Abstract
Objective:
To identify perinatal clinical diseases and treatments that are associated with the development of objectively diagnosed diffuse white matter abnormality (DWMA) on structural MRI at term-equivalent age in very preterm infants.
Study Design:
A prospective cohort of 392 very preterm infants (<33 weeks gestational age) were enrolled from five level III/IV NICUs between September 2016 and November 2019. Brain MRIs were collected at 39 to 45 weeks postmenstrual age (PMA) to evaluate DWMA volume. A pre-defined list of pertinent maternal characteristics, pregnancy/delivery data, and neonatal ICU data was collected for enrolled patients to identify antecedents of objectively diagnosed DWMA.
Results:
Of the 392 infants in the cohort, 377 (96%) had high quality MRI data. Their mean (SD) gestational age was 29.3 (2.5) weeks. In multivariable linear regression analyses, pneumothorax (p=.027), severe bronchopulmonary dysplasia (BPD) (p=.009), severe retinopathy of prematurity (ROP) (p<0.001), and male sex (p=.041) were associated with increasing volume of DWMA. The following factors were associated with decreased risk of DWMA: postnatal dexamethasone therapy for severe BPD (p=.004), duration of caffeine therapy for severe BPD (p = 0.009), and exclusive maternal milk diet at NICU discharge (p=.049).
Conclusions:
Severe ROP and BPD exhibited the strongest adverse association with development of DWMA. We also identified treatments and nutritional factors that appear protective against the development of DWMA that also have implications for the clinical care of very preterm infants.
Keywords: Magnetic Resonance Imaging, infant, preterm, epidemiology, etiology
Introduction
Improvements in the care of preterm infants have resulted in fewer instances of severe injury such as periventricular leukomalacia (PVL) and intraventricular hemorrhage (IVH)1. Even so, the prevalence of neurodevelopmental impairment (NDI) remains unacceptably high2,3. Brain MRI studies in preterm infants have identified diffuse, subtle abnormalities in brain maturation and signal abnormality that may be the modern antecedents of NDI4–8. Diffuse excessive high signal intensity (DEHSI) is defined as the presence of higher than normal signal intensity in the developing white matter, as seen on T2-weighted MRI1. It is detected in 50-80% of MRI scans of very preterm infants at approximately term-equivalent age9. Detailed diffusion MRI studies have identified myelination and axonal microstructural abnormalities in regions of the brain affected by DEHSI10,11. The only post-mortem analysis of infants with DEHSI identified pathology consistent with PVL, such as microgliosis and astrocytosis, in addition to distinct findings such as diffuse vacuolations and lack of necrosis12. It remains unclear if DEHSI represents a milder form of PVL or a distinct abnormality13,14. Volpe has speculated that DEHSI may be the imaging manifestation of underlying diffuse white matter gliosis without focal necrosis14.
Despite these pathological radiographic and histologic findings, only a few studies have explored which perinatal clinical factors contribute to the development of DEHSI, in order to further our understanding of this prevalent signal abnormality1,9,10,15,16. In one study, surgical ligation of patent ductus arteriosus (PDA) was identified as a potential risk factor for DEHSI10. Several other studies could not identify any antecedents of DEHSI; however, these studies were likely underpowered1,9,15. Additionally, these negative results may have stemmed from qualitative diagnosis of DEHSI, which is subjective and cannot be made with high reliability17–19. Not surprisingly, a recent meta-analysis of all DEHSI prognostic studies found that it was not significantly correlated with NDI20. The authors concluded that “additional studies using validated objective tools to define and quantify DEHSI are needed.” We reached the same conclusion in the past and developed an automated algorithm that can objectively and accurately quantify DEHSI21,22. Importantly, we compared the performance of subjectively and objectively diagnosed DEHSI at term with NDI at age 2 and found that objectively defined DEHSI was significantly correlated with cognitive and language development, whereas subjectively defined DEHSI was not6,8. Considering these key differences in correlation with important clinical outcome and the early neuropathological changes summarized above, we refer to the diffuse high signal abnormalities in periventricular and subcortical white matter, when objectively diagnosed, as diffuse white matter abnormality (DWMA). It is defined as the total volume of white matter exhibiting elevated signal intensity (details below) as compared to the surrounding white matter on term T2-weighted MRI21,22.
Previously, we identified severe retinopathy of prematurity (ROP) and severe bronchopulmonary dysplasia (BPD) as antecedent risk factors of objectively diagnosed DWMA16,23. However, these prior studies lacked sufficient power to examine less common antecedent factors and common pathophysiologic pathways (e.g. inflammation). Our objective was to identify antenatal and neonatal clinical factors that are independently associated with the development of objectively diagnosed DWMA at term in a large geographically-defined cohort of very preterm infants. We hypothesized that several modifiable clinical antecedent factors would be associated with the development of DWMA, as diagnosed on term MRI.
Designs & Methods
We enrolled a multicenter prospective cohort of 392 very preterm infants from five neonatal intensive care units (NICU) in the greater Cincinnati area. This included all four academic and community level III/IV NICUs in Cincinnati: 1) Cincinnati Children’s Hospital Medical Center (CCHMC), the primary academic referral center for high-risk neonates; 2) University of Cincinnati Medical Center, the primary academic referral center for high-risk pregnancies; 3) Good Samaritan Hospital; 4) St. Elizabeth’s Healthcare; and one community level III NICU in Dayton, Ohio, Kettering Medical Center. All very preterm infants – born at or before 32 weeks gestational age (GA) – who were cared for in one of these NICUs between September 2016 and November 2019 were eligible for inclusion. Infants were excluded if they met any of the following criteria: 1) known chromosomal or congenital anomalies affecting the central nervous system; 2) cyanotic heart disease; or 3) hospitalization and mechanical ventilation with greater than 50% supplemental oxygen at 45 weeks postmenstrual age (PMA) (because such sicker infants are less able to handle transport to MRI scanner). The Cincinnati Children’s Hospital Institutional Review Board approved the study, and the review boards of the other hospitals approved the study based on an established reliance agreement. Written informed consent was given by a parent or guardian of each study infant, after they were given at least 24 hours in which to review the consent and ask questions of the investigators.
