Abstract
Objective: Our aim was to identify multiple endometrial receptivity related factors by applying non-invasive, repeatable multimodal ultrasound methods. We further established a practical prediction model for pregnancy prediction. Materials and Methods: Our study included 152 participants from Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, and the Third Affiliated Hospital of Wenzhou Medical University. Clinical information including age and ultrasonographic data were collected. By applying t-test and Wilcoxon rank sum tests, we obtained endometrial receptivity related factors, and by using logistic regression, we established a prediction model for possibility of successful pregnancy. Results: Among all the factors associated with endometrial receptivity, uterine peristaltic wave frequency, uterine spiral artery resistant index, endometrial flow index, ultrasound elastography strain radio (SR), and age showed significant statistical difference between nonpregnant and pregnant volunteers. Consequently, we developed and validated a nomogram prediction model with its value of area under the receiver operating curve up to 0.949 for predicting pregnancy by using age and ultrasonographic factors including uterine peristalsis, uterine spiral artery, and ultrasound elastographic features. The sensitivity was 0.83 and specificity was 0.96. In addition, its performance was better than that of a direct scoring system. Conclusion: By employing the pregnancy prediction model with endometrial receptivity associated ultrasonographic factors, clinicians can give a quantitative evaluation and a real time screen of the uterus condition as well as optimal guiding, treatment, and management recommendations for infertility-related patients.
Keywords: Endometrial receptivity, female, infertility, pregnancy, ultrasonographic prediction
Introduction
Pregnancy is a complex process that comprises series of events including ovulation, implantation, decidualization, placentation, and birth of offspring through the process of parturition. Any problem that occurs in process of pregnancy may result in infertility that is defined officially by the Word Health Organization (WHO) as a disease of failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse or as a result of an impairment of a person’s capacity to reproduce, either as an individual or with his or her partner [1,2]. In spite of the advancement of diagnostic technique, infertility is still estimated to affect 186 million people, and about 8% to 12% of reproductive-aged couples suffer infertility in recent years worldwide. It is estimated that infertility among women of childbearing age accounts for 1/7 couples in the Western countries and 1/4 couples in developing countries. Infertility rates are likely to reach 30% in some parts of the world, including Central Asia, South Asia, some countries in sub-Saharan Africa, the Middle East and North Africa, and Central and Eastern Europe [1,3].
WHO statistics reveal that 37% infertile couples in developed countries are caused by female factors [4]. Female factors including advanced women’s age and the uterine condition, especially the condition of the endometrium, may affect endometrial receptivity of the embryo through dysfunction of endometrial development or maturation, or immune and endocrinal local environment, resulting in the failure of embryo implantation [4,5]. In the case of in vitro fertilization (IVF), the endometrial receptivity and the uterine receptivity probably become the most important factor contributing to a successful implantation.
Endometrial receptivity refers to a state in which the endometrium allows blastocysts to locate, adhere, invade, and change the endometrial stroma, leading to embryo implantation [6]. In the treatment of infertility, the receptivity of endometrium has attracted much attention. Synchronous development of the endometrium and oocytes is a prerequisite for sperm-egg binding, embryo implantation, and the receptivity of endometrium. Moreover, the mathematical model of embryo implantation after IVF showed that the contribution rate of endometrium to reproductive success rate was between 31% and 64% [7,8]. We can assume that, although embryo quality is one of the most important factors, adequate endometrial receptivity is also a key factor in successful pregnancy. Application of endometrial receptivity related factors has important guiding significance for the treatment plan so as to effectively improve the success rate of treatment, whereas one of the main problems is the lack of practical clinical tests to assess endometrial receptivity in vivo.
There are many clinical methods to evaluate endometrial receptivity such as immunohistochemistry and endometrial biopsy. Ultrasound-based methods had been given increasingly more attention in the treatment of infertility because of their simplicity, non-invasiveness, and repeatability [9].
Nevertheless, at present, the efficiency of ultrasonic detection of endometrial receptivity is still inconclusive. In recent years, many researchers have used such methods in their studies as applying colour Doppler flow imaging to measure the hemodynamic parameters of uterine artery and spiral artery and employing three-dimensional energy Doppler ultrasound to measure sub-endometrial blood flow parameters, among other methods. Additionally, there is still no widely accepted standardized process for the evaluation of endometrial receptivity worldwide [9-11].
