Abstract
The global burden of vector-borne diseases accounts for more than 17% of infectious diseases in humans. Rapid global expansion of previously obscure pathogens, such as Zika and chikungunya viruses in recent years highlights the importance of understanding how anthropogenic changes influence emergence and spillover of vector-borne diseases. Deforestation has been identified as one anthropogenic change that influences vector-borne disease prevalence, although contrasting pictures of the effects of deforestation on vector-borne disease transmission have been reported. These conflicting findings are likely attributable to the inherent complexity of vector-borne disease systems, which involve diverse groups of vectors, hosts and pathogens, depending on geography. The current study represents a quantitative exploration of the link between deforestation and mosquitoes, the most important common constituents of vector-borne disease systems. Analysis of data compiled from published field studies for 87 mosquito species from 12 countries revealed that about half of the species (52.9%) were associated with deforested habitats. Of these species that are favored by deforestation, a much larger percentage (56.5%) are confirmed vectors of human pathogens, compared to those negatively impacted by deforestation (27.5%). Moreover, species that serve as vectors of multiple human pathogens were all favored by deforestation, including Anopheles bancroftii, Anopheles darlingi, Anopheles farauti, Anopheles funestus s.l., Anopheles gambiae s.l., Anopheles subpictus, Aedes aegypti, Aedes vigilax, Culex annulirostris, and Culex quinquefasciatus. Our quantitative analysis of vector and non-vector species, demonstrates that the net effect of deforestation favors mosquitoes that serve as vectors of human disease, while the obverse holds true for non-vectors species. These results begin to unify our understanding of the relationship between deforestation and vector mosquitoes, an important step in quantifying how land use change, specifically deforestation, affects human risk of vector-borne disease.
Keywords: Deforestation, Vector, Mosquito, Landscape, Land use, Habitat
Introduction
The global burden of vector-borne diseases is substantial, accounting for more than 17% of infectious diseases in humans (WHO, 2016a). The most prevalent mosquito-borne virus, dengue virus, is estimated to infect 390 million people per year (Bhatt et al., 2013). Despite reductions in incidence due to intensification of intervention efforts, malaria causes more than 400,000 deaths per year (WHO, 2016b). Furthermore, recent pandemics resulting from rapid global expansion of previously obscure pathogens, such as Zika, chikungunya, dengue and West Nile viruses, have underscored the importance of vector-borne diseases, and the need to understand the drivers of their emergence.
One such possible driver of virus emergence is anthropogenic land use and land cover change (Jones et al. 2008; Foley et al. 2005). There has been a substantial rise in the number of studies examining the relationship between environmental degradation and infectious diseases in the past two decades (Gottdenker, Streicker, Faust, & Carroll 2014), allowing for an improved understanding of the mechanisms by which such forces give rise to changing vector-borne disease incidence. Of the types of land use and land cover changes studied, deforestation in the tropics is the most frequently examined (Gottdenker et al. 2014), and will be the focus of this study.
Elucidating links between deforestation and vector-borne diseases has been challenging and even controversial (Valle & Clark 2013; Hahn, Gangnon, Barcellos, Asner & Patz 2014; Gottdenker et al. 2014). While most studies have shown that human prevalence of vector-borne disease is greatest in deforested areas (Takken, Vilarinhos, Schneider, & Dos Santos 2005; Vittor et al. 2006; Hahn et al. 2014), some studies have concluded that forest conservation efforts may increase disease burden in humans (Valle & Clark 2013). The disparity between these conflicting studies is likely due to oversimplification of the vector-borne disease paradigm, without recognizing the inherent complexity of vector-borne disease episystems. Vector-borne diseases are extremely diverse with respect to their vectors, hosts, and pathogens. Understanding how the individual constituents of vector-borne disease are affected by deforestation is paramount to meaningful analysis of deforestation and human risk of vector-borne pathogens.
While deforestation does not likely have a uniform effect on the overall prevalence of all vector-borne pathogens, it may be valuable to investigate broad patterns in the relationships between deforestation and specific constituent groups. Mosquitoes, for example, are considered the most important vectors of human pathogens (http://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/). Quantifying relationships between deforestation and vector mosquito abundance is an important step towards building a framework for understanding overall links between deforestation and human disease. Deforestation greatly alters the breeding, abundance, and species composition of mosquitoes (Norris 2004). This is mediated by changes in the availability of breeding sites for the immature stages (Vittor et al. 2009), as well as differences in resources, predation (Yanoviak, Lounibos, & Weaver 2006; Kweka et al. 2011), survival (Afrane, Zhou, Lawson, Githeko, & Yan 2007; Kweka, Kimaro, & Munga 2016), and fecundity (Afrane et al. 2007). Following deforestation, the canopy is no longer present to reduce the direct force of rainfall onto the soil, and the loss of forest litter and plant roots allows for the ready flow of sediment (Birkinshaw, Bathurst, Iroume, & Palacios 2011). This, in turn, leads to soil erosion and altered water quality (Calder, Hofer, Vermont, & Warren, 2008). Whereas the forest floor tends to be shaded, sunlight reaches aquatic habitats unimpeded once the terrain is deforested. Aquatic vegetation and algae then emerge (Neill et al. 2006). Aquatic and terrestrial mosquito predator composition changes, as do the array of pathogens that impact mosquito survival. Survival is also impacted by microclimate changes (Afrane, Zhou, Lawson, Githeko, & Yan 2006), and in particular, the decrease in humidity that accompanies deforestation (Afrane et al. 2006).
Whether these changes favor or disfavor vectors of disease, however, depends on the vector’s specific ecology. To examine this, we systematically reviewed the literature pertaining to the effect of deforestation on mosquitoes, generating a database to test hypotheses related to vector status and deforestation. By including studies of vectors and non-vectors alike, we demonstrate that the net effect of deforestation favors mosquitoes that serve as vectors of human disease, while the obverse holds true for non-vectors.
