Abstract
Lipid–oligonucleotide conjugates (LONs) are powerful molecular-engineering materials for various applications ranging from biosensors to biomedicine. Their unique amphiphilic structures enable the self-assembly and the conveyance of information with high fidelity. In particular, LONs present remarkable potential in measuring cellular mechanical forces and monitoring cell behaviors. LONs are also essential sensing tools for intracellular imaging and have been employed in developing cell-surface-anchored DNA nanostructures for biomimetic-engineering studies. When incorporating therapeutic oligonucleotides or small-molecule drugs, LONs hold promise for targeted therapy. Moreover, LONs mediate the controllable assembly and fusion of vesicles based on DNA-strand displacements, contributing to nanoreactor construction and macromolecule delivery. In this review, we will summarize the general synthesis strategies of LONs, provide some characterization analysis and emphasize recent advances in bioanalytical and biomedical applications. We will also consider the relevant challenges and suggest future directions for building better functional LONs in nanotechnology and materials-science applications.
Keywords: lipid–oligonucleotide, DNA nanostructure, biosensor, biomedicine, bioanalysis, nanoreactor
INTRODUCTION
Besides carrying genetic information, the programmability and self-recognition properties of DNA have shown great promise in promoting DNA nanotechnology for applications in biochemistry and materials sciences. The only limitation to building desired systems is the necessary reliance on pure DNA base pairing. Therefore, the combination of DNA and hydrophobic molecules has attracted attention as an important approach to increase the complexity of built DNA architectures. Here, hydrophobic groups could non-covalently protrude into the DNA backbone and form attractive bulk films, liquid crystals or hydrogels [1]. At the same time, chemically coupling oligonucleotides (ONs) with functional hydrophobic groups, such as polymers and lipids, have also been widely demonstrated [2].
Lipids are indispensable cell bilayer components in biological living systems and are essential for nutrient transport and cell-signaling processes. Lipid formulations are known to increase the solubilization of anticancer agents and have been utilized as carriers in dozens of drugs approved by the US Food and Drug Administration (FDA) [3]. For example, some peptides have been improved with better pharmacokinetics by using lipid carriers as protection against degradation in the gastrointestinal tract. By taking advantage of lipids, lipid-tagged ONs have also represented unique properties in the design of various molecular materials since the first publication in 1989 [4]. They could form multiple structures with different compositions and sizes by tuning experimental conditions and self-properties of the two moieties [5]. Relying on their molecular-recognition ability, information-transfer capability and self-assembly property, the developed lipid–oligonucleotide conjugates (LONs) have been used as membrane-anchored biosensors [6] and also synthetic lipid membrane channels [7]. Research examining their applications in therapeutic strategies [8] and controllable nanoreactors [9] has also been reported.
In this review, we will highlight recent advances in employing lipids for ON modifications to realize a variety of molecular-engineering nanostructures. We will first introduce the current major strategies for preparing LONs. This will be followed by a description of their basic structure and characterization of their properties. Finally, we will discuss future directions for applying LON probes in the bioanalytical and biomedical areas.
DESIGN OF LIPID–OLIGONUCLEOTIDE PROBES
Preparation: strategies for the synthesis of lipid–oligonucleotide conjugates
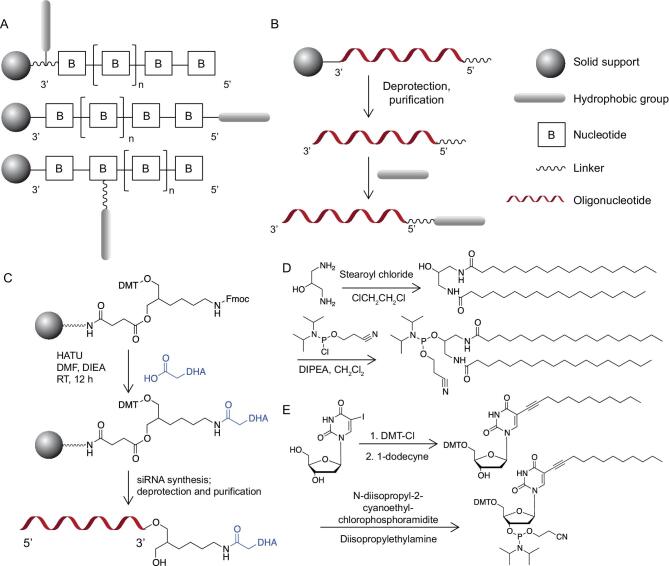
To achieve efficient synthesis of LONs, various approaches have been reported. Basically, two major strategies include the presynthetic approach and the postsynthetic approach (Fig. 1, Table 1). In the presynthetic-conjugation approach, the hydrophobic moiety-modified nucleotides are incorporated during the stepwise ON synthesis process. In the postsynthetic approach, the modification of lipid moiety is achieved after the complete synthesis and purification of the ON sequence. Since the postsynthetic conjugation is usually conducted in solution phase, allowing the separate lipid and ON strand to be assembled after respective syntheses, solid-phase-based presynthesis has a distinct advantage in terms of easier purification. However, the solubility and stability requirements and challenges encountered when introducing hydrophobic moieties directly into solid-phase ON synthesis have restricted its development. Herein, we discuss the recent efforts to construct LONs involving the two strategies. Advances in the preparation of LONs have also been highlighted in some recent reviews [5,10,11].
Figure 1.
(A) Schematic illustration of the presynthetic-conjugation approach. Hydrophobic segments can be incorporated into the oligonucleotide at the 3′-termini or 5′-termini, or between consecutive nucleotides during solid-phase synthesis. (B) Schematic illustration of the postsynthetic-conjugation approach. (C)–(E) Revised schemes showing that the specific linker-modified oligonucleotides can be covalently coupled with the hydrophobic group after their respectively accomplished synthesis and purification. (C) DHA conjugation at the 3′-termini of ONs. (D) Synthesis of lipid phosphoramidite for 5′-termini conjugation of ONs. (E) Synthesis of DMT-on dodec-1-ynyl uridine for intrachain modification of ONs.
Table 1.
Summarized synthesis strategies of LONs.
| Synthesis strategies | Characteristics | Hydrophobic-segment location | Conjugation methods | Selected references |
|---|---|---|---|---|
| Presynthetic approach | During ON synthesis; solid-phase synthesis | 3′-end of ON | Specific linkers (e.g. amide bond) | [13,14] |
| 5′-end of ON | Phosphoramidite chemistry | [17] | ||
| Between consecutive nucleotides | DMT-on phosphoramidite chemistry | [22–24] | ||
| Postsynthetic approach | After ON synthesis; solution-phase synthesis | 5′-end of ON | Linkers (e.g. thioether, phosphoester, triazole) | [27–29] |
Presynthetic approach
So far, the conjugation of hydrophobic segments with ONs has mostly relied on solid-phase synthesis (SPS). Yet, the difficulties and cost of the required organic chemistries have posed challenges to hydrophobic modifications on ONs, essentially because such modifications need to withstand different conditions carried out during SPS. During the elongation of the ON sequence from the 3′-end to the 5′-end, substantial efforts have been made to incorporate hydrophobic modifications anywhere by using the three following methods (Fig. 1A): (i) hydrophobic parts are first functionalized on the 3′-terminal solid support; (ii) hydrophobic phosphoramidite building blocks are coupled onto the 5′-end of the ONs; and (iii) hydrophobic group-prefabricated nucleotides, or non-nucleoside building blocks, are inserted among consecutive nucleotides.
It is easier to introduce the hydrophobic group as a 5′-end phosphoramidite reagent as the ON chain extends from the 3′-end to the 5′-end, but many examples have been reported for the preparation of 3′-lipid-conjugated ONs [4,12]. The first report in the literature regarding 3′-cholesteryl-conjugated ONs was described by Letsinger and co-workers, in which the premade 3′-solid support-bound H-phosphonate derivative was tethered with a cholesteryl group by oxidative phosphoramidation [4]. Afterwards, more efforts were directed toward prefabricating solid supports and hydrophobic conjugates with specific linkers before their assembly [13,14]. One related work was reported recently by Nikan et al., who conjugated docosahexaenoic acid (DHA) to the 3′-termini of siRNA sense strand via an amide bond, as shown in Fig. 1C [13]. This DHA conjugation enhanced the siRNA retention and silencing effect in mouse brain, showing the potential of modifying siRNA with hydrophobic groups for the treatment of neurodegenerative disease [13].
Besides 3′-conjugates bearing hydrophobic groups, it is noteworthy that many hydrophobic phosphoramidite building blocks are now available for coupling onto the 5′-end of ONs [15,16]. Since these phosphoramidites for 5′-conjugates typically do not possess a 4,4′-dimethoxytrityl (DMT) group, which is important for consecutive synthesis of ONs in the 3′–5′ direction, they could only be introduced at the 5′-terminus. For example, Nishina and co-workers prepared α-tocopherol phosphoramidite, combined it with siRNA at the 5′-termini and demonstrated efficient downregulation of the target mRNA in vivo [17]. Another straightforward procedure for creating 5′-LONs was reported by Godeau et al. Here, a dT-modified lipid phosphoramidite was synthesized by conjugating alkyne-labeled lipid with azido-dT via a ‘click-chemistry’ reaction and the resulting lipid phosphoramidite was further coupled to the 5′-end of the ON using SPS [18]. Our group has also been working on LONs for a long time. We have designed amphiphilic ON molecules by coupling constructed diacyllipid phosphoramidite onto the 5′-termini of ONs during SPS, as shown in Fig. 1D [16]. Importantly, the amphiphilic LONs-formed micelles are feasible for intracellular imaging and targeted gene therapy [19–21], which will be discussed further in the following sections.
Incorporation of the hydrophobic moieties into interchain positions of ONs is also an attractive method to construct LONs with the help of hydrophobic-group-premodified nucleoside or non-nucleoside DMT-on phosphoramidites. For instance, Guzaev et al. developed a non-nucleoside DMT-on phosphoramidite for multiple modifications of ON with octyl groups via potentially biodegradable ester moieties. These ester bonds could withstand the alkaline condition of DNA deprotection because of the proximate phosphate residues [22]. Additionally, Durand synthesized a dT-modified cholesterol phosphoramidite that could be positioned at any thymidine monomer site in the ON without interfering with base pairing [23]. The Herrmann group introduced another interesting prefabricated nucleoside phosphoramidite. As shown in Fig. 1E, the DMT-on dodec-1-ynyluracil nucleobase was synthesized and the resulting DNA amphiphiles were then assembled into micelles for virus capsid loading [24]. Other approaches were exploited as well, with many new hydrophobic group-functionalized phosphoramidites developed [25,26].