In our present study, we employed a series of multimodal ultrasound methods to evaluate endometrial receptivity related uterus condition to achieve complementary advantages and better evaluate the optimal period of endometrial implantation. Through multidimensional comprehensive evaluation, we aimed to find an ultrasonic standard of individualized endometrial receptivity evaluation to predict the pregnancy outcome more quickly and accurately.
Materials and methods
Patients and clinical information
Our study included 152 participants from Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, and the Third Affiliated Hospital of Wenzhou Medical University, including 117 cases of infertility and 35 cases of pregnancy with the ages ranging from 24 to 40 years and the infertility period ranging from 2 to 12 years.
All subjects met the following criteria: (1) laboratory examination confirmed that their spouse’s semen analysis was in the normal range; (2) ultrasound or laparoscopy confirmed that there were no organic lesions in the uterus and ovary of the patient; (3) bilateral (or at least unilateral) fallopian tube patency confirmed by hysterosalpingography; (4) no application of estrogen or progesterone within 3 months; and (5) no history of pelvic surgery within 6 months.
Ethical approval
This study was approved by the Medical Ethics Committee of Wenzhou People’s Hospital, Wenzhou Maternal and Child Health Care Hospital, and the Third Affiliated Hospital of Wenzhou Medical University (NCT04014453). Additionally, all the clinical information was obtained with informed consent.
Ultrasonographic methods
We applied Voluson™ E10 (GE, Boston, MA, USA) to perform color Doppler ultrasound diagnosis, by volume transducer with frequency of 5 to 9 MHz. All cases were examined according to the standard transvaginal examination.
Endometrial thickness, endometrial morphology, and peristaltic wave were obtained by conventional transvaginal two-dimensional ultrasonography. In addition, we applied two-dimensional colour Doppler flow imaging to detect sub-endometrial blood flow distribution, from which we observed a subendometrial blood flow pattern. Two-dimensional power Doppler imaging was employed to detect uterine spiral artery resistance and uterine artery pulsation. Moreover, endometrial volume, endometrial flow index, vascularization index, and vascularization flow index were measured by three-dimensional power Doppler imaging, whereas the quantitative evaluation of endometrial microvascular perfusion was detected by contrast-enhanced ultrasound imaging, and the endometrial elasticity was evaluated by Shear Wave Elasticity Imaging (SWEI).
As a result, clinical information such as age and ultrasonographic data such as endometrial thickness, endometrial echo pattern, uterine peristaltic wave frequency (UPF), sub-endometrial blood flow pattern, uterine spiral artery resistant index (RI), uterine artery pulsative index (PI), endometrial volume, endometrial flow index (FI), and ultrasound elastography strain radio (SR) were collected for analysis.
Pregnancy diagnosis
A urine pregnancy test was performed, of which its positive result reflected a biochemical pregnancy. Ultrasonic examination was performed at 6 to 7 weeks after menopause, and clinical pregnancy was confirmed by the existence of an intrauterine pregnancy sac with embryo and fetal heartbeat.
Statistical analysis
Except for total score (mean ± standard deviation [SD]), all continuous variables were shown and calculated using median ± SD (Table 1). For univariate analysis, Wilcoxon rank sum test was conducted for abnormally distributed continuous variables, whereas t-test was performed for normally distributed variables.
Table 1.