Materials and methods
Data search and inclusion criteria
We searched Google Scholar and the PubMed databases using search terms “mosquito” AND “habitat” OR “forest” OR “landscape” OR “deforestation”, and from citations within publications. The last search was performed February 28, 2017. Inclusion criteria for retrieved studies included: (1) field studies reporting some metric of mosquito abundance, (2) simultaneous sampling from forested and deforested sites, (3) at least 100 mosquitoes per species were sampled and (4) values were reported for individual species. Data that compared abundances from forested and deforested areas across years (longitudinal) were not considered, since year-to-year variation in mosquito populations are often naturally quite large (Wolda & Galindo 1981; Chase & Knight 2003; Reisen et al. 2008). Only data from forested and human-altered habitats were compiled, i.e., data from natural marshes, grasslands, scrub or other natural open habitat types were not considered, as these do not necessarily constitute deforestation.
Information was compiled primarily from tables within publications, or figures (one instance), or solicited from authors (one instance). Study meta-data included geographical region, country, coordinates (when available), metric (e.g., larvae/dip, females/trap), and habitats compared to forest (e.g., village-outdoor, urban, suburban, rice field) (Table 1).
Table 1.
Summary of studies included in quantitative analysis of deforestation effects on mosquitoes.
| Region | Location | Mosquito community | Metric | Source |
|---|---|---|---|---|
| Africa | Senegal | Sylvatic chikungunya vectors | Females/Person/Evening | Diallo et al. 2012 |
| Africa | Kenya | African treehole mosquitoes | Williams’ mean of larval abundance | Lounibos 1981 |
| Africa | Kenya | Anophelines | Percentage of sites containing larvae | Minakawa et al. 2005 |
| Africa | Kenya | Anopheles gambiae s.l. | Proportion of eggs in each substrate | Munga et al. 2005 |
| Africa | Kenya | Malaria vectors | Emerging adults | Munga et al. 2006 |
| Africa | Kenya | Anophelines | No. anopheline-positive habitats (percent) | Mushinzimana et al. 2006 |
| Africa | Madagascar | Anophelines | Total females | Zohdy et al. 2016 |
| Asia | Sri Lanka | Surface-water breeding mosquitoes | Larval density | Amerasinghe & Ariyasena 1990 |
| Asia | Thailand | Anophelines | Females per man-hour | Overgaard et al. 2003 |
| Asia | Republic of Korea | Anophelines | Percentage of sites containing larvae | Sithiprasasna et al. 2005 |
| Asia | Thailand | Arbovirus vectors | Females/trap | Thongsripong et al. 2013 |
| North America | USA | All mosquitoes | Total larvae in tires | Kling et al. 2007 |
| South America and Caribbean | Brazil | Anopheles darlingi | Larval densities/1,000 dips August | Barros & Honó;rio 2015 |
| South America and Caribbean | Puerto Rico | Dengue vectors | Larvae/ovitrap | Cox et al. 2007 |
| South America and Caribbean | Peru | Anopheles darlingi | Average human-biting rate | Vittor et al. 2006 |
| South Pacific and Australia | Indonesia | Malaria vectors | Females/person/evening | Harbach et al. 1987 |
| South Pacific and Australia | Australia | All mosquitoes | Total females | Steiger et al. 2012 |
Data synthesis and analysis
Index of change (IOC) (Amerasinghe & Ariyasena 1990), IOC = (P2 − P1)/(P2 + P1) where P2 = deforested, P1 = forest, was used to assess the relative difference in mosquito numbers found between forested and deforested areas, by species. IOC ranges from −1 to +1, representing a relative increase or decrease in abundance in paired forested and deforested sites. Species included in the dataset were categorized as a vector of human pathogen(s) or not based upon inclusion in text, table, or index in Mullen & Durden (2009).
Tukey’s student t-test and logistic regression were employed to explore relationships between mosquitoes and deforestation. One-sample t-tests (PROC TTEST, SAS 9.4) were performed for individual mosquito species, using IOC as the dependent variable. The null hypothesis tested was that abundance (IOC) was not significantly different as would be expected if deforestation had no effect on abundance (H0 = 0). Simple logistic regression was run (PROC LOGISTIC, SAS 9.4) on the entire set of mosquito species to determine whether vector species were associated with deforested habitat. Since mosquitoes that transmit anthroponotic pathogens (no wildlife reservoir) may be affected differently by deforestation than mosquitoes that transmit zoonotic pathogens (feed upon a wider variety of hosts), we conducted a second logistic regression analysis excluding vectors of anthroponoses. Mosquito abundance in deforested habitats (IOC) was the sole predictor variable in logistic models, with vector status (coded 1 for vector, 0 for non-vector) serving as binary outcome. Alpha for all comparisons was 0.05.
Results
Data from seventeen published studies met the criteria for the current analysis. A majority (n= 15) of studies reported only upon selected species, genera or vectors of specific pathogens, and did not attempt to report on the entire mosquito community at a given study location (see Appendix A in Supplementary material). The most commonly reported group were anophelines in general (n = 5) and vectors of human malaria (n = 5), such that ten studies (Harbach, Baimai, & Sukowati 1987; Overgaard, Ekbom, Suwonkerd, & Takagi 2003; Minakawa et al. 2005; Munga et al. 2005, 2006; Sithiprasasna, Lee, Ugsang, & Linthicum 2005; Mushinzimana et al. 2006; Vittor et al. 2006; Barros & Honorio 2015; Zohdy et al. 2016) reported on these groups to the exclusion of all culicine mosquitoes. Only two studies (Kling, Juliano, & Yee 2007; Steiger et al. 2012) attempted to report on the abundance of the entire mosquito community at a location. Other studies that were considered quantified African treehole mosquitoes (Lounibos 1981), arbovirus vectors (Thongsripong et al. 2013), dengue vectors (Cox, Grillet, Ramos, Amador, & Barrera 2007), surface water-breeding mosquitoes (Amerasinghe & Ariyasena 1990), or sylvatic vectors of chikungunya virus (Diallo et al., 2012).