Postsynthetic approach
When using the postsynthetic-conjugation approach, it is required that certain reactive groups be attached to both ONs and lipids. The assembly of the ONs and lipid groups is then obtained after independent synthesis and purification. Some general methods have been developed to form different linkages connecting ONs and lipids in buffered solution, including thioether [27], phosphoester [28] and triazole [29] linkers. Raouane et al. developed an approach for the incorporation of the maleimide-modified natural lipid squalene with the thiolated siRNA sense strand via maleimide-sulfhydryl chemistry [27]. It was found that these thioether-linked amphiphilic molecules self-assembled into nanoparticles, showing strong inhibition towards tumor growth in vivo [27]. Recently, the Mirkin group demonstrated the synthesis of LONs using ‘click-chemistry’, in which the purified DBCO (Dibenzocyclooctyne)-terminated ON was reacted with azide-derivatized lipid chains [29]. Relying on the thermoresponsive ability of Pluronic F127, the authors cross-linked the micellar LONs for enhanced immunostimulation, while the non-cross-linked amphiphiles were removed by temperature transition [29]. A similar click-reaction-based strategy was reported by Awino and co-workers for the preparation of nucleic-acid nanocapsules with responsive drug-release ability [30]. They first let the alkyne-modified lipid moieties form a micellar structure. An esterified diazido cross-linker was then added to stabilize the micelle via ‘click-chemistry’. Finally, the thiolated ONs were coupled onto the remaining alkynes through a thiol-yne reaction [30].
Characterization: analysis of LONs properties
Various designs have been introduced in the previous section to describe the efforts in conjugating hydrophobic groups with ONs. Yet, irrespective of the conjugation approach, each LON analogue has similar characteristics in a broad sense. Consisting of a specific ON head for target recognition (ONs, proteins or cells) and a hydrophobic tail for anchoring membranes or driving self-assembly, LONs have special properties owing to their two-part structures. Recognition ability and stability are the two main properties, as well as prerequisites, for LONs to function well in different applications and, to demonstrate this, several related studies will be discussed in this section.
Recognition property
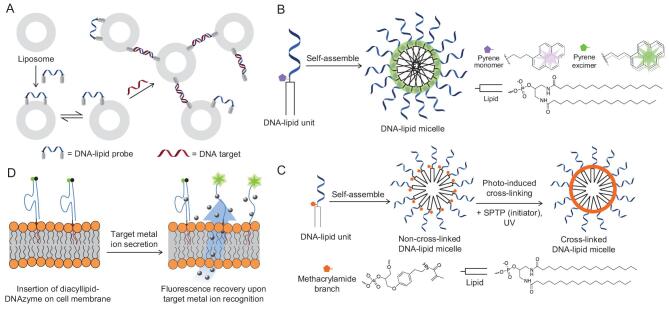
ONs are unique information elements capable of specific recognition towards targets. Although fabricated with hydrophobic building blocks, the ON parts still retain their recognition property to aid in storing or transferring information with high fidelity in LONs. For example, Jacobsen et al. reported a lipophilic DNA-controlled liposome assembly for target-DNA detection by forming a specific DNA duplex via a Watson-Crick base pairing. As shown in Fig. 2A, by insertion of the four palmityl chain-labeled DNA strands in the same liposomes, the rigid dsDNA would favorably anchor on interliposomal membranes after hybridization with the external target-DNA sequence. The remarkable thermal transition and increase in particle size allowed accurate discrimination for targeted DNA, even with a single mismatch [31].
Figure 2.
(A) Revised schematic illustration of liposome assembly controlled by the hybridization between DNA–lipid probes (blue) and targeted DNA (red). (B) Revised scheme illustrating the self-assembly of DNA–lipid micelles. The incorporated pyrene monomers aggregated and generated excimer-type fluorescence as a reporter in the micelle system. (C) Revised scheme illustrating the cross-linking of self-assembled DNA–lipid micelles after UV irradiation. (D) Revised schematic representation of DNAzyme sensor anchored on the cell membrane for real-time monitoring of metal ions in the cellular microenvironment.
A similar idea was used for tethering lipid vesicles based on the recognition property of ON heads [32]. For instance, as reported by the Boxer group, the information encoded in the DNA–lipid conjugate in each preformed vesicle allowed the specific attachment to the complementary DNA sequence on the surface of a fluid-support lipid bilayer. This sequence-specific tethering model could be useful for the study of interactions between native membranes or cells with other components [32].
Most LONs could self-assemble in the water phase as a result of the hydrophobic effect caused by the lipid segments. Depending on the length of the ON part and the number of hydrophobic units, i.e. the balance between the electronic repulsions and hydrophobic effect, LONs could assemble to shift between spherical vesicles and micelles [33]. As presented by Thompson, LONs were demonstrated as programmable smart nanomaterials in response to the additional DNA strand. The recognition property of LONs enabled reversible hybridization with target-DNA strands, thereby driving the reversible vesicle–micelle phase switches [33].
Interestingly, the information-transfer ability of LONs not only facilitated the phase transitions in LONs-programmed vesicles, but could also help in the construction of lipid vesicles with designed geometry and controlled size. The Lin group recently published two papers reporting on the generation of liposomes by using DNA nanotemplating [34,35]. Basically, multiple single-stranded DNA handles that displayed on the DNA-origami ring or cage (DNA origami is a recent technique of folding DNA oligonucleotides to create various 3D nanoscale architectures for developing programmable DNA materials [36]) were hybridized with lipid-modified complementary DNA sequences. These lipid molecules then nucleated liposome formation with extra lipids added. As a result, liposomes with different complex membrane structures were generated based on defined DNA templates [34]. By applying this reconfigurable-membrane-engineering technique, a capable platform for studies of membrane mechanics, drug delivery across cell barriers and artificial organelles would be constituted in the future.
In addition to complementary sequences, nucleic-acid aptamers, known as chemical antibodies, are also widely used as target ligands because of their specific recognition property [37,38]. Aptamers are short, single-stranded DNA or RNA oligonucleotides generated by a process called SELEX (Systematic Evolution of Ligands by EXponential Enrichment) [38]. Over the past 30 years, many aptamers have been identified and could be approached via an aptamer database (e.g. Aptagen) and they have high binding affinity and specificity to many target molecules, including ions, small molecules, proteins and cells [39,40]. Aptamer–lipid conjugates have been exploited in our group over the years. One classical example to represent the recognition property of aptamer–lipid was from Wu et al., who reported that amphiphiles could self-assemble into micelles and detect cancer cells with high specificity through the selective recognition of aptamers towards target cells [19]. More research about aptamer–lipid conjugates will be discussed in the following section.
Stability
In order to evaluate the stability of LONs, one of the easiest methods is to monitor the melting temperature (Tm) of the duplex formed between the target LON and its complementary sequence, as a higher Tm usually indicates the higher complementarity of the duplex [41]. One related study was reported by Letsinger et al., who found that the appropriate positions of hydrophobic groups could facilitate base pairing between LONs. In particular, they found that a high Tm was exhibited when the base pairing happened in an unfavorable orientation, as both the poly-A and poly-T were modified with cholesteryl at their 5′-ends, indicating the strong hydrophobic forces required to stabilize the formation of a ‘wrong’ duplex [41].
Critical micelle concentration (CMC) is commonly used to characterize the assembly stability of LONs. When the concentration of LONs is above the CMC, LONs become thermodynamically willing to aggregate into the micelle structure to reduce free energy in the system. And it has been previously reported that a decrease in the CMC could help to enhance micelle stability, usually due to the longer hydrophobic lipid domain, which, however, would increase the micellar size and lower encapsulated drug performance in vivo, as the thermodynamic equilibrium of the micelle assembly would be hard to achieve [42]. Our group has utilized the fluorescence spectrum of pyrene molecules to determine the CMC of DNA micelle flares [16,43]. Basically, a pyrene phosphoramidite was incorporated into each LON amphiphile (Fig. 2B). Since multiple pyrene units in self-assembled ONs tend to aggregate and produce excimer-type fluorescence, the shift of fluorescence spectra from pyrene monomer to excimer versus the lowest LON concentration indicates the upper limit of the CMC. By using this method, it was determined that our micelle flares had a very low CMC, at ∼10 nM, suggesting the superior stability of the micelles independently of the effects of the ON length and temperature [43].
Apart from physical stability, the biostability of LONs is also significant, since many designs aim to apply the modified LONs to real biological environments. Accordingly, nuclease-resistance studies have been carried out to examine the stability of DNA–lipid micelles compared to single-stranded DNA using deoxyribonuclease I (DNase I) or serum proteins [43]. Free ON has been known to be quickly degraded in the presence of serum nucleases, with a half-life ranging from a few minutes to several hours. However, modifications on ONs such as peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) have rendered significant nuclease resistance due to their unnatural entities-mediated low recognition from nuclease, with an increased half-life from hours to days [44,45]. In terms of LONs, fast micelle formation was also able to prevent degradation by nuclease, possibly resulting from the steric hindrance of the DNA corona. Subsequent studies in the Tan group further improved the physical and biological stability of DNA–lipid micelles [46–48]. Our most recent study reported the introduction of a methacrylamide unit between the DNA and lipid segments, which served to cross link the amphiphiles after self-assembly via photoinduced polymerization (Fig. 2C) [46]. Compared with traditional non-cross-linked micelles, the resulting micelles showed enhanced structural stability, as demonstrated by electrophoresis analysis, as well as excellent biostability after evaluation by DNase I and serum. This efficient covalent cross-linking method facilitated the construction of micelles and rigidified the structures without disturbing the specific recognition ability of the DNA aptamer [46].
With excellent recognition ability and stability, LONs and related micelle structures could be applied to various bioanalytical and biomedical applications. But, in order to achieve the desired goals, it is critical to consider the balance between increasing structural and probe stabilities (lowering CMC) and enhancing drug-loading or targeted-recognition abilities. As a consequence, better LONs should be designed with attempts at (i) stabilizing LONs by using covalent or non-covalent cross-linkers, or complexing with other fatty acids, or modifying ON heads with other architectures (e.g. cyclization [49]); (ii) improving targeting the hydrophilic head by either increasing quantities or exploiting more complex structures (e.g. molecular beacons giving ‘turn-on’ recognition signals); (iii) exploring more drug-loading possibilities, or combining drugs in the LON synthesis or cross-linking (e.g. nucleoside analogue clofarabine drug [50]), in terms of LON-based therapeutics.
BIOANALYTICAL AND BIOMEDICAL APPLICATIONS OF DNA–LIPID PROBES
Cell-membrane-anchored biosensors
The cell surface and cellular microenvironment comprise molecules expressed on the cell surface and various local biological species. This is the hub of cell–cell communication and cell signaling [51]. Extracellular signals from metal ions, pH, cytokines, growth factors and biomolecules themselves influence the activities of a key set of cell-surface receptors and downstream pathways, all constituting a complex network for sensing environmental changes, regulating cellular activities and maintaining homeostasis [52]. This microenvironment plays a pivotal role in regulating cellular activities, including metabolism, growth, development, differentiation and apoptosis [53]. Disturbance of the microenvironment may contribute, or relate, to disease progression, as manifested by inflammation, onset of tumorigenesis and metabolic diseases [54]. Insight into biomolecule distribution and interaction patterns in the local surroundings or on the live-cell membranes is critical to biological studies about cellular functions, disease diagnosis, as well as drug discovery. Powerful biosensing and bioimaging techniques would significantly improve our understanding and ability to manipulate biological mass dynamics and functions.
