Abstract
Objectives
Argentina is a low and middle-income country (LMIC) with a highly fragmented healthcare system that conflicts with access to healthcare stated by the country’s Universal Health Coverage plan. A tele-mammography network could improve access to breast cancer screening decreasing its mortality. This research aims to conduct an economic evaluation of the implementation of a tele-mammography program to improve access to healthcare.
Methods
A cost-utility analysis was performed to explore the incremental benefit of annual tele-mammography screening for at-risk Argentinian women over 40 years old. A Markov model was developed to simulate annual mammography or tele-mammography screening in two hypothetical population-based cohorts of asymptomatic women. Parameter uncertainty was evaluated through deterministic and probabilistic sensitivity analysis. Model structure uncertainty was also explored to test the robustness of the results.
Results
It was estimated that 31 out of 100 new cases of breast cancer would be detected by mammography and 39/100 by tele-mammography. The model returned an incremental cost-effectiveness ratio (ICER) of £26 051/quality-adjusted life-year (QALY) which is lower than the WHO-recommended threshold of £26 288/QALY for Argentina. Deterministic sensitivity analysis showed the ICER is most sensitive to the uptake and sensitivity of the screening tests. Probabilistic sensitivity analysis showed tele-mammography is cost-effective in 59% of simulations.
Discussion
Tele-mammography should be considered for adoption as it could improve access to expertise in underserved areas where adherence to screening protocols is poor. Disaggregated data by province is needed for a better- informed policy decision. Telemedicine could also be beneficial in ensuring the continuity of care when health systems are under stress like in the current COVID-19 pandemic.
Conclusion
There is a 59% chance that tele-mammography is cost-effective compared to mammography for at-risk Argentinian women over 40- years old, and should be adopted to improve access to healthcare in underserved areas of the country.
Keywords: public health, COVID-19, information systems, health equity, BMJ health informatics
Summary.
What is already known?
Telemedicine has been shown to be cost-effective in improving access to health services in rural areas and for chronic conditions by improving monitoring of patients and adherence to treatments.
What does this paper add?
With telemedicine having a crucial role in the screening and monitoring of chronic conditions during the COVID-19 pandemic, this would be the first paper that shows telemedicine—specifically telemammography—is cost-effective for breast cancer screening, and can guarantee the continuity of care and managing the surge caused by health emergencies like the current COVID-19 pandemic.
It also opens up the possibility to explore the cost-effectiveness of telemammography in other national healthcare systems, particularly for other low and middle-income countries.
Introduction
Breast cancer is the leading cause of death from cancer in women from all social strata. Seventy per cent of the global-reported deaths were in low and middle-income countries (LMIC).1
Population screening for breast cancer is strongly recommended by most guidelines, as it is highly curable if detected at early stages. The main goal is to allow diagnosis in asymptomatic women. The Surveillance, Epidemiology, and End Results programme of the US National Cancer Institute showed a good correlation of the stage at diagnosis with the 5-year survival rates: 62.5% of women are diagnosed at stage I/II, accounting for a 5-year survival rate of 85.5%–98.8%, contrasting with those diagnosed at stage III/IV, where the 5-year survival rate falls up to 30%.2
The Argentinian National Cancer Institute ranks breast cancer as the most common cancer in the country with 19 000 cases diagnosed yearly. It represents 17% of all malignancies with an incidence of 71 per 100 000 and 32% of cancer in women.3 It is the leading cause of death from cancer in women, with an estimated mortality rate of 18.0 per 100 000. According to the Pan American Health Organization (PAHO), Argentina ranks the second region for breast cancer mortality.3
Argentina is a low to middle-income country where universal healthcare is guaranteed by the government. However, considerable inequalities in accessibility exist among different regions in the country, which challenge the adoption of mammography or ultrasonography for breast cancer screening and make it unaffordable for public and private healthcare providers in remote geographies.
Telemammography networks are an affordable and scalable way of improving treatment and prevention of breast cancer. They usually operate with a main centre and strategically located digital mammography facilities, particularly in remote or in-need areas. Women go to these facilities to have their mammography taken, then the images are sent to the main centre to be interpreted and sent back to the facility in less than 24 hours. This could reduce the access barriers to early diagnosis by, first, improving access to state-of-the-art technology like digital mammography; second, improving diagnostic accuracy by a remote interpretation by trained physicians; third, increasing the breast cancer awareness through involvement of government bodies, civil societies and the private sector; and creating a pathway for national and regional cancer control programmes.