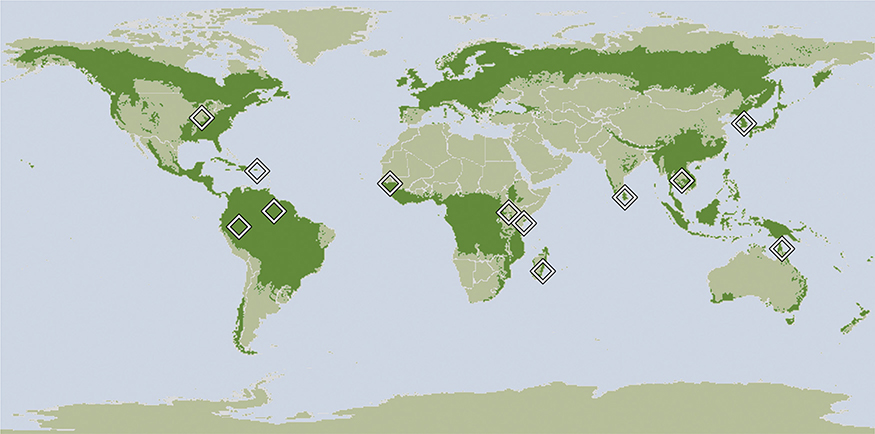
The geographical coverage of studies was large, and included at least one representative study from all major forested biogeographical regions (Fig. 1), excepting nearctic and paleartic forest biomes. A relatively large number of studies (n = 5) were from Kenya, reporting mainly upon anopheline vectors of malaria in highland areas (Minakawa et al. 2005; Munga et al. 2005, 2006; Mushinzimana et al. 2006) or treehole mosquitoes from the coast (Lounibos 1981). Other studies from Africa were from Senegal (Diallo et al. 2012) and Madagascar (Zohdy et al. 2016). Studies from Asia and the South Pacific region reported on mosquitoes from Sri Lanka (Amerasinghe & Ariyasena 1990), Thailand (Overgaard et al. 2003; Thongsripong et al. 2013), Republic of Korea (Sithiprasasna et al. 2005), Australia (Steiger et al. 2012) and Indonesia (Harbach et al. 1987). In the western hemisphere, studies were from USA (Kling et al. 2007), Puerto Rico (Cox et al. 2007), Peru (Vittor et al. 2006), and Brazil (Barros & Honório 2015).
Fig. 1.
Map showing global distribution of forest biomes and locations of field data summarized in the current study. Forest distributions from Potapov et al. (2008).
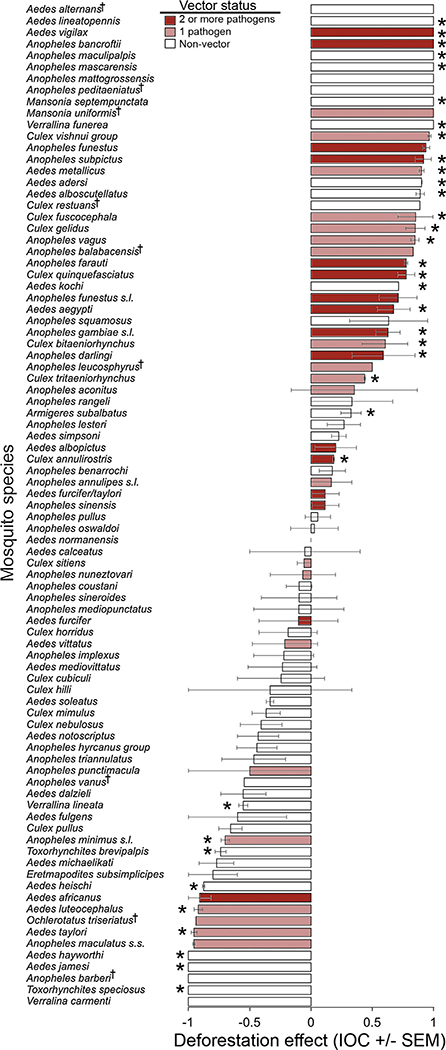
The combined diversity of mosquitoes from studies was large, constituting 498 observations on 87 species or species groups from 8 genera. Overall, the numbers of IOC-values were not significantly different between species with positive and negative IOC values (40 vs. 46; X2 = 0.42; P = 0.548), while the distribution of significant results was significantly different between the numbers of species with positive and negative IOC values (25/21 vs. 9/31; X2 = 9.08; P = 0.0026). Of 46 species that were more abundant in deforested areas, average IOC was significantly greater than the null hypothesis (no change) for 25 species (54.3%; Fig. 2). Of 40 species negatively impacted by deforestation, average IOC was significantly lesser (Fig. 2) than the null hypothesis (no change) for 9 species (22.5%).
Fig. 2.
Association of 87 mosquito species with forested and deforested habitats. Data are compiled from 17 published studies from 12 countries, reported in Appendix A in Supplementary material. Positive IOC-values indicate higher abundances in deforested habitats. Bars represent average index of change (IOC) and associated standard error. Asterisks denote species for which abundance is greater than no change (Ho = 0) as determined by Student’s t-test. The symbol † denotes species for which only one observation was available.