Univariate analysis of all the involved factors
| Items | Non-pregnant | Pregnant | Statistics | P value |
|---|---|---|---|---|
| Endometrial thickness (mm) | 2.00±0.72 | 2.00±0.59 | Z = 1.68 | 0.0932# |
| Endometrial echo pattern (mm) | 2.00±0.75 | 2.00±0.71 | Z = 1.92 | 0.0545# |
| Uterine peristaltic wave frequency (UPF) | 2.00±0.73 | 2.00±0.68 | Z = 2.94 | 0.0033*,# |
| Subendometrial blood flow pattern (ml/min) | 2.00±0.70 | 2.00±0.68 | Z = 0.28 | 0.7815 |
| Uterine spiral artery resistant index (RI) | 1.00±0.56 | 3.00±0.70 | Z = 6.72 | <0.0001*,# |
| Uterine artery pulsative index (PI) | 3.00±0.78 | 3.00±0.65 | Z = 0.49 | 0.6224 |
| Endometrial volume (ml) | 2.00±0.85 | 2.00±0.78 | Z = 1.82 | 0.0680# |
| Endometrial flow index (FI) | 1.00±0.48 | 2.00±0.70 | Z = 6.56 | <0.0001*,# |
| Ultrasound elastography strain radio (SR) | 1.00±0.50 | 3.00±0.56 | Z = 7.67 | <0.0001*,# |
| Age (years) | 33.00±5.58 | 31.00±4.55 | Z = -0.68 | 0.4996*,# |
| Total score (score) | 15.92±2.85 | 20.29±2.79 | t = -7.97 | <0.0001* |
Except for overall score (using Mean ± SD by t-test), all variables are shown and calculated by Wilcoxon rank sum tests using Median ± SD. There is no missing data from the 117 non-pregnant and 35 pregnant participants.
showing significance of factors;
factors involved in logistic regression model.
To establish a prediction model, we applied both the total score method and logistic regression analysis. ROCs were drawn to calculate the optimal cut-off point and area under curve (AUC) for total score as a single predictor as well as a logistic regression model. Except for total score, variables with P values less than 0.1 in univariate analysis were included into logistic regression with the stepwise selection method. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used to describe the predictive properties.
A fivefold cross validation method was conducted when evaluating the predictive effect of the model via the caret package (https://cran.r-project.org/web/packages/caret/index.html) [12]. A nomogram was formulated based on the results of multivariate analysis using the rms package. The performance of the nomogram was measured by the calibration curve with 1000 Bootstrap resample. Significant level was set as 0.05 in all of the statistical analyses that were performed by using SAS 9.4 and R software.
Results
Screening and testing potential endometrial receptivity associated factors that are closely related to successful pregnancy
Of the 152 participants who were included in this study, 117 (76.97%) were not pregnant and 35 (23.03%) were pregnant. The description and univariate analysis are listed in Table 1. Among all the factors, UPF, uterine spiral artery RI, endometrial FI, ultrasound elastography SR, age, and total score showed significant statistical difference between nonpregnant and pregnant volunteers.
Establishment of logistic regression-based prediction model
The total score showed good independent prediction property with a high AUC (0.867), and the cut-off value was 19 (Table 2). For the logistic regression model, seven variables including endometrial thickness, endometrial echo pattern, UPF, uterine spiral artery RI, endometrial FI, ultrasound elastography SR, and age were involved. As a result, four variables (UPF, uterine spiral artery RI, ultrasound elastography SR, and age) were selected as key factors in the logistic regression model (Table 3), which lead to a high predictive efficiency of the logistic model with AUC up to 0.949. The sensitivity, specificity, PPV, and NPV were 0.83, 0.96, 0.85, 0.94, respectively (see Table 2). The algorithm established by the logistic regression model is:
Table 2.
Prediction properties of internal validation
| Method | Internal Validation | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| AUC | Cutoff | Sensitivity | Specificity | PPV | NPV | |
| Logistic Regression* | 0.949 | 0.43 | 0.83 | 0.96 | 0.85 | 0.94 |
| Total score | 0.867 | 19 | 0.80 | 0.82 | 0.57 | 0.93 |
Based on the combination of uterine peristalsis (Uterine peristaltic wave frequency, UPF), uterine spiral artery (Uterine spiral artery resistant index, RI), ultrasound elastography (Ultrasound elastography strain radio, SR) and age.
Table 3.
The results of 5-fold cross validation of the predictive model
| Dataset | AUC | 95% CI | Cutoff | 95% CI | SEN | 95% CI | SPE | 95% CI | PPV | 95% CI | NPV | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||||||
| Low | Up | Low | Up | Low | Up | Low | Up | Low | Up | Low | Up | |||||||
| Training data | 0.9500 | 0.9486 | 0.9515 | 0.3517 | 0.3340 | 0.3694 | 0.8573 | 0.8489 | 0.8657 | 0.9233 | 0.9149 | 0.9318 | 0.9745 | 0.9719 | 0.9770 | 0.6673 | 0.6547 | 0.6799 |
| Validation data | \ | \ | \ | \ | \ | \ | 0.8920 | 0.8720 | 0.9120 | 0.7948 | 0.7498 | 0.8399 | 0.9351 | 0.9206 | 0.9496 | 0.7180 | 0.6705 | 0.7654 |
| All data | 0.9497 | 0.4278 | 0.8286 | 0.9573 | 0.9839 | 0.6392 | ||||||||||||
SEN: sensitivity; SPE: specificity.
