Abstract
Background: A recent study showed that OVX-induced osteoporosis was reversed after injection of osteoblasts cultured from the bone marrow in rats. The present study evaluated the effect of injecting MSCs, osteoblasts, and exosomes isolated from osteoblasts for the treatment of osteoporosis in the rabbit model. Methods: Osteoporosis was created in 40 rabbits by performing ovareictomy at 6 months of age, and 1 mg/kg body weight of methyl prednisolone sodium succinate was injected daily for 8 weeks. Animals were fed twice daily and were given water ad libitum. MSCs and osteoblasts were grown from the bone marrow as per the methodology described earlier. From osteoblasts, exosomes were extracted. After the 15th day, MSCs (Group 2), osteoblasts (Group 3), and exosomes (Group 4) were injected into 5 animals each, and 0.5 ml of normal saline were injected into the control group (Group 1). After 12 weeks (11 months of age), all the animals were euthanized. The whole femur and the lumbar vertebrae 3-5 were dissected out and were subjected to radiological assessment using high-resolution peripheral quantitative computerized tomography (HRpQCT). All parameters of the bone volume, trabecular number, thickness, and spacing were assessed using SPSS (Statistical Package for the Social Sciences), version 21.0, Chicago, Illinois. A p value of <0.05 was considered Statistically significant with a confidence interval (CI) of 95%. Results: Structural indices of the osteoblasts-injected animals were significantly better than the control group for the distal femur. The most significant improvement was seen in the osteoblasts, MSCs, and exosomes group in that order. The p value of all parameters was <0.0001 in the osteoblasts group, whereas the total and bone volume had a lower p value in the MSCs group. In the osteoblasts group, the positive changes were similar in the distal femur and lumbar vertebrae, but with MSCs and exosomes, the changes were more pronounced in the vertebral spine than the distal femur. Conclusions: This study shows that autologous bone marrow-derived osteoblasts have the robust influence of reversing OVX-induced osteoporosis in rabbits.
Keywords: Osteoporosis, osteoblasts, mesenchymal stem cells (MSCs), exosomes
Introduction
Secondary to the loss of estrogen, osteoporosis occurs in the postmenopausal age group, in which bone loss exceeds bone formation [1-4]. Osteoporosis is a serious metabolic bone disease in the world that causes enormous morbidity and mortality due to fragility fractures that are secondary to osteoporosis [5]. In the US, the financial cost due to fragility fractures was $22 billion in 2008, and this might have increased much more since then [6]. The osteoporotic-related fractures in Saudi Arabia are the least-studied subject; hence, the real prevalence is still unknown. It was assessed that, by 2050, the lifetime cost of managing the fragility fractures of femurs in Saudi Arabia may be US$9.34 billion annually [7] due to the increasing prevalence of osteoporosis in the population [8-12].
The world’s population is living longer compared to previous decades, and it has been reported that this may increase in the near future [13,14]. The increased longevity of the Saudi Arabian population is no different from the rest of the world. The increased care for osteoporosis patients in the coming years will add to the economic burden. The management of osteoporosis and fragility fractures prevention has remained the same since the last decade, and we still rely on old medications with complications [15-20]. The new approach to treating osteoporosis with customized treatment by stem cell therapy was achievable in animal studies and showed promising results in experimental animals [21-24].
The aim of this study is to assess the effect of MSCs, osteoblasts, and exosomes derived from osteoblasts in the rabbit model.
Methods
Institutional Review board of the Imam AbdulRahman Bin Faisal University, Dammam, Saudi Arabia gave the ethical approval and the deanship of the scientific research funded this study. Osteoporosis was induced in 40 rabbits by performing ovareictomy at 6-month age and 1 mg/kg body weight of methyl prednisolone sodium succinate was injected daily for 8 weeks. Animals were fed twice daily as well as water ad libitum. Autologous bone marrow was used to isolate MSCs, and osteoblasts were separated as described [25]. Osteoblasts exosomes were extracted as described earlier [1]. On 15th day, 106 cells in 0.5 mL normal saline of MSCs (Group 2) and osteoblasts (Group 3) were respectively injected, in the exosomes (Group 4) 100 µg protein and in the control 0.5 ml of normal saline was injected in the saphenous veins.
