Abstract
Objectives
To compare the impact of respirator extended use and reuse strategies with regard to cost and sustainability during the COVID-19 pandemic.
Design
Cost analysis.
Setting
USA.
Participants
All healthcare workers within the USA.
Interventions
Not applicable.
Main outcome measures
A model was developed to estimate usage, costs and waste incurred by several respirator usage strategies over the first 6 months of the pandemic in the USA. This model assumed universal masking of all healthcare workers. Estimates were taken from the literature, government databases and commercially available data from approved vendors.
Results
A new N95 respirator per patient encounter would require 7.41 billion respirators, cost $6.38 billion and generate 84.0 million kg of waste in the USA over 6 months. One respirator per day per healthcare worker would require 3.29 billion respirators, cost $2.83 billion and generate 37.22 million kg of waste. Decontamination by ultraviolet germicidal irradiation would require 1.64 billion respirators, cost $1.41 billion and accumulate 18.61 million kg of waste. H2O2 vapour decontamination would require 1.15 billion respirators, cost $1.65 billion and produce 13.03 million kg of waste. One reusable respirator with daily disposable filters would require 18 million respirators, cost $1.24 billion and generate 15.73 million kg of waste. Pairing a reusable respirator with H2O2 vapour-decontaminated filters would reduce cost to $831 million and generate 1.58 million kg of waste. The use of one surgical mask per healthcare worker per day would require 3.29 billion masks, cost $460 million and generate 27.92 million kg of waste.
Conclusions
Decontamination and reusable respirator-based strategies decreased the number of respirators used, costs and waste generated compared with single-use or daily extended-use of disposable respirators. Future development of low-cost, simple technologies to enable respirator and/or filter decontamination is needed to further minimise the economic and environmental costs of masks.
Keywords: COVID-19, health economics, health services administration & management, infectious diseases
Strengths and limitations of this study.
Describes the current economic and environmental impact of several mask reuse strategies on a national scale among healthcare workers.
Estimates cost and waste specific to respirator use in order to meet the demands of COVID-19.
Explores respirator reuse strategies to reduce the economic and environmental toll during COVID-19 and beyond.
Only a few respirator strategies and decontamination methods are evaluated in this study.
Conducted from a US perspective only; parameters are not applicable to other countries and did not include ancillary costs.
Introduction
The COVID-19 pandemic has led to personal protective equipment (PPE) shortages worldwide, including shortage of N95 respirators and surgical masks.1–3 In order to maximise resources, many hospitals have adopted extended use of masks or decontamination and reuse strategies, particularly of N95 respirators.1 4 5 Prior to the pandemic, a new N95 respirator was typically used for each patient encounter and then discarded.5 6 In light of the PPE shortage, some hospitals have now moved to using one respirator per several encounters or even several days.4 6 Decontamination strategies such as hydrogen peroxide vapour (H2O2) and ultraviolet germicidal irradiation (UVGI) are being adopted and thus far appear effective, but concerns about decontamination reducing mask fit and integrity remain, as well as concerns regarding cost of the technology.5–9
The US government awarded a $415 million contract to Battelle in April 2020 to deploy 60 hydrogen peroxide vapour decontamination sites across the country.6 7 While this may be feasible in resource-rich settings, the hydrogen peroxide system requires significant infrastructure and trained personnel, limiting its translation to resource-constrained areas.7 9 There is therefore a need for simpler methods of respirator decontamination that can be deployed on a large scale.10 Investigations into heat, steam and detergent decontamination are ongoing; however, these have thus far been shown to compromise mask integrity.3 5 Nebraska Medicine piloted a UVGI system that has been approved by the US Centers for Disease Control and Prevention (CDC), which may be easier to deploy for hospitals that already have UV decontamination systems in place.11
Reusable respirators designed for prolonged use such as half-mask elastomeric respirators are available, but have not been heavily adopted due to challenges with sterilisation, cost and bulky size.10 Several scalable, less expensive reusable respirators have been recently developed that can be easier to decontaminate using standard hospital equipment to try to address the respirator shortage.10 12 The Pneumask project, for example, which repurposes snorkel masks, has already distributed more than 23 000 masks internationally.12–15 Other types of reusable masks that aim to address barriers to communication, such as the Jelli M1 mask16 and ClearMask, have recently been developed.17 Potential benefits of reusable respirators compared with disposable respirators could include reduced cost and waste. The use of innovative filtration techniques and antimicrobial nanoparticles (NPs) could also reduce viral spread, and when incorporated into reusable respirators, reduce cost and waste even further.18 Introducing novel mask types, such as a variety of reusable masks, presents an opportunity to diversify the market, and in turn provide more flexibility within supply chains. This has the potential to increase efficiency and reduce cost, waste and energy consumption associated with supply chain disruption.19
The global increase in the use of plastics for mask and PPE production has drastically increased medical waste, with countries such as Spain and China reporting increases of 350% and 370%, respectively.20 21 As of February 2020, the production rate of face masks in China alone increased by 12-fold.22 23 Rough estimates have shown the COVID-19 pandemic could generate up to 7200 tons a day in medical waste, a sizeable portion of which comes from masks.21 24 A reusable respirator could be a more sustainable alternative to disposable respirators, particularly if respirator and mask usage becomes more commonplace post-pandemic, such as in Asia.25–27 Already environmentalists have noted a surge in plastic pollution from discarded masks in the ocean and continued heavy use of disposable PPE is unlikely to be sustainable.21 24 28
The optimal respirator use strategy that maximises supply, minimises cost and minimises waste is unknown. This analysis estimates respirator use, cost and waste generation in the USA over the course of the first 6 months of the COVID-19 pandemic to explore the optimal strategy for respirator use. For the purpose of this study, we used the following terms to describe the different respirator use and reuse strategies: single use refers to the use of one disposable respirator per patient encounter, followed by disposal; extended use refers to extended use of a disposable respirator for an entire day, followed by disposal; and reuse refers to strategies to decontaminate respirators or use of non-disposable respirators for longer term.