logit (P) = -18.8623 + 0.9532X (Uterine peristalsis) + 1.5737X (Uterine spiral artery) + 3.9092X (Ultrasound elastography) + 0.154X (Age)
Validation of the prediction model
The result of the validation process by conducting a fivefold cross validation method is shown in Table 4, in which the AUC for training data was pretty high at 0.9500 (95% CI, 0.9486 to 0.9515). The ROC (Figure 1) shows the prediction property of both the total score and the logistic regression model. The prognostic nomogram that integrated all significant independent factors for pregnancy is shown in Figure 2, and the calibration curve showed good agreement between prediction and observation in the probability of metastasis (Figure 3).
Table 4.
The results of logistic regression model
| Parameter | β | Wald χ2 | OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Intercept | -18.8623 | 18.758 | \ | \ | \ | <0.0001 |
| Uterine peristalsis | 0.9532 | 4.3373 | 2.594 | 1.058 | 6.361 | 0.0373 |
| Uterine spiral artery | 1.5737 | 8.6038 | 4.825 | 1.686 | 13.808 | 0.0034 |
| Ultrasound elastography | 3.9092 | 13.5489 | 49.857 | 6.219 | 399.69 | 0.0002 |
| Age | 0.154 | 4.5351 | 1.167 | 1.012 | 1.344 | 0.0332 |
Figure 1.
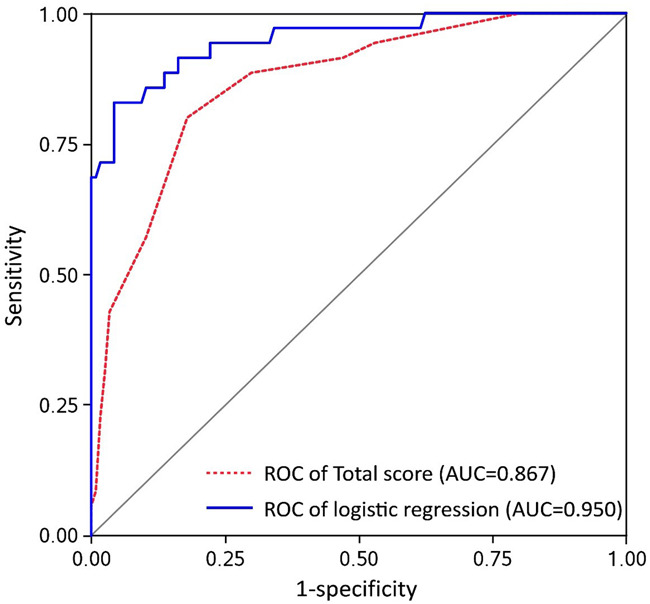
The receiver operating characteristic curve.
Figure 2.
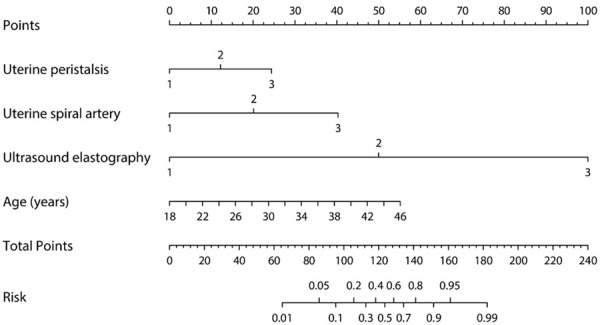
Nomogram of uterine peristalsis, uterine spiral artery, ultrasound elastography and age.
Figure 3.
