation were adopted as appropriate. Rehabilitation training was performed according to Bobath’s exercise therapy, twice a day, 30 min/time, for three consecutive months.
Research group: First, the patients were treated with scalp acupuncture at motor area, balance area, sensory area, tremor-control area, foot motor-sensory area, vision area, speech area (II and III), Baihui and Sishencong. Following disinfection of the acupoints, acupuncture was performed according to patient’s condition (Huatuo filiform needles with a diameter of 30-60 mm and a length of 25 mm). Ten needles were punctured in parallel and retained for 30-60 min (rotated 5 times). In body acupuncture, conception vessel, governor vessel, hand-Yangming, hand-Jueyin, foot-Yangming and foot-taiyin were selected. Children lied in supine position, and the selected meridians were punctured every 10 mm from top to bottom, with a depth of 10 mm. The needles were not retained. Massage therapy took governor vessel, bladder meridian and large intestine meridian as main meridians. Massage techniques, such as rolling, kneading, patting and pushing, were used to press the main peripheral acupoints along the meridians. Meanwhile, massage was performed around the scapula, sternocleidomastoid muscle, limb muscle, and lumbar and gluteal muscles to relax the spasm muscles. The massage was performed 5 times per week, 1 time/d, 20 min/time, for three consecutive months.
Outcome measures
Main outcome measures: Treatment efficacy was observed. The Peabody Developmental Motor Scale (PDMS, 26-item grasping and 72-item visual-motor integration subtests) was used to evaluate fine and gross motor skills. Moreover, gross motor function measure (GMFM-88, the total score ranges from 0 to 264) was employed for the assessment of motor function (sitting, standing, and walking). The scores are positively correlated with the motor function. Also, the development (normal development, critical state, and delayed development) of children in the two groups was monitored.
Secondary outcome measures: Levels of inflammatory factors interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) were quantified by ELISA. Changes in muscle tension were monitored with the Ashworth scale (AS, Grade I-IV, higher scores indicate increased muscular tension) [19]. Children’s daily activities, language, fine motor skills, and adaptability were assessed by modified Barthel index (MBI, the total score ranges from 0-100, the lower the score, the more severe the disability), Gesell’s Developmental Schedule (GDS, the higher the score the better the language ability) [20,21], and other scales.
Statistical methods
All the statistical analyses were carried out with SPSS24.0, and Chi-square test was used for between-group comparisons. The continuous data were expressed by mean ± standard deviation. Independent samples t test was used for between-group comparisons, and one-way ANOVA and LSD post-hoc test were used for multi-group comparisons. Values of P<0.05 indicated statistically significant differences.
Results
General data
There was no difference in age, sex, BMI, living environment, parental history of smoking, parental history of alcohol drinking, family medical history, nationality and delivery mode between the two groups (P>0.05) (Table 1).
Table 1.
General data [n (%)]
| Research group (n=63) | Control group (n=50) | t or χ2 | P | |
|---|---|---|---|---|
| Age (years) | 3.8±1.8 | 3.5±1.9 | 0.859 | 0.392 |
| Sex | 0.085 | 0.771 | ||
| Male | 26 (41.27) | 22 (44.00) | ||
| Female | 37 (58.73) | 28 (56.00) | ||
| BMI (kg/cm2) | 13.52±3.05 | 13.76±4.72 | 0.327 | 0.744 |
| Living environment | 0.266 | 0.606 | ||
| Rural | 42 (66.67) | 31 (62.00) | ||
| Urban | 21 (33.33) | 19 (38.00) | ||
| Parental history of smoking | 0.724 | 0.125 | ||
| Yes | 46 (73.02) | 35 (70.00) | ||
| No | 17 (26.98) | 15 (30.00) | ||
| Parental history of alcohol drinking | 0.226 | 0.635 | ||
| Yes | 43 (68.25) | 32 (64.00) | ||
| No | 20 (31.75) | 18 (36.00) | ||
| Family medical history | 0.156 | 0.693 | ||
| Yes | 18 (28.57) | 16 (32.00) | ||
| No | 45 (71.43) | 34 (68.00) | ||
| Delivery mode | 0.225 | 0.635 | ||
| Vaginal delivery | 28 (44.44) | 20 (40.00) | ||
| Caesarean section | 35 (55.56) | 30 (60.00) | ||
| Nationality | 1.080 | 0.299 | ||
| Han nationality | 58 (92.06) | 43 (86.00) | ||
| Minority nationalities | 5 (7.94) | 7 (14.00) |
Clinical efficacy after treatment
Evaluations of clinical efficacy demonstrated that the treatment was markedly effective in 32 children (50.79%), effective in 26 (41.27%), and ineffective in 5 (7.94%), with a total effective rate of 92.06% in research group; whereas the corresponding numbers in the control group were 15 (30.00%), 23 (46.00%), and 12 (24.00%), with an overall response rate of 76.00%. Therefore, the research group had a higher overall response rate than the control group (P=0.018) (Table 2).