This research aims to conduct a cost-utility analysis of the implementation of a telemammography programme to provide a summary measure of efficiency that can later be used to compare interventions across different healthcare programmes.4
Methods
Screening strategy
Argentinian guidelines recommend annual screening with mammography for all women between 40 and 74 years old.5 Yet, access to the test is limited in rural areas where the uptake is as low as 39%.6
In this study, the current strategy was compared with a telemammographic approach. Both screening methods use the same machines (direct digital mammography and 3D mammography for women with denser breast tissue) which met the technical standards of the European guidelines for quality assurance in breast cancer screening and diagnosis.7 The number of devices used was 1 for each group. Both tests have a high sensitivity to detect a subset of the population who should have confirmatory biopsy to determine the presence or absence of disease.8
Modelling strategy
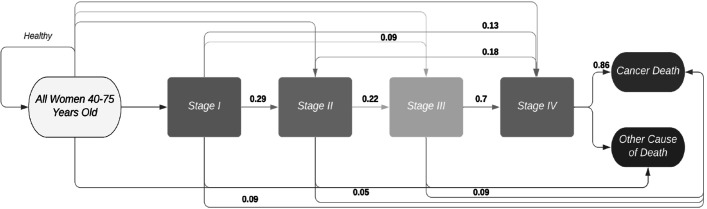
A state-transition Markov model was developed on Microsoft Excel V.16.38 to inform a long-term decision model (figure 1). In this study, we simulated annual mammography or telemammography screening methods for breast cancer in two hypothetical population-based cohorts of 1000 asymptomatic Argentinian women over 40 years old who were assumed to be at risk of breast cancer but had not yet been diagnosed.
Figure 1.
State-transition Markov model for breast cancer progression adapted for this study population, with transition probabilities.
Natural history of disease
Figure 1 illustrates the natural history of the disease used in this evaluation, and was adapted from a published study.9 In each cycle, women could either remain cancer free, die from all-cause death or progress into stage I. From here, women could either subsequently transition into stages II, III, IV and cancer-related death or go directly into more severe stages (eg, I to III or I to IV directly). From each stage, women could die from other causes or cancer-related causes. Stage I cases detected by either screening test were treated accordingly if confirmed. The false negatives or missed cases by both tests that later on developed the disease were assumed to debut as ‘new cases’ progressing from healthy directly to stage II, III or IV.
Epidemiological and clinical data
The invasive breast cancer incidence standardised by age was combined from four different provinces in Argentina: Bahia Blanca, Córdoba, Mendoza and Tierra del Fuego.10–12 The stage distribution data with telemammography were provided directly by a private telemammography firm in Argentina to improve the comparability of the results. These data are not publicly available. Non-detected breast cancer cases in stage I are assumed to be included as ‘new cases’ that appeared directly in later stages II, III and IV, which would add to the ones transitioned from previous stages.
Characteristics of the tests
For this model, a baseline sensitivity of 78% (95% CI 75% to 90%) was used for both comparators, with a specificity of 64.5%, a positive predictive value of 89% and a diagnostic accuracy of 89.3%.13 14 To validate that the same sensitivity could be used, we based our case in a study comparing 26 radiology services in Argentina, Bolivia, Colombia, Cuba and Mexico, funded by the PAHO.15 Argentina has shown the highest quality measures of all the participating countries as it had the greatest number of doctors working full time on breast cancer diagnosis and the quality of the image and certainty of radiological interpretation was the highest of the region.15
Quality-adjusted life-years gained
Argentina adheres to the MERCOSUR economic evaluation guidelines which recommend quality-adjusted life-year (QALY) as the best measure of health outcomes in cost-effectiveness analyses.4 The calculation formula used for this study was ‘Years of Life’ × ‘Utility Value’=number of QALYs. Utility scores used were taken from published literature.16 The state of death had a value of zero and no disutility for screening or false-positive results were considered.
Costs
Resource use was calculated from a societal perspective to account for the underlying concept of opportunity cost.17 To account for currency issues, the real conversion factor from the National Bank was used for costs in Argentine pesos (ARS). For non-ARS costs, the World Bank’s purchasing power parity (PPP) index was used. Adjustment for inflation was done using the gross domestic product (GDP) deflator index as all costs were updated to 2020 figures.18 Future costs and future benefits were discounted at 3%.4
Direct screening costs
The direct screening costs were divided into two main categories: medical and non-medical. The medical costs include: the cost of the screening tests and, in the case of mammography, an additional medical appointment with a mastologist.19 The price used for mammography screening (£47.85/test) was calculated as an average of the public (£24.23/test) and private (£71.47/test) sector costs, while the price for telemammography screening was reported directly by two private clinics in Argentina, including the human resources cost (£8.72/test).
A ‘set-up cost’ of an additional £0.38/test was considered for the telemammography group calculated by dividing the new centre’s yearly ‘maintenance fee’ of £2250/year by the average number of tests per centre.