More vector species were affected positively by deforestation than were affected negatively by deforestation (Fig. 2). Logistic regression analysis revealed a significant relationship between IOC and vector status (X2 = 298.2, P < 0.0001). When vectors of anthroponoses were excluded, a weaker yet statistically significant association between IOC and vector status (X2 = 9.9, P = 0.0016) was still observed. A large percentage (56.5%) of species that were positively affected by deforestation are confirmed vectors of human pathogens, compared to those negatively impacted by deforestation (27.5%). Eleven mosquito species that were found to be significantly positively affected by deforestation are vectors of multiple human pathogens. For example, Anopheles bancroftii, Anopheles darlingi, Anopheles farauti, Anopheles funestus s.l., Anopheles gambiae s.l., and Anopheles subpictus, all considered important vectors of both human malaria and lymphatic filariasis, were found to be significantly positively affected by deforestation (Fig. 2). In addition, several vectors of multiple arboviruses, including Aedes aegypti, Aedes vigilax, Culex annulirostris and Culex quinquefasciatus were found to be significantly positively affected by deforestation. No multiple-pathogen vectors and just three single pathogen vectors were found to be significantly negatively affected by deforestation (Fig. 2).
Discussion
In the present study we synthesize the literature on the abundances of mosquito species in relation to deforestation. The picture that emerges illustrates that vectors of human pathogens are more abundant in deforested than forested areas, whereas non-vectors display the opposite tendency. This may reflect evolutionary processes that drive the parasite, host, and vector to converge in space. An efficient vector of human disease preferentially feeds on humans, survives long enough to have repeated blood meals, is abundant, and allows the pathogen to undergo the extrinsic portion of its life cycle such that the pathogen is excreted into a new host. It is therefore in the interest of the pathogen and the vector to adapt to anthropogenic landscapes.
Such coevolution is exemplified by Anopheles gambiae, the dominant vector of malaria in sub-Saharan Africa. Anopheles gambiae s.l. is a highly polymorphic species complex, adapted to a wide range of anthropogenic habitats. Genetic and phenotypic analyses of Anopheles gambiae indicate that acquisition of traits such as larval heat tolerance and adult resistance to desiccation have facilitated the dispersal of this mosquito species from rainforest into drier savanna environments (Coluzzi, Sabatini, della Torre, Di Deco, Petrarca 2002; White, Collins, & Besansky 2011), possibly around the same time human farming activity contributed to the collapse of the central African rainforest and conversion into savannah (Lehmann & Diabate 2008; Bayon et al. 2012).
One of the primary New World malaria vectors, Anopheles darlingi, may have similarly become adapted to humans. The preponderance of data suggests that this species takes advantage of aquatic habitats that are on the forest-deforested interface, as well as large, man-made water bodies (Charlwood 1996; Roberts et al. 2002; Vittor et al. 2009; Barros & Honório 2015). The presence of this mosquito predated the appearance of humans by millions of years (Marinotti et al. 2013), and its anthropophilic behavior therefore represents a relatively recent adaptation.
The evolutionary advantage for the adaptation of zoonotic vector-borne diseases to deforested habitat is less clear. Examining the host preference for such mosquito species that are more abundant in deforested habitats, it becomes apparent that many of these feed on livestock (e.g. Culex sp. vishnui subgroup, Culex fuscocephala, Culex gelidus, Aedes vigilax, Anopheles subpictus) (Amerasinghe & Amerasinghe 1999; Samuel, Arunachalam, Hiriyan, & Tyagi 2008; Jansen et al. 2009). Thus conversion of forest to agricultural land may bolster zoonotic diseases utilizing cattle, swine, or poultry as hosts. This is exemplified by Japanese encephalitis virus, which is maintained in wading birds and amplified in swine. Thongsripong et al. (2013) demonstrated that the Japanese encephalitis virus vector, Culex sp. vishnui subgroup, was scarce in forest but abundant in rice paddies. The members of this subgroup tend to feed on pigs and cattle (Samuel et al. 2008). The spatial convergence of human settlements, rice and pig farming in Southeast Asia has thus led to a proliferation of prime JEV vectors and concomitant amplification of virus in pigs, thereby increasing risk of incidental infection to humans (Le Flohic, Porphyre, Barbazan, & Gonzalez 2013).
There were several challenges encountered in synthesizing the literature on mosquito abundance by deforestation. The mosquito sampling strategies varied greatly by study, and included human landing catches (Harbach et al. 1987; Overgaard et al. 2003; Vittor et al. 2006; Diallo et al. 2012), light traps (Sithiprasasna et al. 2005; Steiger et al. 2012; Thongsripong et al. 2013), chemical lure traps (Thongsripong et al. 2013), resting adult mosquito aspiration (Thongsripong et al. 2013), larval collections (Sithiprasasna et al. 2005; Minakawa et al. 2005; Mushinzimana et al. 2006; Cox et al. 2007), and a variety of experimental designs (Cox et al. 2007; Munga et al. 2006; Afrane et al. 2007). Each method samples a different subset of the mosquito population; for example, larval collections may yield zoophilic mosquito species that would not appear in human landing catches. Attractant traps and light traps employ a wide array of attractants, including light, CO2 and chemical lures that are also likely to select for a specific subset of mosquito species. Lord et al. (2016) demonstrated that light traps and resting mosquito collections yield different distributions and abundances of mosquito species in a Japanese encephalitis virus endemic region, with greater mosquito species diversity seen in the resting collections.