Objective DWMA Identification and Quantification on MRI
Brain MRI was obtained between 39 and 45 weeks PMA. Images were collected during natural sleep with a 3T Philips Ingenia scanner and a 32-channel head coil located at Cincinnati Children’s Hospital, as previously described24. We used our feed and wrap technique to avoid the use of sedation. Axial T2-weighted image parameters were as follows: echo time (TE) 166 ms, repetition time (TR) 18567 ms, flip angle (FA) 90°, and voxel dimensions 1.0×1.0×1.0 mm; MPRAGE T1-weighted images (3D FFE): TR/TE/TI = 8.5/3.4/1610 ms, FA 13°, in-plane resolution = 1 × 1 × 1 mm; susceptibility weighted imaging: TE 5.4 ms, TE 55 ms, FA 13°, and voxel dimensions 1.0×1.0×1.5 mm.
Volume of DWMA was objectively quantified using our well-established published algorithm (Figure 1)22. Briefly, we aligned the T2-weighted images, acquired using 1 mm resolution, via the anterior and posterior commissures, performed bias field and signal inhomogeneity correction in SPM1225, and segmented brain tissues using a unified segmentation algorithm with spatial priors obtained from a neonatal probabilistic atlas. This produced voxel labels and volumes for the three main tissue classes. The central, subcortical, and periventricular white matter regions in which DWMA is typically visible were isolated between the brain slice immediately above the third ventricle inferiorly and superiorly above the lateral ventricles up to the most superior slice where white matter remains contiguous (i.e. not interrupted by gray matter) (on axial orientation). Restricted to these brain regions, we labeled DWMA as any white matter voxels with signal intensity more than 1.8 SD above the mean intensity for all grey and white matter voxels4. Due to the poor neonatal image contrast-to-noise ratio, our automated software is prone to falsely labeling a few isolated voxels as DWMA, typically one to three voxels in white matter-gray matter border regions. We manually removed these false positives to improve accuracy. Finally, to correct for the effect of varying head sizes, we computed normalized DWMA volume by dividing total DWMA volume by total white matter volume.
Figure 1. Semiautomated segmentation of diffuse white matter abnormality (DWMA) in the periventricular and central white matter.
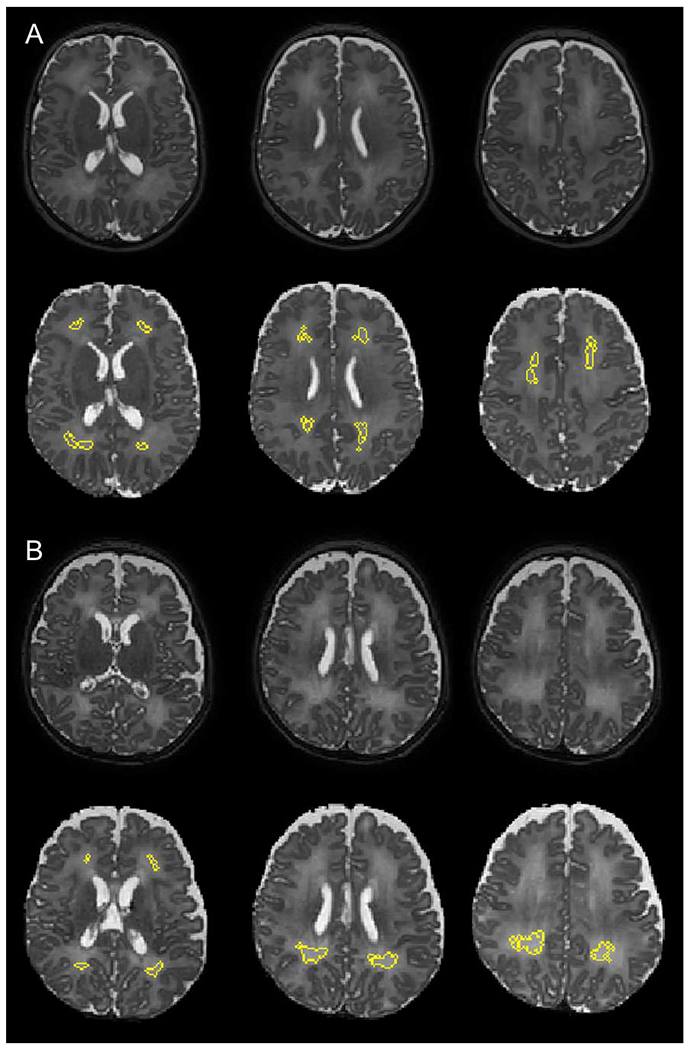
A. Top three panels display raw axial T2-weighed magnetic resonance images through the central part of the brain from a 28 week very preterm boy. Higher signal intensity can be appreciated in the white matter from the surrounding gray matter. Bottom three panels display the segmented (yellow) moderate degree of DWMA white matter regions. B. Top three panels display raw axial T2-weighed MR images from a 30 week very preterm boy. Higher signal intensity can be appreciated in the periventricular and central white matter from the surrounding gray matter. Bottom three panels display the segmented (yellow) severe DWMA.
MRI Scoring
A single pediatric neuroradiologist, while masked to clinical history, performed all qualitative and quantitative assessments of MR images. Brain abnormalities were defined using a standardized scoring system developed by Kidokoro et al26 , as previously described with high reliability24. Cerebral white matter abnormality was graded on a scale between zero and four for: 1) cystic degeneration, 2) focal signal abnormalities, 3) delayed myelination, 4) thinning of the corpus callosum, 5) dilated lateral ventricles, and 6) reduction of WM volume.