Non-medical screening costs measured were mainly transportation to the centre and cost of a family caretaker for the test day.20 21
Direct treatment costs
Direct medical costs associated to cancer treatment include surgery, chemotherapy, radiotherapy and ‘other procedures’ including medical visits and treatment for adverse events and were valued for each cancer stage. The costs for Argentina were derived from published studies in Mexico, Colombia and Brazil; weighted by the sample size of the study, accounted for inflation and currency conversion. So for this study, the average standard lifetime treatment costs resulted in £21 482, £32 683, £38 406 and £44 508 for stages I, II, III and IV, respectively. All parameters used for the base case as well as their public sources are displayed in table 1.
Table 1.
Summary of input variables used for the base model analysis
| Variable description | Source | |||||
| Sample size | 1000 | – | ||||
| Discount factor for costs and outcomes | 3% (95% CI 2% to 6%) | WHO guidelines17 | ||||
| Age-specific probabilities | ||||||
| Age band | Incidence of breast cancer | All-cause mortality | ||||
| 40–44 | 0.001220 | 0.00168 | CI5 Vol X* National Institute of Statistics and Census (INDEC), Argentina10 11 |
|||
| 45–49 | 0.002019 | 0.00266 | ||||
| 50–54 | 0.002271 | 0.00412 | ||||
| 55–59 | 0.003085 | 0.00629 | ||||
| 60–64 | 0.003142 | 0.00930 | ||||
| 65–69 | 0.003135 | 0.01410 | ||||
| 70–74 | 0.003223 | 0.02228 | ||||
| 75–79 | 0.002971 | 0.03812 | ||||
| 80–84 | 0.003095 | 0.07289 | ||||
| 85+ | 0.003204 | 0.23 153 | ||||
| Stage distribution of breast cancer | ||||||
| Population | Mammography | Telemammography | ||||
| Stage I | 0.244 | 0.132 | 0.428 | MoH official figures (Argentina) Published ‘Dr. Jose Ramon Vidal’ Hospital Data (Corrientes, Argentina)12 |
||
| Stage II | 0.449 | 0.283 | 0.326 | |||
| Stage III | 0.231 | 0.299 | 0.167 | |||
| Stage IV | 0.043 | 0.278 | 0.080 | |||
| Disease progression-transition probabilities | ||||||
| Stage I | Stage II | Stage III | Stage IV | Cancer death | ||
| Stage I | 0.4 | 0.29 | 0.09 | 0.13 | 0.09 | Mexican Social Security Institute (case study)30 |
| Stage II | – | 0.55 | 0.22 | 0.18 | 0.05 | |
| Stage III | – | – | 0.22 | 0.7 | 0.09 | |
| Stage IV | – | – | – | 0.14 | 0.86 | |
| Characteristics of the tests | ||||||
| Mammography | Telemammography (TM) | |||||
| Specificity | 0.865 (95% CI 0.83 to 0.90) | MoH official figures (Argentina)6 Private (TM centre) |
||||
| Sensitivity | 0.78 (95% CI 0.75 to 0.90) | |||||
| Uptake | 0.39 (95% CI 0.32 to 0.46) | 0.5 (95% CI 0.43 to 0.57) | ||||
| Utility scores | ||||||
| Point estimate | 95% CI | |||||
| Stage I | 0.91 | 0.314 to 1.00 | Pataky et al16 | |||
| Stage II | 0.75 | 0.320 to 0.983 | ||||
| Stage III | 0.51 | 0.272 to 0.745 | ||||
| Stage IV | 0.45 | 0.203 to 0.557 | ||||
| COSTS | ||||
| Variable description | Cost in ARS | Year | Cost in GBP | Source |
| Direct screening costs | ||||
| Direct medical costs | ||||
| Mammography (public sector) | $2279 | 2020 | £ 24.23 | Official figures (Argentina)19 |
| Medical consults (specialist) | $470 | 2020 | £ 5 | |
| Mammography (private sector) | $6721 | 2020 | £ 71.47 | Private data |
| Telemammography | $810 | 2020 | £ 8.72 | |
| Mammography—average | $4500 | 2020 | £ 47.85 | Calculation |
| Set-up costs (telemammography) | ||||
| Online campaigns | 2019 | £ 10 073.08 | Private data | |
| Offline campaigns | 2019 | £ 9 258.21 | ||
| Opening a new clinic | 2019 | £ 73 500.00 | ||
| Number of tests per clinic (year) | 2019 | 6000 | ||
| Additional screening cost/test | 2019 | £ 16.58 | Calculation | |
| Leasing model (8-year contract) | 2020 | £ 2 250 | Private data | |
| Additional screening cost/test | 2020 | £ 0.38 | Calculation | |
| Direct non-medical costs | ||||
| Distance to mammography centre | 2020 | 200 km | Private data | |
| Distance to telemammography centre | 2020 | 50 km | ||
| Price for transport/km | 2019 | £ 0.17 | Official figures (Argentina)20 21 | |
| Average daily cost of informal care (caretaker) | 2019 | £ 11.31 | ||
| Total non-medical costs of mammography | 2020 | £ 39.39 | Calculation | |
| Total non-medical costs of telemammography | 2020 | £ 10.83 | Calculation | |
| Direct treatment costs | ||||
| Stage I | 2018 | £ 21 482.39 | Average treatment costs: Brazil, Colombia and Mexico30 | |
| Stage II | 2018 | £ 32 683.87 | ||
| Stage III | 2018 | £ 38 406.00 | ||
| Stage IV | 2018 | £ 44 508.32 | ||
*Cancer Incidence in Five Continents (CI5) Vol X report by the International Agency for Research on Cancer (IARC).