Human landing catches are employed for a variety of reasons; it has been argued that this method is the most appropriate for determining human disease risk (Diallo et al. 2012). Furthermore, there are mosquito species that are not effectively sampled by other means (Vittor et al. 2006; Diallo et al. 2012). Since humans are used as the attractant, it follows then that the sample will contain mostly anthropophilic species and will exclude zoophilic species. Thus, non-vectors are underrepresented in these studies. Furthermore, only two studies reported data on the entire mosquito community, while others focused on selected species, typically nuisance species or vectors of human disease. This reporting bias could bias our analysis regarding the number of mosquito species positively or negatively affected by deforestation, with non-vector and non-nuisance species being underrepresented.
Another bias may have been introduced by sampling at a single elevation. All of the adult mosquitoes sampled in these studies were collected at ground level (approximately up to 1.5 m). In forest habitat, some mosquito species are canopy dwellers (Andrews et al. 2014; Lord et al. 2016), and these would therefore also be underrepresented in this data set. While some of the studies presented here did trap at canopy elevations (Lounibos 1981), no traps were operated at equivalent elevations in deforested areas, therefore no comparative data were available at higher elevation between forested and deforested sites. Without comparative data, canopy trap data were unfortunately excluded from our analysis.
The different ways in which land use and land cover were determined and classified in these studies posed another challenge. Several studies used Landsat Thematic Mapper satellite images to classify vegetation types followed by field observation to validate the remote sensing classifications (Sithiprasasna et al. 2005; Mushinzimana et al. 2006; Vittor et al. 2006; Cox et al. 2007; Diallo et al. 2012). Others used aerial photography (Overgaard et al. 2003), Google Earth (Thongsripong et al. 2013), or simple field observation (Harbach et al. 1987; Steiger et al. 2012; Munga et al. 2006). Furthermore, the category “forest” ranged from lowland tropical rainforests to deciduous highland forests with varying amounts of human perturbation. Deforestation, in turn, is a dynamic process that may give rise to a wide spectrum of land uses such as small- or large-scale agriculture, irrigation schemes, successive vegetation types, villages and cities. In order to identify the trends in mosquito species abundance resulting from deforestation, we have grouped these various land use/land cover types by natural (e.g. forest) vs. anthropogenically altered habitat types.
The seemingly low number of studies that met the inclusion criteria (n = 17) is a startling reflection of how few studies have actually measured vector abundance in forested and deforested areas, highlighting the need for future studies in areas where deforestation rates are highest (southeast Asia, Latin America and Africa). Nonetheless, 87 species from 8 genera were included in the analysis, representing diverse taxa from vector and non-vector groups.
The finding of higher vector abundance in altered habitats may be explained by a variety of mechanisms, such as differences in predation, nutrients, microclimate, and parasitism compared to forest. Predation of immature and mature mosquitoes may contribute to differences in mosquito species assemblages and abundances observed, and is thought to be an important regulatory mechanism in long-lasting habitats (Kweka et al. 2011). Predators of mosquito larvae include many species of fish (Louca et al. 2009), amphibians (Ohba et al. 2010), micro-crustaceans (Kroeger, Liess, & Duquesne 2014), larvae of the Toxorhynchites genus (Murrell & Juliano 2013), wolf spiders (Futami, Sonye, Akweywa, Kaneko, & Minakawa 2008), and other invertebrates, while adult mosquitoes are predated upon by birds (Poulin, Lefebvre, & Paz 2010), bats (Gonsalves, Law, Webb, & Monamy 2013), and certain invertebrates (e.g. the jumping spider Evarcha culicivora, which feeds indirectly on vertebrate blood by predating blood-fed Anopheles (Jackson & Nelson 2012)). Interestingly, our own analysis showed that two mosquito species of the genus Toxorhynchites, whose larvae prey preferentially on larvae of blood-feeding mosquitoes in tree cavities, were negatively impacted by deforestation (Fig. 2). Assessing the impact of deforestation on mosquito predators and mosquitoes may require a community ecology approach, a field of study that yearns to be developed.
Resources also vary according to habitat and may influence not only mosquito abundance but also vectorial capacity (Stone, Jackson, & Foster 2012). While adult female mosquitoes require blood for oviposition, most species also require nectar for energy. In a series of experiments in which An. gambiae females were provided with a sugar-rich or sugar-poor environment, it was established that while An. gambiae survival was greater in sugar rich plant environment, the mosquitoes tended to have higher biting rates in the sugar-poor environment (Gary & Foster 2001; Stone et al. 2012). These factors influenced vectorial capacity in a temperature-dependent fashion, and at 27 °C, a net increase in vectorial capacity was predicted for the sugar-poor environments. In contrast, An. sergentii exhibited higher survival rates and shorter gonotrophic cycles in sugar-rich oases compared to sugar-poor oases in Israel, resulting in an estimated 250-fold higher vectorial capacity in the sugar-rich site (Gu, Müller, Schlein, Novak, & Beier 2011).
Aquatic food sources for immature forms include algae and pollen. Differences in the development of immature forms by land cover were elucidated in a study on the emergence of adult An. gambiae from experimental aquatic habitats (Munga et al. 2006). Forest, farmland, and natural swamp habitats in Kenya were seeded with An. gambiae larvae. Only the farmland aquatic habitats yielded adult mosquitoes, and it was hypothesized that the maize pollen from the farms may have contributed to emergence. In a separate study, An. gambiae and An. funestus larval presence was negatively associated with the percent canopy cover (Minakawa et al. 2005). Increased shade reduces with the amount of algae, which constitutes an important food source for mosquito larvae (Brouard et al. 2011). Furthermore, sunlight inhibits the growth of various entomophagic fungi. For example, Aedes aegypti larval survival was reduced in shaded breeding sites due to mortality conferred by the fungus Leptolegnia chapmanii (Rueda Páramo, López Lastra, & García 2015). Infection and mortality due to this fungus was significantly lower in sunlit habitats.