Methods
Data sources
We estimated respirator usage, cost and waste from late March 2020 to late September 2020. The input parameters for the model are found in tables 1–3. Data were sourced and adapted from the scientific literature or USA national databases. Base case respirator cost and waste estimates used the 3M 1860 disposable respirator as well as a recently published reusable respirator.10 29
Table 1.
Parameters used to estimate respirator usage, cost and waste generation
| Parameter | Value | Reference |
| US population as of 2019 | 328.2 million | 73 |
| Total number of healthcare and frontline workers in the USA as of 2020 | 18 (17–19) million | 34–36 |
| Weight of one 3M 1860 N95 respirator | 11.3 g | 29 |
| Weight of one 3-ply disposable personal protective (PPE-100–50) surgical mask | 8.5 g | 54 |
| Total cost of assembled reusable transparent elastomeric adaptable long-lasting (TEAL) respirator, minus filters | US$6.11 ($4.42 GBP; $5.20 Euro) | 10 |
| Weight of one TEAL reusable respirator | 46.5 g | 10 |
| Weight of one reusable respirator filter | 2.26 g | 10 |
| Cost of one pair of filters required per reusable respirator | US$0.34 ($0.25 GBP; $0.29 Euro) | 10 |
| Cost of one 3-ply surgical mask (Fluidshield Level 1) | US$0.14 ($0.10 GBP; $0.12 Euro) | 47 |
| Cost of one 3M 1860 N95 respirator | US$0.86 ($0.62 GBP; $0.73 Euro) | 46 |
| Cost of the National Battelle System funded by the US Food and Drug Administration (FDA) | US$415 million ($300.23 million GBP; $352.93 Euro) | 6 |
| Reduction in the number of respirators required for HCW population in the USA by the use of H2O2 vapour decontamination | 20-fold | 6 |
| Reduction in the number of respirators required for HCW population in the USA by the use of UVGI | 5-fold | 11 |
HCW, healthcare worker; UVGI, ultraviolet germicidal irradiation.
Table 2.
Hospitalisation-specific parameters used to estimate number of respirators required by the one respirator per patient encounter strategy over 6 months
| Parameter | Total | Reference |
| Number of hospital admissions | 14 227 773 | 49 |
| Number of patients admitted to the general ward | 12 583 927 | 37 49 |
| Number of patients admitted to theIntensive Care Unit (ICU) | 1 643 846 | 37 49 |
| Number of hospitalisations due to COVID-19 | 396 355 | 41 |
| Average length of stay for general ward patients | 4.6 days | 38 |
| Average length of stay for patients admitted to the ICU | 3.3 days | 38 |
| Median length of stay for non-ICU COVID-19 patients | 10.1 days | 40 |
| Median length of stay for COVID-19 patients admitted to the ICU | 10.5 days | 40 |
| Number of respirators required per day for interactions with general ward patients | 8 | 34 |
| Number of respirators required per day for interactions with ICU patients | 14 (12–16) | 34 |
Table 3.
HCW-specific parameters used to estimate number of respirators required by the one respirator per patient encounter strategy
| Parameter | Total | Number of workers with patient contact | Number of workers without patient contact | Reference |
| Number of nursing home workers | 3 427 000 | 856 750 | 2 570 250 | 34 |
| Number of emergency medicine service workers | 297 000 | 267 300 | 29 700 | 34 |
| Number of emergency department workers | 132 000 | 132 000 | 0 | 34 |
| Number of hospital workers | 6 053 000 | 1 997 490 | 4 055 510 | 34 |
| Number of outpatient workers | 3 206 000 | 2 148 020 | 1 057 980 | 34 |
| Number of other healthcare workers in other occupations | 6 000 000 | 0 | 6 000 000 | 34 35 |
HCW, healthcare worker.
Respirator usage
We considered seven respirator usage strategies: one disposable respirator per patient encounter (single-use respirators), extended use of one disposable respirator per healthcare worker (HCW) per day, reuse of one respirator per HCW per day enabled by daily UVGI decontamination, reuse of one disposable respirator per HCW per day enabled by daily H2O2 vapour decontamination, one reusable respirator with disposable filters per HCW, one reusable respirator with H2O2 vapour-decontaminated filters per HCW and one disposable surgical mask per HCW per day. We assumed that HCWs would be masked for all patient encounters (universal masking) given limited access to rapid COVID-19 testing nationally.30–32 For the H2O2 and UVGI decontamination strategies, we accounted for a 30% respirator discard rate due to soiled or damaged respirators as has previously been reported.33 For each usage strategy, we considered low, average and high estimates for the size of the HCW population (17–19 million) based on estimates from the CDC, the Bureau of Labor Statistics and published literature.34–36
For the one respirator per patient encounter strategy, we estimated respirators required by HCWs with exposure to patients and those without. The number of respirators required for HCWs due to patient contact was based on the number of hospitalised patients (COVID-19 and non-COVID-19), average length of stay (LOS) and average number of visits from HCWs per day (table 2).34 Data for the number of respirators required per patient per day, LOS per patient and the number of ICU and hospital admissions were extracted from the recent COVID-19 literature, government reports and a previous influenza study estimating respirator usage to prevent aerosol transmission.34 37–40 To estimate the number of overall hospitalised patients, we incorporated drops in hospital admission rates due to the pandemic, which were as high as 42.8% below usual rates of admissions in April 2020 before rebounding down to 15.9% below usual rates in June/July 2020.41 In addition, HCWs with patient contact were estimated to be using four respirators per day in between direct patient care.34 42 HCWs without patient contact were assumed to be using one respirator per day given universal masking (table 3).
We then used these results to infer estimates for extended use and reuse of respirators enabled by the alternate respirator strategies. For our one disposable respirator per HCW per day strategy, we assumed that each HCW (with or without patient contact) would use one, new respirator per day.
