Abstract
Hepatic fibrosis is a chronic liver injury process, and its continuous development can lead to cirrhosis, hepatic failure and even hepatocellular carcinoma (HCC). Autophagy has attracted much attention because of its controversial role in the course of hepatic fibrosis. In this review, we introduce the mechanism related to noncoding RNAs and some of the signaling pathways that promote or inhibit fibrosis by affecting autophagy. Finally, we list some targets related to autophagy that enable hepatic fibrosis therapy and forecast its prospect in hepatic fibrosis. This review will provide new ideas in diagnosing and treating hepatic fibrosis, which will be helpful to reduce the incidence of cirrhosis and its complications.
Keywords: Hepatic fibrosis, autophagy, hepatic stellate cell, noncoding RNA, exosomes
The development of hepatic fibrosis
Hepatic fibrosis is a chronic liver injury process with many causes, such as long-term alcoholism, viral infection, obesity, and familial hereditary diseases [1,2]. Studies have shown that various cells participate in the progression of hepatic fibrosis, such as hepatocytes, cholangiocytes, bone marrow-derived cells, and especially hepatic stellate cells (HSCs) [3-5]. In this pathological process, pro-inflammatory and pro-fibrotic factors promote the activation and proliferation of these cells into myofibroblasts. These myofibroblasts can produce excessive extracellular matrix (ECM) [6,7] and finally lead to the occurrence of hepatic fibrosis, and eventually cause liver cirrhosis, liver failure and even HCC (Figure 1).
Figure 1.
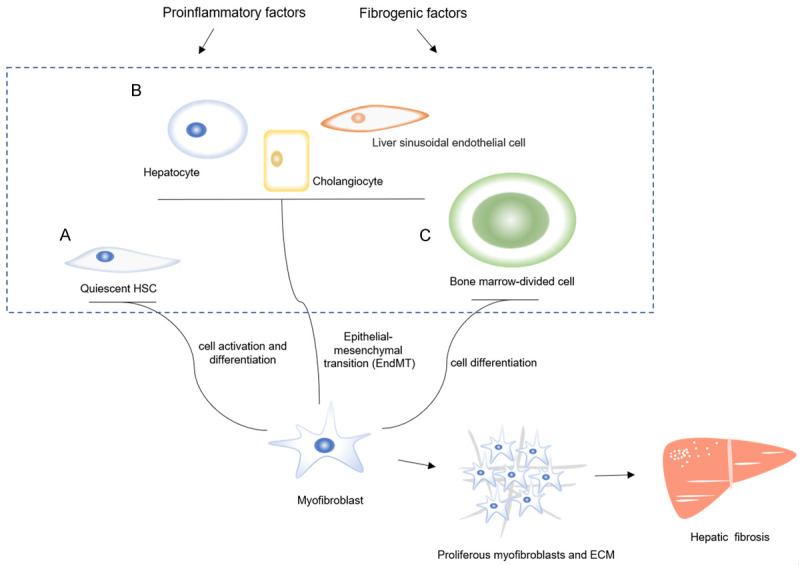
The development of hepatic fibrosis. The pro-inflammatory and pro-fibrotic factors could activate many of the different cell types, which include (A) the activation of quiescent HSCs, (B) the enhanced ability to acquiring myofibroblast characteristics (endothelial-mesenchymal transition, EndMT) in hepatocytes, cholangiocytes and liver sinusoidal endothelial cells, and (C) the differentiation of bone marrow-divided cells, which leads to these cells proliferation into myofibroblasts, and finally the myofibroblasts secret a large quantity of ECM. This process promotes the hepatic fibrosis.
Previous studies have suggested that hepatic fibrosis is a static process [8]. However, this finding is contrary to that of Dawood, who argues that hepatic fibrosis is a dynamic and reversible process [6]. Subsequently, a study analyzed the role of the retinoic acid signaling pathway in regulating the fibrogenic capacity of HSCs and found that the retinoic acid and PPAR-γ signaling pathways synergistically reverse hepatic fibrosis [9], suggesting that there is a reversible repair process occurring in hepatic fibrosis. Whether hepatic fibrosis is irreversible or reversible, it is the main pathological process of the irreversible liver diseases, such as cirrhosis and HCC. Thus, it is vital to inhibit the emergence of hepatic fibrosis.
Hepatic fibrosis and autophagy
Previous investigations have shown that autophagy plays an important role in the development of hepatic fibrosis [10]. To date, the role of autophagy in promoting or inhibiting hepatic fibrosis is still controversial. It is believed that, on the one hand, autophagy could participate in the digestion of lipid droplets and provide energy for the activation of HSCs, which plays a direct impact on promoting fibrosis; on the other hand, autophagy could inhibit the emergence of hepatic fibrosis through anti-inflammatory effects [11]. An increasing number of studies have suggested that there is a complex relationship between autophagy and hepatic fibrosis.
Autophagy promotes hepatic fibrosis
TGF-β1 is considered to be an essential factor in promoting HSC activation [12]. Ye et al. [13] showed that the expression of the autophagy-related protein LC3II/I was increased in HSCs after TGF-β1 treatment, which prompted HSC activation to induce autophagy and ultimately accelerate the process of hepatic fibrosis. In addition, a research compared the degree of hepatic fibrosis in mice with ATG5 deficiency and found that the mice without ATG5 deficiency had obvious hepatic fibrosis, which was not observed in the mice with ATG5 deficiency [14]. Therefore, it is speculated that autophagy can promote the emergence of hepatic fibrosis, while inhibiting autophagy may alleviate this disease.