Clinical Antecedents
Trained research staff collected a pre-defined list of maternal characteristics, pregnancy/delivery data, and infant data beginning at birth and ending at NICU discharge or study MRI examination, whichever occurred first. All variables were as previously defined (Table 3: online only)16. We could not examine the effect of surgical ligation for PDA, as this was performed in only three subjects. All brain MRI scans were read by a pediatric neuroradiologist as previously described24, using an established neonatal MRI quantification system to derive a global abnormality score26.
Table 3:
Definition of antepartum, intrapartum, and postnatal clinical and demographic maternal or infant factors examined for their relationship with objectively diagnosed diffuse white matter abnormality.
| Variable | Definition |
|---|---|
| Antepartum | |
| Prenatal care | Did mother receive three or more visits and prenatal care started prior to third trimester |
| Maternal progesterone | Maternal progesterone therapy use during current pregnancy |
| Maternal hypertension | Chronic or pregnancy induced hypertension recorded in the mother’s chart or if maternal systolic blood pressure above 140 or diastolic over 90 mmHg was recorded prior to or during the present pregnancy on at least 2 occasions. |
| Gestational diabetes | Any gestational diabetes diagnosed during current pregnancy |
| Antepartum hemorrhage | Any placenta previa, abruption or threatened abortion resulting in bleeding documented after 20 weeks of pregnancy. |
| Multiple birth | Multiple gestation pregnancy (twins or greater, live or stillborn) |
| Intrapartum/Birth | |
| Clinical chorioamnionitis | Clinical chorioamnionitis documented in mother’s medical records |
| Histologic chorioamnionitis | Chorioamnionitis documented on the placental pathology report or findings defined by the Stillbirth Collaborative Research Network pathology protocol. |
| Funisitis | Inflammation of umbilical cord documented on the placental pathology report. |
| Amniotic fluid inflammation | If an amniocentesis was done within a week of delivery that showed biomarkers of inflammation/inflammatory cells (e.g. IL-6, IL-8, CRP, TNF-alpha, TGF-beta). |
| Antenatal steroids | Exposure to one or more antenatal doses of corticosteroids (e.g. betamethasone, dexamethasone) during this pregnancy. |
| Maternal antibiotics within 72 hours prior to birth | Any maternal antibiotics used within 72 hours prior to birth |
| Maternal magnesium | Mother administration of magnesium sulfate during the admission prior to this delivery. |
| Delayed cord clamping | If infant had documented delayed cord clamping. |
| Cord milking | If infant had documented cord milking. |
| Gestational age (GA) | Gestational age in completed weeks and days by best obstetric estimate in the following hierarchy – early prenatal ultrasound, last menstrual period, second trimester ultrasound; the best neonatologist estimate if best obstetric estimate was unavailable. |
| Birth weight z-score | Calculated using the Fenton 2013 Growth Calculator for Preterm Infants: https://peditools.org/fenton2013/ |
| Small for gestational age (SGA) | Gestational age below the 10th percentile for birth weight as defined by Fenton 2013 Growth Calculator for Preterm Infants: https://peditools.org/fenton2013/ |
| Resuscitation/stabilization in delivery room requiring intubation | Tracheal intubation in delivery room to provide positive pressure ventilation. |
| Intrapartum/Birth | |
| Resuscitation/stabilization in delivery room requiring epinephrine | Epinephrine delivered intravenously or intratracheally for resuscitation. |
| Sex | Male, female, or ambiguous sex of the infant |
| Low Apgar score at 5 minutes | Five minute Apgar score of <5 |
| Postnatal/NICU | |
| Pneumothorax | Documented pneumothorax or collection of air in the pleural space with displacement of the lung away from the chest wall. |
| Pulmonary hemorrhage | Bright red blood per endotracheal tube associated with clinical deterioration. |
| Postnatal dexamethasone for bronchopulmonary dysplasia | Systemic dexamethasone to prevent or treat bronchopulmonary dysplasia. Does not include steroids for extubation and/or stridor, or inhaled steroids. |
| Patent ductus arteriosus (PDA) | Echocardiographic evidence of PDA with documentation of left to right shunting or clinical evidence by continuous murmur, hyperdynamic precordium, bounding pulses, wide pulse pressure, congestive heart failure, chest X-ray changes, and/or increase oxygen requirement. |
| PDA closure surgical procedure | A cardiac catheterization or surgical ligation for PDA closure |
| Prophylactic indomethacin | Use of indomethacin within 24 hours of birth |
| Cyclooxygenase inhibitor therapy | Receipt of one or more doses of treatment ibuprofen or indomethacin to close a diagnosed PDA. |
| Caffeine therapy | Use of caffeine during NICU stay, regardless of indication |
| Duration of caffeine | Duration of caffeine use prior to brain MRI |
| Pulmonary hypertension | Clinical or echocardiographic diagnosis of pulmonary hypertension |
| Lowest mean blood pressure in the first 24 hours | the lowest mean arterial blood pressure in the first 24 hours after birth in mm Hg |
| Transitional hypotension requiring therapy | If infant was treated for low blood pressure in the first 24 hours after birth with volume or vasoactive medications |
| Type of nutrition near term-equivalent age | Record type of infant nutrition - maternal milk only, formula only, or both at time of NICU discharge or MRI scan, whichever came first |
| Late onset sepsis | Culture positive blood or CSF infection after the first week of life |
| Total days of total parenteral nutrition support | Number of days in which the infant received parenteral alimentation including amino acids or lipid solution |