ARS, Argentine peso; GBP, British pound sterling; MoH, Ministry of Health.
Base case analysis
The model followed a cohort of 1000 at-risk 40-year-old Argentinian women over a lifetime horizon. The economic evaluation output was the incremental cost-effectiveness ratio (ICER), and cost per life-year gained was also reported. A threshold of three times the GDP of Argentina was used to decide on whether telemammography was cost-effective compared with mammography, following a general recommendation of the WHO guidelines.17 According to the World Bank in 2018, the GDP per capita in Argentina was (in US$) $11 633, which when converted to British pound sterling using the PPP index is £8762.6; therefore, in this study, a cost-effectiveness threshold of £26 288/QALY was used.18
Uncertainty and sensitivity analysis
Deterministic and probabilistic sensitivity analysis
We carried out one-way and probabilistic sensitivity analyses (PSA) to explore parameter uncertainty. In the deterministic sensitivity analysis (DSA), we varied the uptake and sensitivity of the tests, discount rate of future costs and outcomes and the quality of life (QoL) within their minimum and maximum scores. The costs included on this DSA were varied by an increase/decrease of 30%. This model was based on a series of structural assumptions, one being all ‘missed cases’ undetected in stage I were lost and not identified in stages II, III and IV; tested by DSA.
PSA was conducted to assess the uncertainty of all relevant parameters simultaneously. Costs followed a gamma distribution whereas QoL, the uptake rates and the sensitivity of the tests followed a beta distribution as suggested in the literature. All the input variables were varied simultaneously, and we could obtain 1000 estimates of incremental costs and effects by sampling from the distribution summarised in two measures of parameter uncertainty: the incremental net monetary benefit and the cost-effectiveness acceptability curves (CEACs). The CEAC was plotted to show the probability of the intervention being cost-effective at different willingness-to-pay (WTP) thresholds.
Results
Our model predicted 100 new cases of breast cancer for every 1000 women over a lifetime, of which 31 cases were detected by mammography and 39 by telemammography, and the rest missed. The estimated lifetime cost was £35 875 for a woman in the mammography cohort gaining an average of 22.61 life-years per annum of which 22.38 were QALYs. For the telemammography group, the lifetime cost was £37 545, gaining 22.68 life-years of which 22.45 were QALYs. The base case returned an ICER of £26 051 and a cost of £21 391 per life-year gained, which are both slightly lower than the WHO-recommended threshold of £26 288/QALY for Argentina. Data are shown in table 2.
Table 2.
Summary of base case analysis
| Comparators | Discounted years | Discounted QALY | Discounted costs |
| Mammography | 22.6066 | 22.3846 | £ 35 874.82 |
| Telemammography | 22.6847 | 22.4487 | £ 37 545.19 |
| Baseline results | Incremental discounted life-years | Incremental discounted QALY | Incremental discounted costs (£) |
| 0.0781 | 0.0641 | £ 1 670.36 | |
| ICER | £ 26 051.32 | ||
| Costs/LYG | £ 21 390.76 | ||
ICER, incremental cost-effectiveness ratio; LYG, life-year gained; QALY, quality-adjusted life-year.
Deterministic sensitivity analysis
As expected, the ICER proved very sensitive to the uptake of mammography (£30 937–£459), telemammography (£6644–£29 887) and the QoL in stage I where a decrease to the lower end of the CI would increase the ICER to £46 752. The ICER was also very responsive to the sensitivity of the tests; when it increased to 90% the ICER plummeted to £13 702. Other parameters that unilaterally impacted the ICER considerably were an increased discount rate of 6%, the QoL in stage II and the direct medical costs of mammography varying the ICER±8% both ways. For model structure robustness, we tested the extreme scenario that all cancer cases not detected in stage I were lost and did not vary the ICER considerably. The tornado diagram in figure 2 shows the results of the DSA.