At 12 weeks (11 months of age), all the animals were euthanized. The whole femur, Lumbar 3-5th vertebrae were dissected out and were subjected to radiological assessment using High Resolution peripheral quantitative computerized tomography (HRpQCT). All the parameters of the bone volume, trabecular number, thickness and spacing were assessed using the VOX = Based on counting voxels; TRI = based on Triangularization of surface. Statistical analysis was performed using the Statistical Package for Social Sciences software, version 21.0 (SPSS Inc, Chicago, IL, USA). Data was presented as a mean standard deviation (±SD). HRpQCT analysis was taken into consideration for each sample and was reproducible; a coefficient of variation (CV) was calculated as the standard deviation of the three repeated measurements divided by the subject mean. Moreover the precision error was calculated as root-mean-square (RMS) averages for each of samples and each parameter assessed. A p value of <0.05 was considered statistical significant with Confidence Interval (CI) of 95%. As a standard policy of the Imam AbdulRahman Bin Faisal University all the studies are monitored by The Monitoring Office for Research and Research Ethics (MORRE) which is instituted by the Ministry of Higher Education, Kingdom of Saudi Arabia.
Results
Osteoblast-treated animals had significant positive differences in most of the parameters compared to the control group. Table 1 gives the comparison of the control to the MSCs group. Most the parameters were significant but the osteoblasts group had a highly significant p value between <0.001 to <0.0001. (Table 2). The exosomes group was only significant in trabecular number and trabecular separation (Table 3).
Table 1.
The comparison between the control and the MSCs group
| Parameter | Control Group | MSCs Group | P Value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 324.761±2.547 | 359.349±24.485 | 0.007 |
| Bone Volume (TV mm^3) (VOX) | 14.308±2.224 | 16.8±5.08 | 0.07 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.044±0.007 | 0.47±0.01 | 0.001 |
| Trabecular Number [1/mm] (VOX) | 0.303±0.032 | 0.498±0.066 | 0.01 |
| Trabecular Thickness [1/mm] (VOX) | 0.171±0.008 | 0.175±0.003 | 0.001 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 0.848±0.137 | 4.643±0.654 | 0.001 |
| Trabecular separation = marrow Thickness [mm] (VOX) | 3.087±0.154 | 1.364±0.453 | 0.001 |
| Total Volume (Tmm^3) (TRI) | 322.547±4.696 | 357.146±24.219 | 0.001 |
| Bone Volume (TV mm^3) (TRI) | 14.260±1.540 | 16.729±5.158 | 0.001 |
| TRI-BS | 107.696±27.559 | 120.488±30.732 | 0.001 |
| TRI-BS/BV | 14.226±0.886 | 16.885±1.030 | 0.002 |
| Trabecular number [1/mm] (TRI) | 0.315±0.039 | 0.453±0.125 | 0.001 |
| Trabecular Thickness [1/mm] (TRI) | 0.062±0.002 | 0.135±0.009 | 0.001 |
| Trabecular Spacing | 3.060±0.215 | 0.590±0.119 | 0.001 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
Table 2.
Comparison between control and osteoblast for structural indices of distal femur
| Parameter | Control Group | Osteoblast Group | P Value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 324.761±2.547 | 89.673±15.504 | 0.001 |
| Bone Volume (TV mm^3) (VOX) | 14.308±2.224 | 29.667±1.141 | 0.001 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.044±0.007 | 0.691±0.071 | 0.001 |
| Trabecular Number [1/mm] (VOX) | 0.303±0.032 | 0.511±0.29 | 0.001 |
| Trabecular Thickness [1/mm] (VOX) | 0.171±0.008 | 2.631±0.461 | 0.001 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 0.848±0.137 | 5.30±0.369 | 0.001 |
| Trabecular separation = marrow thickness [mm] (VOX) | 3.087±0.154 | 1.124±0.186 | 0.001 |
| Total Volume (Tmm^3) (TRI) | 322.547±4.696 | 456.241±15.50 | 0.001 |
| Bone Volume (TV mm^3) (TRI) | 14.260±1.540 | 19.224±7.27 | 0.001 |
| TRI-BS | 107.696±27.559 | 176.055±12.024 | 0.001 |
| TRI-BS/BV | 14.226±0.886 | 15.619±1.074 | 0.001 |
| Trabecular number [1/mm] (TRI) | 0.315±0.039 | 0.510±0.24 | 0.001 |
| Trabecular Thickness [1/mm] (TRI) | 0.062±0.002 | 0.148±0.009 | 0.001 |
| Trabecular Spacing | 3.060±0.215 | 0.427±0.127 | 0.001 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
Table 3.