Noncoding RNAs participate in autophagy and promote hepatic fibrosis
The lncRNA/miRNA/autophagy related-gene axis
Studies have shown that DNA methyltransferase 3A (DNMT3A) is involved in the regulation of autophagy and contains binding sites for miR-29b [15-17]. Xie et al. [18] found that the knockout of lncRNA-SNHG7, which is highly expressed in HCC, downregulated the expression of miR-29b and upregulated DNMT3A, which promoted HSC activation and autophagy. This result suggested that the lncRNA-SNHG7/miR-29b/DNMT3A axis was involved in autophagy-induced hepatic fibrosis. Previous studies found that endothelial cells also had the ability to acquire myofibroblast characteristics (endothelial-mesenchymal transition, EndMT), which further promoted the occurrence of fibrosis [19]. Studies found that the downregulation of lncRNA-Tug1, which has high expression in hepatic fibrosis, increased the level of miR-142-3p and decreased the autophagy gene ATG5, which weakened EndMT and autophagy [20,21]. This result indicated that the lncRNA-Tug1/miR-142-3p/ATG5 axis is involved in autophagy-induced hepatic fibrosis. According to the previous research, the levels of lncRNA-NEAT1 and the autophagy gene ATG9a were increased, but miR-29b was decreased in IGFBPrP1-induced hepatic fibrosis [22]. Moreover, lncRNA-NEAT1 also increased HSC autophagy. These results indicate that the lncRNA-NEAT1/miR-29b/ATG9a regulatory axis is involved in IGFBPrP1-induced HSC autophagy and activation. Studies have indicated that lncRNA-XIST is highly expressed in HCC [23]. High-mobility group box-1 (HMGB1) was discovered to induce autophagy [24]. And another research found that the activation of the lncRNA-XIST/miR-29b/HMGB1 axis promoted alcoholic liver fibrosis (ALF) injury and autophagy in the ALF mouse model [25].
MiRNAs/autophagy related-gene axis
MiR-29a is reduced in patients with liver cirrhosis [26]. A study found that overexpressed miR-29a is able to alleviate autophagy by lessening the levels of LC3BII and p-ULK, which leads to reduced liver damage and fibrosis in BDL mice [27]. MiR-30a is an important regulator in fibrosis and is downregulated in NASH [28-30]. There is research pointing out that the overexpression of miR-30a suppresses autophagy by inhibiting Beclin1 directly in TGF-β1-induced activated HSCs, which leads to the restraint of HSC proliferation and remits the extent of fibrosis [31]. In addition, guanine nucleotide-binding α-subunit (Gα) proteins promote G protein-coupled receptor (GPCR) activation, and the activation of autophagic processes requires many ligands of GPCRs that interact with Gα12 [32,33]. The overexpression of Gα12 activated HSCs and promoted ATG12-5 conjugation, which accelerated autophagy, but this effect was reversed by upregulating miR-16 in HSCs [34]. A previous study showed that miR-223 participated in various types of liver diseases, and the autophagy gene ATG7 is the direct target of miR-223 [35]. In particular, Wang et al. [36] found that exosomes derived from natural killer (NK) cells (NK-Exos) with high miR-223 expression inhibited the activation of HSCs and restrained autophagy by decreasing the level of ATG7.
In conclusion, the expression of these noncoding RNAs is changed in unhealthy livers. Further, noncoding RNAs have the ability to affect the level of autophagy and promote the degree of fibrosis by the lncRNA/miRNA/autophagy-related gene axis or miRNA/autophagy-related gene axis. The discovery of these noncoding RNAs provides more targets in diagnosing and treating hepatic fibrosis. Interestingly, we found that noncoding RNAs from exosomes mitigate fibrosis, which seems to provide a new method for clinical drug delivery in the treatment of hepatic fibrosis (Figure 2).
Figure 2.
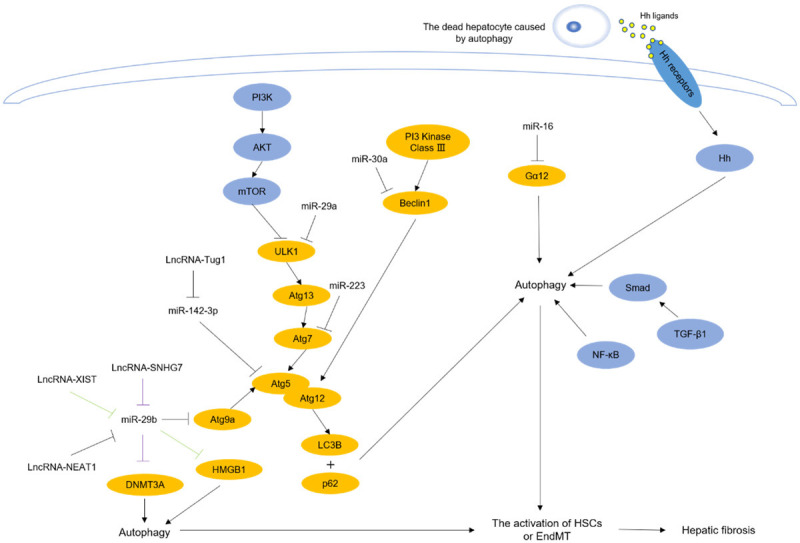
Non-coding RNAs and signaling pathways participate in autophagy-induced hepatic fibrosis. The expression of these non-coding RNAs and signaling pathways are altered in different liver diseases, and they could lead to the autophagy-related genes changed and promote the activation of HSCs or EndMT, which could induce hepatic fibrosis. The pathway marked in purple is LncRNA-SNHG7/miR-29b/DNMT3A, and the pathway marked in green is LncRNA-XIST/miR-29b/HMGB1.
Signaling pathways participate in autophagy-induced hepatic fibrosis
The TGF-β1/Smad signaling pathway
TGF-β1 is a key factor in inflammation, and TGF-β1/Smad signaling is a major pathway for fibrosis [12,37,38]. GNS561, an antifibrotic drug, inhibited LX-2 cell autophagy by destroying lysosomal function, and the phosphorylation of Smad2 (p-smad2) and Smad3 was reduced [39]. Another investigation [40] found that levo-tetrahydropalmatine (L-THP) reduced the formation of ECM and the autophagy biomarker levels of LC3 and Beclin1 by downregulating the TGF-β1/Smad pathway in CCl4-induced hepatic fibrosis mouse models. Another study discovered that isorhamnetin decreased the deposition of collagen and the expression of Beclin1 in bile duct ligation (BDL)-induced hepatic fibrosis mouse models by inhibiting massive macrophage recruitment and downregulating the TGF-β1/Smad signaling pathway [41]. Based on these studies, we conclude that inhibition of the TGF-β1/Smad signaling pathway can inhibit autophagy and ameliorate liver fibrosis.