Recently, such technologies as mass spectrometry, Western blot and enzyme-linked immunosorbent assay (ELISA) have been developed for analysing purified molecules from cell-culture media and cell lysate [55]. However, most of these methods can only detect and measure bulk samples. While valuable, developing biosensing tools for molecules in native forms found in the live-cell microenvironment still encounters challenges. To address these engineering challenges, well-designed molecular sensors have been anchored onto the cell membrane, resulting in the performance of live-cell studies with high spatio-temporal resolution [56]. We recently developed varied LON structures in which the lipid moiety allows facile and efficient engineering of DNA-based sensors onto the cell phospholipid bilayer [6,56,57]. Compared to previous methods, lipid modification can be considered as a universal strategy without affecting cell physiology or sensor performance and no complicated processes are required.
Real-time monitoring of substances in the extracellular microenvironment
Some cellular substances, including protons, metal ions, small molecules and proteins, can affect cell functions and biological processes. Changes in the concentration of these substances are often associated with disease progression. Consequently, studying and monitoring these target molecules in real time would be beneficial to improve our ability to understand and manipulate biological systems. Currently, various technologies, such as flow cytometry and confocal laser-scanning microscopy, have been proposed to probe and image many kinds of events in the extracellular microenvironment. Qiu et al. [57] first reported a simple and universal strategy for real-time monitoring of extracellular metal ions based on a one-step insertion of a DNAzyme sensor on the cell-membrane surface after having modified a diacyllipid tail on DNA via SPS (Fig. 2D) [58]. Compared with other methods, this modification process does not require applying toxic chemical reactions [59], specific sites [60] or complicated manipulations [61]. Furthermore, the lipid-insertion strategy rarely affects the cell's physiological performance or sensing characteristics (stability and reliability). The experimental results also show that the self-assembled lipid-aptamer anchored on the cell surface has higher surface coverage and higher efficiency than can be achieved by covalent chemistry methods. The decoration strategy was shown to be desirable and reliable, offering a convenient tool for the detection of various targets by modifying different functional DNA probes on the cell membrane. However, irreversible cleavage of the substrate strand induced by the target does not readily accommodate a fluctuating monitoring signal.
In fact, most analytical methods were designed to track target concentrations under ‘static’ conditions, while only a few have been designed to monitor the dynamics of the cell microenvironment [62]. Some reversible reactions, e.g. the formation of a quadruplex structure combined with potassium ions and a pH-sensitive i-motif sequence, may provide a strategy for dynamic monitoring in real time. Xiong et al. [63] designed a fluorescent probe based on a thrombin-binding aptamer (TBA). It could form a chair-type G-quadruplex structure in the presence of potassium ions and dynamically monitor them (Fig. 3A). The C18 diacyllipid linked with the TBA sequence from SPS was anchored to the cell membrane through hydrophobic interaction between the lipophilic tail and the cellular phospholipid layer. A FRET (Förster resonance energy transfer) pair offered signal information by the K+-induced structural change of the TBA. As the coordination between the TBA and K+ is fast and reversible, the fluorescence probe could be applied to real-time and reversible measurement of the fluctuation level of K+ in the complex cell microenvironment.
Figure 3.
(A) Schematic illustration of the reversible membrane-immobilized K+ probe. Reprinted with permission from [63]. Copyright (2016) Royal Society of Chemistry. (B) Schemes of cell membrane-anchored DNA tweezers for extracellular pH monitoring. Reprinted with permission from [64]. Copyright (2018) American Chemical Society. (C) Reprinted with permission from [65]. Copyright (2018) American Chemical Society. (D) Schematic illustration of the T-cell-aptamer sensor for cytokine-secretion measurement. Reprinted with permission from [67]. Copyright (2017) Royal Society of Chemistry.
Similar designs were also applied to build some DNA-nanomachine-based biosensors, such as DNA tweezers, walkers and motors. Recently, DNA tweezers with a FRET pair as the signal reporter and cholesterol as the anchoring unit on the cell surface for extracellular pH monitoring were reported by both the Yao group (Fig. 3B) [64] and the Jiang group (Fig. 3C) [65]. In particular, the Jiang group investigated the number of cholesterol moieties anchoring on the cell surface. They found that multisite cholesterol anchoring could improve stability and prevent detachment of the DNA-sensing system from the cell-membrane surface. Moreover, they tuned the pH responsiveness by regulating the content of the C-G-C triplet and found that an enhanced response to higher acidity could be obtained with a higher percentage of the C-G-C triplet in the triplex motif. These cell-surface-anchored DNA tweezers have demonstrated a fast and reversible response upon extracellular pH change in real time. The strategies discussed here have potential for pH-related disease diagnostics and biomedical research.
In order to better understand the individual cellular, cell-to-cell variation and related processes, extensive interest has been generated in the field of single-cell research. Unlike ELISA, showing the advantage of analysing collective cellular properties [66], flow-cytometry assays could provide single-cell cytokine readouts. However, they could not be used to monitor cytokine production with high spatio-temporal resolution. In combination with a droplet-microfluidic system, Qiu et al. addressed this drawback by developing a simple, high-throughput and signal-on sensor to specifically monitor cytokine secretion (IFN γ) at the single-cell level, as shown in Fig. 3D [67]. The droplet-microfluidic system could generate picoliter water-in-oil droplets, which provided a versatile platform for the single-cell assay with low cost and high throughput. A cholesterol tail was attached to an aptamer probe and the two ends of the hairpin structure were separately labeled with a fluorophore and a quencher for recognition and sensing. They claimed that this strategy could be applied as a powerful and reliable molecular tool for studying immune response at the single-cell level.
Measuring biophysical events on cell surfaces
The ability to quantitate the dynamic and transient molecular encounters of proteins and lipids is essential for understanding cell-membrane biophysics. However, transient encounter events between different cell-surface components are too fast (between the microsecond and millisecond ranges) to be detected by traditional analytical tools. Given the importance and challenge of such detection, a facile and efficient molecular probe compatible with standard fluorescence microscopy and flow cytometry is highly desired. This challenge has been overcome by a DNA walker reported by the Tan group (Fig. 4A) [6]. As biological programmable macromolecules, DNA-based walkers, which are able to walk directionally and progressively, have been recently developed to mimic biological protein motors in cargo transportation and biosynthesis. The DNA walker has also been found to be useful for probing dynamic and transient encounter events among biomolecules on the live-cell surface. To accomplish this, the DNA walker is anchored on the cell surface by lipid molecules on DNA strands; it then follows a track (cell membrane) while walking. DNA translocation on the DNA walker happens through a toehold-mediated strand displacement after two anchor sites (lipids) encounter by diffusion on the cell membrane. Because the accumulation signal is linearly correlated with the rate of lipid encounters, the walker has been successfully applied to measure the rapid encounter dynamics of two lipid molecules. By adopting a second-order-reaction model to calculate the encounter rates and 2D comparison of three kinds of membrane lipid-molecule anchors, the same lipid domains were found to have generally higher collision rates compared with heterogeneous encounters. The same strategy has also been extended to study cell-surface-receptor-encounter dynamics by using an aptamer-modified DNA nanowalker.
Figure 4.
(A) Probing the dynamic and transient biomolecule-encounter events on a live cell surface with a DNA nanowalker. Reprinted with permission from [6]. Copyright (2017) Springer Nature. (B) Measuring tensile forces among cells with DNA-based membrane molecular probes. Reprinted with permission from [68]. Copyright (2017) American Chemical Society. (C) Assembly/disassembly actions of photoswitchable DNA-origami assembly. Reprinted with permission from [69]. Copyright (2014) American Chemical Society. (D) Reversible assembly processes of DNA triangular prisms on cell-mimicking giant membrane vesicles. Reprinted with permission from [70]. Copyright (2017) American Chemical Society.
Mechanical forces regulate behavior such as cell migration, proliferation and differentiation, as well as guiding cell fate and many important cell-developmental processes. To date, however, force studies of intercellular and extracellular matrices and measurement at the cell–cell interface with traditional methods, such as monolayer stress microscopy and microfabricated cantilever array, have proven very challenging. Zhao et al. reported an efficient cell-membrane DNA probe to measure tensile forces among cells (Fig. 4B) [68]. Lipid-modified DNAs have been applied to efficiently anchor the tension probe onto the cell surface with high stability, while the other end of the probe was modified with cyclic Arg-Gly-Asp-d-Phe-Lys (cRGDfk) ligand, which readily linked to the second cell with the receptor. By using a distance-dependent FRET mechanism, the probe would have fluorescence output change when experiencing a change in forces between neighboring cells. The change of fluorescence resulted from the unfolding of the FRET pair on the DNA probe. With standard fluorescence microscopy, the probe's function has been successfully demonstrated and could be a potential approach to measuring various cellular processes.
DNA-nanoassembly behavior on cell-membrane mimicry
Thus far, DNA nanostructures, such as DNA walkers, tweezers and logic circuits, have been applied on the cell surface to perform intelligent tasks. In the meantime, massive studies have investigated the dynamic behavior of various DNA nanostructures on various types of cell-mimicking membranes, which may help to develop better understanding of membrane physicochemical properties and provide a significant impact on improving cell-surface-anchored DNA nanostructures. Suzuki et al. reported on photoswitchable DNA-origami assembly/disassembly using atomic-force microscopy (Fig. 4C) [69]. Their DNA origami was placed on a lipid bilayer surface with moderate mobility by cholesterol modification on the outer edges. One edge of the DNA origami was modified with sequence complementary azobenzene DNA strands and then used for dimer–monomer transition under UV- and visible-light irradiation. Assembly/disassembly behavior was successfully monitored by high-speed atomic-force microscopy in real time. Peng et al. investigated the assembly and disassembly processes of DNA triangular prisms on cell-mimicking giant membrane vesicles derived from mammalian cells (Fig. 4D) [70]. One arm of the 3D DNA prism was conjugated with a cholesterol anchor so that the construct could be efficiently localized on the giant vesicle surface. DNA-mediated strand hybridization and displacement were used to manipulate the dynamic behavior, as demonstrated by FRET. By regulating membrane-anchored DNA nanostructures, such methods can be applied for artificial-cell engineering and biomimetic nanotechnology.
Targeted cancer theranostics
Owing to the similarity between conjugated lipid groups in LONs and the lipid bilayers in cell membranes, LONs have been recognized as having excellent biocompatibility and membrane permeability. After labeling specific fluorophores, LONs have shown compelling feasibility for intracellular imaging. Furthermore, effective delivery of encapsulated drugs or therapeutic ONs in the form of LONs has favored some administration concerns, including carrier safety and pharmacokinetic behavior. Thus, with versatile modifications, these hybrid LONs have been successfully extended to a larger perspective of applications in tumor imaging and targeted therapeutic strategies.
Imaging
Real-time imaging of living cells is essential for understanding life science—more specifically, the study of cancer progression. In this case, imaging tools with special properties would allow the visualization of both tumor sites in the body and the level of tumor behavior [71]. Yet, challenges remain owing to the lack of specific imaging probes against targeted cancer cells. As we discussed in the previous section, hydrophobic segments are good materials with which to penetrate cells with high affinity by having structures similar to those of cell membranes [72]. LONs could also incorporate nucleic-acid aptamers for specific target recognition. Therefore, efforts have been made to develop aptamer–lipid conjugate-based imaging probes for targeted cancer bioimaging.