Comparison between control and exosome for structural indices of distal femur
| Parameter | Control Group | Exosome Group | P Value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 324.761±2.547 | 287.513±2.909 | 0.1 |
| Bone Volume (TV mm^3) (VOX) | 14.308±2.224 | 14.214±1.630 | 0.1 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.044±0.007 | 0.28±0.005 | 0.01 |
| Trabecular Number [1/mm] (VOX) | 0.303±0.032 | 0.369±0.05 | 0.01 |
| Trabecular Thickness [1/mm] (VOX) | 0.171±0.008 | 0.100±0.008 | 0.1 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 0.848±0.137 | 0.885±0.109 | 0.1 |
| Trabecular separation = marrow thickness [mm] (VOX) | 3.087±0.154 | 0.933±0.137 | 0.001 |
| Total Volume (Tmm^3) (TRI) | 322.547±4.696 | 181.508±2.878 | 0.1 |
| Bone Volume (TV mm^3) (TRI) | 14.260±1.540 | 14.202±6.128 | 0.1 |
| TRI-BS | 107.696±27.559 | 118.503±19.062 | 0.1 |
| TRI-BS/BV | 14.226±0.886 | 12.750±1.522 | 0.1 |
| Trabecular number [1/mm] (TRI) | 0.315±0.039 | 0.310±0.030 | 0.1 |
| Trabecular Thickness [1/mm] (TRI) | 0.062±0.002 | 0.101±0.003 | 0.01 |
| Trabecular Spacing | 3.060±0.215 | 0.966±0.233 | 0.1 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
MSCs group was weakly significant in half of the parameters compared (Table 4).
Table 4.
Comparison between Control and MSCs for structural indices of lumbar spine
| Parameter | Control Group | MSCs Group | P value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 21.251±5.980 | 28.352±1.450 | 0.001 |
| Bone Volume (TV mm^3) (VOX) | 2.446±0.783 | 2.776±0.424 | 0.001 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.086±0.028 | 0.098±0.015 | 0.01 |
| Trabecular Number [1/mm] (VOX) | 0.996±0.002 | 1.339±0.387 | 0.01 |
| Trabecular Thickness [1/mm] (VOX) | 0.105±0.009 | 0.114±0.027 | 0.3 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 1.949±0.486 | 4.850±2.907 | 0.001 |
| Trabecular separation = marrow thickness [mm] (VOX) | 1.024±0.052 | 0.795±0.0280 | 0.02 |
| Total Volume (Tmm^3) (TRI) | 21.936±1.101 | 27.943±0.002 | 0.001 |
| Bone Volume (TV mm^3) (TRI) | 2.392±0.792 | 2.732±0.424 | 0.01 |
| TRI-BS | 41.725±13.101 | 60.761±17.74 | 0.001 |
| TRI-BS/BV | 17.711±1.635 | 21.951±4.025 | 0.001 |
| Trabecular number [1/mm] (TRI) | 0.747±0.181 | 1.087±0.318 | 0.008 |
| Trabecular Thickness [1/mm] (TRI) | 0.083±0.03 | 0.093±0.017 | 0.09 |
| Trabecular Spacing | 1.266±0.345 | 0.898±0.314 | 0.004 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
While osteoblast group was again highly significant in all parameters between p<0.01 to <0.0004 (Table 5). For the exosome group (Table 6), most of the parameters were significant except the total volume and relative bone volume and bone surface assessed.
Table 5.