| Highest bilirubin level | Highest level of total bilirubin recorded. |
| Necrotizing enterocolitis | Proven Bell Stage II or III necrotizing enterocolitis (NEC) |
| NEC requiring surgery | Bell Stage IIIB NEC requiring surgery |
| Surgery for spontaneous intestinal perforation or NEC | Spontaneous gastrointestinal perforation or NEC requiring surgery |
| Surgery requiring general anesthesia | Gastrointestinal surgery for NEC or spontaneous perforation, PDA ligation surgery, or any other major surgery (e.g. fundoplication, ventricular shunt) requiring general anesthesia and performed prior to brain MRI. |
| Postnatal/NICU | |
| Retinopathy of prematurity | If ROP diagnosed prior to discharge or MRI (any stage) in either eye in any of the examinations |
| Bronchopulmonary dysplasia | Any need for respiratory support (nasal cannula or higher) as defined by Jensen EA et al. (Am J Respir Crit Care Med. 2019; 200(6): 751–759) at 36 weeks postmenstrual age. |
| Severe retinopathy of prematurity (ROP) | ROP stage 3 or worse or plus disease (enlargement of the posterior veins of the retina and tortuous arterioles) noted in either eye or need for treatment, including laser, surgery, or Avastin injections. |
| Severe bronchopulmonary dysplasia | Grade 2, nasal cannula >2 L/min or noninvasive positive airway pressure or higher BPD level as defined by Jensen EA, et al (Am J Respir Crit Care Med. 2019; 200(6): 751–759) at 36 weeks postmenstrual age. |
| Total days of mechanical ventilation | Number of days of conventional or high frequency mechanical ventilation administration prior to NICU discharge or time of MRI, whichever came first. |
| Total days of positive pressure | Number of days on which the infant received nasal continuous positive airway pressure, nasal intermittent positive airway pressure, high flow nasal cannula (define as flow 3L or higher), and/or mechanical ventilation prior to NICU discharge or time of MRI, whichever came first. |
| Abnormal head ultrasound after 35 weeks postmenstrual age | Any parenchymal abnormality (e.g., hemorrhage, white matter injury, ventriculomegaly, persistent echodensity or echolucency) in cerebral or cerebellar regions on cranial ultrasound performed after 35 weeks postmenstrual age. |
| White matter abnormality score on term MRI | White matter abnormality was graded on a scale between zero and four for six variables per Kidokoro H, et al. 2013 (http://dx.doi.org/10.3174/ajnr.A3521): 1) cystic degeneration, 2) focal signal abnormalities, 3) delayed myelination, 4) thinning of the corpus callosum, 5) dilated lateral ventricles, and 6) reduction of WM volume. |
Statistical Analysis
DWMA volume data was skewed and was thus transformed by taking its cubic root. As described previously16, we examined the association between approximately 50 antenatal, intrapartum, and postnatal clinical factors with normalized DWMA volume in bivariate linear regression analyses. Variables that were correlated with DWMA volume (p<0.10) in bivariate analyses were entered into a multivariable linear regression model in a manual backward stepwise fashion, to evaluate their independent association with DWMA. In addition, we used knowledge of prior literature and biological plausibility to guide variable selection. Because postnatal covariates can overshadow antepartum or intrapartum variables that may be causative, we created multivariable regression models in which we ordered clinical factors temporally, so that the earliest occurring factors were entered first and could not be displaced by later occurring covariates16,27.
To control for variation in clinical care practices between the five NICUs, we included NICU/Center as a covariate in the final model. Main effects and interactions were evaluated. All analyses were adjusted for postmenstrual age at MRI scan. Two-sided p values <0.05 were considered to indicate statistical significance. We performed all analyses using STATA 16.0 (Stata Corp., College Station, TX).
Results
Of the original cohort of 392 infants, 15 could not be accurately segmented, eight because of excessive motion artifacts and seven due to moderate-severe ventriculomegaly/brain injury. Therefore, accurate DWMA data was available for 377 infants (96%). The mean (SD) gestational age was 29.31 (2.50) weeks. On term structural MRI, 33 (8.8%) infants had moderate-severe brain abnormality (global abnormality score >7) and 84 (22.3%) had mild abnormality (global abnormality score 4–7). The median (IQR) volume of normalized DWMA was 394.0 (89.0 – 1006.9) mm3 on term-equivalent age MRI. Twenty-four infants had no quantifiable DWMA (6.4%).
Table 1 summarizes key baseline antepartum, intrapartum, and postnatal maternal and infant clinical characteristics for our final cohort. In bivariate analyses (as a first step for selecting candidate variables for the multivariate model), adjusting for PMA at MRI scan, several antecedents were associated with normalized DWMA volume (p<.10), including maternal progesterone therapy (p=.053), male sex (p=.069), pneumothorax (p=.030), prophylactic indomethacin (p<.001), cyclooxygenase inhibitor therapy (ibuprofen/indomethacin) for PDA (p=.004), any BPD (p=.086), severe BPD (p=.041), postnatal dexamethasone for BPD (p<.001), duration of caffeine therapy (p=.085), severe ROP (p=.042), surgery requiring general anesthesia (p=.073), white matter abnormality score (p=.002), and NICU/Center (p=.014). Additionally, we identified a significant interaction between postnatal dexamethasone therapy and severe BPD (p<.001) and between duration of caffeine therapy and severe BPD (p=.006).
Table 1.
Distributions of important antenatal, intrapartum, and postnatal clinical factors prior to MRI at term-equivalent age in a multicenter cohort of very preterm infants.