Aptamers in LONs can be functionalized with fluorophores to realize fluorescence-based optical imaging [21,46]. For example, our lab has labeled TDO5 aptamer with fluorescein to monitor the binding ability of aptamer–lipid micelles with targeted Ramos cells (a B-cell lymphoma cell line) (Fig. 5A) [19]. Interestingly, densely packed aptamers in the micelles resulted in a much better binding profile because of the multivalent effect; at the same time, the lipid part helped to achieve more rapid cell internalization. Thus, the substantially enhanced selective binding signal at a physiological temperature proved that the attachment of lipid groups to aptamers could generate unique nanomaterials for targeted cancer-cell imaging.
Figure 5.
(A) Scheme for TDO5 aptamer–lipid micelle formation and flow-cytometry assay for binding profiles of aptamer only and aptamer micelles against target Ramos cells or control HL60 cells. Reprinted with permission from [19]. Copyright (2010) National Academy of Sciences. (B) Scheme for the synthesis of EGFR aptamer–lipid nanoparticles with quantum dots and loaded siRNAs. Reprinted with permission from [74]. Copyright (2017) Nature Publishing Group.
However, fluorescence imaging suffers from high background noise and superficial tissue penetration. To solve these problems, the utilization of quantum dots would be beneficial, as they were shown to have lower autofluorescence and deep inner-tissue penetration [73]. Kim et al. have built tumor-targeted lipid nanoparticles by combining EGF (epidermal growth factor) receptor (EGFR) aptamer–lipid conjugates and hydrophobic quantum dots responsible for target-cell recognition and fluorescence observation, respectively (Fig. 5B) [74]. The remarkable fluorescence signal from EGFR-positive cells, as well as in vivo tumor tissues, implied the potential of using this system for bioimaging in real surgery. By loading anticancer siRNAs, cancer-specific treatment was also expected [74]. Moreover, the Tang group fabricated aptamer-decorated self-assembled organic dots with aggregation-induced emission characteristics to enhance photostability and biocompatibility for better cancer-cell targeting and imaging [75].
Therapy
To realize targeted tumor therapeutics, studies involving the engineering of different drugs and drug-carrier systems have been carried out. The modes of delivering therapeutic agents must maintain efficacy while, at the same time, escaping potential off-target effects and immunogenicity [76]. LONs exhibit low cytotoxicity, controllable material size and available drug-loading sites; thus, by incorporating specific targeting ligands, different generations of LONs-based materials have been developed as efficient delivery vehicles for targeted cancer therapy.
Targeted drug delivery.
Small-molecule drugs have played the dominant role in new drug development in the pharmaceutical industries. And, in order to improve personalized treatment for patients, different combinations, formulations and delivery routes have been studied, as well as the incorporation of targeting ligands to provide drug delivery with specificity [77]. The ultimate goal of targeted drug delivery is to deliver a certain amount of therapeutic drugs with a prolonged circulation time, but smaller dosage frequency, to the targeted tumor area. One study direction has been focused on designing LONs-based nanoparticles as drug carriers, since hydrophobic side chains usually provide enough cavities to encapsulate small hydrophobic drug molecules.
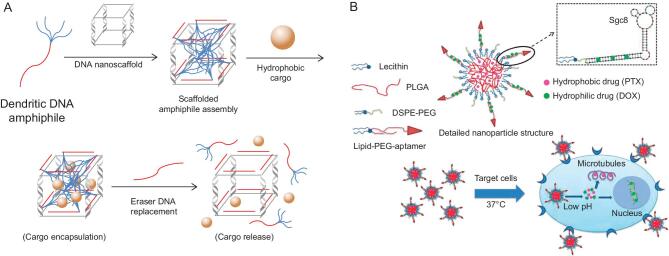
For example, the Sleiman group has built different LON assemblies by applying dendritic alkyl chain-based DNA amphiphiles to specific DNA nanoscaffolds [8,78]. As shown in Fig. 6A, guest molecules such as the hydrophobic dye Nile Red could be loaded in the inner space of the scaffolded assemblies and then be released upon specific DNA-sequence recognition and replacement. These LON amphiphiles-based 3D nanostructures were, therefore, capable of encapsulation and release of hydrophobic small-molecule drug cargos for future targeted-drug-delivery applications [8].
Figure 6.
(A) Revised scheme showing spontaneous scaffolded dendritic chain–DNA amphiphile assembly upon adding specific DNA nanoscaffolds. The formed DNA cage could encapsulate small hydrophobic guest molecules and release them once destroyed by an eraser DNA sequence. (B) Schematic representation of self-assembled hybrid micelles for targeted co-delivery of two anticancer drugs. Reprinted with permission from [81]. Copyright (2014) Royal Society of Chemistry.
Studies employing real drug molecules were also reported. Here, either specific hydrophobic drugs targeting different diseases were loaded inside the hydrophobic core after LONs assembly or hydrophilic drugs bound with ONs [79–81]. Recently, Liu et al. delivered budesonide (sold as SYMBICORT, a glucocorticoid for treating chronic inflammation) through a UU11mer DNA–lipid micelle system, showing better drug solubility in the water phase and good anti-inflammatory activity [82]. In another study from Charbgoo et al., doxorubicin (DOX), a widely used hydrophilic chemotherapy medication for treating multiple types of cancer, preferentially intercalated into double-stranded GC-rich regions in cholesteryl-modified MUC1 aptamer micelles, resulting in enhanced specific therapeutic effects with reduced DOX dosage in both in vitro and in vivo studies [80].
Our group has further demonstrated the co-delivery of two drugs into targeted cancer cells via aptamer–lipid micelles. In this construct, DOX was intercalated into modified sgc8 aptamer and the hydrophobic anticancer drug paclitaxel (PTX) was loaded into the lipid core (Fig. 6B) [81]. The hybrid nanoparticles with drug pairs were selectively transported into target CEM cells, rather than control Ramos cells, and demonstrated significant synergistic anticancer efficacy in the cytotoxicity assay [81]. Consequently, we could explore more advanced targeted-drug-delivery systems with other hydrophobic and hydrophilic anticancer drugs.
Gene therapy.
Gene therapy has offered promise for treating gene-related diseases. However, the introduction of specific genetic materials into targeted tumor sites is limited by several difficulties, such as cellular-membrane barriers, self-instability of materials and poor circulatory profiles [83]. To overcome these challenges, a variety of approaches have been reported, including the use of viral or non-viral vectors [84,85] and physical instruments, such as microinjection, electroporation and sonoporation [86]. Nevertheless, the toxicity, delivery efficacy and expense of these methods have been priority issues for further improvement before clinical applications.
Synthesis and modification simplicity, physiological safety and stability are some of the merits that have contributed to the success of LONs in the delivery of gene molecules for the induction or knock-down of specific gene expression. Our group has previously reported the synthesis of DNA–lipid micelle flares for intracellular mRNA detection and gene therapy [20]. The diacyllipid–molecular beacon conjugates self-assembled into micelle flares and showed efficient cellular uptake. Upon target-mRNA binding, the fluorophore on the molecular beacon was separated from the quencher molecule, resulting in the ‘turn-on’ fluorescence signal. The hybridization with target mRNA even induced a remarkable inhibition of cancer-cell proliferation, suggesting that simple modification with lipid afforded molecular beacons with enhanced cellular entry and targeted gene therapy [20].
Some researchers have directly attached hydrophobic domains to the antisense ONs in order to improve cell internalization and inhibition efficiency of target mRNA in the absence of additional transfection materials [15,87]. For instance, Rush and co-workers conjugated LNA with a hydrophobic tail to build spherical micellar nanoparticles, achieving rapid cell uptake and efficient target-mRNA regulation [15]. It is noteworthy that the densely displayed nucleic acids on the nanoparticle surface restricted degradation from enzymes and significantly increased the depletion ability towards targeted mRNA.
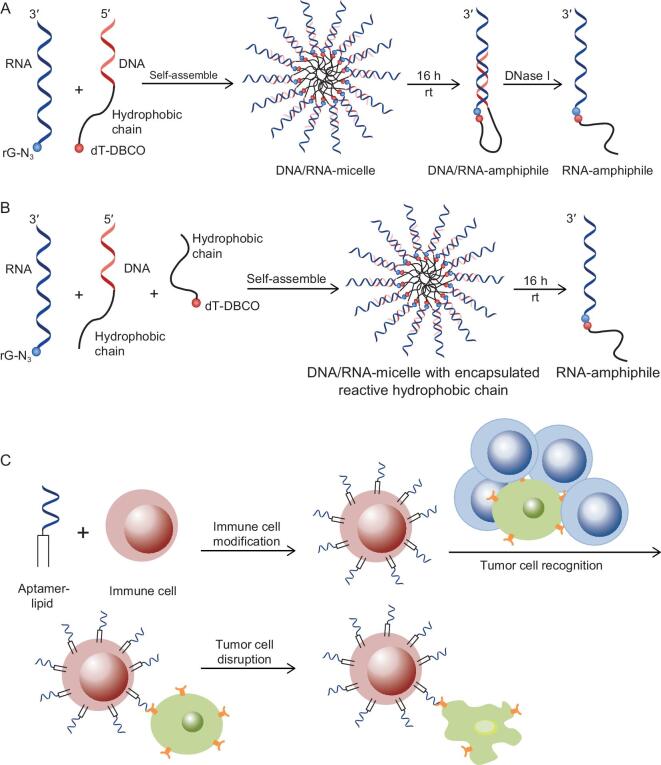
A more recent study from the Sleiman group reported two methods for conjugating specific RNA strands with hydrophobic chains, allowing the formation of RNA-amphiphiles and subsequent efficient gene-silencing capability [88]. As shown in Fig. 7A and B, by employing dibenzocyclooctyne group (DBCO)-labeled DNA-amphiphile as a template, azide-modified RNA was directly attached to the hydrophobic chain via ‘click-chemistry’ and the hybridized DNA could then be removed by adding DNase I. This interesting method provided a novel solution to the challenge of linking RNA strands with hydrophobic molecules, and the outcome has improved the efficacy of RNA therapeutics [88]. To further increase therapeutic efficiency, another study has also reported that the co-delivery of small-molecule anticancer drugs and nucleotide molecules simultaneously could show a synergistic effect for specific tumor treatment [89].
Figure 7.
(A) and (B) Revised schemes of two methods for RNA-amphiphile synthesis. (A) RNA-N3 was hybridized with complementary DNA-amphiphile–DBCO, followed by the formation of DNA/RNA-amphiphile after self-assembly via ‘click-chemistry’. (B) Reactive DBCO-labeled hydrophobic chain was encapsulated in the DNA/RNA micelle, facilitating conjugation. (C) Revised scheme showing modification of immune cells with aptamers for specific recognition towards targeted cancer cells (green), rather than normal cells (blue), and subsequent cell–cell adhesion-induced cancer-cell disruption.
Immunotherapy.
Cancer immunotherapy, which is based on an antitumor immune response to regulate tumor behavior, has shown therapeutic efficacy in the fight against various malignancies [90]. Many approaches have been reported to boost the development of cancer immunotherapy, ranging from vaccine-based [91] or immunoregulatory cytokine-related designs [92] to engineering immune cells with specific ligands [29,93,94]. Among these strategies, immunoregulatory ONs represent strong immunostimulatory ability as a tumor suppressor, such as unmethylated cytosine-guanosine (CpG) DNA motifs and double-stranded RNA motifs [95]. Liu et al. in our group previously introduced the combination of synthetic CpG oligodeoxynucleotides (ODNs) with lipid domains where the lipid tail helped constructs to insert into cell membranes and promoted the association of CpG motifs with tumor cells over a prolonged time [94]. This strategy was investigated with both in vitro and in vivo studies and exhibited potential for localized immunotherapy.