For the daily H2O2 vapour decontamination strategy, using currently available data on respirator integrity and efficiency after multiple cycles of H2O2 vapour decontamination, we assumed that a respirator could be decontaminated for up to 20 cycles, with a 30% discard rate per day due to damaged or visibly soiled respirators after each cycle of decontamination.33 Therefore, to form our estimates for H2O2 vapour decontamination-enabled reuse of respirators, we divided the one respirator per HCW worker per day usage estimates by 20 and assumed 30% of respirators would need to be replaced after each decontamination cycle/per day to account for the estimated discard rate. Given uncertainty regarding discard rates and consistency in maximum number of cycles of decontamination nationally, we performed sensitivity analyses using 10% and 50% discard rates, as well as a maximum of 10 cycles of H2O2 decontamination per respirator.
To model usage estimates for reuse of respirators enabled by daily UVGI decontamination, we used currently available data on respirator integrity and efficiency after multiple cycles of UVGI. Based on these estimates, we assumed that a respirator could be decontaminated for up to five cycles.43 Therefore, to form our estimates for UVGI-enabled reuse of respirators, we divided the one respirator per HCW per day usage estimates by 5 and assumed 30% of respirators would need to be replaced after each decontamination cycle/per day due to the estimated discard rate.33 44 45 Given uncertainty regarding discard rates and consistency in maximum number of cycles of decontamination nationally, we performed sensitivity analyses using 10% and 50% discard rates, as well as a maximum of 2 cycles of UVGI decontamination per respirator.
For the reusable respirators with disposable or H2O2 vapour-decontaminated filter strategies, we assumed that every HCW in the USA will use one reusable respirator and replace or decontaminate the filters daily. Based on a recently published low-cost reusable respirator, we estimated costs and waste from a pair of filters to be approximately ⅖ of the cost and waste generated from an N95 respirator.10 If filters were to be decontaminated using H2O2 vapour, we also assumed that filters could be reused for a maximum of 20 days (20 decontamination cycles).
Cost estimate
To estimate the cost accumulated by each usage method, we used the following costs, which were found in the literature and converted to 2020 US dollars: 3M respirator, $0.86 (converted from $0.79 USD 2014 to USD 2020), multiplied by the number of respirators required46; one surgical mask, $0.14, multiplied by the number of surgical masks required47; reusable respirator, $6.11, multiplied by the number of reusable respirators required10; a pair of filters for reusable respirators, $0.34, multiplied by the number of pairs of filters required; and nationally distributed H2O2 vapour decontamination systems across 60 sites, $415 million.6 Due to variation in implementation and maintenance costs for Battelle H2O2 vapour decontamination systems across sites, it was difficult to estimate exact costs.48 We performed a sensitivity analysis to estimate lower and upper-bound costs based on data from the Battelle decontamination centre in Somerville, Massachusetts, and added them to the total cost of the respirators themselves. This decontamination centre is capable of decontaminating 80 000 respirators per day and servicing up to roughly 157 hospitals. There are currently 6090 hospitals across the USA.49 For the lower bound, we estimated that if each site were able to service 157 hospitals, this would require approximately 39 decontamination centres and only 65% of the 415 million dollars to fund 60 sites across the USA. For the upper bound, we used a decontamination cost per respirator of $3.25 and multiplied that by the respirator usage required for the first 6 months of the pandemic.50 We performed the sensitivity analysis varying different parameters for the lower and upper bounds in order to test the widest range for the cost of the H2O2 decontamination strategy. In addition, we estimated the shipping costs from a large academic hospital in Boston, Massachusetts (Massachusetts General Hospital) to the local Battelle decontamination centre in Somerville, Massachusetts. The shipping costs for 1 day per each hospital were estimated to be $114 to and from the site (for a total of $228 in shipping costs; based on the estimated weight of 11.33 kg for shipping 1000 masks over a distance of roughly 5632.7 meters).51 We scaled this cost by the number of hospitals and Battelle sites across the USA over the course of the first 6 months of the pandemic and arrived at a total nationwide shipping cost of $250 million. We added this to the overall costs for lower, base-case and upper bound costs. For the cost of the UVGI system, we assumed the base-case cost of the UVGI system to only include the cost of the respirators required as the literature suggests that UV systems are more readily available on site in many hospitals in comparison to H2O2 vapour decontamination systems.32 This may be because UV systems require significantly less space and personnel than H2O2 vapour decontamination systems.33 However, we also performed a sensitivity analysis to account for the varying costs and sophistication of UVGI systems, ranging from the installation of a brand new, high volume system11 to a less expensive, lower volume system that uses repurposed materials.9 52 In addition, we explored a range of UVGI system costs which do not include installation, maintenance, distribution, energy or personnel costs.9 11 43 52 53 We also estimated the average cost generated per patient for each strategy by dividing the total cost by the total number of hospitalised patients with COVID-19 during the first 6 months of the pandemic.
Waste estimate
Waste estimates for each usage method measured the mass of the total respirators, surgical masks and filters used and disposed of through the 6-month duration. The mass of 3M’s 1860 respirator, a standard surgical mask and a reusable respirator are 11.3, 8.5 and 46.5 g, respectively.10 29 54 Single filters for reusable respirators were estimated using ⅕ of a respirator (2.26 g per a single filter, 4.53 g per pair of filters).10 29 Thus, to form our waste estimates, we multiplied respirator, surgical mask and reusable respirator usage by their respective masses. We estimated the average waste generated per patient for each strategy by dividing the total amount of waste by the total number of hospitalised patients with COVID-19 during the first 6 months of the pandemic. We also performed an additional sensitivity analyses using an alternate disposable respirator.
Ethics approval statement
This study did not require ethics approval as it did not involve human participants.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research. It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting or dissemination plans of our research.