The NF-κB signaling pathway
The NF-κB signaling pathway is widely regarded to participate in inflammation, and there is research indicating that the NF-κB signaling pathway is involved in the regulation of autophagy to reduce inflammation in the lung [42,43]. However, studies on the role of NF-κB in mediating autophagy to regulate hepatic fibrosis are limited. 3-Methyladenine (3-MA) is an autophagy inhibitor that inhibits NF-κB translocation into the nucleus, decreasing the infiltration of inflammatory cells and the deposition of collagen in CCl4-induced hepatic fibrosis models, which ameliorates hepatic fibrosis [44]. Ghrelin is discovered to suppress the level of LC3 and maintain the balance between matrix metalloproteinases-2 (MMP2) and tissue inhibitor of matrix metalloproteinases (TIMP1) in HSCs by decreasing the expression of NF-κB, which attenuates hepatic fibrosis [45]. Astaxanthin is an anti-inflammatory medicine and is found to inhibit autophagy by downregulating the expression and translation of NF-κB, which restrains the provision of energy for HSCs, playing a protective effect against hepatic fibrosis [46]. According to these studies, we conclude that downregulation of the NF-κB signaling pathway can inhibit autophagy and alleviate liver fibrosis.
The PI3K/AKT/mTOR signaling pathway
The PI3K/AKT signaling pathway is associated with various inflammatory diseases [47-49]. As a downstream signaling molecule of this pathway, mammalian target of rapamycin (mTOR) directly and negatively regulates cell autophagy [50]. A growing number of scholars are devoted to exploring the role of PI3K/AKT/mTOR signaling in the relationship between autophagy and hepatic fibrosis. Ginsenoside Rg3 (G-Rg3) reduces the level of p62 and the conversion from LC3a to LC3b in inflammatory inducer lipopolysaccharide (LPS)-induced HSC-T6 cells by enhancing the phosphorylation of PI3K and AKT, which promotes regression from hepatic fibrosis through inhibiting the survival of activated HSC-T6 [51]. Quercetin is an anti-inflammatory drug that can inhibit the number of autophagosomes in CCl4-induced hepatic fibrosis mouse models by activating the PI3K/AKT/mTOR signaling pathway, decreasing the formation of ECM, and preventing hepatic fibrosis [52]. Insulin-like growth factor binding protein-related protein 1 (IGFBPrP1) is a new profibrotic factor that interacts with TGF-β1, and it increased the expression of LC3B and Beclin1 in HSCs by downregulating the PI3K/AKT/mTOR signaling pathway [53]. As we have described, the enhancement of the PI3K/AKT/mTOR signaling pathway can inhibit cell autophagy, protect the liver and ameliorate liver fibrosis.
The Hedgehog signaling pathway
The Hedgehog (Hh) signaling pathway is reported to be involved in the activation of HSCs in nonalcoholic fatty liver disease (NAFLD) [54]. There is crosstalk between Hh and mTORC1, which indicates that the Hh signaling pathway is associated with cell autophagy [55]. Tao et al. [56] proposed that because of autophagy, dead hepatocytes can release a large number of Hh ligands, and those ligands bind to the relevant receptors on HSCs, ultimately activating the Hh signaling pathway and HSCs, leading to the development of hepatic fibrosis. This shows that the inhibition of the Hh signaling pathway could be a new target in the treatment of hepatic fibrosis; however, the study of the Hh signaling pathway in autophagy and hepatic fibrosis is limited.
We found that the aforementioned pathways mostly involve inflammation, and the activation of these pathways is able to enhance cell autophagy, thus inducing hepatic fibrosis. However, the role of the PI3K/AKT/mTOR signaling pathway is different from the others we introduced. This suggests that we could reduce the inflammatory response and inhibit autophagy by regulating these signaling pathways, which could be helpful in relieving hepatic fibrosis (Figure 2).
Autophagy inhibits hepatic fibrosis
Although many investigations have shown that autophagy promotes the development of hepatic fibrosis, some studies have pointed out that it could also inhibit hepatic fibrosis. Autophagic flux was used to measure the level of autophagy. An investigation observed the relationship of oroxylin A with hepatic fibrosis, and it was found that this drug increased autophagic flux, inhibited ECM deposition in HSCs, and alleviated hepatic fibrosis in rats induced by CCl4 [57]. In addition, increased autophagic flux could reduce the IL-1 secreted by Kupffer cells and further inhibited the activation of HSCs [58]. Thus, it is likely that autophagy also participates in the inhibition of hepatic fibrosis.
Noncoding RNA participates in autophagy-inhibited hepatic fibrosis
Contrary to the noncoding RNA that we described above, there are others involved in autophagy-inhibited hepatic fibrosis.
MiR-125a expression is higher in patients with liver cirrhosis than in those with fibrosis [59,60]. As its target, vitamin D receptor (VDR) has been proven to bind with its ligand 1, 25-(OH)2D3 and adjust autophagy by accommodating Beclin1 expression [61]. He et al. [62] observed that the silencing of miR-125a decreased α-SMA by restoring the level of VDR and autophagic flux in CCl4-induced fibrotic mice. Deficiency of phosphatase and tensin homolog (PTEN) aggravates oxidative damage in hepatic fibrosis by increasing p62, which indicates that PTEN is associated with autophagy [63]. In addition, miR-20a is boosted in hepatic fibrosis and has a binding site for PTEN [64,65]. A research observed that decreased miR-20a increases the expression of PTEN and promotes the levels of Beclin1 and ATG7, which reduces the levels of AST, ALT and collagen in hepatic fibrosis mice [66]. Furthermore, exosomes from adipose-derived mesenchymal stem cells (ADSCs) with high expression of miR-181-5P have been found to downregulate the levels of collagen I, vimentin, α-SMA and fibronectin in the liver by enhancing HSC-T6 cell autophagy [67].