In another interesting study, Xiong et al. engineered immunological effector cells with an aptamer for targeted cancer-cell recognition, bringing killer cells specifically to attack cancer cells, while, at the same time, minimizing side effects (Fig. 7C) [93]. The immune cells gained recognition ability after modification with aptamers through lipid-tail linkage. Then, cell–cell adhesion allowed an effective cytotoxic immune response to kill targeted cancer cells. This simple, but effective, method served as a redirected immunotherapy approach and was expected to employ more aptamers for a variety of cell-based immunotherapies in the future [93].
LONs-mediated assembly and fusion
The hydrophobic tail of LON conjugates gives rise to hydrophobic–hydrophobic interactions among different types of membranes, such as cell membranes, vesicle membranes and hydrophobic-droplet-phase membranes. This sort of non-covalent interaction was depicted as an anchoring phenomenon and it garnered tremendous attention as it enabled facile decoration of nucleic acids on the cell, liposome and emulsion-droplet surface. By virtue of DNA reactions, including hybridization and displacement, functionalized vesicles, or droplets, are endowed with controllable assembly and fusion behaviors. In the past two decades, many studies have contributed to broadening the application of LONs to control vesicle or droplet interactions, including programmable assembly, controllable nanoreactors and membrane-fusion-induced delivery and biosensing [96–98].
Sequential assembly
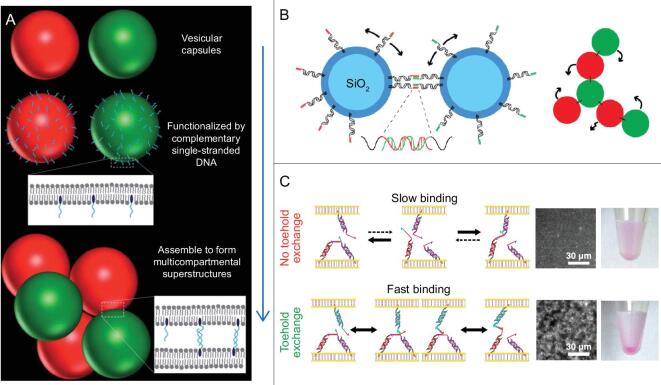
Self-assembly behavior of nano/microscale-particle suspensions led to the design and creation of materials with tunable properties, especially through surface non-covalent interaction of particles in the bulky solution. DNA strands with specific hybridization and thermal reversibility have become a potent motif for particle-surface modification to enable assembly or aggregation. Soft materials, such as liposomes and oil-emulsion droplets, have a hydrophobic membrane, or surface, in aqueous buffer, which can then interact with the hydrophobic tail in LONs. In Vanderlick's work (Fig. 8A), lipid–DNA was first applied to the liposome surface in order to control vesicle association and population in solution [99,100]. Lately, this lipid–DNA-mediated interaction strategy has also been extended to hard nanomaterials, such as silicon [101]. A new solid colloidal system composed of hard and non-deformable particles was created by Mirjam et al. (Fig. 8B). The particles were modified with highly movable cholesterol–DNA conjugates on the surface. Thus, their colloids performed good association/dissociation transition under the control of DNA thermal reversible interaction.
Figure 8.
(A) Liposome assembly induced by specific hybridization of membrane-anchored DNA. Reprinted with permission from [100]. Copyright (2011) Royal Society of Chemistry. (B) Solid colloidal system composed of cholesterol-DNA-modified hard particles. Reprinted with permission from [101]. Copyright (2013) American Chemistry Society. (C) Kinetic liposome assembly mediated by DNA toehold exchange reaction. Reprinted with permission from [103]. Copyright (2016) American Chemistry Society.
In addition, the programmability of DNA has become a striking characteristic in the design and engineering of smart materials. Kinetic DNA hybridization, such as toehold reactions and strand-replacement reactions, allows sequential initiation, signaling and logical programming. The programmable potential of DNA by rational strand design is exemplified by nanodevices that employ DNA logic gates, DNA robotic motion and DNA computation circuits, as well as related concepts [102]. This attractive property was also transplanted into the design and engineering of smart materials, especially DNA-coated colloidal-system complexes. Assembly/disassembly behavior could also be programmed by lipid–DNA-mediated interaction by using DNA strands to connect the vesicles, or droplets, and by introducing sequential DNA-strand reactions. The complex phase behavior endorsed by DNA sequential displacement reactions in colloidal systems has enabled many innovative studies, such as ultrasensitive detectors and nanorobotic walkers. For example, Mognetti et al. designed a kinetic pathway to realize liposome assembly based on a toehold exchange mechanism among multiple types of surface-anchored lipid–DNA conjugates, as shown in Fig. 8C. The rearrangement of liposomes was enabled by cascade DNA interaction without the need to thermally denature DNAs [103].
Bioinspired fusion mediated by LONs
Although LONs can be applied to manipulate the complex assembly of compartmentalized vesicles, or droplets, to form colloidal architecture, another interesting model inspired by natural membrane fusion has attracted scientists’ attention to expand the function of lipid–DNA. Membrane fusion is a common phenomenon in cell communication, which plays an especially important role in neurotransmission, endocytosis and exocytosis. In the biological-membrane-fusion process, SNARE (Soluble N-ethylmaleimide-sensitive factor activating protein receptor) primarily mediates vesicle fusion whereby vesicles fuse with their target membrane-bound compartments, e.g. lysosome, according to the SNARE hypothesis [104]. It has been widely accepted that the SNARE complex was formed by a four-helix bundle between two membrane compartments (Fig. 9A), which could physically facilitate membrane interaction in the docking process [105]. Inspired by this zipper model, Boxer et al. developed a vesicle-fusion method mediated by lipid–DNA, as shown in Fig. 9B [106]. The fusion process operates via a mechanism similar to that of a LON-mediated assembly, which relies on hydrophobic tail-membrane anchoring and DNA hybridization, but it requires different membrane-anchoring geometry of strand pairs. Unlike the lipid–DNA-induced assembly in the previous section, whereby hydrophobic tails are always coupled on the 5′-end of DNA strands, the DNA hybrid in the fusion complex must have one strand coupled with the lipid at the 5′-end and the other at the 3′-end. Thus, hybridization can form into a zipper-like structure to pull the two vesicles into close apposition.
Figure 9.
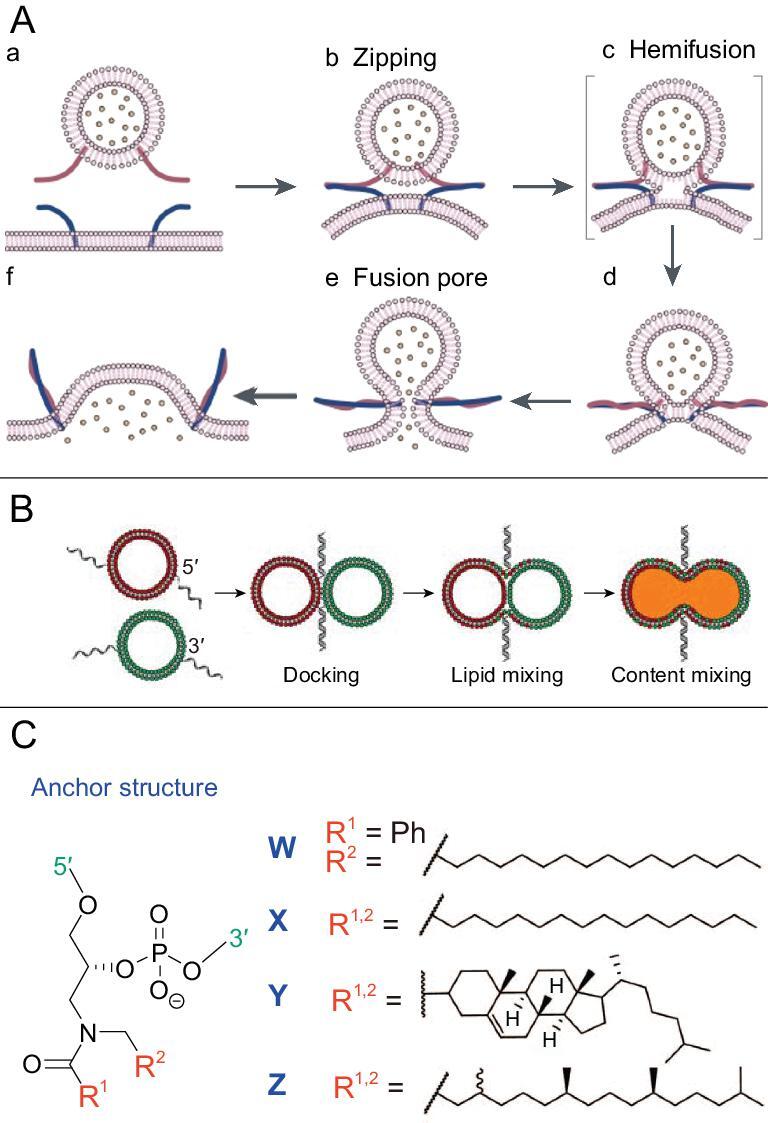
(A) Membrane-fusion process facilitated by SNARE protein. Reprinted with permission from [104]. Copyright (2001) Springer Nature. (B) Controllable vesicle fusion mediated by lipid DNA. Reprinted with permission from [106]. Copyright (2008) Springer-Verlag. (C) Different types of anchors in hydrophobically modified DNA. Reprinted with permission from [107]. Copyright (2017) Royal Society of Chemistry.
Boxer's controllable specific fusion method established the basis for using lipid–DNA to realize vesicle fusion and content mixing. Subsequent studies have dissected the physical process whereby factors, including membrane lipid components, temperature, DNA-strand length, linkers and hydrophobic anchors, were explored to improve this fusion model with higher fusion and content-mixing efficiency. Incorporation of a triethylene glycol spacer between the hydrophobic anchor and DNA was discovered to significantly increase fusion and content-mixing efficiency [107]. The most important factor was temperature, for which content-mixing efficiency was remarkable at 50°C with negligible leakage [107]. Cholesterol is another widely used alternative hydrophobic anchor owing to the commercial availability of cholesterol-coupling reagents for DNA modification. It was revealed that two cholesterol anchors are necessary to prevent DNA strands from shuttling to the same membrane, which could lead to membrane separation, rather than fusion. Other lipid anchors, such as phosphatidylcholine, phosphatidylethanolamine and sphingomyelin, were also explored, indicating the diversity of modification methods to enrich the lipid–DNA-mediated fusion model (Fig. 9C) [108,109].