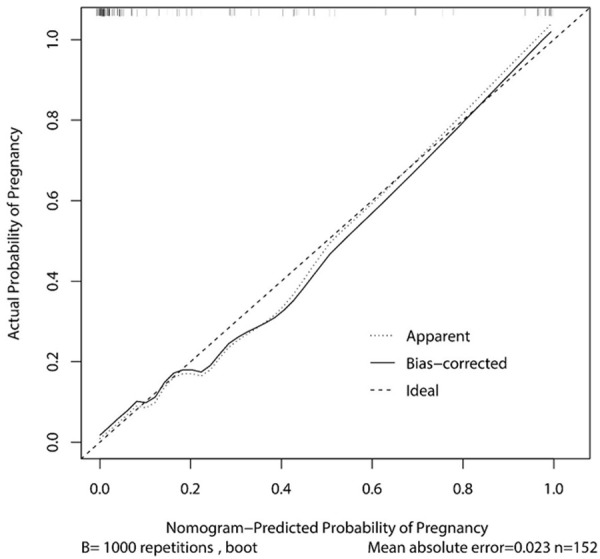
The calibration curve for the predictive model and nomogram.
Discussion
The endometrium of the uterus plays a crucial role in implantation in a successful fertility and pregnancy, which is like soil to the seeds (embryo). Thus, any disease, even functional abnormalities influenced by immune and endocrine factors, that affects the acceptance of an embryo or endometrial receptivity will result in pregnancy impairment. Ultrasound-based imaging techniques can reflect multiple physiological and pathological conditions of the uterus. Potential endometrial receptivity factors included endometrial thickness, endometrial echo pattern, UPF, subendometrial blood flow pattern, uterine spiral artery RI, uterine artery PI, endometrial volume, endometrial FI, and ultrasound elastography SR. Among them, UPF, RI, FI, and SR showed a significantly statistical difference between nonpregnant and pregnant participants. From these factors, uterine peristalsis (reflected by UPF), uterine spiral artery (reflected by RI), and ultrasound elastography (reflected by SR) were eventually included as parameters (combined with age) of the nomogram prediction model. To our knowledge, this is the first nomogram model for pregnancy success possibility.
Aging is undoubtedly a strong risk factor for infertility not only because of the continuous loss of oocytes stored in the ovaries and the decline of oocyte quality or ovulation function [1], but also because of the senescence and degeneration of cells and tissues of endometrium that greatly affect endometrial receptivity. Therefore, age can be used as one of the indicators to predict endometrial receptivity and pregnancy probability.
This study also showed that UPF is closely related to the success of endometrial receptivity and pregnancy (P = 0.0033). Transvaginal ultrasound can be used to monitor endometrial wavy movement in female patients. Endometrial peristaltic wave is an important indicator of uterine physiology [12-14]. The frequency and form of endometrial peristaltic wave changed periodically during the menstrual cycle. Ijland et al. [14] divided the endometrial movement of women within a natural menstrual cycle into five types: fundus-to-cervix (FC) waves, cervix-to-fundus (CF) waves, opposing (OPP) waves, random waves, and no activity. It is considered that the frequency of endometrial movement in the follicular stage increased, with the form of movement becoming increasingly more diverse and reaching the highest level of diversity in the late follicular stage. In the follicular phase the direction of the endometrial peristaltic wave was mainly FC movement, whereas in the early luteal phase, there was a small amount of CF movement and more OPP movement, but the FC movement disappeared completely. The frequency of endometrium movement in the middle luteal phase was significantly decreased to facilitate the implantation of fertilized eggs [12]. Retrograde uterine contraction or a relatively static uterine environment may promote embryo retention and implantation. Therefore, the direction and frequency of the peristalsis of the endometrium are important factors affecting embryo implantation of endometrium in patients.
A large number of studies suggest that ultrasonic monitoring of blood flow of the uterus may be related to endometrial receptivity [15-18]. A normal uterine artery and subendometrial blood perfusion are of great necessity for embryo implantation. In this study, uterine spiral artery RI, endometrial FI, and subendometrial blood flow pattern were studied as uterine artery blood flow parameters in which RI and FI were statistically different between patients in the pregnant group and those in the nonpregnant group (both P<0.001), suggesting that the decrease of uterine perfusion may be one of the causes of pregnancy failure. The branch artery of the endometrial spiral artery is the main nutrient source of the endometrium and is an ideal index to evaluate endometrial receptivity. The blood flow state of the uterine artery in adult women is high resistance and low flow type, and the blood flow resistance of the uterine artery shows typical periodic change during the whole menstrual cycle [19]. When the resistance of uterine artery blood flow increases, the endometrial blood supply is poor and the development of endometrium is abnormal, which makes the endometrial receptivity decrease. The results of this study showed that the RI value of the uterine spiral artery in pregnant patients was significantly lower than that in nonpregnant patients. Therefore, RI can also be included as one of the factors to predict endometrial receptivity and pregnancy probability.