In conclusion, we summarized numerous noncoding RNAs that are related to autophagy-inhibited hepatic fibrosis. Similarly, they have an abnormal change in the liver with fibrosis and target these noncoding RNAs by reversing their aberrant expression in the liver might accelerate autophagy and cure hepatic fibrosis (Figure 3).
Figure 3.
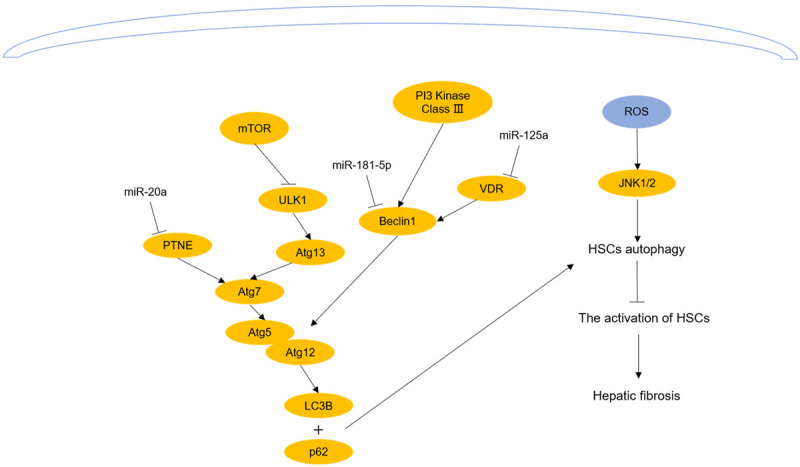
The non-coding RNAs and signaling pathways participate in autophagy-inhibited activation of HSCs. Reversing the expression of no-ncoding RNAs which are altered in different liver diseases or enhancing ROS could promote autophagy and inhibit the activation of HSCs, which could alleviate hepatic fibrosis.
Signaling pathways participate in autophagy-inhibited hepatic fibrosis
The ROS signaling pathways
Because ROS are important signaling molecules that regulate metabolism and inflammation, several studies have suggested that ROS participate in many kinds of inflammation [68-71]. Furthermore, ROS are connected with the early stage of autophagy [72]. There is evidence indicating that dihydroartemisinin (DHA) induces the cellular microenvironment with high ROS, enhances the phosphorylation of JNK1/2, which is the downstream target of ROS, increases autophagic flux in activated HSCs, decreases the secretion of inflammatory factors in the cellular supernatant, such as IL-4 and IL-6, and further inhibits inflammation-induced activation of HSCs [73]. However, in L02 human hepatocytes, glycochenodeoxycholic acid (GCDCA) promoted fibrosis by increasing ROS and inhibiting autophagic flux, which indicates that the low level of ROS in L02 human hepatocytes relieves hepatic fibrosis by increasing autophagy [74]. It is interesting that the role of autophagy in hepatic fibrosis is related not only to ROS concentration but also to cell type (Figures 3 and 4).
Figure 4.
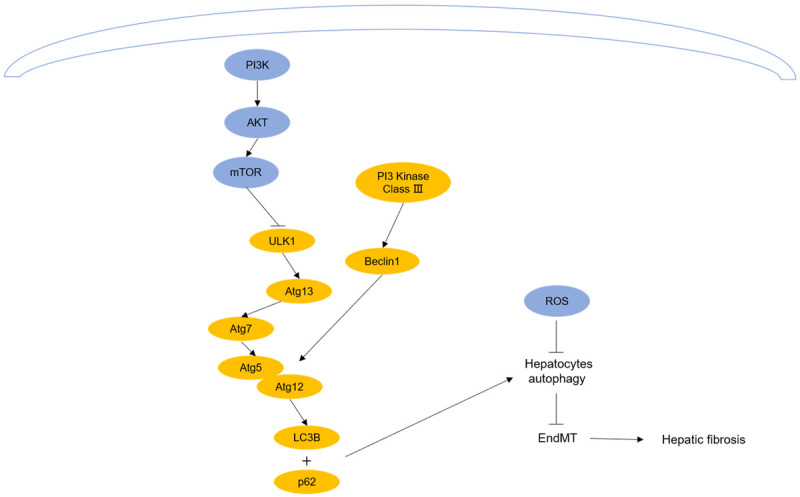
The non-coding RNAs and signaling pathways regulate autophagy and inhibit the EndMT in hepatocytes. Inhibiting PI3K/AKT/mTOR and ROS pathway could promote autophagy and inhibit the activation of HSCs, which could alleviate hepatic fibrosis.
The PI3K/AKT/mTOR signaling pathway
Moreover, there is other evidence confirming that the PI3K/AKT/mTOR signaling pathway participates in autophagy-inhibited fibrosis. Kong et al. [75] revealed that curcumin increased autophagic vacuoles in hepatocytes by downregulating the PI3K/AKT/mTOR signaling pathway, which led to the inhibition of the EndMT in hepatocytes and alleviation of hepatic fibrosis. Another investigation substantiated that the strengthening of hepatocellular autophagy was able to reduce the level of AST in fibrotic mice by inhibiting the PI3K/AKT/mTOR signaling pathway [66]. Unlike studies showing that the PI3K/AKT/mTOR signaling pathway mediates autophagy and promotes the activation of HSCs, this pathway may remit fibrosis by regulating autophagy in hepatocytes (Figure 4).
Targets in hepatic fibrosis therapy
At present, there is no effective treatment for hepatic fibrosis, but autophagy can be a new target. According to previous studies, we can identify some targets related to autophagy in hepatic fibrosis therapy.
Targets in noncoding RNAs
Many noncoding RNAs related to autophagy could be targets in the treatment of hepatic fibrosis.
Downregulation of the lncRNA/miRNA/autophagy-related gene axis, such as the lncRNA-SNHG7/miR-29b/DNMT3A axis, lncRNA-Tug1/miR-142-3p/ATG5 axis, lncRNA-NEAT1/miR-29b/ATG9a axis, and lncRNA-XIST/miR-29b/HMGB1 axis, and upregulation of the miR-30a/Beclin1 axis, miR-16/Gα12, and miR-29a/LC3B are capable of relieving hepatic fibrosis by inhibiting autophagy. The overexpressed miR-223 derived from the exosomes of NK cells also has the ability to treat hepatic fibrosis by inhibiting HSC autophagy.