| Perinatal Clinical Factors* | All Infants (N=377) |
|---|---|
| Maternal diabetes | 44 (11.7%) |
| Histologic chorioamnionitis† | 104 (30.6%) |
| Maternal progesterone therapy | 73 (19.4%) |
| Antenatal steroids (any) | 348 (92.3%) |
| Antenatal magnesium therapy | 316 (83.8%) |
| Gestational age (weeks), median (range) | 29.7 (23.0–32.9) |
| Birth weight (grams), mean (SD) | 1302 (452) |
| Male sex | 198 (52.5%) |
| Apgar score <5 at 5 minutes§ | 48 (12.9%) |
| Pneumothorax | 25 (6.6%) |
| Prophylactic indomethacin therapy | 15 (4.0%) |
| Cyclooxygenase inhibitor therapy | 36 (9.6%) |
| Caffeine therapy | 266 (70.6%) |
| Duration of caffeine therapy in infants with severe BPD, median (range) | 62 (0–89) |
| Lowest mean blood pressure in first 24 hours (mm Hg), mean (SD)** | 31.4 (7.4) |
| Transitional hypotension requiring volume or vasoactive therapy | 15 (4.0%) |
| Medical NEC | 11 (2.9%) |
| Surgery for necrotizing enterocolitis or spontaneous intestinal perforation | 9 (2.4%) |
| Culture positive late onset sepsis | 41 (10.9%) |
| Patent ductus arteriosus | 94 (24.9%) |
| Severe retinopathy of prematurity | 18 (4.8%) |
| Bronchopulmonary dysplasia (any severity) | 154 (40.9%) |
| Severe bronchopulmonary dysplasia | 65 (17.2%) |
| Postnatal dexamethasone for severe bronchopulmonary dysplasia | 31 (8.2%) |
| Surgery requiring general anesthesia | 42 (11.1%) |
| White matter abnormality score on term MRI, median (range) | 1 (0-15) |
| Moderate-severe abnormality on term MRI | 33 (8.8%) |
| Postmenstrual age at MRI scan (weeks), mean (SD) | 42.7 (1.4) |
All values are N (%), unless otherwise noted.
Pathology not performed in 27 infants;
Data missing for 4 infants;
Data missing for 7 infants
In multivariable linear regression analyses, controlling for PMA at MRI scan and NICU/Center, several postnatal variables remained significant in the final model, including infant sex, pneumothorax, severe ROP, severe BPD, postnatal dexamethasone for severe BPD (interaction term), duration of caffeine therapy for severe BPD (interaction term), exclusive maternal breast milk nutrition at NICU discharge, and white matter abnormality score (Table 2). Both severe ROP and severe BPD increased the risk of DWMA. Conversely, postnatal steroid and caffeine therapy for severe BPD reduced the risk of DWMA (Table 2). The addition of gestational age (p=.854) did not change the significance of the other variables in the final model. Replacing our primary outcome variable – DWMA volume normalized by white matter volume – with either DWMA volume normalized by combined white and gray matter volume or uncorrected DWMA volume did not change the significance level or regression parameters of the covariates by more than 5%.
Table 2.
Final multivariable linear regression model displaying the coefficients of several clinical antecedents that were associated with the development of diffuse white matter abnormality (DWMA), which was objectively-defined on structural brain MRI at term-equivalent age.
| Clinical Antecedent | Coefficient (95% CI)* | P Value |
|---|---|---|
| Pneumothorax | 0.0328 (0.0038, 0.0618) | 0.027 |
| Severe bronchopulmonary dysplasia (BPD) | 0.0570 (0.0141, 0.0999) | 0.009 |
| Severe retinopathy of prematurity | 0.0659 (0.0305, 0.1014) | <0.001 |
| Dexamethasone*severe BPD (interaction) | −0.0551 (−0.0928, −0.0174) | 0.004 |
| Duration of caffeine*severe BPD (interaction) | −0.0008 (−0.0016, −0.0001) | 0.029 |
| Exclusive maternal milk at NICU discharge | −0.0237 (−0.0472, −0.0002) | 0.049 |
| White matter abnormality score on term MRI | −0.0047 (−0.0081, −0.0014) | 0.006 |
| Male sex | 0.0151 (0.0006, 0.0296) | 0.041 |
| Postmenstrual age at MRI scan | −0.0266 (−0.0322, −0.0211) | <0.001 |
| Center | −0.0098 (−0.0173, −0.0023) | 0.011 |
Normalized volume of DWMA was transformed by taking its cubic root. Therefore, the coefficients represent approximately the cubic root change in DWMA volume for one unit change in the clinical antecedent, while holding the other independent variables constant.
Of the 65 infants with severe BPD, 39 were treated with postnatal dexamethasone, all using the published Dexamethasone A Randomized Trial (DART) protocol, which results in a cumulative dosing of 0.89 mg/kg of dexamethasone administered over 10 days28. Nine of these infants received a second course of DART, and one infant received three total courses. The median (range) duration of caffeine therapy was 62 (0-89) days for infants with severe BPD. At NICU discharge or study MRI, whichever came first, 42 (11.1%) were receiving their mother’s breast milk exclusively, 165 (43.8%) were receiving a combination of mother’s milk and preterm infant formula, and the remaining 170 (45.1%) were exclusively receiving infant formula. Replacing exclusive mother’s milk in the model with exclusive formula at discharge resulted in a beta coefficient of .01431 (95% CI: −.00036, .02898; p=.056), which represents a significant trend towards an opposite effect on DWMA volume.
We did not find a significant relationship in bivariate or multivariable analyses between DWMA and any of the following key clinical variables: antenatal corticosteroid therapy, antenatal magnesium therapy, clinical or histologic chorioamnionitis, delayed cord clamping, low 5-minute Apgar score, transitional hypotension requiring volume or vasoactive therapy, PDA, sepsis, antibiotics, surgery due to necrotizing enterocolitis (NEC) or spontaneous intestinal perforation (SIP), or duration of total parenteral nutrition.
Discussion
Studying a well-characterized and geographically-defined cohort of very preterm infants, we identified several new independent perinatal clinical factors that antecede the development of DWMA at term-equivalent age. We also externally validated the previously-reported association between both severe ROP and severe BPD and increased DWMA volume16. These two diseases exhibited the largest effect on DWMA volume. Interestingly, postnatal dexamethasone therapy and caffeine therapy, the two most effective drugs for reducing BPD risk, were associated with a decreased DWMA risk when administered to very preterm infants with severe BPD. These novel associations have not been reported previously. They further strengthen the observed adverse association between severe BPD and DWMA. Pneumothorax and an exclusive diet of maternal milk represent two additional factors that have not been previously reported. Each of the new antecedents we uncovered has been associated with NDI, but the way in which they are mediated by early brain development/injury has not been fully delineated.