Figure 2.
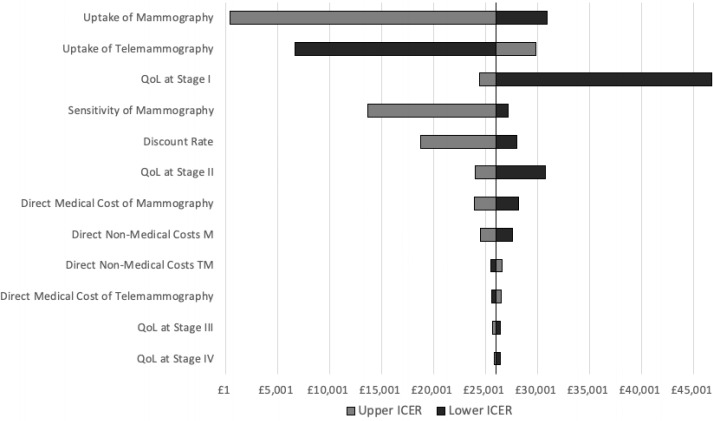
Tornado diagram—deterministic sensitivity analysis (one way). The incremental cost-effectiveness ratio (ICER) proved very sensitive to the uptake of mammography (£30 937–£459), telemammography (£6 644–£29 887) and the QoL in stage I where a decrease to the lower end of the CI would increase the ICER to £46 752. The ICER was also very responsive to the sensitivity of the tests; when it increased to 90% the ICER plummeted to £13 702.table 1 M, mammography; QoL, quality of life; TM, telemammography.
Probabilistic sensitivity analysis
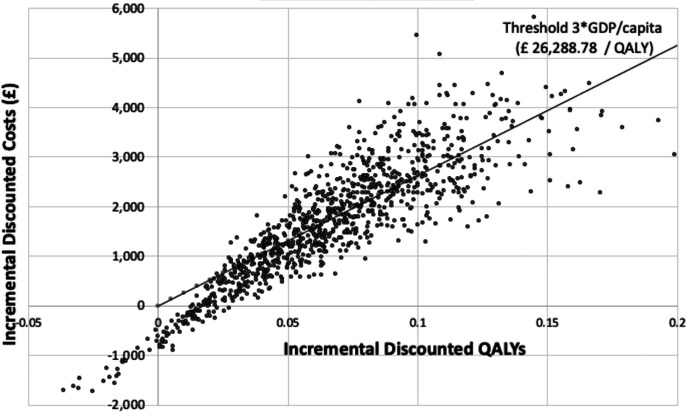
The cost-effectiveness plane in figure 3 shows the ICER result of 1000 simulations in the PSA. The majority of the dotted cloud fell in the—north and south—eastern quadrants, meaning that most of the simulations resulted in a greater increase in QALY.
Figure 3.
Cost-effectiveness plane—probabilistic sensitivity analysis results. The cost-effectiveness plane shows the incremental cost-effectiveness ratio (ICER) result of 1000 simulations in the probabilistic sensitivity analysis. The majority of the dotted cloud fell in the—north and south—eastern quadrants, meaning that most of the simulations resulted in a greater increase in quality-adjusted life-year (QALY). table 1 GDP, gross domestic product.
The CEAC presented in figure 4 showed that at the WHO-recommended threshold for Argentina of £26 288/QALY, there is a 59% chance of telemammography being cost-effective.
Figure 4.
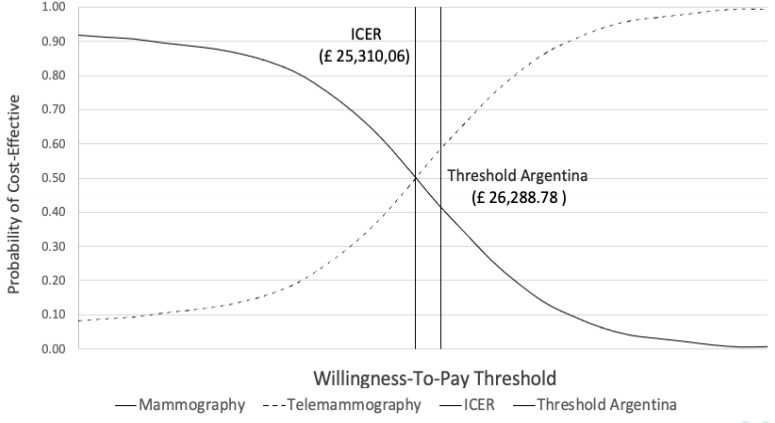
Cost-effectiveness acceptability curve—probabilistic sensitivity analysis (PSA) results. The cost-effectiveness acceptability curve shows that at the WHO-recommended threshold for Argentina of £26 288/quality-adjusted life-year (QALY), there is a 59% chance of telemammography being cost-effective.table 1 ICER, incremental cost-effectiveness ratio.