Moreover, the downregulation of the miR-125a/VDR axis and miR-20a/PTNE axis could play a role in remitting hepatic fibrosis by promoting autophagy. However, the high expression of miR-181-5P in adipose-derived mesenchymal stem cell exosomes could suppress hepatic fibrosis by enhancing autophagy.
Targets in pathways
We found that the downregulation of the TGF-β1/Smad signaling pathway, NF-κB signaling pathway and Hedgehog signaling pathway can reduce liver inflammation, inhibit HSC autophagy and further improve hepatic fibrosis. On the basis of the distinction in cell types, we have also discovered that the inhibition of autophagy by upregulating the PI3K/AKT/mTOR signaling pathway is able to restrain the activation of HSCs, but the suppression of this pathway in hepatocytes could inhibit the EndMT or inflammation by enhancing autophagy. In addition, accelerating the ROS pathway in HSCs could facilitate autophagy and relieve hepatic fibrosis, which is contrary to the ROS pathway in L02 cells.
Expectations
Approximately 2,000,000 people die from liver disease each year worldwide, of which 1 million die from cirrhosis and its complications [1]. As the early stage of liver cirrhosis, hepatic fibrosis is reversible. Therefore, inhibiting hepatic fibrosis has become a way to prevent serious liver disease. However, many investigations indicate that there is a compact relationship between autophagy and hepatic fibrosis, which indicates that autophagy could be a target in curing hepatic fibrosis.
From the aforementioned studies, we found that autophagy plays contradictory roles in the progression of hepatic fibrosis, and it is generally recognized that autophagy not only promotes fibrosis by digesting lipids and providing energy for the activation of HSCs but also inhibits fibrosis by resisting inflammation. Furthermore, we found that the activation of some inflammatory pathways, such as the TGF-β1/Smad and NF-κB signaling pathways, increases the partial inflammatory response, activates autophagy, induces the aggravation of liver injury and promotes the occurrence of hepatic fibrosis. However, there are few investigations on signaling pathways related to autophagy inhibiting fibrosis, but these pathways may still be related to inflammation. ROS are closely related to inflammation, and autophagy plays an antifibrotic role in HSCs with high ROS, whereas it plays an antifibrotic effect in L02 cells with low ROS. Interestingly, the inhibition of the PI3K/AKT/mTOR signaling pathway could activate HSC autophagy and accelerate hepatic fibrosis, yet this pathway also has the ability to facilitate hepatocyte autophagy, reduce the levels of serum AST and ALT, alleviate the degree of liver injury or the EndMT, and ease fibrosis. This suggests that the dual role of autophagy in hepatic fibrosis is not only associated with different signaling pathways but also depends on the type of effector cells.
Moreover, various liver diseases contribute to abnormal changes in different noncoding RNAs, and these noncoding RNAs could regulate the expression of autophagy-related genes directly, alter the level of cell autophagy, and subsequently affect the progression of hepatic fibrosis, especially the noncoding RNAs in exosomes. Exosomes secreted from cells are membranous structures that contain the specific proteins and RNAs from parent cells and transmit intercellular information [40]. In the latest investigations, exosomes have been found to be involved in adjusting the process of hepatic fibrosis. Because exosomes are a part of the human body itself, it will be a new mode of administration in antifibrosis, which could increase the bioavailability of the drug.
Liver biopsy is the gold standard for the diagnosis of hepatic fibrosis, but it does not have extensive application due to the invasiveness and cost, and there is still a lack of effective drugs to treat hepatic fibrosis, which creates a serious challenge in diagnosing and curing hepatic fibrosis. Although autophagy may have two distinct effects on hepatic fibrosis, an increasing number of studies indicate that autophagy could be a new target in the diagnosis and treatment of hepatic fibrosis, which will improve grievous clinical problems in the liver.
Disclosure of conflict of interest
None.
References
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Wang X, Zhang Y, Chen L, Hua L, Xu W. Pien-Tze-Huang ameliorates hepatic fibrosis via suppressing NF-κB pathway and promoting HSC apoptosis. J Ethnopharmacol. 2019;244:111856. doi: 10.1016/j.jep.2019.111856. [DOI] [PubMed] [Google Scholar]
- 3.Pan X, Shao Y, Wang F, Cai Z, Liu S, Xi J, He R, Zhao Y, Zhuang R. Protective effect of apigenin magnesium complex on H(2)O(2)-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm Biol. 2020;58:553–560. doi: 10.1080/13880209.2020.1772840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydın MM, Akçalı KC. Liver fibrosis. Turk J Gastroenterol. 2018;29:14–21. doi: 10.5152/tjg.2018.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Dawood RM, El-Meguid MA, Salum GM, El Awady MK. Key players of hepatic fibrosis. J Interferon Cytokine Res. 2020;40:472–489. doi: 10.1089/jir.2020.0059. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Ding C, Liu W, Liu X, Zhao Y, Zheng Y, Dong L, Khatoon S, Hao M, Peng X, Zhang Y, Chen H. Abscisic acid ameliorates oxidative stress, inflammation, and apoptosis in thioacetamide-induced hepatic fibrosis by regulating the NF-κB signaling pathway in mice. Eur J Pharmacol. 2020;891:173652. doi: 10.1016/j.ejphar.2020.173652. [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939–962. xi. doi: 10.1016/j.cld.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panebianco C, Oben JA, Vinciguerra M, Pazienza V. Senescence in hepatic stellate cells as a mechanism of liver fibrosis reversal: a putative synergy between retinoic acid and PPAR-gamma signalings. Clin Exp Med. 2017;17:269–280. doi: 10.1007/s10238-016-0438-x. [DOI] [PubMed] [Google Scholar]