An intriguing emerging area of study is now shedding light on the influence of ecological degradation on insect immunity (Muturi, Kim, Alto, Berenbaum, & Schuler 2011). A study examining the impact of larval stressors on virus susceptibility experimentally demonstrated that maintaining Ae. aegypti larvae at elevated temperatures (32° C vs 25 °C) led them to become more susceptible to Sindbis virus infection as adults but had no effect on mosquito survival. It was hypothesized that increased susceptibility may be the result of poor development of tissues, breakdown of the midgut barrier, or an upregulation of antimicrobial peptides that disrupt the protective midgut microbiome (Muturi et al. 2011). Since microclimate changes leading to higher temperatures in deforested habitat are well documented (Afrane et al. 2006; Li et al. 2015), this may be yet another mechanisms by which deforestation gives rise to greater disease risk.
In conclusion, in this systematic review of the literature and analysis of mosquito abundance by species and habitat, we demonstrate that mosquito vectors of human disease are disproportionately represented in deforested habitats. Various mechanisms may contribute to the decreased abundance of such vectors in forested landscapes, including increased predation, increased mortality due to fungi in sunlit aquatic habitats, and decreased larval resources (algae, pollen). Furthermore, cooler temperatures in forest (at least in subtropical and tropical climes) lead to slower larval development, longer pathogen extrinsic cycles, and possibly decreased susceptibility of the vector to the pathogen. However, the association between deforestation and mosquito-borne diseases is far from uniform or certain. Incorporation of ecological theory (such as metapopulation theory and landscape ecology) and evolutionary perspectives would expand our understanding of the dynamics that drive changes in mosquito species assemblages. Given the rapid pace of global land use change and deforestation, it is imperative that these dynamics are better understood to mitigate disease risk and guide land use policy.
Supplementary Material
Acknowledgements
The authors would like to thank T. Tscharntke and T. Schowalter for editing the special issue of BAAE and inviting this submission. The authors thank P. Lounibos for sharing data (Lounibos 1981) on treehole mosquitoes from Kenya. We thank K. Sloyer for producing the map in Fig. 1. Three anonymous reviewers provided thoughtful criticisms that greatly improved the final draft. This work was supported by NIFAFLA-VME-005446.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.baae.2017.09.012.
References
- Afrane YA, Zhou G, Lawson BW, Githeko AK, & Yan G (2006). Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. The American Journal of Tropical Medicine and Hygiene, 74(5), 772–778. [PubMed] [Google Scholar]
- Afrane YA, Zhou G, Lawson BW, Githeko AK, & Yan G (2007). Life-table analysis of Anopheles arabiensis in western Kenya highlands: Effects of land covers on larval and adult survivorship. The American Journal of Tropical Medicine and Hygiene, 77(4), 660–666. [PubMed] [Google Scholar]
- Amerasinghe PH, & Amerasinghe FP (1999). Multiple host feeding in field populations of Anopheles culifacies and An. subpictus in Sri Lanka. Medical and Veterinary Entomology, 13(2), 124–131. [DOI] [PubMed] [Google Scholar]
- Amerasinghe FP, & Ariyasena TG (1990). Larval survey of surface water-breeding mosquitoes during irrigation development in the Mahaweli Project, Sri Lanka. Journal of Medical Entomology, 27(5), 789–802. [DOI] [PubMed] [Google Scholar]
- Andrews ES, Schoeler GB, Gozalo AS, Carbajal F, Lopez-Sifuentes V, & Turell MJ (2014). Species diversity, seasonal, and spatial distribution of mosquitoes (Diptera: Culicidae) captured in Aotus monkey-baited traps in a forested site near Iquitos, Peru. Journal of Medical Entomology, 51(6), 1127–1135. [DOI] [PubMed] [Google Scholar]
- Barros FS, & Honorio NA. (2015). Deforestation and malaria on the Amazon frontier: Larval clustering of Anopheles darlingi (Diptera: culicidae) determines focal distribution of malaria. The American Journal of Tropical Medicine and Hygiene, 93(5), 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayon G, Dennielou B, Etoubleau J, Ponzevera E, Toucanne S, & Bermell S (2012). Intensifying weathering and land use in Iron Age Central Africa. Science, 335(6073), 1219–1222. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL,… & Myers MF. (2013). The global distribution and burden of dengue. Nature, 496(7446), 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw SJ, Bathurst JC, Iroume A, & Palacios H (2011). The effect of forest cover on peak flow and sediment discharge — An integrated field and modelling study in central-southern Chile. Hydrological Processes, 25(8), 1284–1297. [Google Scholar]; Brouard O, Le Jeune AH, Leroy C, Cereghino R, Roux O, Pelozuelo L, … & Carrias, J. F. (2011). Are algae relevant to the detritus-based food web in tank-bromeliads? PLoS One, 6(5), e20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder I, Hofer T, Vermont S, & Warren P (2008). Towards a new understanding of forests and water. UNASYLVA-FAO-229., 3 [Google Scholar]
- Chase JM, & Knight TM (2003). Drought-induced mosquito outbreaks in wetlands. Ecology Letters, 6(11), 1017–1024. [Google Scholar]
- Charlwood JD (1996). Biological variation in Anopheles darlingi root. Memórias do Instituto Oswaldo Cruz, 91, 391–398. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, della Torre A, Di Deco MA, & Petrarca V (2002). A polytene chromosome analysis of the Anopheles gambiae species complex. Science, 298(5597), 1415–1418. [DOI] [PubMed] [Google Scholar]
- Cox J, Grillet ME, Ramos OM, Amador M, & Barrera R (2007). Habitat segregation of dengue vectors along an urban environmental gradient. The American Journal of Tropical Medicine and Hygiene, 76(5), 820–826. [PubMed] [Google Scholar]