Comparison between control and osteoblast for structural indices of lumbar spine
| Parameter | Control Group | Osteoblast Group | P Value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 21.251±5.980 | 29.592±4.317 | 0.001 |
| Bone Volume (TV mm^3) (VOX) | 2.446±0.783 | 4.367±2.363 | 0.001 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.086±0.028 | 0.154±0.083 | 0.01 |
| Trabecular Number [1/mm] (VOX) | 0.996±0.002 | 1.553±0.651 | 0.01 |
| Trabecular Thickness [1/mm] (VOX) | 0.105±0.009 | 0.130±0.013 | 0.0002 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 1.949±0.486 | 6.371±4.910 | 0.001 |
| Trabecular separation = marrow thickness [mm] (VOX) | 1.024±0.052 | 0.727±0.385 | 0.03 |
| Total Volume (Tmm^3) (TRI) | 21.936±1.101 | 31.945±0.001 | 0.001 |
| Bone Volume (TV mm^3) (TRI) | 2.392±0.792 | 4.301±6.157 | 0.001 |
| TRI-BS | 41.725±13.101 | 113.65±15.90 | 0.001 |
| TRI-BS/BV | 17.711±1.635 | 19.852±7.217 | 0.01 |
| Trabecular number [1/mm] (TRI) | 0.747±0.181 | 1.390±0.0642 | 0.001 |
| Trabecular Thickness [1/mm] (TRI) | 0.083±0.03 | 0.117±0.011 | 0.0004 |
| Trabecular Spacing | 1.266±0.345 | 0.750±0.409 | 0.003 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
Table 6.
Comparison between control and exosome for structural indices of lumbar spine
| Parameter | Control Group | Exosome Group | P value |
|---|---|---|---|
| Total Volume (TV mm^3) (VOX) | 21.251±5.980 | 23.004±6.644 | 0.42 |
| Bone Volume (TV mm^3) (VOX) | 2.446±0.783 | 3.304±0.003 | 0.001 |
| BV/TV (Vox) Relative Bone Volume (%) | 0.086±0.028 | 0.117±0.033 | 0.5 |
| Trabecular Number [1/mm] (VOX) | 0.996±0.002 | 1.130±0.145 | 0.008 |
| Trabecular Thickness [1/mm] (VOX) | 0.105±0.009 | 0.132 ±0.002 | 0.001 |
| Connectivity density, normed by TV [1/mm^3] (VOX) | 1.949±0.486 | 4.127±0.125 | 0.001 |
| Trabecular separation = marrow thickness [mm] (VOX) | 1.024±0.052 | 0.889±0.129 | 0.01 |
| Total Volume (Tmm^3) (TRI) | 21.936±1.101 | 27.678±6.591 | 0.001 |
| Bone Volume (TV mm^3) (TRI) | 2.392±0.792 | 3.237±1.011 | 0.01 |
| TRI-BS | 41.725±13.101 | 63.451±0.935 | 0.001 |
| TRI-BS/BV | 17.711±1.635 | 19.605±0.257 | 0.1 |
| Trabecular number [1/mm] (TRI) | 0.747±0.181 | 1.135±0.017 | 0.001 |
| Trabecular Thickness [1/mm] (TRI) | 0.083±0.03 | 0.102±0.001 | 0.001 |
| Trabecular Spacing | 1.266±0.345 | 0.779±0.014 | 0.001 |
VOX = Based on counting voxels; TRI = based on Triangularization of surface.
Figure 1 shows the HRpQCT for 4 groups of the distal femur. In the control group, the number of trabeculae is fewer and quantity of bone is less compared to MSCs treated, Osteoblasts and exosome treated rabbits. HRpQCT scans for Lumbar Spine for four Groups. In the control group, the number of trabeculae is fewer and quantity of bone is less compared to MSCs treated, Osteoblasts and exosome treated rabbits. The maximum bone quantity was observed in the osteoblasts group (Figure 2).
Figure 1.