Table 2.
Clinical efficacy after treatment
| Research group (n=63) | Control group (n=50) | χ2 | P | |
|---|---|---|---|---|
| Markedly effective | 32 (50.79) | 15 (30.00) | ||
| Effective | 26 (41.27) | 23 (46.00) | ||
| Ineffective | 5 (7.94) | 12 (24.00) | ||
| Overall response rate | 58 (92.06) | 38 (76.00) | 5.628 | 0.018 |
Alterations of inflammatory factors before and after treatment
There was no significant difference in levels of inflammatory factors (IL-6 and TNF-α) between the two groups before treatment (P>0.05), but a significant decrease was seen in both groups after treatment, especially in the research group (P<0.05) (Figure 1).
Figure 1.
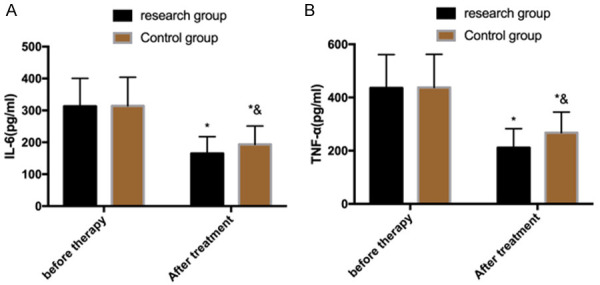
Alterations of inflammatory factors before and after treatment. A. Alterations of inflammatory factor IL-6 before and after treatment. B. Alterations of inflammatory factor TNF-α before and after treatment. Note: *P<0.05 vs. before treatment, &P<0.05 vs. research group. IL-6: interleukin 6; TNF-α: tumor necrosis factor-α.
Assessment of motor ability before and after treatment
The PDMS was used to evaluate the motor ability of children before and after treatment. The results showed that there was no significant difference between the two groups before treatment (P>0.05); the scores reduced in both groups after treatment, and the reduction was greater in the research group (P<0.05) (Figure 2).
Figure 2.
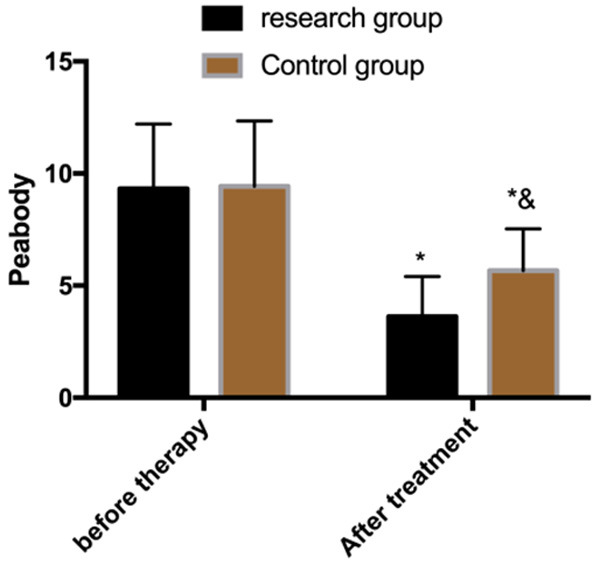
Assessment of motor ability before and after treatment. Note: *P<0.05 vs. before treatment, &P<0.05 vs. research group.
Evaluation of gross motor function before and after treatment
The GMFM-88 compared the gross motor function in sitting, standing and walking positions between the two groups before and after treatment. It turned out that there was no significant difference between the two groups before treatment (P>0.05), but the scores increased in both groups after treatment, and the increase was greater in the research group (P<0.05) (Figure 3).
Figure 3.