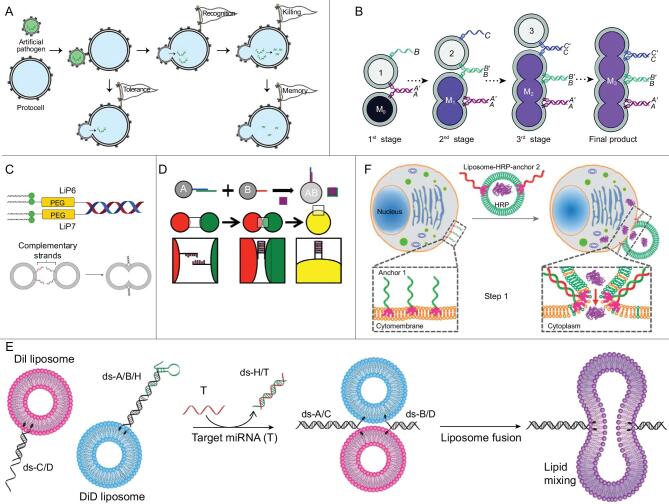
The liposome-based nanometer-sized bioreactor emerged as an artificial way to mimic biological compartmental synthesis in complex physical environment. However, the biggest limitation of the liposome nanoreactor is the impermeability of the bilayer membrane. For the standard liposome bulk solution, liposome vesicles must be homogeneously suspended with minimum association. On the contrary, to accelerate the reaction, fast aggregation and fusion among liposome units are desired at specific conditions. LON conjugates could perfectly resolve the conflict by enabling the specific recognizable liposome interaction so that on-demand reaction could occur when components from different liposome populations were mixed in the fusion process. Our group's recent work applied this strategy in building up the protocell communication, which successfully mimicked pathogen-cell immune response under cholesterol-DNA-mediated artificial protocell-membrane fusion (Fig. 10A) [110].
Figure 10.
(A) Protocell communication mediated by cholesterol–DNA conjugate. Reprinted with permission from [110]. Copyright (2018) American Chemical Society. (B) DNA-controlled programmable liposome-fusion cascade. Reprinted with permission from [111]. Copyright (2017) John Wiley and Sons. (C) Lipidated peptide–DNA conjugate in the membrane-fusion model. Reprinted with permission from [112]. Copyright (2017) Royal Society of Chemistry. (D) Stimulus-responsive droplet fusion for biosensing in an emulsion system. Reprinted with permission from [113]. Copyright (2014) American Chemical Society. (E) MicroRNA detection by DNA-mediated liposome fusion. Reprinted with permission from [114]. Copyright (2018) John Wiley and Sons. (F) Protein delivery guided by DNA-programmed liposome–cell fusion. Reprinted with permission from [115]. Copyright (2018) Royal Society of Chemistry.
However, most bottom-up biological synthesis involves multistep reactions in which sequential mixing events from multiple compartments are required beyond the single-fusion event. To overcome this barrier, Vogel's team developed a DNA-programmed liposome-fusion cascade by introducing six different lipid–DNA conjugates [111]. As shown in Fig. 10B, four functionalized liposome populations were programmed to merge with three orthogonally hybridizing duplex sequences. The step-by-step fusion events allowed efficient content mixing and transfer of recognition units for the following stage. This artificial fusion cascade, as endorsed by DNA-coding versatility, is a promising platform for diverse applications, such as artificial protocell systems and liposome nanoreactor-based synthesis. In their next work, lapidated PNA were innovatively utilized to improve mismatch discrimination and stability toward enzymatic and chemical degradation of the liposome system (Fig. 10C) [112].
A similar strategy was also applied to the oil-in-water emulsion system to achieve rapid and controlled mixing of droplets in bulk solution. In Goodwin's work, a biosensor for thrombin was designed. Here, lipid–DNA-mediated fusion was triggered by competitive thrombin–aptamer binding [113]. Fluorescent-signal changes that resulted from component mixing, along with droplet fusion, could perform with high sensitivity down to 100 nM in only 15 minutes (Fig. 10D). Last year, another biosensing work using lipid–DNA liposome fusion successfully realized highly sensitive microRNA detection [114]. The flu biomarker miR-29a worked as a trigger to hybridize with the blocked hairpin, enabling the sticky ends of lipid–DNA to be released to hybridize with the complementary strands on another liposome population in a zipper-like manner. Based on the resulting increase in FRET signals from the liposome-fusion process, a low detection limit of 18 nM for the target miRNA was obtained (Fig. 10E).
With an increasing number of studies on DNA-mediated liposome fusion, the application of this model was further pushed into macromolecule delivery to increase cellular uptake and endosome release. The Fan group reported their efficient intracellular protein delivery using this lipid–DNA-programmed fusion strategy [115]. Encapsulated cargos could bypass the endocytic pathway and go directly into the cytoplasm via DNA-directed membrane fusion between liposomes and cells (Fig. 10F). This strategy presented potential in macromolecule delivery, gene editing and various translational applications.
CONCLUSION AND FUTURE PERSPECTIVES
Since the first demonstration in the literature ∼30 years ago, LONs have become increasingly common in materials science and biotechnology (Table 2). Compared with other types of ONs, such as LNAs, PNAs and ON-polymers, LONs are more readily and efficiently synthesized and modified with lower cost using conventional chemistry; and they have better water solubility, drug-loading capability and penetration ability through the cell membrane, which are beneficial for theranostic applications without demanding extra drug vehicles or cellular-delivery strategies. With a diverse range of synthetic methods, lipids can be coupled onto ONs at different sites. The SPS approach enables automated and facile fabrication of LONs, while still requiring specific lipid phosphoramidites. In contrast, when combined with enzymatic reactions or click reactions, modified ONs and lipids could be linked in buffered solution, but may result in low yields with high cost. Therefore, efforts are still needed to overcome challenges in the preparation of LONs on a large scale with excellent reproducibility.
Table 2.
Summarized bioapplications of LON probes.
| Bioanalytical and biomedical applications of DNA–lipid probes | DNA structure | Targets/goals | Cell types | Selected references | |
|---|---|---|---|---|---|
| Cell-membrane-anchored biosensors | Real-time monitoring of substances in the extracellular microenvironment | DNAzyme | Mg2+ | CEM | [57] |
| Quadruplex | K+ | CEM | [63] | ||
| i-motif/Triplex | H+/OH− | CEM/HepG2 | [64,65] | ||
| Aptamer | Cytokine | T Cells | [67] | ||
| Measuring biophysical events on cell surfaces | DNA walker | Transient encounter events measurement | Ramos/CEM | [6] | |
| DNA hairpin | Tensile-forces measurement | 3T3 | [68] | ||
| DNA-nanoassembly behavior on cell-membrane mimicry | DNA origami | DNA assembly/disassembly investigation | Lipid bilayer surface | [69] | |
| Triangular prism | Cell-mimicking giant vesicles | [70] | |||
| Targeted cancer theranostics | Cancer imaging | Aptamer/micelle | Cancer-cell detection | Ramos | [19] |
| Cancer therapy | DNA cage | Small-molecule drug delivery | − | [8] | |
| Micelle | Gene therapy | HeLa/HepG2 | [88] | ||
| Aptamer | Immunotherapy | NK/K562 | [93] | ||
| LONs-mediated assembly and fusion | Sequential assembly | Complementary DNA | Liposome assembly | − | [100] |
| Bioinspired fusion mediated by LONs | DNA-reaction network | Protocell communication | Artificial cells | [110] | |
| Complementary DNA | Nanoreactor | Giant unilamellar vesicles | [111,112] | ||
Solving the limitations involved in LONs stability and specificity remains another future task. Lipid digestion and ON degradation under specific enzymes or pH environments should be minimized to enhance the in vivo bioavailability and pharmacokinetics of transported therapeutic agents. Optimized LONs could be blended or engineered with other materials, such as biocompatible polymers and nanoparticles, to realize synergistic functionalities, which may further inhibit nanostructure disaggregation and drug precipitation. Besides increasing material types, combination with DNA-origami structures may facilitate the overall structural stiffness as well. Constructing covalent or stimuli-responsive linkers-based networks among LONs through different cross-linking methods may also provide a solution to stall the digestion of the formulation.
Although LONs have already been equipped with outstanding cellular permeability and drug-encapsulation capability, owing to the hydrophobicity and cell-membrane-mimicking property of lipids, continuous work to design more innovative LONs-based nanostructures with molecular-recognition and catalytic abilities is imperative to accelerate LONs applications in biosensing, biomedical diagnosis and treatment. The incorporation of functional ONs such as aptamer, siRNA and CpG could improve the targeting ability and help to increase the efficacy of therapeutic performance. Moreover, some other functional ON structures, including DNAzyme, G-quadruplex and i-motif, could be combined with lipids to develop new flexible composites to enable multiple and stimuli-responsive functionalities (e.g. precise drug release) by utilizing their conformational change and relationship with biomass in the microenvironment.
In addition to the theranostic applications of LONs, by adjusting the parameters of ONs and lipids, more future studies could emphasize on (i) investigating membrane biophysics, e.g. vesicle-fusion energy, which would facilitate the understanding of making biomimetic organelles and artificial cells; (ii) monitoring cellular behaviors, with improvement in cell-membrane surface-anchored DNA nanostructures to predict cellular processes and functions; (iii) exploring interactions between lipids and other molecules, such as proteins and sugars, to study their mechanisms, structures and functions in real cell membranes by generating biologically relevant membranes using specific LON structures; (iv) constructing nanoreactors, as LONs could form appealing containers for different chemical reactants to perform reactions more easily after the fusion process; (v) manipulating the assembly/disassembly of nanomaterials, as LONs have been used as potent templates to enable certain assembly through specific DNA-strand hybridization. Furthermore, studies investigating the length, coupling number and other molecular parameters of LONs should also be compared and optimized more deeply to construct a better nanostructure for different purposes.
It remains to be seen which LON formulation would be suitable for scale-up manufacture and for efficacy in different applications. More systematic studies from in vivo assessments in pharmacokinetics regarding the long-term toxicity of the physicochemical properties of LONs will further broaden LONs-based bioapplications. Improving LON constructs for clinical use should become a major future trend. Evaluations in animal models will also give guidance for new and better LON designs. It is believed that molecular engineering enabled by improved LON structures will lead to a promising expansion in bioanalytical and biomedical applications in the future.
Acknowledgements
The authors thank Dr. K.R. Williams for manuscript review.
Contributor Information
Xiaowei Li, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Kejun Feng, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA; School of Chemistry and Materials Engineering, Huizhou University, Huizhou 516007, China.
Long Li, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Lu Yang, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Xiaoshu Pan, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Hoda Safari Yazd, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Cheng Cui, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA; Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory for Chemo/Bio- Sensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Biology, and Aptamer Engineering Center of Hunan Province, Hunan University, Changsha 410082, China; Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences; The Cancer Hospital of the University of Chinese Academy of Sciences, Hangzhou 310022, China.
Juan Li, Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory for Chemo/Bio- Sensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Biology, and Aptamer Engineering Center of Hunan Province, Hunan University, Changsha 410082, China.
Leonid Moroz, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Yujia Sun, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA; Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory for Chemo/Bio- Sensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Biology, and Aptamer Engineering Center of Hunan Province, Hunan University, Changsha 410082, China.
Bang Wang, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Xiang Li, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Tong Huang, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA.