Discussion
The main findings of this societal cost-utility analysis indicate that with a total medical and non-medical screening cost of £19.92, annual telemammography leads more QALY per additional costs than annual mammography for breast cancer screening in at-risk Argentinian women over 40 years old.
This study provides the first evidence that, from a societal perspective, telemammography is cost-effective compared with mammography screening in an LMIC and it could be a way to improve access to health services in remote areas. With telemedicine having a crucial role in the screening and monitoring of chronic conditions during the COVID-19 pandemic, this would be the first paper that shows telemedicine is cost-effective for breast cancer screening.
The results of this study are aligned with published literature looking at cost-effectiveness of general telemedicine. A systematic review of 21 economic evaluations concluded that telemedicine was cost-effective in most medical fields for diagnosis and management of chronic conditions, but not for acute care.22 As in this study, the literature concludes that the cost-effectiveness of telemedicine is related to three major factors: cost sharing (patient volume and lowering infrastructure costs); effectiveness of telemedicine in terms of patient utility and successful clinical consultations; and indirect societal cost savings by decreasing cost of patients’ lost productivity.23 Advancements in automation technologies like artificial intelligence and machine learning are allowing tasks such as diagnosing through imaging to be done more cost-efficiently and potentially more accurately, but more research is required to accurately gauge the extent of these benefits and understand the risks of reliance on these technologies.
Barriers to implementing a breast cancer screening programme
Despite rarely incurring direct out-of-pocket expenses at the point of care, Argentina sees a very low level of uptake of breast cancer screening due to various personal and cultural barriers. Telemammography has the potential to improve access to expert diagnoses by overcoming these barriers by combining elements, including reduced travel times and improved education regarding the procedure.
These barriers must be explored to understand why national-level screening programmes are failing in some countries. A literature review including 35 articles concerning beliefs and attitudes towards breast cancer screening in Latin America concluded that, apart from resources and personnel, ‘competent community educational interventions across all aspects of breast cancer’ are vital to ensure a successful population screening programme.24 In some LMICs, the lack of breast cancer awareness could cause a 29-month delay on average in medical care after self-detecting a breast lump, which resulted in diagnoses at more advanced stages.25 Therefore, a screening programme with a strong educational component is key to ensure high participation rates in Argentina.
Other deterrents of care-seeking behaviour included: fear of disease, pain and embarrassment of the examination, self-neglect (from Spanish adjective ‘flojera’), fatalism about testing positive, lack of family and social support (taboo), language barriers and geographical barriers (transportation); the last one being vital in such a large country as Argentina. Perception of the staff’s clinical experiences also proved to be a barrier for test acceptance.24
These unmet needs are magnified by the current COVID-19 pandemic where cancer diagnostics and surgery have been disrupted by the response to the pandemic with reduction in survival rates of 13% (stage II) and 18% (stage III) as a consequence.26 Telemammography, usually having smaller, less crowded centres separated from hospitals, can be a way of reducing exposure to the virus while continuing screening. More research needs to be done, but a telemedicine model of healthcare delivery may offer systemic cost reduction through reduced levels of infection for patients and health professionals.
Limitations of the study and difference with other studies
Data and model structure
Data were imported from other studies that may not be representative or generalisable to the Argentinian population. Nevertheless, careful consideration was put into using data from comparable countries (middle income or Latin America where possible) and updating the costs accordingly. An analysis stratified by province could improve accuracy, but no regional-level data were available, and is also out of the scope of this research. Additionally, no disutility for screening or false-positive results were considered.
Critique of the threshold
There is little theoretical justification regarding setting such a hard rule as the threshold is not linked to the country’s affordability, budget impact, feasibility of implementation or the cost compared with other necessary and feasible interventions.27 Also, hard thresholds do not consider ethical implications, judgements of social value or equity issues. Not contemplating respects of local settings jeopardises generalisability to all decision-making contexts as countries are interested in other macro aspects, and not in efficiency alone.
Comparators
Having a no-screening arm could be more representative of the screening reality of most regions where this programme is being considered, as uptake of mammography is less than 50%. The negative impact of overdiagnosis must be considered for the additional cost to the health system in confirmatory testing and follow-ups, and because it negatively impacts the QoL of those patients. Furthermore, effectiveness of a screening programme is challenging to measure because of selection bias (higher risk perception of those being screened), lead time bias (longer ‘survival’ time observed due to an earlier date of diagnosis) and length time bias.28 More research could include other comparators like targeted risk screening, ultrasound or adjuvant endocrine therapy.