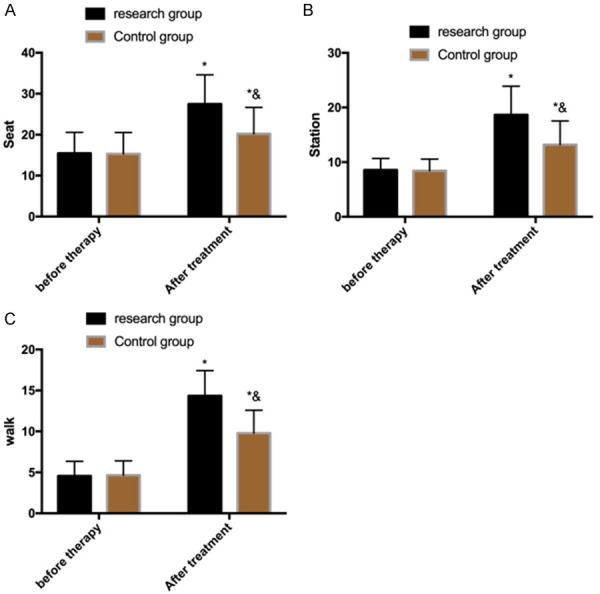
Evaluation of gross motor function before and after treatment. A. Scores of gross motor function in a sitting position. B. Scores of gross motor function in a standing position. C. Scores of gross motor function in a walking position. Note: *P<0.05 vs. before treatment, &P<0.05 vs. research group.
Changes in muscle tension before and after treatment
The AS showed that there was no significant difference in scores of muscle tension between the two groups before treatment (P>0.05), but the scores decreased in both groups after treatment, and the research group was lower than the control group (P<0.05) (Figure 4).
Figure 4.
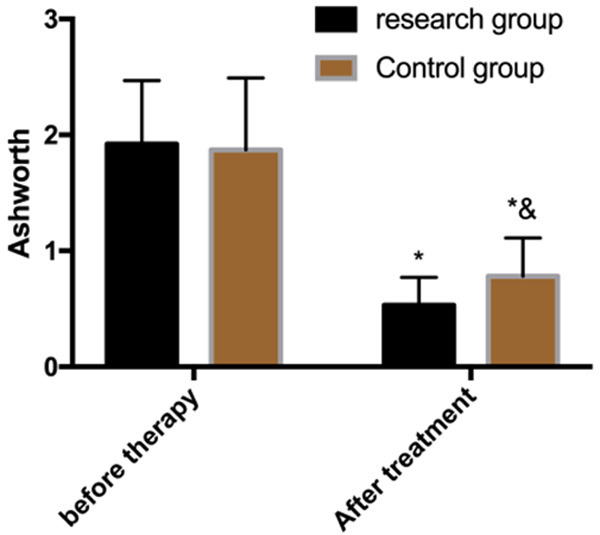
Changes in muscle tension before and after treatment. Note: *P<0.05 vs. before treatment, &P<0.05 vs. research group.
Comparison of scores of MBI and GDS
The MBI and GDS scores revealed that no significant differences in language, fine motor skills and adaptability were observed between the two groups before treatment (P>0.05), but after treatment, the scores increased, and the scores in the research group were higher than those in the control group (P<0.05) (Figure 5).
Figure 5.
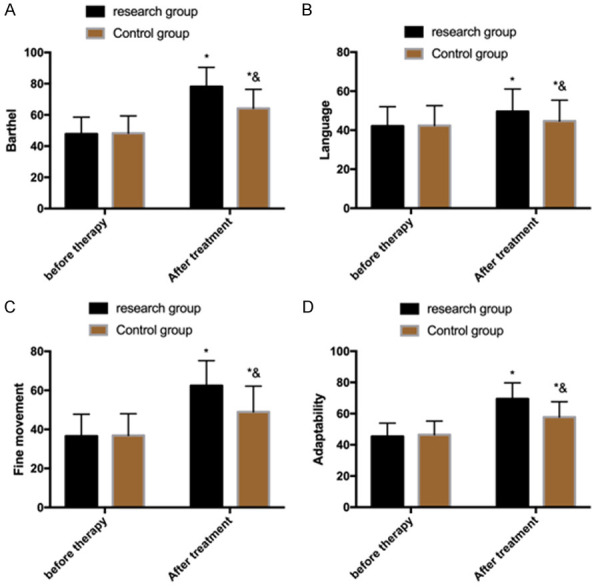
Comparison of scores of MBI and GDS. A. MBI scores in the two groups. B. Language scores of children in the two groups. C. Scores of fine motion skills of children in the two groups. D. Adaptability score of children in the two groups. Note: *P<0.05 vs. before treatment, &P<0.05 vs. research group. MBI: modified Barthel index; GDS: Gesell’s Developmental Schedule.