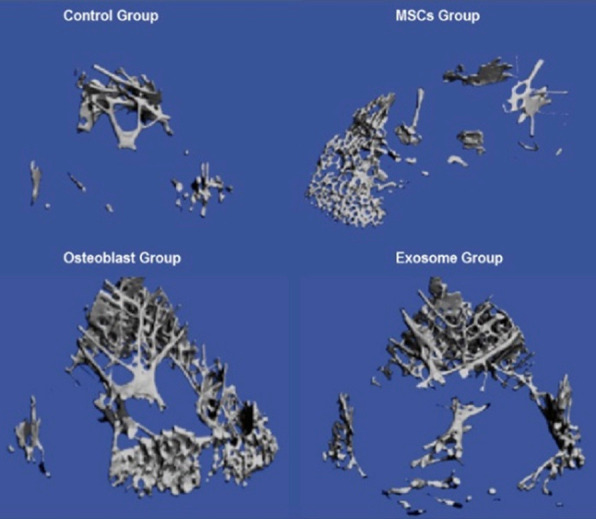
HRpQCT scans for Distal femur for four Groups. In the control group, the number of trabeculae was fewer and quantity of bone was less compared to MSCs treated, Osteoblasts and exosome treated rabbits. The maximum bone quantity was observed in the osteoblasts group.
Figure 2.
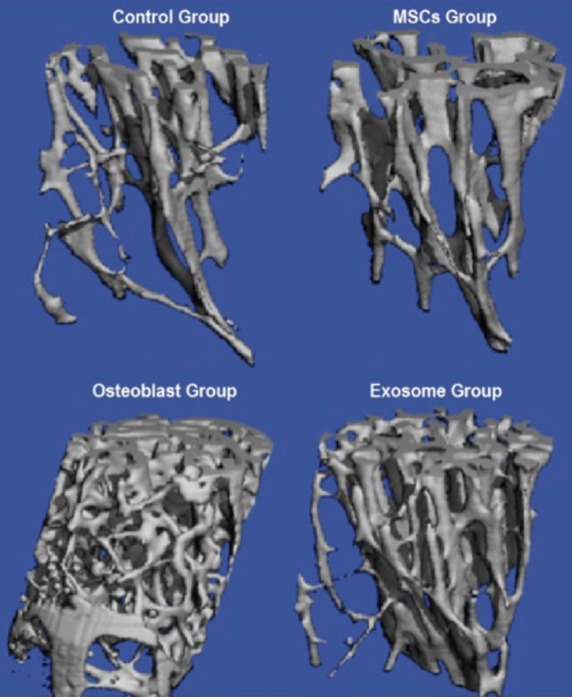
HRpQCT scans for Lumbar Spine for four Groups. In the control group the number of trabeculae are few in number and quantity of bone is less compared to MSCs treated, Osteoblasts and exosome treated rabbits. The maximum bone quantity was observed in the osteoblasts group.
Discussion
This study shows a reversal of osteoporosis in rabbits using bone marrow-derived MSCs, osteoblasts, and osteoblast-derived exosomes. The effect was robust in the osteoblasts therapy when compared to the MSCs and osteoblast-derived exosomes. Exosome was the least effective in the reversal of osteoporosis. In the exosomes group, it appears that 100 µg protein extracted from the osteoblasts injected may not be enough for complete reversal of osteoporosis, as seen in the osteoblasts group.
Complications of osteoporosis in terms of fragility fractures are still causing a worldwide health care issue in the aging population [26,27]. Oral medications for osteoporosis are very effective, but the compliance is quite bad [28,29]. Drugs do not work if they are not taken, and this leads to an increase in the incidence of fragility fractures [30,31]. One way to prevent the escalating numbers of fragility fractures is to develop newer, safer, and less-expensive medications with minimal complications. The new approach to treat osteoporosis with bone marrow-derived osteoblast therapy in animals appears promising.
The US FDA issued in 1994 [32] and the 2016 [33] guidelines for preclinical and clinical evaluation of agents in the treatment and prevention of postmenopausal osteoporosis have suggested two animal models (e.g., a rodent (rat) and a non-rodent larger animal) in which the efficacy and safety can be demonstrated. This study has shown the effect of osteoblasts in rats [21,22]. Rabbits were used in this study, and the effect was similar and positive.