- 10.Hung TM, Hsiao CC, Lin CW, Lee PH. Complex cell type-specific roles of autophagy in liver fibrosis and cirrhosis. Pathogens. 2020;9:225. doi: 10.3390/pathogens9030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Liu R, Wu J, Li X. Self-eating: friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics. 2020;10:7993–8017. doi: 10.7150/thno.47826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Yang Y. Activation of Nrf2/AREs-mediated antioxidant signalling, and suppression of profibrotic TGF-β1/Smad3 pathway: a promising therapeutic strategy for hepatic fibrosis - a review. Life Sci. 2020;256:117909. doi: 10.1016/j.lfs.2020.117909. [DOI] [PubMed] [Google Scholar]
- 13.Ye HL, Zhang JW, Chen XZ, Wu PB, Chen L, Zhang G. Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy. Life Sci. 2020;242:117175. doi: 10.1016/j.lfs.2019.117175. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy LD, Young AR, Pérez-Mancera PA, Nimmervoll B, Jaulim A, Chen HC, McIntyre DJO, Brais R, Ricketts T, Pacey S, De La Roche M, Gilbertson RJ, Rubinsztein DC, Narita M. A novel Atg5-shRNA mouse model enables temporal control of autophagy in vivo. Autophagy. 2018;14:1256–1266. doi: 10.1080/15548627.2018.1458172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Cai M, Wang J, Gao Q, Guo X, Jia X, Xu S, Zhu H. Decreased ovarian function and autophagy gene methylation in aging rats. J Ovarian Res. 2020;13:12. doi: 10.1186/s13048-020-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page A, Paoli P, Moran Salvador E, White S, French J, Mann J. Hepatic stellate cell transdifferentiation involves genome-wide remodeling of the DNA methylation landscape. J Hepatol. 2016;64:661–673. doi: 10.1016/j.jhep.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, Havelange V, Volinia S, Blum W, Rush LJ, Perrotti D, Andreeff M, Bloomfield CD, Byrd JC, Chan K, Wu LC, Croce CM, Marcucci G. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Wu Y, Liu S, Lai Y, Tang S. LncRNA-SNHG7/miR-29b/DNMT3A axis affects activation, autophagy and proliferation of hepatic stellate cells in liver fibrosis. Clin Res Hepatol Gastroenterol. 2021;45:101469. doi: 10.1016/j.clinre.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Ma K, Li C, Xu J, Ren F, Xu X, Liu C, Niu B, Li F. LncRNA Gm16410 regulates PM(2.5)-induced lung endothelial-mesenchymal transition via the TGF-β1/Smad3/p-Smad3 pathway. Ecotoxicol Environ Saf. 2020;205:111327. doi: 10.1016/j.ecoenv.2020.111327. [DOI] [PubMed] [Google Scholar]
- 20.Han X, Hong Y, Zhang K. TUG1 is involved in liver fibrosis and activation of HSCs by regulating miR-29b. Biochem Biophys Res Commun. 2018;503:1394–1400. doi: 10.1016/j.bbrc.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Huang XQ, Jiang YY, Li N, Wang J, Chen SY. LncRNA TUG1 regulates autophagy-mediated endothelial-mesenchymal transition of liver sinusoidal endothelial cells by sponging miR-142-3p. Am J Transl Res. 2020;12:758–772. [PMC free article] [PubMed] [Google Scholar]
- 22.Kong Y, Huang T, Zhang H, Zhang Q, Ren J, Guo X, Fan H, Liu L. The lncRNA NEAT1/miR-29b/Atg9a axis regulates IGFBPrP1-induced autophagy and activation of mouse hepatic stellate cells. Life Sci. 2019;237:116902. doi: 10.1016/j.lfs.2019.116902. [DOI] [PubMed] [Google Scholar]
- 23.Chang S, Chen B, Wang X, Wu K, Sun Y. Long non-coding RNA XIST regulates PTEN expression by sponging miR-181a and promotes hepatocellular carcinoma progression. BMC Cancer. 2017;17:248. doi: 10.1186/s12885-017-3216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zeng C, Zheng B, Liu C, Tang M, Jiang Y, Chang Y, Song W, Wang Y, Yang C. HMGB1-induced autophagy facilitates hepatic stellate cells activation: a new pathway in liver fibrosis. Clin Sci (Lond) 2018;132:1645–1667. doi: 10.1042/CS20180177. [DOI] [PubMed] [Google Scholar]
- 25.Xie ZY, Wang FF, Xiao ZH, Liu SF, Lai YL, Tang SL. Long noncoding RNA XIST enhances ethanol-induced hepatic stellate cells autophagy and activation via miR-29b/HMGB1 axis. IUBMB Life. 2019;71:1962–1972. doi: 10.1002/iub.2140. [DOI] [PubMed] [Google Scholar]
- 26.Yang YL, Wang FS, Lin HY, Huang YH. Exogenous therapeutics of microrna-29a attenuates development of hepatic fibrosis in cholestatic animal model through regulation of phosphoinositide 3-Kinase p85 alpha. Int J Mol Sci. 2020;21:3636. doi: 10.3390/ijms21103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YH, Yang YL, Huang FC, Tiao MM, Lin YC, Tsai MH, Wang FS. MicroRNA-29a mitigation of endoplasmic reticulum and autophagy aberrance counteracts in obstructive jaundice-induced fibrosis in mice. Exp Biol Med (Maywood) 2018;243:13–21. doi: 10.1177/1535370217741500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Niu X, Wang Y, Kong L, Wang R, Zhang Y, Zhao S, Nan Y. MiR-146a-5p suppresses activation and proliferation of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis through directly targeting Wnt1 and Wnt5a. Sci Rep. 2015;5:16163. doi: 10.1038/srep16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan CT, Li XX, Cheng QJ, Wang YH, Wang JH, Liu CL. MiR-30a regulates the atrial fibrillation-induced myocardial fibrosis by targeting snail 1. Int J Clin Exp Pathol. 2015;8:15527–15536. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Yang M, Lan H, Yu X. miR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am J Pathol. 2013;183:808–819. doi: 10.1016/j.ajpath.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Yu Y, Li S, Liu Y, Zhou S, Cao S, Yin J, Li G. MicroRNA-30a ameliorates hepatic fibrosis by inhibiting Beclin1-mediated autophagy. J Cell Mol Med. 2017;21:3679–3692. doi: 10.1111/jcmm.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–496. doi: 10.1152/ajpcell.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CL, Ho MC, Lee PH, Hsu CY, Huang WP, Lee H. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am J Physiol Cell Physiol. 2009;297:C451–458. doi: 10.1152/ajpcell.00586.2008. [DOI] [PubMed] [Google Scholar]