This is the third study to identify severe ROP as a key antecedent of DWMA16,23. Development of severe ROP is among the most prominent neonatal factors associated with NDI, independent of structural injury on cranial ultrasound29–32. Even qualitative structural MRI studies have not been able to mechanistically explain this association. Recently, we identified a negative association between ROP and automatically-quantified sulcal depth, a measure of brain maturation, on term MRI33. The same factors that appear to damage the immature retina in infants that develop ROP – oxidant injury and inflammation – are likely also injuring the immature brain34–36. The association with DWMA suggests an additional mechanism by which ROP is causally associated with NDI29,30.
Similar to severe ROP, we previously reported a robust association between severe BPD and DWMA in a separate independent cohort16. Unlike this prior report, which was likely underpowered for less common conditions/treatments, in our current study we identified an interaction between severe BPD and postnatal dexamethasone therapy and an interaction between severe BPD and duration of caffeine therapy, both of which were associated with reduced DWMA volume. These findings are highly relevant for the clinical care of very preterm infants as they contribute to our understanding of how these drugs may improve long-term neurodevelopmental outcomes, particularly when administered to infants at high risk for severe BPD. For example, postnatal dexamethasone use, even at the relatively low doses used in our population (e.g. DART protocol28), has remained low since 2003, when the American Academy of Pediatrics recommended a moratorium due to concerns regarding NDI following dexamethasone therapy37. However, it soon become clear that NDI occurred primarily in treated infants at low baseline risk of BPD (<50%), typically during the first week of age, when such risk is difficult to estimate38. Conversely, treatment of infants at high baseline risk of BPD (>50-67%) results in a lower risk of cerebral palsy or death. Our finding of an association between dexamethasone treatment in infants with severe BPD and DWMA development suggests a beneficial mechanism of this corticosteroid.
The association of DWMA with duration of caffeine therapy in infants with severe BPD suggests that the proven long-term beneficial effects of caffeine on motor development39 in very preterm infants may be mediated through an early neuroprotective effect on DWMA development. Mechanistically, this benefit may be directly mediated by caffeine’s weak diuretic effects, via protection against intermittent hypoxemia40, and/or through adenosine receptor blockage and secondary anti-inflammatory effects41,42. Conversely, it may act indirectly by reducing the risk of BPD43. At the macroscopic level, the neuroprotective effects of caffeine and dexamethasone may be mediated through reduction of DWMA, which is an independent predictor of motor development at 3 years of age44. Our novel findings suggest that DWMA volume could potentially be used as a surrogate outcome measure in quality improvement efforts or new randomized trials, in order to assess the expanded use of new therapeutic dosing of caffeine or dexamethasone in infants with severe BPD.
The cognitive benefits of human milk in preterm infants were demonstrated in a meta-analysis of 11 cohort studies with short-term outcomes45 and in studies of preterm infants at school age and in adolescence46–48. We identified a significant protective effect of an exclusive maternal milk diet during the NICU period on DWMA development at term. These findings have not been reported previously and add to our growing understanding of the neuronal mechanisms by which maternal milk confers neurocognitive benefits. In quantitative MRI studies, Pogribna et al.49 identified dose-dependent improved microstructural maturation of the corpus callosum at term in breast milk-fed infants, while Belsa et al.50 reported significant benefits on the corpus callosum and several other white matter regions, including the periventricular white matter, in a smaller cohort of very preterm infants.
The bright signal abnormalities that are the hallmark of DWMA begin to decrease between 42 and 50 weeks PMA51. We found a similar pattern of decrease in DWMA with advancing age at MRI scan. Interestingly, we also observed a negative association between objectively-defined DWMA and a semi-quantitative measure of WM injury/abnormality. This association has not been previously reported and suggests that other white matter abnormalities/injuries may result from factors different that those associated with DWMA. Importantly, as with white matter abnormalities such as PVL and ventriculomegaly, objectively-defined DWMA is predictive of cognitive, language, and motor deficits at 2 to 3 years corrected age, a finding that has now been reported in two independent cohorts of preterm infants6,8,44. As stated above, a recent meta-analysis of subjectively diagnosed DEHSI did not find a significant association with NDI20. We speculate that subjective diagnosis is not predictive of NDI because studies: 1) exhibited poor inter- and intra-rater reliability; 2) used categorical rather than continuous outcome; 3) employed modest sample sizes17–19. Each of these factors reduces statistical power and biases study results towards the null. Furthermore, these studies were unable to validate their DEHSI diagnosis with any ground truth. We addressed these key limitations by using computer simulations to objectively quantitate DWMA and confirm its high accuracy and further validated our algorithm by correlating DWMA with several clinically meaningful outcomes, including long-term cognitive, language, and motor scores.
Both ROP and BPD share systemic inflammation, hypoxia, and hyperoxia as common mechanisms that could directly or indirectly result in brain injury. In addition to the well-known risk factors of hypoxia and hyperoxia, recent evidence from large epidemiologic investigations and experimental models implicate antenatal inflammation and postnatal infection/inflammation as strong risk factors for the development of ROP35,36,52–54. The large reduction in BPD incidence noted in randomized trials of postnatal corticosteroids and the lack of benefit observed following antioxidant therapies or tighter control of oxygen saturation, suggests that inflammation may be a significant causal factor in the development of BPD55–59. Our findings of reduced DWMA following treatment with dexamethasone, a potent anti-inflammatory drug, and caffeine, which also has anti-inflammatory properties41,42, further supports inflammation as a key mechanism in the development of DWMA. Conversely, we did not observe an association between DWMA and other pro-inflammatory conditions such as chorioamnionitis or postnatal sepsis. Human milk also has anti-inflammatory properties, as it contains molecules such as secretory immunoglobulin A, transforming growth factor beta, lactoferrin, and interleukin-10 that can protect the newborn against inflammation60,61. We also did not find an association with delayed cord clamping, low Apgar scores, transitional hypotension, or PDA, factors more closely associated with hypoxia-ischemia, suggesting that this may not be the predominant underlying mechanism in the development of DWMA.