This study was limited by the fact that a larger animal (i.e., sheep) should have been used to assess the reversal of osteoporosis because its bone loss with an estrogen deficiency is quite similar to that of women and is proven to be ideal [34,35]. As strength, the study used a smaller mammal, and the researchers made sure that total osteoporosis was induced with OVX in addition to methyl prednisolone injections to block the extra ovarian estrogen. The present and previous studies in rats have confirmed that it is time to start thinking about monitoring bone mineral density and bone-turnover markers in phase I human trials in order to test the efficacy of autologous-derived osteoblasts.
Acknowledgements
The authors thank the Deanship of Scientific Research, Imam AbdulRahman Bin Faisal University, Dammam, Saudi Arabia fro providing the grant vide #2017-077.
Disclosure of conflict of interest
None.
References
- 1.Rosen CJ. Restoring aging bones. Sci Am. 2003;288:70–77. doi: 10.1038/scientificamerican0303-70. [DOI] [PubMed] [Google Scholar]
- 2.Marcus R. Post-menopausal osteoporosis. Best Prac Res Clin Obstet Gynaecol. 2002;16:309–327. doi: 10.1053/beog.2002.0284. [DOI] [PubMed] [Google Scholar]
- 3.Fleisch H. Pathophysiology of osteoporosis. Bone Mineral. 1993;22(Suppl):S3–36. [Google Scholar]
- 4.Nichols KJ. Evaluation of osteoporosis. J Am Osteopath Assoc. 2000;100(Suppl):S4–7. doi: 10.7556/jaoa.2000.100.1.4s. [DOI] [PubMed] [Google Scholar]
- 5.Chopra A. Osteoporosis: a new understanding of its impact and pathogenesis. J Am osteopath Assoc. 2000;100(Suppl):S1–4. doi: 10.7556/jaoa.2000.100.1.1s. [DOI] [PubMed] [Google Scholar]
- 6.Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22:1835–1844. doi: 10.1007/s00198-010-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadat-Ali M, Al-Dakheel DA, Azam MQ, Al-Bluwi MT, Al-Farhan MF, AlAmer HA, Al-Meer Z, Al-Mohimeed A, Tabash IK, Karry MO, Rassasy YM, Baragaba MA, Amer AS, AlJawder A, Al-Bouri KM, ElTinay M, Badawi HA, Al-Othman AA, Tayara BK, Al-Faraidy MH, Amin AH. Reassessment of osteoporosis-related femoral fractures and economic burden in Saudi Arabia. Arch Osteoporos. 2015;10:37. doi: 10.1007/s11657-015-0240-5. [DOI] [PubMed] [Google Scholar]
- 8.Sadat-Ali M, AlZamami JF, Al-Naimi SN, Al-Naimi DA, Al-Dakheel DA, AlTwejry AM. The Increasing Prevalence of Osteoporosis in Eastern Saudi Arabia. Annals of Afr Med (Accepted for Publications) [Google Scholar]
- 9.Sadat-Ali M, Al-Habdan I, Al-Mulhim F, Yousef A. Bone mineral density among postmenopausal Saudi Arabian women. Saudi Med. 2004;25:1623–1625. [PubMed] [Google Scholar]
- 10.El-Desouki MI, Sherafzal MS, Othman SA. Comparison of bone mineral density with dual energy X-ray absorptiometry, quantitative ultrasound and single energy X-ray absorptiometry. Saudi Med J. 2005;26:1346–1350. [PubMed] [Google Scholar]
- 11.Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporosis Int. 2005;16:43–55. doi: 10.1007/s00198-004-1639-9. [DOI] [PubMed] [Google Scholar]
- 12.Sadat-Ali M, Al-Habdan I, Marwah S. Bone mineral density measurements of distal radius in Saudi Arabian females. Annals of Saudi Med. 1996;16:414–416. doi: 10.5144/0256-4947.1996.414. [DOI] [PubMed] [Google Scholar]
- 13.Woodward A, Blakely T. The healthy country? A history of life and death in New Zealand (Auckland University Press) 2014 [Google Scholar]
- 14.Cassel CK. Successful aging. How increased life expectancy and medical advances are changing geriatric care. Geriatrics. 2001;56:35–39. [PubMed] [Google Scholar]
- 15.Graham DY. What the gastroenterologists should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci. 2002;47:1665–1678. doi: 10.1023/a:1016495221567. [DOI] [PubMed] [Google Scholar]
- 16.De Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–1021. doi: 10.1056/NEJM199610033351403. [DOI] [PubMed] [Google Scholar]