- 34.Kim KM, Han CY, Kim JY, Cho SS, Kim YS, Koo JH, Lee JM, Lim SC, Kang KW, Kim JS, Hwang SJ, Ki SH, Kim SG. Gα(12) overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J Hepatol. 2018;68:493–504. doi: 10.1016/j.jhep.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye D, Zhang T, Lou G, Liu Y. Role of miR-223 in the pathophysiology of liver diseases. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Wang Y, Quan J. Exosomal miR-223 derived from natural killer cells inhibits hepatic stellate cell activation by suppressing autophagy. Mol Med. 2020;26:81. doi: 10.1186/s10020-020-00207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bestion E, Jilkova ZM, Mège JL, Novello M, Kurma K, Pour STA, Lalmanach G, Vanderlynden L, Fizanne L, Bassissi F, Rachid M, Tracz J, Boursier J, Courcambeck J, Serdjebi C, Ansaldi C, Decaens T, Halfon P, Brun S. GNS561 acts as a potent anti-fibrotic and pro-fibrolytic agent in liver fibrosis through TGF-β1 inhibition. Ther Adv Chronic Dis. 2020;11:2040622320942042. doi: 10.1177/2040622320942042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Q, Cheng P, Wu J, Guo C. PPARγ/NF-κB and TGF-β1/Smad pathway are involved in the anti-fibrotic effects of levo-tetrahydropalmatine on liver fibrosis. J Cell Mol Med. 2021;25:1645–1660. doi: 10.1111/jcmm.16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu N, Feng J, Lu X, Yao Z, Liu Q, Lv Y, Han Y, Deng J, Zhou Y. Isorhamnetin inhibits liver fibrosis by reducing autophagy and inhibiting extracellular matrix formation via the TGF-β1/Smad3 and TGF-β1/p38 MAPK pathways. Mediators Inflamm. 2019;2019:6175091. doi: 10.1155/2019/6175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fordjour FA, Asiedu E, Larbi A, Kwarteng A. The role of nuclear factor kappa B (NF-κB) in filarial pathology. J Cell Commun Signal. 2021;15:185–193. doi: 10.1007/s12079-021-00607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying Y, Sun CB, Zhang SQ, Chen BJ, Yu JZ, Liu FY, Wen J, Hou J, Han SS, Yan JY, Yang ZS, Xiong L. Induction of autophagy via the TLR4/NF-κB signaling pathway by astragaloside IV contributes to the amelioration of inflammation in RAW264.7 cells. Biomed Pharmacother. 2021;137:111271. doi: 10.1016/j.biopha.2021.111271. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Yang H, Fan Y, Yang Y, Cao W, Jia Y, Cao Y, Sun K, Pang Z, Du H. 3-Methyladenine ameliorates liver fibrosis through autophagy regulated by the NF-κB signaling pathways on hepatic stellate cell. Oncotarget. 2017;8:107603–107611. doi: 10.18632/oncotarget.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao Y, Zhang S, Yu F, Li H, Guo C, Fan X. Ghrelin attenuates liver fibrosis through regulation of TGF-β1 expression and autophagy. Int J Mol Sci. 2015;16:21911–21930. doi: 10.3390/ijms160921911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen M, Chen K, Lu J, Cheng P, Xu L, Dai W, Wang F, He L, Zhang Y, Chengfen W, Li J, Yang J, Zhu R, Zhang H, Zheng Y, Zhou Y, Guo C. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-β1 expression and autophagy. Mediators Inflamm. 2014;2014:954502. doi: 10.1155/2014/954502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma R, Choi D, Chen AJ, Harrington CA, Wilson DJ, Grossniklaus HE, Dailey RA, Ng J, Steele EA, Planck SR, Korn BS, Kikkawa D, Czyz CN, Foster JA, Kazim M, Harris GJ, Edward DP, Al-Hussain H, Maktabi AMY, Alabiad C, Garcia A, Rosenbaum JT. Enrichment of IGF-1R and PPARγ signalling pathways in orbital inflammatory diseases: steps toward understanding pathogenesis. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2020-318330. bjophthalmol-2020-318330. [DOI] [PubMed] [Google Scholar]
- 48.Dou D, Liang J, Zhai X, Li G, Wang H, Han L, Lin L, Ren Y, Liu S, Liu C, Guo W, Li J. Oxytocin signalling in dendritic cells regulates immune tolerance in the intestine and alleviates DSS-induced colitis. Clin Sci (Lond) 2021;135:597–611. doi: 10.1042/CS20201438. [DOI] [PubMed] [Google Scholar]
- 49.Calvier L, Xian X, Lee R, Sacharidou A, Mineo C, Shaul PW, Kounnas MZ, Tsai S, Herz J. Reelin depletion protects against atherosclerosis by decreasing vascular adhesion of leukocytes. Arterioscler Thromb Vasc Biol. 2021;41:1309–1318. doi: 10.1161/ATVBAHA.121.316000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rashidi S, Mansouri R, Ali-Hassanzadeh M, Mojtahedi Z, Shafiei R, Savardashtaki A, Hamidizadeh N, Karimazar M, Nguewa P, Manzano-Román R. The host mTOR pathway and parasitic diseases pathogenesis. Parasitol Res. 2021;120:1–16. doi: 10.1007/s00436-021-07070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang S, Wang Y, Chen C, Li W. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis. 2020;11:454. doi: 10.1038/s41419-020-2597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu L, Zhang Q, Mo W, Feng J, Li S, Li J, Liu T, Xu S, Wang W, Lu X, Yu Q, Chen K, Xia Y, Lu J, Xu L, Zhou Y, Fan X, Guo C. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Zhang Q, Kong Y, Guo X, Zhang H, Fan H, Liu L. Insulin-like growth factor binding protein-related protein 1 activates primary hepatic stellate cells via autophagy regulated by the PI3K/Akt/mTOR signaling pathway. Dig Dis Sci. 2020;65:509–523. doi: 10.1007/s10620-019-05798-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Tsuchida T. Mechanisms of hepatic stellate cell activation as a therapeutic target for the treatment of non-alcoholic steatohepatitis. Nihon Yakurigaku Zasshi. 2019;154:203–209. doi: 10.1254/fpj.154.203. [DOI] [PubMed] [Google Scholar]