- Diallo D, Sall AA, Buenemann M, Chen R, Faye O, Diagne CT, … & Weaver SC. (2012). Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Neglected Tropical Diseases, 6(6), e1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR,… & Helkowski JH. (2005). Global consequences of land use. Science, 309(5734), 570–574. [DOI] [PubMed] [Google Scholar]
- Futami K, Sonye G, Akweywa P, Kaneko S, & Minakawa N (2008). Diving behavior in Anopheles gambiae (Diptera: Culicidae): Avoidance of a predacious wolf spider (Araneae: Lycosidae) in relation to life stage and water depth. Journal of Medical Entomology, 45(6), 1050–1056. [DOI] [PubMed] [Google Scholar]
- Gary RE, & Foster WA (2001). Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae). Journal ofMedical Entomology, 38(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Gonsalves L, Law B, Webb C, & Monamy V (2013). Foraging ranges of insectivorous bats shift relative to changes in mosquito abundance. PLoS One, 8(5), e64081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottdenker NL, Streicker DG, Faust CL, & Carroll CR (2014). Anthropogenic land use change and infectious diseases: A review of the evidence. Ecohealth, 11(4), 619–632. [DOI] [PubMed] [Google Scholar]
- Gu W, Müller G, Schlein Y, Novak RJ, & Beier JC (2011). Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS One, 6(1), e15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Gangnon RE, Barcellos C, Asner GP, & Patz JA (2014). Influence of deforestation, logging, and fire on malaria in the Brazilian Amazon. PLoS One, 9(1), e85725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbach RE, Baimai VISUT, & Sukowati SUPRATMAN (1987). Some observations on sympatric populations of the malaria vectors Anopheles leucosphyrus and Anopheles balabacensis in a village-forest setting in South Kalimantan. The Southeast Asian Journal of Tropical Medicine and Public Health, 18(2), 241–247. [PubMed] [Google Scholar]
- Jackson RR, & Nelson XJ (2012). Evarcha culicivora chooses blood-fed Anopheles mosquitoes but other East African jumping spiders do not. Medical and Veterinary Entomology, 26(2), 233–235. [DOI] [PubMed] [Google Scholar]
- Jansen CC, Webb CE, Graham GC, Craig SB, Zborowski P, Ritchie SA,… & van den Hurk, A. F. (2009). Blood sources of mosquitoes collected from urban and peri-urban environments in Eastern Australia with species-specific molecular analysis of avian blood meals. American Journal of Tropical Medicine and Hygiene, 81(5), 849–857. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, & Daszak P (2008). Global trends in emerging infectious diseases. Nature, 451(7181), 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling LJ, Juliano SA, & Yee DA (2007). Larval mosquito communities in discarded vehicle tires in a forested and unforested site: Detritus type, amount, and water nutrient differences. Journal of Vector Ecology, 32(2), 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger I, Liess M, & Duquesne S (2014). Temporal and spatial habitat preferences and biotic interactions between mosquito larvae and antagonistic crustaceans in the field. Journal of Vector Ecology, 39(1), 103–111. [DOI] [PubMed] [Google Scholar]
- Kweka EJ, Zhou G, Gilbreath TM, Afrane Y, Nyindo M, Githeko AK, & Yan G (2011). Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasites & Vectors, 4(1), 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka EJ, Kimaro EE, & Munga S (2016). Effect of deforestation and land use changes on mosquito productivity and development in Western Kenya Highlands: Implications for malaria risk. Frontiers in Public Health, 4, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Flohic G, Porphyre V, Barbazan P, & Gonzalez JP (2013). Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Neglected Tropical Disease, 7(9), e2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, &Diabate A (2008). The molecular forms of Anopheles gambiae: A phenotypic perspective. Infection, Genetics and Evolution, 8(5), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao M, Motesharrei S, Mu Q, Kalnay E,&Li S. (2015). Local cooling and warming effects of forests based on satellite observations. Nature communications, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JS, Al-Amin HM, Chakma S, Alam MS, Gurley ES, & Pulliam JR (2016). Sampling design influences the observed dominance of Culex tritaeniorhynchus: Considerations for future studies of Japanese encephalitis virus transmission. PLoS Neglected Tropical Diseases, 10(1), e0004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca V, Lucas MC, Green C, Majambere S, Fillinger U, & Lindsay SW (2009). Role of fish as predators of mosquito larvae on the floodplain of the Gambia River. Journal of Medical Entomology, 46(3), 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP (1981). Habitat segregation among African treehole mosquitoes. Ecological Entomology, 6(2), 129–154. [Google Scholar]
- Marinotti O, Cerqueira GC, de Almeida LGP, Ferro MI, Loreto EL, Zaha A, … Vasconcelos AT, et al. (2013). The genome of Anopheles darlingi, the main neotropical malaria vector. Nucleic acids research, 41(15), 7387–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, & Yan G (2005). Spatial distribution of anopheline larval habitats in Western Kenyan highlands: Effects of land cover types and topography. The American Journal of Tropical Medicine and Hygiene, 73(1), 157–165. [PubMed] [Google Scholar]
- Mullen GR, & Durden LA. (Eds.). (2009). Medical and veterinary entomology. Academic Press. [Google Scholar]
- Munga S, Minakawa N, Zhou G, Barrack OOJ, Githeko AK, & Yan G (2005). Oviposition site preference and egg hatchability of Anopheles gambiae: Effects of land cover types. Journal of Medical Entomology, 42(6), 993–997. [DOI] [PubMed] [Google Scholar]
- Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OOJ, Githeko AK, & Yan G (2006). Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. The American Journal of Tropical Medicine and Hygiene, 74(1), 69–75. [PubMed] [Google Scholar]
- Murrell EG, & Juliano SA (2013). Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia, 173(3), 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinzimana E, Munga S, Minakawa N, Li L, Feng CC, Bian L, … & Githeko, A. K. (2006). Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malaria Journal, 5(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Kim CH, Alto BW, Berenbaum MR, & Schuler MA (2011). Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Tropical Medicine & International Health, 16(8), 955–964. [DOI] [PubMed] [Google Scholar]
- Neill C, Deegan LA, Thomas SM, Haupert CL, Krusche AV, Ballester VM, & Victoria RL (2006). Deforestation alters the hydraulic and biogeochemical characteristics of small lowland Amazonian streams. Hydrological Processes, 20(12), 2563–2580. [Google Scholar]
- Norris DE (2004). Mosquito-borne diseases as a consequence of land use change. EcoHealth, 1(1), 19–24. [Google Scholar]
- Ohba SY, Kawada H, Dida GO, Juma D, Sonye G, Minakawa N, & Takagi M (2010). Predators of Anopheles gambiae sensu lato (Diptera: Culicidae) larvae in wetlands, western Kenya: Confirmation by polymerase chain reaction method. Journal of Medical Entomology, 47(5), 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard HJ, Ekbom B, Suwonkerd W, & Takagi M (2003). Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landscape Ecology, 18(6), 605–619. [Google Scholar]
- Poulin B, Lefebvre G, & Paz L (2010). Red flag for green spray: Adverse trophic effects of Bti on breeding birds. Journal of Applied Ecology, 47(4), 884–889. [Google Scholar]
- Potapov P, Yaroshenko A, Turubanova S, Dubinin M, Laestadius L, Thies C, … & Zhuravleva I. (2008). Mapping the World’s intact forest landscapes by remote sensing. Ecology and Society, 13(2). [Google Scholar]
- Reisen WK, Cayan D, Tyree M, Barker CM, Eldridge B, & Dettinger M (2008). Impact of climate variation on mosquito abundance in California. Journal of Vector Ecology, 33(1), 89–98. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Manguin S, Rejmankova E, Andre R, Harbach RE, Vanzie E,… & Polanco J. (2002). Spatial distribution of adult Anopheles darlingi and Anopheles albimanus in relation to riparian habitats in Belize, Central America. Journal of Vector Ecology, 27, 21–30. [PubMed] [Google Scholar]
- Rueda Páramo ME, López Lastra CC, & García JJ (2015). Persistence and pathogenicity of a native isolate of Leptolegnia chapmanii against Aedes aegypti larvae in different anthropic environments. Biocontrol Science and Technology, 25(2), 238–243. [Google Scholar]
- Samuel PP, Arunachalam N, Hiriyan J, & Tyagi BK (2008). Host feeding pattern of Japanese encephalitis virus vector mosquitoes (Diptera: Culicidae) from Kuttanadu, Kerala, India. Journal of Medical Entomology, 45(5), 927–932. [DOI] [PubMed] [Google Scholar]
- Sithiprasasna R, Lee WJ, Ugsang DM, & Linthicum KJ (2005). Identification and characterization of larval and adult anopheline mosquito habitats in the Republic of Korea: Potential use of remotely sensed data to estimate mosquito distributions. International Journal of Health Geographies, 4(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger DM, Johnson P, Hilbert DW, Ritchie S, Jones D, & Laurance SG (2012). Effects of landscape disturbance on mosquito community composition in tropical Australia. Journal of Vector Ecology, 37(1), 69–76. [DOI] [PubMed] [Google Scholar]
- Stone CM, Jackson BT, & Foster WA (2012). Effects of plant–community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae. The American Journal of Tropical Medicine and Hygiene, 87(4), 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Vilarinhos PDTR, Schneider P, & Dos Santos F (2005). Effects of environmental change on malaria in the Amazon region of Brazil. Frontis, 9, 113–123. [Google Scholar]
- Thongsripong P, Green A, Kittayapong P, Kapan D, Wilcox B, & Bennett S (2013). Mosquito vector diversity across habitats in central Thailand endemic for dengue and other arthropod-borne diseases. PLoS Neglected Tropical Diseases, 7(10), e2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D, & Clark J (2013). Conservation efforts may increase malaria burden in the Brazilian Amazon. PLoS One, 8(3), e57519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields TIM, Lozano WS, … & Patz JA. (2006). The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene, 74(1), 3–11. [PubMed] [Google Scholar]
- Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, … & Patz JA. (2009). Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darling. The American Journal of Tropical Medicine and Hygiene, 81(1), 5–12. [PMC free article] [PubMed] [Google Scholar]
- White BJ, Collins FH, & Besansky NJ (2011). Evolution of Anopheles gambiae in relation to humans and malaria. Annual Review of Ecology, Evolution, and Systematics, 42, 111–132. [Google Scholar]
- WHO (2016a) [cited 2017 February 23]. Available from: http://www.who.int/mediacentre/factsheets/fs094/en/.
- WHO (2016b) Vector-bornediseases [cited 2017 February 23]. Available from: http://www.who.int/mediacentre/factsheets/fs387/en/.
- Wolda H, & Galindo P (1981). Population fluctuations of mosquitoes in the non-seasonal tropics. Ecological Entomology, 6(1), 99–106. [Google Scholar]
- Yanoviak SP, Lounibos LP, & Weaver SC (2006). Land use affects macroinvertebrate community composition in phytotelmata in the Peruvian Amazon. Annals of the Entomological Society of America, 99(6), 1172–2281. [Google Scholar]
- Zohdy S, Derfus K, Headrick EG, Andrianjafy MT, Wright PC, & Gillespie TR (2016). Small-scale land-use variability affects Anopheles spp. distribution and concomitant Plasmodium infection in humans and mosquito vectors in southeastern Madagascar. Malaria Journal, 15(1), 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.