Results
Mask usage
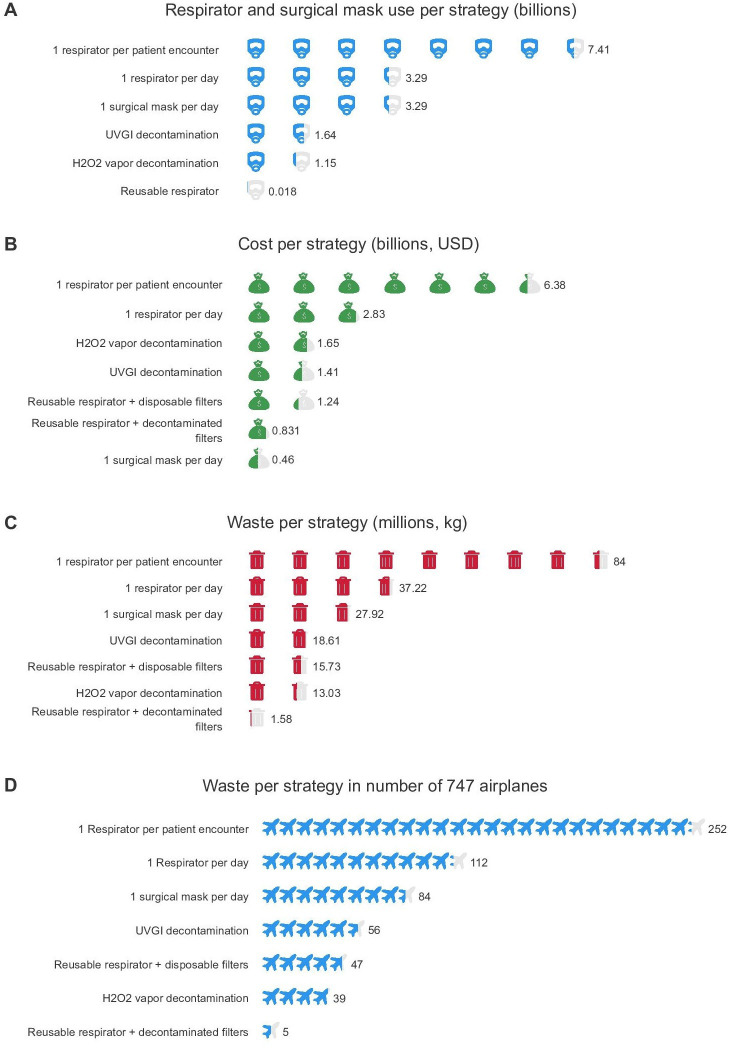
The estimated numbers of respirators required in the USA for each strategy are shown in figure 1A, table 4. The use of a new respirator per patient encounter in the USA would require 7.41 billion respirators. An extended-use strategy of one respirator per day per HCW would reduce need by over 50% to 3.29 billion respirators. Decontamination by UVGI would further reduce the need to 1.64 billion respirators. Employing a H2O2 vapour decontamination strategy would further reduce need by 84% to only 1.15 billion respirators. A reusable respirator strategy (with either disposable or decontaminated filters), where one respirator is assigned to each HCW for the duration of the pandemic, would further reduce need to approximately 18 million respirators, for a total reduction in respirator need by over 99%. Using a new surgical mask daily would require 3.29 billion surgical masks.
Figure 1.
Comparison of the following per respirator reuse strategies: (A) number of respirators or surgical masks used, (B) costs in billions of USD, (C) waste generated in millions of kg, (D) waste generated per strategy in the equivalent number of 747 airplanes by mass (mass of one 747 aeroplane, 333 000 kg). Values are estimated for the first 6 months of the pandemic. UVGI, ultraviolet germicidal irradiation.
Table 4.
Numbers of respirators, cost accumulated and waste generated per strategy over a duration of 6 months per base, low and high number of estimated HCWs
| Respirator strategy | Number of respirators required | Cost accumulated (US$) | Cost accumulated (US$) per patient | Waste generated (kg) | Waste generated (kg) per patient |
| 1 per patient encounter | 7.41 (7.22–7.59) billion | $6.38 (6.21–6.52) billion | $16.09 (15.67–16.46) thousand | 84.0 (81.79–85.96) million | 211.94 (206.38–216.88) |
| 1 per day | 3.29 (3.10–3.47) billion | $2.83 (2.67–2.98) billion | $7.13 (6.73–7.52) thousand | 37.22 (35.15–39.29) million | 93.90 (88.69–99.12) |
| UVGI-decontaminated 3M 1860 N95 respirators | 1.64 (1.55–1.73) billion | $1.41 (1.33–1.49) billion | $3.56 (3.37–3.76) thousand | 18.61 (17.58–19.64) million | 46.95 (44.34–49.56) |
| H2O2 decontaminated 3M 1860 N95 respirators | 1.15 (1.09–1.21) billion | $1.65 (1.60–1.71) billion | $4.17 (4.03–4.31) thousand | 13.03 (12.30–13.75) million | 32.87 (31.04–34.69) |
| Reusable TEAL respirator+disposable filters | 0.018 (0.017–0.019) billion | $1.24 (1.17–1.31) billion | $3.13 (2.96–3.30) thousand | 15.73 (14.85–16.60) million | 39.68 (37.47–41.88) |
| Reusable TEAL respirator+decontaminated filters | 0.018 (0.017–0.019) billion | $0.831 (0.822–0.841) billion | $2.10 (2.07–2.12) thousand | 1.58 (1.49–1.67) million | 3.99 (3.77–4.21) |
| Surgical mask, 1 per day | 3.29 (3.10–3.47) billion (surgical masks) | $0.460 (0.434–0.485) billion | $1.16 (1.10–1.23) thousand | 27.92 (26.37–29.47) million | 70.45 (66.53–74.36) |
HCW, healthcare worker; TEAL, transparent elastomeric adaptable long-lasting; UVGI, ultraviolet germicidal irradiation.