- 55.Larsen LJ, Møller LB. Crosstalk of hedgehog and mTORC1 pathways. Cells. 2020;9:2316. doi: 10.3390/cells9102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao Y, Wang N, Qiu T, Sun X. The role of autophagy and NLRP3 inflammasome in liver fibrosis. Biomed Res Int. 2020;2020:7269150. doi: 10.1155/2020/7269150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, Zhang Z, Yao Z, Wang L, Zhang F, Shao J, Chen A, Zheng S. Activation of autophagy is required for Oroxylin A to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. Int Immunopharmacol. 2018;56:148–155. doi: 10.1016/j.intimp.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 58.Lodder J, Denaës T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coppola N, Onorato L, Panella M, de Stefano G, Mosca N, Minichini C, Messina V, Potenza N, Starace M, Alessio L, Farella N, Sagnelli E, Russo A. Correlation between the hepatic expression of human microRNA hsa-miR-125a-5p and the progression of fibrosis in patients with overt and occult HBV infection. Front Immunol. 2018;9:1334. doi: 10.3389/fimmu.2018.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coppola N, Potenza N, Pisaturo M, Mosca N, Tonziello G, Signoriello G, Messina V, Sagnelli C, Russo A, Sagnelli E. Liver microRNA hsa-miR-125a-5p in HBV chronic infection: correlation with HBV replication and disease progression. PLoS One. 2013;8:e65336. doi: 10.1371/journal.pone.0065336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tavera-Mendoza LE, Westerling T, Libby E, Marusyk A, Cato L, Cassani R, Cameron LA, Ficarro SB, Marto JA, Klawitter J, Brown M. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc Natl Acad Sci U S A. 2017;114:E2186–E2194. doi: 10.1073/pnas.1615015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He W, Ni W, Zhao L, Wang X, Liu L, Fan Z. MicroRNA-125a/VDR axis impaired autophagic flux and contributed to fibrosis in a CCL4-induced mouse model and patients with liver cirrhosis. Life Sci. 2021;264:118666. doi: 10.1016/j.lfs.2020.118666. [DOI] [PubMed] [Google Scholar]
- 63.Petersen DR, Saba LM, Sayin VI, Papagiannakopoulos T, Schmidt EE, Merrill GF, Orlicky DJ, Shearn CT. Elevated Nrf-2 responses are insufficient to mitigate protein carbonylation in hepatospecific PTEN deletion mice. PLoS One. 2018;13:e0198139. doi: 10.1371/journal.pone.0198139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Zheng L, Ding Y, Li Q, Wang R, Liu T, Sun Q, Yang H, Peng S, Wang W, Chen L. MiR-20a induces cell radioresistance by activating the PTEN/PI3K/Akt signaling pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:1132–1140. doi: 10.1016/j.ijrobp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Dhar S, Kumar A, Rimando AM, Zhang X, Levenson AS. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget. 2015;6:27214–27226. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L, Mou Q, Wang Y, Zhu Z, Cheng M. Resveratrol contributes to the inhibition of liver fibrosis by inducing autophagy via the microRNA-20a-mediated activation of the PTEN/PI3K/AKT signaling pathway. Int J Mol Med. 2020;46:2035–2046. doi: 10.3892/ijmm.2020.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quan W, Liu HX, Zhang W, Lou WJ, Gong YZ, Yuan C, Shao Q, Wang N, Guo C, Liu F. Cardioprotective effect of rosmarinic acid against myocardial ischaemia/reperfusion injury via suppression of the NF-κB inflammatory signalling pathway and ROS production in mice. Pharm Biol. 2021;59:222–231. doi: 10.1080/13880209.2021.1878236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L, Chen J, Zhou Y, Zhang J, Chen L. Artesunate alleviates diabetic retinopathy by activating autophagy via the regulation of AMPK/SIRT1 pathway. Arch Physiol Biochem. 2021:1–8. doi: 10.1080/13813455.2021.1887266. [DOI] [PubMed] [Google Scholar]
- 70.Zhu W, Fang T, Zhang W, Liang A, Zhang H, Zhang ZP, Zhang XE, Li F. A ROS scavenging protein nanocage for in vitro and in vivo antioxidant treatment. Nanoscale. 2021;13:4634–4643. doi: 10.1039/d0nr08878a. [DOI] [PubMed] [Google Scholar]
- 71.Bajgai J, Xingyu J, Fadriquela A, Begum R, Kim DH, Kim CS, Kim SK, Lee KJ. Effects of mineral complex material treatment on 2,4-dinitrochlorobenzene-induced atopic dermatitis like-skin lesions in mice model. BMC Complement Med Ther. 2021;21:82. doi: 10.1186/s12906-021-03259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Guo M, Zhao S, Shao J, Zheng S. ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic Biol Med. 2016;101:272–283. doi: 10.1016/j.freeradbiomed.2016.10.498. [DOI] [PubMed] [Google Scholar]
- 74.Lan W, Chen Z, Chen Y, Tan M, Chen Y, Chen J, Chi X, Chen Y. Glycochenodeoxycholic acid impairs transcription factor E3 -dependent autophagy-lysosome machinery by disrupting reactive oxygen species homeostasis in L02 cells. Toxicol Lett. 2020;331:11–21. doi: 10.1016/j.toxlet.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 75.Kong D, Zhang Z, Chen L, Huang W, Zhang F, Wang L, Wang Y, Cao P, Zheng S. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy. Redox Biol. 2020;36:101600. doi: 10.1016/j.redox.2020.101600. [DOI] [PMC free article] [PubMed] [Google Scholar]