- 17.Rupel K, Ottaviani G, Gobbo M, Contardo L, Tirelli G, Vescovi P, Di Lenarda R, Biasotto M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ) Oral Oncol. 2014;50:1049–57. doi: 10.1016/j.oraloncology.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Holzinger D, Seemann R, Matoni N, Ewers R, Millesi W, Wutzl A. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2014;72:1937. doi: 10.1016/j.joms.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Kharazmi M, Hallberg P. Bisphosphonate-associated atypical femoral fractures and one-year mortality. Ups J Med Sci. 2014;18:1–2. doi: 10.3109/03009734.2014.959213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhadada SK, Sridhar S, Muthukrishnan J, Mithal A, Sharma DC, Bhansali A, Dhiman V. Predictors of atypical femoral fractures during long term bisphosphonate therapy: a case series & amp; review of literature. Indian J Med Res. 2014;140:46–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Sadat-Ali M, Al-Dakheel DA, AlMousa SA, AlAnii FM, Ebrahim WY, AlOmar HK, AlSayed HN, Acharya S, AlHawaj H. Stem-cell therapy for ovariectomy-induced osteoporosis in rats: a comparison of three treatment modalities. Stem Cells Cloning. 2019;12:17–25. doi: 10.2147/SCCAA.S204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadat-Ali M, Al-Turki HA, Acharya S, Al-Dakheel DA. Bone marrow-derived osteoblasts in the management of ovariectomy induced osteoporosis in rats. J Stem Cells Regen Med. 2018;14:63–68. doi: 10.46582/jsrm.1402010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiernan J, Hu S, Grynpas MD, Davies JE, Stanford WL. Systemic mesenchymal stromal cell transplantation prevents functional bone loss in a mouse model of age-related osteoporosis. Stem Cells Transl Med. 2016;5:683–693. doi: 10.5966/sctm.2015-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ocarino Nde M, Boeloni JN, Jorgetti V, Gomes DA, Goes AM, Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51:426–433. doi: 10.3109/03008201003597049. [DOI] [PubMed] [Google Scholar]
- 25.Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW, Bora-Sohn , Kim D, Cho MC, Lee MM, Park YB. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur J Heart Fail. 2005;7:730–738. doi: 10.1016/j.ejheart.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Burge R, Dawson-Hughes B, Solomon D, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 27.Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26:1929–1937. doi: 10.1007/s00198-015-3093-2. [DOI] [PubMed] [Google Scholar]
- 28.Yood RA, Emani S, Reed JI, Lewis BE, Charpentier M, Lydick E. Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int. 2003;14:965–968. doi: 10.1007/s00198-003-1502-4. [DOI] [PubMed] [Google Scholar]
- 29.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271–277. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 30.Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 31.Caro JJ, Ishak KJ, Kf H, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapies on fracture rates in actual practice. Osteoporos Int. 2004;15:1003–1008. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 32.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone. 1995;17(Suppl):125S–133S. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 33. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. Assessed December 2020.
- 34.Chavassieux P, Garnero P, Duboeuf F, Vergnaud P, Brunner-Ferber F, Delmas PD, Meunier PJ. Effects of a new selective estrogen receptor modulator (MDL 103,323) on cancellous and cortical bone in ovariectomized ewes: a biochemical, histomorphometric, and densitometric study. J Bone Miner Res. 2001;16:89–96. doi: 10.1359/jbmr.2001.16.1.89. [DOI] [PubMed] [Google Scholar]
- 35.Hornby SB, Ford SL, Mase CA, Evans GP. Skeletal changes in the ovariectomised ewe and subsequent response to treatment with 17-beta oestradiol. Bone. 1995;17:389S–394S. doi: 10.1016/8756-3282(95)00316-6. [DOI] [PubMed] [Google Scholar]