Development of children after treatment
After treatment, the research group reported more normally developing children than the control group (P=0.008), and developmental delay occurred less frequently (P=0.023). Children in the research group were better developed than those in the control group (P<0.05) (Table 3).
Table 3.
Development of children after treatment
| Research group (n=63) | Control group (n=50) | χ2 | P | |
|---|---|---|---|---|
| Normal development | 36 (57.14) | 16 (32.00) | 7.094 | 0.008 |
| Critical state | 24 (38.10) | 25 (50.00) | 1.609 | 0.205 |
| Developmental delay | 3 (4.76) | 9 (18.00) | 5.147 | 0.023 |
Discussion
CP is the most common, severe and costly dyskinesia in childhood [22]. It has always been the focus of social public health and prevention. Because of its complex etiology, there are still controversies about its treatment [23]. It has been reported that 40% of the affected children can’t walk independently, one third develop epilepsy, and most suffer different degrees of cognitive impairment, seriously endangering healthy development of children and causing a heavy burden on the family and the society [24]. The incidence of CP has been increasing with social development and changes in living habits [25]. Therefore, in addition to reducing the risk factors, it is also important to refine and optimize its treatments. TCM has been widely used in clinical practice and made remarkable achievements [26]. We hypothesize that it may contribute to the treatment of CP in children. TCM believes that CP is mainly induced by obstruction of channels and collateral, and phlegm obstructing orifices [27]. The present study focuses on the clinical efficacy of acupuncture plus massage and its influence on motor development in children with SCP. The results were as follows:
First of all, evaluations of clinical efficacy demonstrated that the treatment was markedly effective in 32 children, effective in 26 and ineffective in 5, with a total effective rate of 92.06% in the research group; whereas the corresponding numbers in the control group were 15, 23, and 12, with a total effective rate of 76.00%. Therefore, the research group had higher total effective rate than the control group. It is suggested that meridian acupuncture plus massage effectively improves the clinical efficacy and has high safety. Acupuncture needles that are inserted into specific meridians at specific angles correct energy imbalance in the body and restore internal dynamic balance [28]. Massage is a series of orderly movements carried out in different parts of the body in a coordinated manner for specific aims, so as to relieve the pressure on muscles and internal organs, and stimulate blood circulation [29]. We suspect that the high efficacy of the combination therapy of meridian acupuncture and massage may be a result of targeted dredging of meridians and relaxation of muscle spasm, thereby more directly improving children’s motor disability and intelligence. Li et al. [30] states that meridian acupuncture is able to enhance the upper limb function of children with spastic hemiplegia cerebral palsy. In addition, in the study of Guo et al. [31], massage is reported to promote blood circulation and improve nerve function of children with CP. All of these support the results of this study. Second, we found that the levels of IL-6 and TNF-α decreased in both groups after treatment, especially in the research group, indicating that acupuncture plus massage downregulates the levels of inflammatory factors and increases treatment efficacy. Inflammatory factors often lead to abnormal blood-brain barrier and brain damage in patients with CP [32]. Those decreased levels may be achieved by enhanced cerebral microcirculation after meridian acupuncture and massage, which reduces inflammatory factors through meridian dredging. Third, Peabody motor development scale was used to evaluate the motor ability of children before and after treatment; the results showed that the scores reduced in both groups after treatment, and the reduction was greater in the research group. The GMFM-88 compared the gross motor function in sitting, standing and walking positions between the two groups before and after treatment. It turned out that the scores increased in both groups, and the increase was greater in the research group The AS showed that the scores of muscle tension in research group were lower than those in the control group after treatment. The MBI and GDS revealed that the scores of language, fine motor skills and adaptability increased after treatment, and the research group was higher than the control group. The above findings suggest that the combination therapy significantly accelerates the recovery of motor function, intelligence and language skills, as well as alleviates muscle spasm and promotes children’s development. Meridian acupuncture has the effect of dilating blood vessels, promoting circulation, activating cells and restoring impaired function by acupuncture at major nerve points in the brain and body [33], Finally, the development of children in the two groups after treatment was observed. The research group reported more normally developing children than the control group, and developmental delay occurred less frequently. Children in the research group were better developed than those in the control group. It further supports our findings, and indicates the high practical value of the combination therapy. However, due to the small sample size, selection of a statistical analysis of a large amount of data is unfeasible; therefore bias cannot be ruled out. In addition, the short experimental period does not allow us to evaluate the long-term prognosis of the patients. More detailed studies and analyses will be conducted to address the above limitations.
To sum up, meridian acupuncture plus massage contributes to a significant improvement of motor development in children with SCP.