Cost estimate
The estimated costs for each respirator use strategy are summarised in figure 1B, table 4. The use of a new respirator per patient per HCW would cost an average of $6.38 billion ($16.09 thousand (k) per patient). Extended use of one respirator per day would reduce the cost to $2.83 billion ($7.13k per patient), saving approximately $3.55 billion. The H2O2 vapour decontamination strategy would reduce cost to $1.65 billion ($4.17k per patient), saving approximately $1.18 billion, although sensitivity analyses estimated the cost of the H2O2 decontamination system could vary between $1.51 and $4.98 billion (online supplemental table 1). The decontamination by UVGI strategy would reduce the cost to $1.41 billion ($3.56k per patient), saving an additional $24 million. A reusable respirator with disposable filters would cost $1.24 billion ($3.13k per patient), although this is almost entirely filter costs ($1.13 billion). A reusable respirator with a decontaminated filter and surgical mask strategies would be the least costly strategies at $831 million dollars and $460 million dollars ($2.10k and $1.16k per patient, respectively), which is a total cost savings of over $5.54 billion (figure 1B). This is more than the amount of money provided by the CARES Act to support the CDC’s pandemic response efforts and programmes.55
bmjopen-2021-048687supp001.pdf (48.9KB, pdf)
Waste estimate
The estimated waste generated by each respirator use strategy is summarised in figure 1C, D, table 4. The use of a new respirator per patient encounter per HCW would generate 84.0 million kg of waste (211.94 kg of waste per patient). Extended use of one respirator per day would reduce waste to 37.22 million kg (93.90 kg per patient). The decontamination by UVGI strategy would reduce waste to 18.61 million kg (46.95 kg per patient). A H2O2 vapour decontamination (with a 30% discard rate) strategy would reduce waste to 13.03 million kg (32.87 kg per patient). A reusable respirator with disposable filters would generate 15.73 million kg of waste (14.89 million kg from filters, 39.68 kg total per patient). Pairing the reusable respirator with a decontaminated filter would significantly reduce generated waste to 1.58 million kg (3.99 kg per patient), for an overall reduction in waste generation by approximately 82.42 million kg, equivalent to going from a mass of 252 Boeing 747 airplanes to five (figure 1D). The surgical mask strategy would generate 27.92 million kg of waste (70.45 kg per patient).
Sensitivity analyses
A sensitivity analysis of a larger commonly used disposable respirator (Gerson 1730) did not significantly change the estimated cost of the strategies or relative amounts of waste generation (online supplemental table 2). An additional sensitivity analysis was conducted looking at a different 3M disposable respirator cost found from the commercial manufacturer 3M to account for variability in market costs ($1.27/respirator). The cost variation did not change the relative rankings of the reuse strategies (online supplemental table 3).46 56 Cost and waste estimates for commercially available reusable half-facepiece elastomeric respirators (3M 7500 series) with P100 filters (assuming that each HCW uses one pair of filters per week) were also explored (online supplemental table 4).57–60 Low and high cost estimates of $2.02 and $2.26 billion were calculated using sources from the commercial manufacturer 3M,57 with reusable respirator costs ranging from $25 to $45 per respirator with a single disposable P100 filter cost of $7.00.58 These cost estimates of $2.02–$2.26 billion were lower than the one respirator per day reuse strategy, but higher than the H2O2 decontamination, UVGI decontamination, reusable respirator, reusable respirator with decontaminated filters and surgical mask strategies (table 4, online supplemental table 4). Low and high waste estimates of 3.22 million kg and 3.59 million kg were calculated using a respirator weight of 135 g and filter weight of 4.54 g (online supplemental table 4). These waste estimates were lower than the one per day reuse strategy, H2O2 decontamination, UVGI, reusable respirator and surgical mask strategies, but higher than the reusable respirator with decontaminated filters strategy (table 4, online supplemental table 4).
A sensitivity analysis of the H2O2 decontamination system costs estimated a range of $1.51–$4.98 billion, with variation in cost driven by differing estimates in the number of decontamination centres required to service all of the hospitals in the USA and in the cost of the decontamination per mask (online supplemental table 1).
Sensitivity analyses of respirator discard rates and maximum cycles of H2O2 decontamination found that a 10% discard rate lowered respirator usage, cost and waste generation by 657 million respirators, $560 million and 7.45 million kg, respectively. A 50% discard rate would increase respirator usage, cost and waste generation by 660 million respirators, $570 million and 7.44 million kg, respectively. Lowering maximum decontamination to 10 cycles increased respirator usage, cost and waste generation by 160 million respirators, $150 million and 1.86 million kg, respectively (online supplemental tables 5 and 6).
A sensitivity analysis of the UVGI decontamination system costs estimated a range of $1.41–1.42 billion, even accounting for variations in sophistication of technology installed (online supplemental table 7). Sensitivity analyses of respirator discard rates and maximum cycles of UVGI decontamination found that a 10% discard rate reduced respirator usage, cost and waste generation by 654 million respirators, $562 million and 7.44 million kg, respectively. A 50% discard rate increased respirator usage, cost and waste generation by 660 million respirators, $570 million and 7.44 million kg, respectively. Lowering maximum decontamination to two cycles increased respirator usage, cost and waste generation by 990 million respirators, $850 million and 11.17 million kg, respectively (online supplemental tables 8 and 9).
Discussion
Principal findings
The COVID-19 pandemic has dramatically increased the demand for respirators across the world, leading to supply shortages, spending in the billions of dollars and generation of large amounts of medical waste. Even after widespread vaccination efforts, masks will likely continue to be required due to factors such as variable vaccine uptake, incomplete vaccinations, lack of knowledge as to who has received a vaccine, the possibility of reinfection and unclear duration of vaccination efficacy.61 62 Additionally, even after the pandemic, respirator and mask usage both in healthcare settings and among the general public may persist.63 The continued use of disposable respirators and masks is unlikely to be sustainable and will have significant environmental consequences.21 With this in mind, it is critical to understand the best strategy to maximise respirator and mask availability while minimising costs and waste generation.