The strengths of our study include a robust, geographically-based cohort, the use of an objective, validated algorithm to diagnose DWMA, and comprehensive examination of important perinatal variables known to be associated with NDI. Our study also has several weaknesses. We did not collect serum biomarkers of inflammation, hypoxia, or ischemia to further elucidate the molecular mechanisms underlying DWMA development. The rates of PDA ligation and surgery for NEC or SIP were low, and therefore we were underpowered to examine their associations with DWMA. Finally, our findings should only be viewed as associations rather than primary causes. Nevertheless, this study represents the largest and most rigorous examination of the antecedents of DWMA development in very preterm infants. The significant relationship between these novel antecedent factors and DWMA enhances our understanding of the mechanisms of their known adverse/protective effects on NDI. It further suggests that quality improvement and research interventions that target the underlying mechanisms that lead to ROP, pneumothorax, and BPD, or increased the therapeutic use of dexamethasone and caffeine in infants at high risk for BPD, may result in a lower burden of DWMA and subsequently in reduced rates of NDI.
Supplementary Material
Acknowledgements
We sincerely thank the Cincinnati Infant Neurodevelopment Early Prediction Study (CINEPS) Investigators (see online Appendix). We also greatly appreciate the support of our NICU fellows, nurses, and staff, and, most importantly, all the study families that made this research possible.
Funding Source:
Supported by National Institutes of Health grants R01-NS094200 and R01-NS096037 from the National Institute of Neurological Disorders and Stroke (NINDS) to Dr. Nehal Parikh.
Abbreviations:
- DWMA
diffuse white matter abnormality
- DEHSI
diffuse excessive high signal intensity
- MRI
magnetic resonance imaging
- ROP
retinopathy of prematurity
- BPD
bronchopulmonary dysplasia
- PVL
periventricular leukomalacia
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to disclose. The funders played no role in the design, analysis, or presentation of the findings. The first author, NAP, wrote the first draft of the manuscript.
Data Statement:
All available data is included within this manuscript. Those wishing to obtain the data directly from the authors, can do so by emailing the corresponding author.
References
- 1.Jeon TY, Kim JH, Yoo SY, Eo H, Kwon JY, Lee J, et al. Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology. 2012;263(2):518–26. [DOI] [PubMed] [Google Scholar]
- 2.Torchin H, Morgan AS, Ancel P-Y. International comparisons of neurodevelopmental outcomes in infants born very preterm. Seminars in Fetal and Neonatal Medicine. 2020;25(3):101109. [DOI] [PubMed] [Google Scholar]
- 3.Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol. 2018;60(4):342–55. [DOI] [PubMed] [Google Scholar]
- 4.Engelhardt E, Inder TE, Alexopoulos D, Dierker DL, Hill J, Van Essen D, et al. Regional impairments of cortical folding in premature infants. Ann Neurol. 2015;77(1):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline JE, Illapani VSP, He L, Altaye M, Logan JW, Parikh NA. Early cortical maturation predicts neurodevelopment in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh NA, He L, Bonfante-Mejia E, Hochhauser L, Wilder PE, Burson K, et al. Automatically quantified diffuse excessive high signal intensity on MRI predicts cognitive development in preterm infants. Pediatr Neurol. 2013;49(6):424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cayam-Rand D, Guo T, Grunau RE, Benavente-Fernandez I, Synnes A, Chau V, et al. Predicting developmental outcomes in preterm infants: A simple white matter injury imaging rule. Neurology. 2019;93(13):e1231–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh NA, He L, Priyanka Illapani VS, Altaye M, Folger AT, Yeates KO. Objectively Diagnosed Diffuse White Matter Abnormality at Term Is an Independent Predictor of Cognitive and Language Outcomes in Infants Born Very Preterm. J Pediatr. 2020;220:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brostrom L, Bolk J, Padilla N, Skiold B, Eklof E, Martensson G, et al. Clinical Implications of Diffuse Excessive High Signal Intensity (DEHSI) on Neonatal MRI in School Age Children Born Extremely Preterm. PLoS One. 2016;11(2):e0149578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skiold B, Horsch S, Hallberg B, Engstrom M, Nagy Z, Mosskin M, et al. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Paediatr. 2010;99(6):842–9. [DOI] [PubMed] [Google Scholar]
- 11.Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–86. [DOI] [PubMed] [Google Scholar]
- 12.Parikh NA, Pierson CR, Rusin JA. Neuropathology Associated With Diffuse Excessive High Signal Intensity Abnormalities on Magnetic Resonance Imaging in Very Preterm Infants. Pediatr Neurol. 2016;65:78–85. [DOI] [PubMed] [Google Scholar]
- 13.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134(3):331–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpe JJ. Dysmaturation of Premature Brain: Importance, Cellular Mechanisms, and Potential Interventions. Pediatr Neurol. 2019;95:42–66. [DOI] [PubMed] [Google Scholar]
- 15.Murner-Lavanchy IM, Kidokoro H, Thompson DK, Doyle LW, Cheong JLY, Hunt RW, et al. Thirteen-Year Outcomes in Very Preterm Children Associated with Diffuse Excessive High Signal Intensity on Neonatal Magnetic Resonance Imaging. J Pediatr. 2019;206:66–71 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh NA, He L, Li H, Priyanka Illapani VS, Klebanoff MA. Antecedents of Objectively Diagnosed Diffuse White Matter Abnormality in Very Preterm Infants. Pediatr Neurol. 2020;106:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart AR, Smith MF, Rigby AS, Wallis LI, Whitby EH. Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol. 2010;40(8):1390–6. [DOI] [PubMed] [Google Scholar]
- 18.Calloni SF, Cinnante CM, Bassi L, Avignone S, Fumagalli M, Bonello L, et al. Neurodevelopmental outcome at 36 months in very low birth weight premature infants with MR diffuse excessive high signal intensity (DEHSI) of cerebral white matter. Radiol Med. 2015;120(11):1056–63. [DOI] [PubMed] [Google Scholar]
- 19.Morel B, Antoni G, Teglas JP, Bloch I, Adamsbaum C. Neonatal brain MRI: how reliable is the radiologist’s eye? Neuroradiology. 2016;58(2):189–93. [DOI] [PubMed] [Google Scholar]