Disclosure of conflict of interest
None.
References
- 1.Gulati S, Sondhi V. Cerebral palsy: an overview. Indian J Pediatr. 2018;85:1006–1016. doi: 10.1007/s12098-017-2475-1. [DOI] [PubMed] [Google Scholar]
- 2.Crowgey EL, Marsh AG, Robinson KG, Yeager SK, Akins RE. Epigenetic machine learning: utilizing DNA methylation patterns to predict spastic cerebral palsy. BMC Bioinformatics. 2018;19:225. doi: 10.1186/s12859-018-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinnon C, White J, Morgan P, Harvey A, Clancy C, Fahey M, Antolovich G. Clinician perspectives of chronic pain management in children and adolescents with cerebral palsy and dyskinesia. Phys Occup Ther Pediatr. 2021;41:244–258. doi: 10.1080/01942638.2020.1847236. [DOI] [PubMed] [Google Scholar]
- 4.Whitney DG, Singh H, Miller F, Barbe MF, Slade JM, Pohlig RT, Modlesky CM. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 2017;94:90–97. doi: 10.1016/j.bone.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park EY, Kim WH. Effect of neurodevelopmental treatment-based physical therapy on the change of muscle strength, spasticity, and gross motor function in children with spastic cerebral palsy. J Phys Ther Sci. 2017;29:966–969. doi: 10.1589/jpts.29.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surana BK, Ferre CL, Dew AP, Brandao M, Gordon AM, Moreau NG. Effectiveness of lower-extremity functional training (LIFT) in young children with unilateral spastic cerebral palsy: a randomized controlled trial. Neurorehabil Neural Repair. 2019;33:862–872. doi: 10.1177/1545968319868719. [DOI] [PubMed] [Google Scholar]
- 7.Novak I, Morgan C, Fahey M, Finch-Edmondson M, Galea C, Hines A, Langdon K, Namara MM, Paton MC, Popat H, Shore B, Khamis A, Stanton E, Finemore OP, Tricks A, Te Velde A, Dark L, Morton N, Badawi N. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20:3. doi: 10.1007/s11910-020-1022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse A, Schranz C, Tilp M, Svehlik M. Muscle and tendon morphology alterations in children and adolescents with mild forms of spastic cerebral palsy. BMC Pediatr. 2018;18:156. doi: 10.1186/s12887-018-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papageorgiou E, Simon-Martinez C, Molenaers G, Ortibus E, Van Campenhout A, Desloovere K. Are spasticity, weakness, selectivity, and passive range of motion related to gait deviations in children with spastic cerebral palsy? A statistical parametric mapping study. PLoS One. 2019;14:e0223363. doi: 10.1371/journal.pone.0223363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble JJ. Estimating muscle volume from two-dimensional measurements: a promising method for assessment. Dev Med Child Neurol. 2018;60:9–10. doi: 10.1111/dmcn.13619. [DOI] [PubMed] [Google Scholar]
- 11.Liao HH, Yen HR, Muo CH, Lee YC, Wu MY, Chou LW, Sun MF, Chang TT. Complementary traditional Chinese medicine use in children with cerebral palsy: a nationwide retrospective cohort study in Taiwan. BMC Complement Altern Med. 2017;17:155. doi: 10.1186/s12906-017-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao J, He L, Yu X, Wang L, Chen H, Zhao B, Jiang Y. Rehabilitation with a combination of scalp acupuncture and exercise therapy in spastic cerebral palsy. Complement Ther Clin Pract. 2019;35:296–300. doi: 10.1016/j.ctcp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee GE, Lee PT, Ran N, Zhou J. Scalp acupuncture for children with cerebral palsy: a protocol for a systematic review. Medicine (Baltimore) 2019;98:e18062. doi: 10.1097/MD.0000000000018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghafoor U, Lee JH, Hong KS, Park SS, Kim J, Yoo HR. Effects of acupuncture therapy on MCI patients using functional near-infrared spectroscopy. Front Aging Neurosci. 2019;11:237. doi: 10.3389/fnagi.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Shi W, Khiati D, Shi B, Shi X, Luo D, Wang Y, Deng R, Huang H, Li J, Yan W, Yang H. Acupuncture treatment on the motor area of the scalp for motor dysfunction in children with cerebral palsy: study protocol for a multicenter randomized controlled trial. Trials. 2020;21:29. doi: 10.1186/s13063-019-3986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Fu X, Dai G, Wang X, Zhang Z, Cheng H, Zheng P, An Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J Transl Med. 2017;15:48. doi: 10.1186/s12967-017-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbasan B, Akaya KU, Akyuz M, Oskay D. Effects of neuromuscular electrical stimulation and Kinesio Taping applications in children with cerebral palsy on postural control and sitting balance. J Back Musculoskelet Rehabil. 2018;31:49–55. doi: 10.3233/BMR-169656. [DOI] [PubMed] [Google Scholar]