Of the strategies compared, we find that all reuse strategies (UVGI decontamination, H2O2 vapour decontamination, reusable respirators with disposable filters or reusable respirators with decontaminated filters) could significantly decrease the number of respirators required compared with single-use or extended-use mask strategies by at least 1.65 billion respirators in the USA alone. This would greatly increase availability and access of respirators worldwide. In addition, reuse strategies could save at least $1.18 billion dollars in costs nationally over the course of the pandemic. Finally, reuse strategies significantly reduce waste generation in the USA by at least 18.61 million kg. These estimates from our study only capture the economic and environmental impact over the first 6 months of the COVID-19 pandemic in the USA and suggest that the long-term and global impact of reuse strategies are even higher, especially when considering respirators and masks used by the general population.
Our analyses found that UVGI decontamination, H2O2 vapour decontamination and reusable respirators with disposable filters were similar in cost and waste generation. Combining the strategies by using a reusable respirator with H2O2 vapour-decontaminated filters was the least costly of all strategies compared and generated the least amount of waste. This finding suggests that even with UVGI and H2O2 vapour decontamination strategies, the adoption of a reusable respirator can have a significant impact in both cost and waste generation, although additional studies are needed to estimate the impact of additional costs, such as shipping to shared decontamination sites, installation costs and time associated with decontamination or cleaning methods. Additional investigation is needed to capture other potential costs and benefits related to each mask-reuse strategy.
In settings where UVGI or H2O2 vapour decontamination is not feasible, such as in resource-constrained settings where installation and maintenance of such systems are challenging, reusable respirators with disposable filters may be preferable to disposable respirators. These respirators may also be decontaminated with standard hospital equipment such as alcohol and bleach wipes, which may be more readily available in settings with limited resources.10 12 Anticipatory investment in a reusable respirator may not only provide access to high-quality PPE for COVID-19 in such settings but also reduce overall waste and injury to our environment. Development of technologies to facilitate decontamination of respirators and/or filters that do not require special equipment, training or infrastructure could even further reduce costs and waste as in the reusable respirator with decontaminated filters strategy.
Limitations
One potential limitation of our study is the assumption that all respirator strategies discussed are equally effective at protecting the user. The decision to employ decontamination methods for reuse should be weighed against the possibility for greater health risks incurred by incomplete decontamination or lowered respirator efficacy, which may incur additional costs. The CDC recommended extended respirator use and reuse strategies for N95 respirators if respirators maintained their fit and function after decontamination.64 Several studies have evaluated the effect of extended use and reuse strategies that require multiple donning on the fit and efficacy of N95 respirators independent of decontamination. One study found that 48% of subjects failed a fit test after only one redonning of an N95 respirator. Additionally, another study found that among test subjects experienced in respirator donning, consecutively donning the same respirator five times was the threshold before mask-fit dropped below 100%.65 Furthermore, both UVGI and H2O2 decontamination methods have shown to reduce filtration and mask performance after three rounds of decontamination in some studies.66 Therefore, it is important to note that the efficacy of each reuse strategy may not be equal and should be considered prior to implementation. Potential costs related to unequal respirator efficiency and protection were not estimated in our analysis.
An additional limitation of our study is that we modelled one strategy for all HCWs, regardless of frequency and type of patient contact. For HCWs at low risk of contact with bodily fluids (including respiratory droplets), it may be possible to deploy alternate strategies such as extended use of disposable respirators or less frequent decontamination. This could potentially further reduce cost and waste and increase respirator availability without sacrificing protection.
We estimated only a few respirator strategies and decontamination methods, and other methods for extended respirator use and reuse across the world were not captured in our analysis. Furthermore, our estimates were performed from a US perspective, and these numbers will be different for other countries depending on parameters such as number of healthcare workers, rates of infection and number of hospitalised patients, although we suspect that the relative benefit of reuse strategies compared with single-use or extended-use respirator strategies will persist. Additionally, the number of COVID-19 hospitalisations was likely underestimated in this study, as only two-thirds of states and territories in the USA have reported this data during the COVID-19 pandemic; however, we suspect that this therefore underestimates the potential impact of mask reuse strategies.42 Furthermore, our cost estimates did not include installation, maintenance, distribution or personnel costs associated with various strategies, and additional studies should be performed. In addition, our analysis measured only the waste generated by masks themselves and did not study the environmental impact of manufacturing, packaging or waste generation from decontamination processes, which some studies estimate could generate up to 90% of greenhouse gas emissions.19 67 Furthermore, the environmental impact of single-use plastics generated from packaging related to mask use, estimated to have increased by up to 40% during the pandemic, may contribute a significant amount of additional environmental waste.68 These aspects were not included in our analyses and require further quantification. Finally, our estimates for the reusable respirator strategy were based on a recently published prototype.10 Updated analyses should be performed as these and other low-cost reusable respirators and masks become more available.12 69
Implications and future research
While our analysis measured the economic and environmental impact of several mask reuse strategies, there are several areas of investigation that may contribute to further reductions in cost and environmental impact. For example, our analysis highlighted the importance of considering not only reusable respirators, but also reusable or decontaminatable filters, as these drove the cost and waste of reusable respirator/disposable filter strategies. Inexpensive, simple methods for filter decontamination are needed. Alternatively, redesign of reusable respirators to require smaller filters or development of fully reusable respirators would greatly reduce cost, waste and potentially the need for single-use plastics. Additionally, the development of novel materials for masks to increase durability of these systems after repeated exposures to H2O2 vapour or other decontamination techniques may increase the lifespan of masks and decrease the volume of masks used. Incorporation of bactericidal or antiviral agents, nanoparticles or nanotechnology into masks may also increase their reusability and potentially decrease the need for cleaning agents in regions where there may be concomitant shortages of these solutions. Antimicrobial agents derived from natural products (tea tree oil, grapefruit seed extract, etc) as well as NPs from different metals and metal compounds (copper, silver, zinc oxide, etc) have also been shown to improve filtration and reduce viral load on mask surfaces.14 15 There are a variety of masks now commercially available that use nanotechnology and range from disposable surgical masks, washable masks and reusable respirators such as Innonix RespoKare (citric acid NPs), Cupron (copper NPs) and Argaman BioBlockX (silver NPs).14 15 18 These strategies may also decrease waste of common hospital-based wipes used to decontaminate masks, which was not included in this analysis. Finally, the development of biodegradable or recyclable materials that provide efficient particle protection may minimise the environmental effects of discarded masks.