However, some other studies stated that it was not correlated with pregnancy outcome and could not predict endometrial receptivity [20], which probably owes to the fact that that there is no uniform standard of the location and range of sub-endometrium [21,22]. The difference in size and location of the sub-endometrium may cause different blood flow parameters. Defining the extent of the sub-endometrium region requires further study.
Ultrasonic elastic imaging is a new technology developed in recent decades. It is a simple, non-invasive method for evaluating the texture softness and hardness of tissue in a region of interest and performs well in the differential diagnosis of uterine diseases. In our study the endometrial elasticity was evaluated by SWEI, which is a new elastic imaging technique developed in recent years and has been widely used in the clinical diagnosis and research of thyroid, breast, liver, prostate, and other diseases [23,24]. When a force is applied to the tissue, the distribution of displacement, strain, and velocity produced by different tissues is different. The hardness information of the microstructure can be analysed qualitatively and quantitatively by encoding the different information produced by it and measuring the corresponding parameters. Elasticity is an important characteristic of biological tissue, which is related to the type and composition of tissue molecules and cells. When pathological or physiological changes occurred in microstructure such as the composition of tissue cells, macroscopically, the hardness of the tissue also change accordingly [23,25]. All of these changes will influence endometrial receptivity and pregnancy dramatically. Our results also exhibited that ultrasound elastography SR had a close link with successful pregnancy (P<0.0001).
Traditionally, endometrial thickness was used as an indicator of uterine receptivity or endometrial receptivity, but there are still a lot of controversies about the practical application value of measuring endometrial thickness to evaluate endometrial receptivity [26-28]. The thickness of a certain area of the endometrium on the sagittal section of the uterus cannot reflect the overall situation of the uterine cavity, especially for those with uneven distribution of endometrial thickness. By contrast, three-dimensional transvaginal ultrasound can effectively and accurately measure endometrial volume, which has gradually become a new estimation index of endometrial receptivity. Nevertheless, in this study, both endometrial thickness and endometrial volume (P = 0.0932 and 0.0680, respectively) showed no statistical difference between nonpregnant and pregnant participants, which is presumably caused by the limited size of sample and could exhibit statistical difference in the larger multicentric follow-up clinical studies.
The evaluation of endometrial echo pattern has limitation that is similar to endometrial thickness and volume, mainly because they are measured at a point in a fixed period. The growth of endometrium is a dynamic process influenced by all kinds of endogenous and exogenous factors, especially the change of hormone level in vivo and the application of hormones or other drugs. The thickness, volume, and echo pattern of endometrium change greatly during the menstrual cycle; thus it is incomplete to reflect endometrial receptivity only by the endometrial state at a certain point and at a certain period of time. Therefore, the dynamic monitoring of these three factors and the adoption of average or comprehensive levels as the prediction indicators may exhibit better results.
Medical imaging and ultrasonography of the uterus can detect and reflect almost all aspects of functional conditions from the thickness or volume of the endometrium, uterine elasticity, and peristalsis to the blood supply of the uterus and endometrium. These factors reflect the capacity, environmental condition and status, and nutrition supply of the soil to an embryo (seed), which we defined as endometrial receptivity. By applying age combined with these ultrasonographic factors that can reflect the functional disorders or abnormalities in the uterus, our model showed excellent performance in the predictive effect of pregnancy and fertilization.
Today, IVF is employed widely in clinical medicine, however, it is still expensive. In some cases, the condition of the uterus cannot give rise to pregnancy even with IVF. Applying this endometrial receptivity related prediction model to evaluate the successful probability of fertilization is of importance and benefit, because it can reduce unnecessary costs, anxiety, depression, and family conflicts.