- 20.Rath CP, Desai S, Rao SC, Patole S. Diffuse excessive high signal intensity on term equivalent MRI does not predict disability: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2020. [DOI] [PubMed] [Google Scholar]
- 21.He L, Parikh NA. Automated detection of white matter signal abnormality using T2 relaxometry: application to brain segmentation on term MRI in very preterm infants. Neuroimage. 2013;64:328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Parikh NA. Atlas-guided quantification of white matter signal abnormalities on term-equivalent age MRI in very preterm infants: findings predict language and cognitive development at two years of age. PLoS One. 2013;8(12):e85475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh NA, Lasky RE, Kennedy KA, McDavid G, Tyson JE. Perinatal factors and regional brain volume abnormalities at term in a cohort of extremely low birth weight infants. PLoS One. 2013;8(5):e62804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamm L, Patel M, Peugh J, Kline-Fath BM, Parikh NA, Cincinnati Infant Neurodevelopment Early Prediction Study G. Early brain abnormalities in infants born very preterm predict under-reactive temperament. Early Hum Dev. 2020;144:104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1994;2(4):189–210. [Google Scholar]
- 26.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34(11):2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315(2):81–6. [DOI] [PubMed] [Google Scholar]
- 28.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, Investigators DS. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117(1):75–83. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124–9. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts RS. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014;311(5):523–5. [DOI] [PubMed] [Google Scholar]
- 31.Msall ME, Phelps DL, DiGaudio KM, Dobson V, Tung B, McClead RE, et al. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Behalf of the Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics. 2000;106(5):998–1005. [DOI] [PubMed] [Google Scholar]
- 32.Beligere N, Perumalswamy V, Tandon M, Mittal A, Floora J, Vijayakumar B, et al. Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Semin Fetal Neonatal Med. 2015;20(5):346–53. [DOI] [PubMed] [Google Scholar]
- 33.Kline JE, Illapani VSP, He L, Altaye M, Parikh NA. Retinopathy of Prematurity and Bronchopulmonary Dysplasia are Independent Antecedents of Cortical Maturational Abnormalities in Very Preterm Infants. Sci Rep. 2019;9(1):19679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LEH. Pathogenesis of retinopathy of prematurity. Growth Hormone & IGF Research. 2004;14:140–4. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay S, Miloudi K, Chaychi S, Favret S, Binet F, Polosa A, et al. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci. 2013;54(13):8125–39. [DOI] [PubMed] [Google Scholar]
- 36.Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67(4):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr. 2014;165(6):1258–60. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG, et al. Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017;171(6):564–72. [DOI] [PubMed] [Google Scholar]
- 40.Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014;168(3):250–7. [DOI] [PubMed] [Google Scholar]
- 41.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23(2):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iris M, Tsou PS, Sawalha AH. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin Immunol. 2018;192:68–77. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21. [DOI] [PubMed] [Google Scholar]
- 44.Parikh NA, Harpster K, He L, Illapani VSP, Khalid FC, Klebanoff MA, et al. Novel diffuse white matter abnormality biomarker at term-equivalent age enhances prediction of long-term motor development in very preterm children. Sci Rep. 2020;10(1):15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;70(4):525–35. [DOI] [PubMed] [Google Scholar]
- 46.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7-8 years. Arch Dis Child Fetal Neonatal Ed. 2001;84(1):F23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, et al. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J Pediatr. 2016;177:133–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67(4):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pogribna U, Burson K, Lasky RE, Narayana PA, Evans PW, Parikh NA. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR Am J Neuroradiol. 2014;35(4):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blesa M, Sullivan G, Anblagan D, Telford EJ, Quigley AJ, Sparrow SA, et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. 2019;184:431–9. [DOI] [PubMed] [Google Scholar]
- 51.de Bruine FT, van den Berg-Huysmans AA, Leijser LM, Rijken M, Steggerda SJ, van der Grond J, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology. 2011;261(3):899–906. [DOI] [PubMed] [Google Scholar]
- 52.Hong HK, Lee HJ, Ko JH, Park JH, Park JY, Choi CW, et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J Neuroinflammation. 2014;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B, et al. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4):e736–40. [DOI] [PubMed] [Google Scholar]
- 54.Chen ML, Allred EN, Hecht JL, Onderdonk A, VanderVeen D, Wallace DK, et al. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52(10):7052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baud O, Maury L, Lebail F, Ramful D, El Moussawi F, Nicaise C, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387(10030):1827–36. [DOI] [PubMed] [Google Scholar]
- 56.Zeng L, Tian J, Song F, Li W, Jiang L, Gui G, et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: a network meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103(6):F506–F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10:CD001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. Cochrane Database Syst Rev. 2001(1):CD001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. 2017;4:CD011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanson LA, Hahn-Zoric M, Berndes M, Ashraf R, Herias V, Jalil F, et al. Breast feeding: overview and breast milk immunology. Acta Paediatr Jpn. 1994;36(5):557–61. [DOI] [PubMed] [Google Scholar]
- 61.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156(2 Suppl):S3–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data is included within this manuscript. Those wishing to obtain the data directly from the authors, can do so by emailing the corresponding author.