Our analysis raises key questions for stakeholders regarding the optimal strategy to both provide sufficient protection for healthcare workers and patients while also ensuring equitable access to PPE and reducing environmental harm. Given our findings that reusable respirator strategies greatly reduce the number of respirators required and medical waste generated, it is interesting that reusable respirators or decontamination strategies have largely not been adopted in the USA prior to the COVID-19 pandemic. We hypothesise that this could be due to a number of reasons including cost and availability of reusable respirators, lack of recognition of the scale of medical waste and its impact on the environment, and individual healthcare systems’ lack of accountability with regard to medical waste. We are hopeful that the first two reasons will be addressed over the course of the pandemic. Given renewed interest in new technologies for PPE, we expect options and availability for reusable respirators to continue to expand.15 We believe our study as well as others will increase public awareness of the environmental impact of disposable PPE, particularly masks.31 68 In order to improve hospital system accountability over medical waste, however, we may need to turn to policymakers to consider nationwide incentives such as subsidies to transition to reusable PPE, taxes to offset medical waste generation, and other incentives as has been used to promote transition to green technologies in other fields.70–72
Conclusions
In summary, respirator reuse technologies are critical to meet the supply demands imparted by COVID-19, especially in low-resource settings. This need is emphasised by the likelihood that respirators will continue to be commonly used even after widespread vaccination and post-pandemic in certain scenarios, such as healthcare and crowded transportation areas, and such technologies can enable more sustainable use of respirators moving forward. Furthermore, these technologies can save billions of dollars that can be redistributed towards other efforts for economic and environmental recovery brought on by the pandemic. Further study is needed regarding reuse fit and filtration efficacy to minimise health risks associated with reuse strategies. Additionally, future development of low-cost, simple technologies to enable respirator and/or filter decontamination is needed to further minimise the economic and environmental costs of respirators.
Supplementary Material
Footnotes
Contributors: J Chu, OG, J Collins, CH and GT conceived and designed the analysis. JB, AW, PRC and FD contributed data. J Chu, OG and J Collins collected data and performed the analysis. J Chu, OG, J Collins and GT wrote the manuscript. J Chu, OG, J Collins, JB, AW, PRC, FD, CH and GT interpreted the data and reviewed and approved the manuscript. The corresponding author, GT, provided supervision over the study and is the guarantor. GT confirms that all authors meet the authorship criteria and no contributing authors have been omitted.
Funding: J Chu: 5T32DK007191; OG: MIT Undergraduate Research Opportunities Programme, N/A; PC: NIH K23DA044874, R44DA051106; GT: Karl van Tassel (1925) Career Development Professorship, Department of Mechanical Engineering, MIT.
Competing interests: AW, JB and GT have filed multiple patent applications surrounding a reusable respirator and sensors that can be integrated into a respirator. In addition, AW, JB and GT have a financial interest in TEAL Bio, Inc., a biotechnology company focused on developing the next generation of personal protective equipment.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data are included in the manuscript and/or supplementary materials, no additional data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Ranney ML, Griffeth V, Jha AK. Critical Supply Shortages - The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N Engl J Med 2020;382:e41. 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020;395:1225–8. 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA 2020;323:1912–4. 10.1001/jama.2020.5317 [DOI] [PubMed] [Google Scholar]
- 4.Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health 2020;5:e002553. 10.1136/bmjgh-2020-002553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubio-Romero JC, Pardo-Ferreira MDC, Torrecilla-García JA, et al. Disposable masks: disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf Sci 2020;129:104830–30. 10.1016/j.ssci.2020.104830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Books B. Final report for the Bioquell hydrogen peroxide vapor (HPV) decontamination for reuse of N95 respirators: Battelle, 2016. Available: https://www.fda.gov/media/136386/download [Accessed June 2020].
- 7.Battelle . Battelle CCDS FAQ 2020. Available: https://www.battelle.org/inb/battelle-ccds-for-covid19-satellite-locations [Accessed May 2020].
- 8.Fisher EM, Shaffer RE. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J Occup Environ Hyg 2014;11:D115–28. 10.1080/15459624.2014.902954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert RM, Donzanti MJ, Minahan DJ, et al. Mask reuse in the COVID-19 pandemic: creating an inexpensive and scalable ultraviolet system for filtering facepiece respirator decontamination. Glob Health Sci Pract 2020;8:582–95. 10.9745/GHSP-D-20-00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne JD, Wentworth AJ, Chai PR, et al. Injection molded Autoclavable, scalable, Conformable (iMASC) system for aerosol-based protection: a prospective single-arm feasibility study. BMJ Open 2020;10:e039120–e20. 10.1136/bmjopen-2020-039120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe J, Paladino K, Farke JD. N95 Filtering Facepiece Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for Decontamination and Reuse. Nebraska Medicine, 2020. Available: https://www.nebraskamed.com/sites/default/files/documents/covid-19/n-95-decon-process.pdf [Accessed June 2020].
- 12.Kroo L, Kothari A, Hannebelle M, et al. Modified full-face snorkel masks as reusable personal protective equipment for hospital personnel. PLoS One 2021;16:2020.04.24.20078907. 10.1371/journal.pone.0244422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pneumask . The Pneumask project, 2020. Available: https://www.pneumask.org/ [Accessed April 2020].