Additionally, different uterus statuses and conditions require different nursing treatment for the patients. The model provides a good quantitative reference and standard for clinical management. Further, clinicians can employ this model as a real time screening method for patients. By adjusting their pregnancy condition and function, improving the receptivity of the endometrium, and seizing the right moment for fertilization, the pregnancy probability can be increased. Our findings are in agreement with the study by Liang et al. [29] who pointed out that the correct evaluation of endometrial receptivity and selection of the timing of transplantation are the key to improving the embryo implantation rate. Endometrial thickness and volume have a strong negative predictive value for pregnancy outcome. Doppler examination of uterine artery cannot reflect the actual blood flow under the endometrium. The measurement of endometrial and subendometrial blood flow is performed by three-dimensional Doppler ultrasound. It is more objective and is the most commonly used non-invasive method for evaluating endometrial receptivity. However, a single ultrasound parameter has limited predictive value for endometrial receptivity.
In conclusion, this study yielded an endometrial receptivity associated scoring method and predictive model for fertilization and pregnancy by using age and ultrasonographic factors including uterine peristalsis, uterine spiral artery, and ultrasound elastographic features. By using the model and the total score of multiple variables, clinicians can give a quantitative estimation and prediction for the probability of pregnancy, a real time screen of the uterus condition, endometrial receptivity, and the status of patients. Given that the establishment and validation of the model were performed in a single medical centre with hundreds of participants. A larger multicentric follow-up clinical study is of necessity to validate the performance of this scoring and model-based pregnancy evaluation method in clinical practice.
Acknowledgements
The work was financially supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2018ZD043), The Project of Wenzhou Science and Technology Bureau (Y20180181), The Project of Zhejiang Public Welfare Technology Research and Social Development (LGF18H180003).
Disclosure of conflict of interest
None.
References
- 1.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong SC, Showell M, Stewart EA, Rebar RW, Vanderpoel S, Farquhar CM. Baseline anatomical assessment of the uterus and ovaries in infertile women: a systematic review of the evidence on which assessment methods are the safest and most effective in terms of improving fertility outcomes. Hum Reprod Update. 2017;23:533–47. doi: 10.1093/humupd/dmx019. [DOI] [PubMed] [Google Scholar]
- 4.Harris-Glocker M, McLaren JF. Role of female pelvic anatomy in infertility. Clin Anat. 2013;26:89–96. doi: 10.1002/ca.22188. [DOI] [PubMed] [Google Scholar]
- 5.Hatasaka H. Clinical management of the uterine factor in infertility. Clin Obstet Gynecol. 2011;54:696–709. doi: 10.1097/GRF.0b013e3182353d68. [DOI] [PubMed] [Google Scholar]
- 6.Raga F, Bonilla-Musoles F, Casan EM, Klein O, Bonilla F. Assessment of endometrial volume by three-dimensional ultrasound prior to embryo transfer: clues to endometrial receptivity. Hum Reprod. 1999;14:2851–2854. doi: 10.1093/humrep/14.11.2851. [DOI] [PubMed] [Google Scholar]
- 7.Walters DE, Edwards RG, Meistrich ML. A statistical evaluation of implantation after replacing one or more human embryos. J Reprod Fertil. 1985;74:557–563. doi: 10.1530/jrf.0.0740557. [DOI] [PubMed] [Google Scholar]
- 8.Rogers PA, Milne BJ, Trounson AO. A model to show human uterine receptivity and embryo viability following ovarian stimulation for in vitro fertilization. J In Vitro Fert Embryo Transf. 1986;3:93–98. doi: 10.1007/BF01139353. [DOI] [PubMed] [Google Scholar]
- 9.Sher G, Dodge S, Maassarani G, Knutzen V, Zouves C, Feinman M. Management of suboptimal sonographic endometrial patterns in patients undergoing in-vitro fertilization and embryo transfer. Hum Reprod. 1993;8:347–349. doi: 10.1093/oxfordjournals.humrep.a138049. [DOI] [PubMed] [Google Scholar]