Weihong Tan, Center for Research at Bio/Nano Interface, Department of Chemistry and Department of Physiology and Functional Genomics, Health Cancer Center, UF Genetics Institute and McKnight Brain Institute, University of Florida, Gainesville, FL 32611–7200, USA; Molecular Science and Biomedicine Laboratory (MBL), State Key Laboratory for Chemo/Bio- Sensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Biology, and Aptamer Engineering Center of Hunan Province, Hunan University, Changsha 410082, China; Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences; The Cancer Hospital of the University of Chinese Academy of Sciences, Hangzhou 310022, China.
Funding
This work was supported by the National Natural Science Foundation of China (21827811), the Science and Technology Project of Hunan Province (2017XK2103) and the Hunan Provincial Key Area R&D Program (2019SK2201).
Conflict of interest statement. None declared.
REFERENCES
- 1. Liu K, Zheng L, Ma Cet al. DNA–surfactant complexes: self-assembly properties and applications. Chem Soc Rev 2017; 46: 5147–72. [DOI] [PubMed] [Google Scholar]
- 2. Kwak M, Herrmann A. Nucleic acid amphiphiles: synthesis and self-assembled nanostructures. Chem Soc Rev 2011; 40: 5745–55. [DOI] [PubMed] [Google Scholar]
- 3. Savla R, Browne J, Plassat Vet al. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm 2017; 43: 1743–58. [DOI] [PubMed] [Google Scholar]
- 4. Letsinger RL, Zhang G, Sun DKet al. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci USA 1989; 86: 6553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patwa A, Gissot A, Bestel Iet al. Hybrid lipid oligonucleotide conjugates: synthesis, self-assemblies and biomedical applications. Chem Soc Rev 2011; 40: 5844–54. [DOI] [PubMed] [Google Scholar]
- 6. You M, Lyu Y, Han Det al. DNA probes for monitoring dynamic and transient molecular encounters on live cell membranes. Nat Nanotechnol 2017; 12: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Langecker M, Arnaut V, Martin TGet al. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 2012; 338: 932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwardson TG, Carneiro KM, McLaughlin CKet al. Site-specific positioning of dendritic alkyl chains on DNA cages enables their geometry-dependent self-assembly. Nat Chem 2013; 5: 868–75. [DOI] [PubMed] [Google Scholar]
- 9. Rabe A, Löffler P, Ries Oet al. Programmable fusion of liposomes mediated by lipidated pna. Chem Commun 2017; 53: 11921–4. [DOI] [PubMed] [Google Scholar]
- 10. Raouane M, Desmaële D, Urbinati Get al. Lipid conjugated oligonucleotides: a useful strategy for delivery. Bioconjugate Chem 2012; 23: 1091–104. [DOI] [PubMed] [Google Scholar]
- 11. Lönnberg H. Solid-phase synthesis of oligonucleotide conjugates useful for delivery and targeting of potential nucleic acid therapeutics. Bioconjugate Chem 2009; 20: 1065–94. [DOI] [PubMed] [Google Scholar]
- 12. D’Onofrio J, Montesarchio D, De Napoli Let al. An efficient and versatile solid-phase synthesis of 5‘-and 3‘-conjugated oligonucleotides. Org Lett 2005; 7: 4927–30. [DOI] [PubMed] [Google Scholar]
- 13. Nikan M, Osborn MF, Coles AHet al. Docosahexaenoic acid conjugation enhances distribution and safety of siRNA upon local administration in mouse brain. Molecular Therapy-Nucleic Acids 2016; 5: e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osborn MF, Coles AH, Biscans Aet al. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Res 2019; 47: 1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rush AM, Nelles DA, Blum APet al. Intracellular mRNA regulation with self-assembled locked nucleic acid polymer nanoparticles. J Am Chem Soc 2014; 136: 7615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, Zhu Z, Kang Het al. DNA-based micelles: synthesis, micellar properties and size-dependent cell permeability. Chem Eur J 2010; 16: 3791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishina K, Unno T, Uno Yet al. Efficient in vivo delivery of siRNA to the liver by conjugation of α-tocopherol. Mol Ther 2008; 16: 734–40. [DOI] [PubMed] [Google Scholar]
- 18. Godeau G, Staedel C, Barthelemy P. Lipid-conjugated oligonucleotides via ‘click chemistry’ efficiently inhibit hepatitis C virus translation. J Med Chem 2008; 51: 4374–6. [DOI] [PubMed] [Google Scholar]
- 19. Wu YR, Sefah K, Liu HPet al. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc Natl Acad Sci USA 2010; 107: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen T, Wu CS, Jimenez Eet al. DNA micelle flares for intracellular mRNA imaging and gene therapy. Angew Chem Int Ed 2013; 52: 2012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu CC, Chen T, Han Det al. Engineering of switchable aptamer micelle flares for molecular imaging in living cells. Acs Nano 2013; 7: 5724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guzaev A, Lönnberg H. Solid support synthesis of ester linked hydrophobic conjugates of oligonucleotides. Tetrahedron 1999; 55: 9101–16. [Google Scholar]
- 23. Durand A, Brown T. Synthesis and properties of oligonucleotides containing a cholesterol thymidine monomer. Nucleosides Nucleotides Nucleic Acids 2007; 26: 785–94. [DOI] [PubMed] [Google Scholar]
- 24. Kwak M, Minten IJ, Anaya D-Met al. Virus-like particles templated by DNA micelles: a general method for loading virus nanocarriers. J Am Chem Soc 2010; 132: 7834–5. [DOI] [PubMed] [Google Scholar]
- 25. Jin J-O, Park H, Zhang Wet al. Modular delivery of CpG-incorporated lipid-DNA nanoparticles for spleen DC activation. Biomaterials 2017; 115: 81–9. [DOI] [PubMed] [Google Scholar]
- 26. Edwardson TG, Carneiro KM, Serpell CJet al. An efficient and modular route to sequence-defined polymers appended to DNA. Angew Chem Int Ed 2014; 53: 4567–71. [DOI] [PubMed] [Google Scholar]
- 27. Raouane M, Desmaele D, Gilbert-Sirieix Met al. Synthesis, characterization, and in vivo delivery of siRNA-squalene nanoparticles targeting fusion oncogene in papillary thyroid carcinoma. J Med Chem 2011; 54: 4067–76. [DOI] [PubMed] [Google Scholar]
- 28. Kabanov AV, Vinogradov SV, Ovcharenko AVet al. A new class of antivirals: antisense oligonucleotides combined with a hydrophobic substituent effectively inhibit influenza virus reproduction and synthesis of virus-specific proteins in MDCK cells. FEBS Lett 1990; 259: 327–30. [DOI] [PubMed] [Google Scholar]
- 29. Banga RJ, Meckes B, Narayan SPet al. Cross-linked micellar spherical nucleic acids from thermoresponsive templates. J Am Chem Soc 2017; 139: 4278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Awino JK, Gudipati S, Hartmann AKet al. Nucleic acid nanocapsules for enzyme-triggered drug release. J Am Chem Soc 2017; 139: 6278–81. [DOI] [PubMed] [Google Scholar]
- 31. Jakobsen U, Simonsen AC, Vogel S. DNA-controlled assembly of soft nanoparticles. J Am Chem Soc 2008; 130: 10462–3. [DOI] [PubMed] [Google Scholar]
- 32. Yoshina-Ishii C, Miller GP, Kraft MLet al. General method for modification of liposomes for encoded assembly on supported bilayers. J Am Chem Soc 2005; 127: 1356–7. [DOI] [PubMed] [Google Scholar]
- 33. Thompson MP, Chien M-P, Ku T-Het al. Smart lipids for programmable nanomaterials. Nano Lett 2010; 10: 2690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, Yang Y, Pincet Fet al. Placing and shaping liposomes with reconfigurable DNA nanocages. Nat Chem 2017; 9: 653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Y, Wang J, Shigematsu Het al. Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat Chem 2016; 8: 476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong F, Zhang F, Liu Yet al. DNA origami: scaffolds for creating higher order structures. Chem Rev 2017; 117: 12584–640. [DOI] [PubMed] [Google Scholar]
- 37. Kaur H, Yung L-YL.. Probing high affinity sequences of DNA aptamer against VEGF165. PLoS One 2012; 7: e31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shangguan D, Li Y, Tang Zet al. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA 2006; 103: 11838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang Z, Shangguan D, Wang Ket al. Selection of aptamers for molecular recognition and characterization of cancer cells. Anal Chem 2007; 79: 4900–7. [DOI] [PubMed] [Google Scholar]
- 40. Wrzesinski J, Ciesiolka J.. Characterization of structure and metal ions specificity of Co2+-binding RNA aptamers. Biochemistry 2005; 44: 6257–68. [DOI] [PubMed] [Google Scholar]
- 41. Owczarzy R, Moreira BG, You Yet al. Predicting stability of DNA duplexes in solutions containing magnesium and monovalent cations. Biochemistry 2008; 47: 5336–53. [DOI] [PubMed] [Google Scholar]
- 42. Lu Y, Zhang E, Yang Jet al. Strategies to improve micelle stability for drug delivery. Nano Res 2018; 11: 4985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang YY, Wu CC, Chen Tet al. DNA micelle flares: a study of the basic properties that contribute to enhanced stability and binding affinity in complex biological systems. Chem Sci 2016; 7: 6041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vester B, Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry 2004; 43: 13233–41. [DOI] [PubMed] [Google Scholar]
- 45. Quijano E, Bahal R, Ricciardi Aet al. Focus: genome editing: therapeutic peptide nucleic acids: principles, limitations, and opportunities. Yale J Biol Med 2017; 90: 583. [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Figg CA, Wang Ret al. Cross-linked aptamer–lipid micelles for excellent stability and specificity in target-cell recognition. Angew Chem 2018; 130: 11763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jin C, Liu X, Bai Het al. Engineering stability-tunable DNA micelles using photocontrollable dissociation of an intermolecular G-Quadruplex. ACS Nano 2017; 11: 12087–93. [DOI] [PubMed] [Google Scholar]
- 48. Lyu Y, Guo Y, Cai Ret al. Spherically directed synthesis and enhanced cellular internalization of metal-crosslinked DNA micelles. Chem 2019; 5: 913–28. [Google Scholar]
- 49. Wang Y, Wu Z, Ma Zet al. Promotion of micelle stability via a cyclic hydrophilic moiety. Polym Chem 2018; 9: 2569–73. [Google Scholar]
- 50. Wang D, Liu B, Ma Yet al. A molecular recognition approach to synthesize nucleoside analogue based multifunctional nanoparticles for targeted cancer therapy. J Am Chem Soc 2017; 139: 14021–4. [DOI] [PubMed] [Google Scholar]
- 51. Barthes J, Özçelik H, Hindié Met al. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. Biomed Res Int 2014; 2014: 921905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016; 1863: 2977–92. [DOI] [PubMed] [Google Scholar]