- 18.Jeon H, Jung JH, Yoon JA, Choi H. Strabismus is correlated with gross motor function in children with spastic cerebral palsy. Curr Eye Res. 2019;44:1258–1263. doi: 10.1080/02713683.2019.1631851. [DOI] [PubMed] [Google Scholar]
- 19.Harb A, Kishner S. Modified Ashworth Scale. Treasure Island (FL): StatPearls; 2020. [PubMed] [Google Scholar]
- 20.Choe YR, Kim JS, Kim KH, Yi TI. Relationship between functional level and muscle thickness in young children with cerebral palsy. Ann Rehabil Med. 2018;42:286–295. doi: 10.5535/arm.2018.42.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher-Pipher S Pt Dpt, Kenyon LK Pt Dpt PhD Pcs, Westman M Pt Dpt. Improving balance, mobility, and dual-task performance in an adolescent with cerebral palsy: a case report. Physiother Theory Pract. 2017;33:586–595. doi: 10.1080/09593985.2017.1323359. [DOI] [PubMed] [Google Scholar]
- 22.Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral palsy-trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr. 2017;5:21. doi: 10.3389/fped.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong AKL, Fitzgerald TL, Doyle LW, Cheong JLY, Spittle AJ. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60:480–489. doi: 10.1111/dmcn.13697. [DOI] [PubMed] [Google Scholar]
- 24.Zuurmond M, O’Banion D, Gladstone M, Carsamar S, Kerac M, Baltussen M, Tann CJ, Gyamah Nyante G, Polack S. Evaluating the impact of a community-based parent training programme for children with cerebral palsy in Ghana. PLoS One. 2018;13:e0202096. doi: 10.1371/journal.pone.0202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber RL, Friden J. Muscle contracture and passive mechanics in cerebral palsy. J Appl Physiol (1985) 2019;126:1492–1501. doi: 10.1152/japplphysiol.00278.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MJ, Yun YJ, Yu SA, Shin YB, Kim SY, Han JH. Integrative medicine rehabilitation for children with cerebral palsy: a study protocol for a multicenter pragmatic randomized controlled trial. Trials. 2020;21:723. doi: 10.1186/s13063-020-04639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon CY, Lee B, Chang GT, Yoon SH. Efficacy of acupotomy for cerebral palsy: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14187. doi: 10.1097/MD.0000000000014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyu R, Gao M, Yang H, Wen Z, Tang W. Stimulation parameters of manual acupuncture and their measurement. Evid Based Complement Alternat Med. 2019;2019:1725936. doi: 10.1155/2019/1725936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo Z, Li D, Zhang R, Chang M, Yang B, Tang S. Comparisons of the effectiveness and safety of tuina, acupuncture, traction, and chinese herbs for lumbar disc herniation: a systematic review and network meta-analysis. Evid Based Complement Alternat Med. 2019;2019:6821310. doi: 10.1155/2019/6821310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LX, Zhang MM, Zhang Y, He J. Acupuncture for cerebral palsy: a meta-analysis of randomized controlled trials. Neural Regen Res. 2018;13:1107–1117. doi: 10.4103/1673-5374.233455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo T, Zhu B, Zhang X, Xu N, Wang H, Tai X. Tuina for children with cerebral palsy: a protocol for a systematic review. Medicine (Baltimore) 2018;97:e9697. doi: 10.1097/MD.0000000000009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magalhães RC, Moreira JM, Lauar AO, da Silva AAS, Teixeira AL, E Silva ACS. Inflammatory biomarkers in children with cerebral palsy: a systematic review. Res Dev Disabil. 2019;95:103508. doi: 10.1016/j.ridd.2019.103508. [DOI] [PubMed] [Google Scholar]
- 33.Jia Y, Qiu Z, Sun X, Shen Y, Zhou Q, Li S. Acupotomy for patients with trigger finger: a systematic review protocol. Medicine (Baltimore) 2019;98:e17402. doi: 10.1097/MD.0000000000017402. [DOI] [PMC free article] [PubMed] [Google Scholar]