- 10.Bonilla-Musoles F, Raga F, Osborne NG, Castillo JC, Bonilla F Jr. Endometrial receptivity: evaluation with ultrasound. Ultrasound Q. 2013;29:3–20. doi: 10.1097/RUQ.0b013e318281b60a. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K, Kato H. Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril. 1997;67:256–260. doi: 10.1016/S0015-0282(97)81907-3. [DOI] [PubMed] [Google Scholar]
- 12.van Gestel I, IJland MM, Hoogland HJ, Evers JL. Endometrial wave-like activity in the non-pregnant uterus. Hum Reprod Update. 2003;9:131–138. doi: 10.1093/humupd/dmg011. [DOI] [PubMed] [Google Scholar]
- 13.Xu A, Li Y, Zhu L, Tian T, Hao J, Zhao J, Zhang Q. Inhibition of endometrial fundocervical wave by phloroglucinol and the outcome of in vitro fertilization. Reprod Biol. 2013;13:88–91. doi: 10.1016/j.repbio.2013.01.165. [DOI] [PubMed] [Google Scholar]
- 14.Ijland MM, Evers JL, Dunselman GA, van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril. 1996;65:746–749. doi: 10.1016/s0015-0282(16)58207-7. [DOI] [PubMed] [Google Scholar]
- 15.Khan MS, Shaikh A, Ratnani R. Ultrasonography and doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer. J Obstet Gynaecol India. 2016;66:377–382. doi: 10.1007/s13224-015-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riad ON, Hak AA. Assessment of endometrial receptivity using Doppler ultrasonography in infertile women undergoing intrauterine insemination. Gynecol Endocrinol. 2014;30:70–73. doi: 10.3109/09513590.2013.859668. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Bahadur A, Mittal S, Malhotra N, Bhatt A. Predictive value of endometrial thickness, pattern and sub-endometrial blood flows on the day of hCG by 2D doppler in in-vitro fertilization cycles: a prospective clinical study from a tertiary care unit. J Hum Reprod Sci. 2011;4:29–33. doi: 10.4103/0974-1208.82357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uysal S, Ozbay EP, Ekinci T, Aksüt H, Karasu S, Işık AZ, Soylu F. Endometrial spiral artery Doppler parameters in unexplained infertility patients: is endometrial perfusion an important factor in the etiopathogenesis? J Turk Ger Gynecol Assoc. 2012;13:169–171. doi: 10.5152/jtgga.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Mazny A, Abou-Salem N, Elshenoufy H. Doppler study of uterine hemodynamics in women with unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2013;171:84–87. doi: 10.1016/j.ejogrb.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Son JB, Jeong JE, Joo JK, Na YJ, Kim CW, Lee KS. Measurement of endometrial and uterine vascularity by transvaginal ultrasonography in predicting pregnancy outcome during frozen-thawed embryo transfer cycles. J Obstet Gynaecol Res. 2014;40:1661–1667. doi: 10.1111/jog.12406. [DOI] [PubMed] [Google Scholar]
- 21.Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. The role of endometrial and subendometrial blood flows measured by three-dimensional power Doppler ultrasound in the prediction of pregnancy during IVF treatment. Hum Reprod. 2006;21:164–170. doi: 10.1093/humrep/dei277. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, He Y, Wang Y, Zhu Q, Yang J, Zhao X, Sun Y. The role of three-dimensional power Doppler ultrasound parameters measured on hCG day in the prediction of pregnancy during in vitro fertilization treatment. Eur J Obstet Gynecol Reprod Biol. 2016;203:66–71. doi: 10.1016/j.ejogrb.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, Cohen-Bacrie C BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur Radiol. 2012;22:1023–1032. doi: 10.1007/s00330-011-2340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, Oliver C. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95:5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 25.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 26.Zollner U, Specketer MT, Dietl J, Zollner KP. 3D-endometrial volume and outcome of cryopreserved embryo replacement cycles. Arch Gynecol Obstet. 2012;286:517–523. doi: 10.1007/s00404-012-2332-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, Jin L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine (Baltimore) 2018;97:e9689. doi: 10.1097/MD.0000000000009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M. Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Steril. 2001;75:361–366. doi: 10.1016/s0015-0282(00)01695-2. [DOI] [PubMed] [Google Scholar]
- 29.Liang J, Li R, Lv XD, Qiao J. Research progress on ultrasonic evaluation of endometrial receptivity. Reprod Contracept. 2015;36:873–878. [Google Scholar]