Difference with other studies
Some studies concluded that in addition to acute conditions, telemedicine was not cost-effective for airway cancer.22 23 Several reasons can explain this difference in results. First, that telemedicine is very sensitive to the volume of patients treated, and incidence of breast cancer in Argentina is five times the incidence of lung and airway cancer in Scotland.29 Also, there is generally more education and public awareness of the need to screen for breast cancer than to diagnose lung cancer cases. Finally, the cost for telemammography infrastructure follows economies of scale logic being easier to reduce via, for example, leasing options than infrastructure costs for tele-endoscopy.
Conclusions
Our findings show that the ICER fell within the WTP threshold currently recommended by the WHO nearly 60% of the time. This means that there is almost a 60% chance that telemammography is actually cost-effective compared with mammography for at-risk Argentinian women over 40 years old, and should be adopted as a population-based screening methodology for breast cancer, especially to improve access to the very poor, underserved areas of the country. However, because of generalisability, affordability and ethical implications, further research is vital to guide making a more beneficial policy decision.
Research in context
Significance of the study
This study provides the first evidence that, from a societal perspective, telemammography is cost effective compared to mammography screening in a Low- and Middle-Income Countryand it could be a way to improve access to health services in remote areas.
Footnotes
Collaborators: Kelly, Richard. Yu, Alejandro.
Contributors: VAMP conceived the idea, performed the initial literature research, contributed to the design of the study, performed the data collection, analysis, interpretation of results and wrote the first manuscript. RL and LS supervised the study design, validated and verified the methodology and oversaw the computations and use of the software, and collaborated on the formal data analysis and interpretation. RG collaborated with the literature research, verified underlying data sources and analysis regarding Latin American countries and supervised the findings. All authors contributed to the review and editing of the different manuscripts, and RL included feedback on data visualisation. Richard Kelly provided invaluable editing assistance. Alejandro Yu imparted critical knowledge of MS Excel functionalities.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open-access repository. All data and links relevant to the study are included in the article (table 1) or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
LSHTM MSc Research Ethics Committee (Ref 15726).
References
- 1.World Health Organization (WHO) . Fact sheets: cancer. WHO, 2018: e609–16. [Google Scholar]
- 2.SEER . Cancer Stat Facts: Female Breast Cancer. Natl. Cancer Inst. (United States) - Surveillance, Epidemiol. End Results Progr, 2019. Available: https://seer.cancer.gov/statfacts/html/breast.html [Accessed 29 Sep 2019].
- 3.Viniegra M, Paolino M, Arrossi S. Cáncer de mama en Argentina: organización, cobertura Y calidad de las acciones de prevención Y control Panamerican Health Organization (PAHO/WHO); 2010. https://iris.paho.org/handle/10665.2/5527 [Accessed 29 Aug 2019]. 9879507101243. [Google Scholar]
- 4.Gilardino R, Ares Lucia, Jorgensen N. Pharmacoeconomic guidelines around the world: MERCOSUR (Argentina, Brazil, Paraguay, Uruguay). ISPOR, 2018. Available: https://tools.ispor.org/PEguidelines/countrydet.asp?c=45&t=1 [Accessed 19 Sep 2019].
- 5.Azar ME. Mamografía. soc. Argentina Mastología, 2019. Available: http://www.samas.org.ar/index.php/cancer-de-mama/mamografia [Accessed 27 Sep 2019].
- 6.Blanco S, Andisco D, Jiménez P. Calidad de la mamografía Y tamizaje del cáncer de mama en Argentina. Rev Panam Salud Publica 2019;43. 10.26633/RPSP.2020.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karsa Lvon, Holland R, Broeders M. European guidelines for quality assurance in breast cancer screening and diagnosis. Publications Office of the EU, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Johns Hopkins Medicine . Screening tests for common diseases, 2019. Available: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/screening-tests-for-common-diseases [Accessed 29 Sep 2019].
- 9.Groot MT, Baltussen R, Uyl-de Groot CA, et al. Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia. Breast J 2006;12 Suppl 1:S81–90. 10.1111/j.1075-122X.2006.00206.x [DOI] [PubMed] [Google Scholar]
- 10.Bray F, Colombet M, Mery L. CI5 XI: cancer incidence in five continents. XI. Lyon, 2017. http://ci5.iarc.fr/CI5-XI/Default.aspx [Google Scholar]
- 11.National Center of Statistics and Information (INDEC) . Censo Nacional de Población, Hogares Y Viviendas 2010, 2010. Available: https://www.indec.gob.ar/indec/web/Nivel4-CensoNacional-3-999-Censo-2010 [Accessed 27 Mar 2019].