- 53. Marinho HS, Real C, Cyrne Let al. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2014; 2: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Landskron G, De la Fuente M, Thuwajit Pet al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014: 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y, Fonslow BR, Shan Bet al. Protein analysis by shotgun/bottom-up proteomics. Chem Rev 2013; 113: 2343–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fu T, Lyu Y, Liu Het al. DNA-based dynamic reaction networks. Trends Biochem Sci 2018; 43: 547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qiu L, Zhang T, Jiang Jet al. Cell membrane-anchored biosensors for real-time monitoring of the cellular microenvironment. J Am Chem Soc 2014; 136: 13090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Plante OJ, Palmacci ER, Seeberger PH. Automated solid-phase synthesis of oligosaccharides. Science 2001; 291: 1523–7. [DOI] [PubMed] [Google Scholar]
- 59. Tokunaga T, Namiki S, Yamada Ket al. Cell surface-anchored fluorescent aptamer sensor enables imaging of chemical transmitter dynamics. J Am Chem Soc 2012; 134: 9561–4. [DOI] [PubMed] [Google Scholar]
- 60. Beigi R, Kobatake E, Aizawa Met al. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol Cell Physiol 1999; 276: C267–78. [DOI] [PubMed] [Google Scholar]
- 61. Giepmans BN, Adams SR, Ellisman MHet al. The fluorescent toolbox for assessing protein location and function. Science 2006; 312: 217–24. [DOI] [PubMed] [Google Scholar]
- 62. Zhao W, Schafer S, Choi Jet al. Cell-surface sensors for real-time probing of cellular environments. Nat Nanotechnol 2011; 6: 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xiong M, Zhu H, Rong Qet al. A membrane-anchored fluorescent probe for detecting K+ in the cell microenvironment. Chem Commun 2016; 52: 4679–82. [DOI] [PubMed] [Google Scholar]
- 64. Zeng S, Liu D, Li Cet al. Cell-surface-anchored ratiometric DNA tweezer for real-time monitoring of extracellular and apoplastic pH. Anal Chem 2018; 90: 13459–66. [DOI] [PubMed] [Google Scholar]
- 65. Liu L, Dou C-X, Liu J-Wet al. Cell surface-anchored DNA nanomachine for dynamically tunable sensing and imaging of extracellular pH. Anal Chem 2018; 90: 11198–202. [DOI] [PubMed] [Google Scholar]
- 66. Schmid A, Kortmann H, Dittrich PSet al. Chemical and biological single cell analysis. Curr Opin Biotechnol 2010; 21: 12–20. [DOI] [PubMed] [Google Scholar]
- 67. Qiu L, Wimmers F, Weiden Jet al. A membrane-anchored aptamer sensor for probing IFNγ secretion by single cells. Chem Commun 2017; 53: 8066–9. [DOI] [PubMed] [Google Scholar]
- 68. Zhao B, O’Brien C, Mudiyanselage APKet al. Visualizing intercellular tensile forces by DNA-based membrane molecular probes. J Am Chem Soc 2017; 139: 18182–5. [DOI] [PubMed] [Google Scholar]
- 69. Suzuki Y, Endo M, Yang Yet al. Dynamic assembly/disassembly processes of photoresponsive DNA origami nanostructures directly visualized on a lipid membrane surface. J Am Chem Soc 2014; 136: 1714–7. [DOI] [PubMed] [Google Scholar]
- 70. Peng R, Wang H, Lyu Yet al. Facile assembly/disassembly of DNA nanostructures anchored on cell-mimicking giant vesicles. J Am Chem Soc 2017; 139: 12410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2010; 2: a003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jia H-R, Wang H-Y, Yu Z-Wet al. Long-time plasma membrane imaging based on a two-step synergistic cell surface modification strategy. Bioconjugate Chem 2016; 27: 782–9. [DOI] [PubMed] [Google Scholar]
- 73. Hong G, Robinson JT, Zhang Yet al. In vivo fluorescence imaging with Ag2S quantum dots in the second near‐infrared region. Angew Chem Int Ed 2012; 51: 9818–21. [DOI] [PubMed] [Google Scholar]
- 74. Kim MW, Jeong HY, Kang SJet al. Cancer-targeted nucleic acid delivery and quantum dot imaging using EGF receptor aptamer-conjugated lipid nanoparticles. Sci Rep-Uk 2017; 7: 9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang P, Zhao Z, Li Cet al. Aptamer-decorated self-assembled aggregation-induced emission organic dots for cancer cell targeting and imaging. Anal Chem 2017; 90: 1063–7. [DOI] [PubMed] [Google Scholar]
- 76. Kwon IK, Lee SC, Han Bet al. Analysis on the current status of targeted drug delivery to tumors. J Control Release 2012; 164: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kidane A, Bhatt PP. Recent advances in small molecule drug delivery. Curr Opin Chem Biol 2005; 9: 347–51. [DOI] [PubMed] [Google Scholar]
- 78. Rahbani JF, Vengut-Climent E, Chidchob Pet al. DNA nanotubes with hydrophobic environments: toward new platforms for guest encapsulation and cellular delivery. Adv Healthcare Mater 2018; 7: 1701049. [DOI] [PubMed] [Google Scholar]
- 79. Bousmail D, Amrein L, Fakhoury JJet al. Precision spherical nucleic acids for delivery of anticancer drugs. Chem Sci 2017; 8: 6218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Charbgoo F, Alibolandi M, Taghdisi SMet al. MUC1 aptamer-targeted DNA micelles for dual tumor therapy using doxorubicin and KLA peptide. Nanomed Nanotechnol Biol Med 2018; 14: 685–97. [DOI] [PubMed] [Google Scholar]
- 81. Huang F, You M, Chen Tet al. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem Commun 2014; 50: 3103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu Y, Bos IST, Oenema TAet al. Delivery system for budesonide based on lipid-DNA. Eur J Pharm Biopharm 2018; 130: 123–7. [DOI] [PubMed] [Google Scholar]
- 83. Navarro G, Pan J, Torchilin VP. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol Pharm 2015; 12: 301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release 2012; 161: 377–88. [DOI] [PubMed] [Google Scholar]
- 85. Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-an overview. J Clin Diagn Res 2015; 9: GE01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mellott AJ, Forrest ML, Detamore MS. Physical non-viral gene delivery methods for tissue engineering. Ann Biomed Eng 2013; 41: 446–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Karaki S, Benizri S, Mejías Ret al. Lipid-oligonucleotide conjugates improve cellular uptake and efficiency of TCTP-antisense in castration-resistant prostate cancer. J Control Release 2017; 258: 1–9. [DOI] [PubMed] [Google Scholar]
- 88. Dore MD, Fakhoury JJ, Lacroix Aet al. Templated synthesis of spherical RNA nanoparticles with gene silencing activity. Chem Commun 2018; 54: 11296–9. [DOI] [PubMed] [Google Scholar]
- 89. Tsouris V, Joo MK, Kim SHet al. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv 2014; 32: 1037–50. [DOI] [PubMed] [Google Scholar]
- 90. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 91. Oberli MA, Reichmuth AM, Dorkin JRet al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett 2016; 17: 1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang L, Zhu G, Mei Let al. Self-assembled DNA immunonanoflowers as multivalent CpG nanoagents. Acs Appl Mater Inter 2015; 7: 24069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xiong X, Liu H, Zhao Zet al. DNA aptamer-mediated cell targeting. Angew Chem Int Ed 2013; 52: 1472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu H, Kwong B, Irvine DJ. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew Chem Int Ed 2011; 50: 7052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Forte G, Rega A, Morello Set al. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol 2012; 188: 5357–64. [DOI] [PubMed] [Google Scholar]
- 96. Alivisatos AP, Johnsson KP, Peng Xet al. Organization of 'nanocrystal molecules' using DNA. Nature 1996; 382: 609–11. [DOI] [PubMed] [Google Scholar]
- 97. Mirkin CA, Letsinger RL, Mucic RCet al. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996; 382: 607–9. [DOI] [PubMed] [Google Scholar]
- 98. Michele LD, Eiser E.. Developments in understanding and controlling self assembly of DNA-functionalized colloids. PCCP 2013; 15: 3115–29. [DOI] [PubMed] [Google Scholar]
- 99. Beales PA, Vanderlick TK.. Application of nucleic acid–lipid conjugates for the programmable organisation of liposomal modules. Adv Colloid Interface Sci 2014; 207: 290–305. [DOI] [PubMed] [Google Scholar]
- 100. Beales PA, Nam J, Vanderlick TK. Specific adhesion between DNA-functionalized ‘Janus’ vesicles: size-limited clusters. Soft Matter 2011; 7: 1747–55. [Google Scholar]
- 101. van der Meulen SAJ, Leunissen ME. Solid colloids with surface-mobile DNA linkers. J Am Chem Soc 2013; 135: 15129–34. [DOI] [PubMed] [Google Scholar]
- 102. Martinez-Veracoechea FJ, Mognetti BM, Angioletti-Uberti Set al. Designing stimulus-sensitive colloidal walkers. Soft Matter 2014; 10: 3463–70. [DOI] [PubMed] [Google Scholar]
- 103. Parolini L, Kotar J, Di Michele Let al. Controlling self-assembly kinetics of DNA-functionalized liposomes using toehold exchange mechanism. ACS Nano 2016; 10: 2392–8. [DOI] [PubMed] [Google Scholar]
- 104. Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2001; 2: 98–106. [DOI] [PubMed] [Google Scholar]
- 105. Robson Marsden H, Elbers NA, Bomans PHHet al. A reduced SNARE model for membrane fusion. Angew Chem Int Ed 2009; 48: 2330–3. [DOI] [PubMed] [Google Scholar]
- 106. Chan Y-HM, van Lengerich B, Boxer SG. Lipid-anchored DNA mediates vesicle fusion as observed by lipid and content mixing. Biointerphases 2008; 3: FA17–21. [DOI] [PubMed] [Google Scholar]
- 107. Ries O, Löffler PMG, Rabe Aet al. Efficient liposome fusion mediated by lipid–nucleic acid conjugates. Org Biomol Chem 2017; 15: 8936–45. [DOI] [PubMed] [Google Scholar]
- 108. Chan Y-HM, van Lengerich B, Boxer SG. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc Natl Acad Sci USA 2009; 106: 979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Simonsson L, Jönsson P, Stengel Get al. Site-specific DNA-controlled fusion of single lipid vesicles to supported lipid bilayers. ChemPhysChem 2010; 11: 1011–7. [DOI] [PubMed] [Google Scholar]
- 110. Lyu Y, Wu C, Heinke Cet al. Constructing smart protocells with built-in DNA computational core to eliminate exogenous challenge. J Am Chem Soc 2018; 140: 6912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Löffler PMG, Ries O, Rabe Aet al. A DNA-programmed liposome fusion cascade. Angew Chem Int Ed 2017; 56: 13228–31. [DOI] [PubMed] [Google Scholar]
- 112. Rabe A, Löffler PMG, Ries Oet al. Programmable fusion of liposomes mediated by lipidated PNA. Chem Commun 2017; 53: 11921–4. [DOI] [PubMed] [Google Scholar]
- 113. Mohan P, Noonan PS, Nakatsuka MAet al. On-demand droplet fusion: a strategy for stimulus-responsive biosensing in solution. Langmuir 2014; 30: 12321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jumeaux C, Wahlsten O, Block Set al. MicroRNA detection by DNA-mediated liposome fusion. ChemBioChem 2018; 19: 434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sun L, Gao Y, Wang Yet al. Guiding protein delivery into live cells using DNA-programmed membrane fusion. Chem Sci 2018; 9: 5967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]