- 12.Macías G, Fattore G, Breit D. RITA-Registro Institucional de Tumores de Argentina. Presentación, avances y resultados : periodo 2012-2015. Buenos Aires, Argentina: Ministerio de Salud, Argentina; 2017. https://bancos.salud.gob.ar/sites/default/files/2018-10/0000000955cnt-2017-04-21-presentacion-avances-y-resultador-periodo-2012-15.pdf [Accessed 27 Sep 2019]. [Google Scholar]
- 13.PDQ Screening and Prevention Editorial Board . Breast cancer screening (PDQ®): health professional version. Bethesda: National Cancer Institute - National Institute of Health, 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389344 [Google Scholar]
- 14.Zeeshan M, Salam B, Khalid QSB, et al. Diagnostic accuracy of digital mammography in the detection of breast cancer. Cureus 2018;10:e2448. 10.7759/cureus.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleitas I, Caspani CC, Borrás C. La calidad de Los servicios de radiología en cinco países latinoamericanos. Rev Panam Salud Pública 2006;20. [DOI] [PubMed] [Google Scholar]
- 16.Pataky R, Ismail Z, Coldman AJ, et al. Cost-effectiveness of annual versus biennial screening mammography for women with high mammographic breast density. J Med Screen 2014;21:180–8. 10.1177/0969141314549758 [DOI] [PubMed] [Google Scholar]
- 17.Edejer T. Who | making choices in health: who guide to cost-effectiveness analysis, 2017. Available: https://www.who.int/choice/publications/p_2003_generalised_cea.pdf [Accessed 27 Sep 2019].
- 18.The world bank. Argentina, 2019. Available: https://data.worldbank.org/country/argentina [Accessed 27 Mar 2019].
- 19.Boletin Oficial Republica Argentina - Ministerio De Salud Y Desarrollo Social Secretaría De Gobierno De Salud . Resolución 2004/2019 - Nomenclador de Prestaciones de Salud. Buenos Aires, Argentina, 2019. Available: https://www.boletinoficial.gob.ar/detalleAviso/primera/217123/20190923 [Accessed 10 Jul 2020].
- 20.Subsecretaría de Gestión Administrativa de Transporte . Dirección Nacional de Gestión Económica. Cálculo de Costos E Ingresos Medios de Los Servicios de Transporte de Pasajeros Urbanos Y Suburbanos de la Región Metropolitana de Buenos Aires. Buenos Aires, 2018. Available: https://www.argentina.gob.ar/sites/default/files/0019_-_if-2018-00239618-apn-dngemtr_0.pdf [Accessed 07 Oct 2019].
- 21.Ministerio de Producción Y Trabajo . Salarios, aportes Y contribuciones | Argentina.gob.ar. Buenos Aires, 2019. Available: https://www.argentina.gob.ar/casasparticulares/trabajador/sueldo [Accessed 07 Oct 2019].
- 22.Delgoshaei B, Mobinizadeh M, Mojdekar R, et al. Telemedicine: a systematic review of economic evaluations. Med J Islam Repub Iran 2017;31:754–61. 10.14196/mjiri.31.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agha Z, Schapira RM, Maker AH. Cost effectiveness of telemedicine for the delivery of outpatient pulmonary care to a rural population. Telemed J E Health 2002;8:281–91. 10.1089/15305620260353171 [DOI] [PubMed] [Google Scholar]
- 24.Doede AL, Mitchell EM, Wilson D, et al. Knowledge, beliefs, and attitudes about breast cancer screening in Latin America and the Caribbean: an in-depth narrative review. J Glob Oncol 2018;4:1–25. 10.1200/JGO.18.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galukande M, Mirembe F, Wabinga H. Patient delay in accessing breast cancer care in a sub Saharan African country: Uganda. Br J Med Med Res 2014;4:2599–610. 10.9734/BJMMR/2014/7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sud A, Jones ME, Broggio J. Collateral damage: the impact on cancer outcomes of the COVID-19 pandemic. SSRN Electron J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertram MY, Lauer JA, De Joncheere K, et al. Cost–effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94:925–30. 10.2471/BLT.15.164418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb P, Bain C, Page A. Essential epidemiology. Cambridge University Press, 2016. [Google Scholar]
- 29.ISD Scotland . Cancer | cancer statistics | lung cancer and mesothelioma | health topics. Available: https://www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Lung-Cancer-and-Mesothelioma/ [Accessed 01 Mar 2021].
- 30.Knaul FM, Arreola-Ornelas H, Velázquez E. El costo de la atención médica del cáncer mamario. El caso del Instituto Mexicano del Seguro Social 2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open-access repository. All data and links relevant to the study are included in the article (table 1) or uploaded as supplementary information.