- 14.Chua MH, Cheng W, Goh SS, et al. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research 2020;2020:1–40. 10.34133/2020/7286735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmieri V, De Maio F, De Spirito M, et al. Face masks and nanotechnology: keep the blue side up. Nano Today 2021;37:101077. 10.1016/j.nantod.2021.101077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M1 J . Smile again JELLI M1, 2021. Available: https://jellim.com/ [Accessed April 2021].
- 17.ClearMask . See the person, not the mask.™ 2021, 2021. Available: https://www.theclearmask.com/ [Accessed April 2021].
- 18.Kumar A, Sharma A, Chen Y, et al. Copper@ZIF‐8 core‐shell nanowires for reusable antimicrobial face masks. Adv Funct Mater 2021;31:2008054. 10.1002/adfm.202008054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemeš JJ, Fan YV, Jiang P. The energy and environmental footprints of COVID-19 fighting measures - PPE, disinfection, supply chains. Energy 2020;211:118701–01. 10.1016/j.energy.2020.118701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prata JC, Silva ALP, Walker TR, et al. COVID-19 pandemic repercussions on the use and management of plastics. Environ Sci Technol 2020;54:7760–5. 10.1021/acs.est.0c02178 [DOI] [PubMed] [Google Scholar]
- 21.Klemeš JJ, Fan YV, Tan RR, et al. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renewable and Sustainable Energy Reviews 2020;127:109883–83. 10.1016/j.rser.2020.109883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkodie SA, Owusu PA. Impact of COVID-19 pandemic on waste management. Environ Dev Sustain 2021;23:7951–60. 10.1007/s10668-020-00956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang P, Klemeš JJ, Fan YV, et al. More is not enough: a deeper understanding of the COVID-19 impacts on healthcare, energy and environment is crucial. Int J Environ Res Public Health 2021;18:684. 10.3390/ijerph18020684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhabria P. Coronavirus:"The masks you throw away could end up killing a whale": BBC, 2020. Available: https://www.bbc.com/news/av/science-environment-53287940 [Accessed August, 2020].
- 25.Leung H. Why wearing a face mask is encouraged in Asia, but shunned in the U.S. time, 2020. Available: https://time.com/5799964/coronavirus-face-mask-asia-us/2020 [Accessed April 2020].
- 26.Jennings R. COVID-19 pandemic: how cultural differences help asian countries beat COVID-19, while US struggles. Voice of America, 2020. Available: https://www.voanews.com/covid-19-pandemic/how-cultural-differences-help-asian-countries-beat-covid-19-while-us-struggles [Accessed August 2020].
- 27.Burgess A, Horii M. Risk, ritual and health responsibilisation: Japan's 'safety blanket' of surgical face mask-wearing. Sociol Health Illn 2012;34:1184–98. 10.1111/j.1467-9566.2012.01466.x [DOI] [PubMed] [Google Scholar]
- 28.Konyn C. Another side effect of COVID-19: the surge in plastic pollution/ Earth.org, 2020. Available: https://earth.org/covid-19-surge-in-plastic-pollution/2020 [Accessed August 2020].
- 29.3M. science. applied to life. 3M™ disposable respirator 1860, 1860s, N95. products, 2020. Available: https://multimedia.3m.com/mws/media/1538979O/3m-disposable-respirator-1860-1860s-technical-data-sheet.pdf [Accessed June 2020].
- 30.Asadi S, Cappa CD, Barreda S, et al. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep 2020;10:15665–65. 10.1038/s41598-020-72798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman JD, Hupert N, Washburne AD. Using influenza surveillance networks to estimate state-specific prevalence of SARS-CoV-2 in the United States. Sci Transl Med 2020;12:eabc1126. 10.1126/scitranslmed.abc1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gostin LO, Cohen IG, Koplan JP. Universal masking in the United States: the role of mandates, health education, and the CDC. JAMA 2020;324:837–8. 10.1001/jama.2020.15271 [DOI] [PubMed] [Google Scholar]
- 33.Czubryt MP, Stecy T, Popke E, et al. N95 mask reuse in a major urban Hospital: COVID-19 response process and procedure. J Hosp Infect 2020;106:277–82. 10.1016/j.jhin.2020.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carias C, Rainisch G, Shankar M, et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis 2015;60 Suppl 1:S42–51. 10.1093/cid/civ141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC . Healthcare Workers The National Institute for Occupational Safety and Health (NIOSH) CDC, 2017. Available: https://www.cdc.gov/niosh/topics/healthcare/default.html [Accessed June 2020].
- 36.Kaisers Family Foundation . Total healthcare employment, 2018. Available: https://www.kff.org/other/state-indicator/total-health-care-employment/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22sc%22%7D [Accessed June 2020].
- 37.UCSF Philip R . Lee Institute for health policy studies. ICU outcomes, 2020. Available: https://healthpolicy.ucsf.edu/icu-outcomes [Accessed August 2020].
- 38.Agency for Healthcare Research and Quality . Overview of U.S. Hospital stays in 2016: variation by geographic region, 2018. Available: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb246-Geographic-Variation-Hospital-Stays.jsp [Accessed August 2020]. [PubMed]
- 39.Hunter A, Johnson L, Coustasse A. Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag 2020;39:109–16. 10.1097/HCM.0000000000000295 [DOI] [PubMed] [Google Scholar]
- 40.Lewnard JA, Liu VX, Jackson ML. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m2205. 10.1136/bmj.m2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The COVID Tracking Project . National Hospitalization.[updated November 16th, 2020; Cumulative hospitilization data was removed from the website], 2020. Available: https://covidtracking.com/data/national/hospitalization [Accessed August 2020].
- 42.Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff 2020;39:101377hlthaff202000426-935C. 10.1377/hlthaff.2020.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hearn K, Gertsman S, Sampson M, et al. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation does not impair mask efficacy and safety. J Hosp Infect 2020;106:163–75. 10.1016/j.jhin.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brickman J, Scott C, Courtad C. Optimization, Validation, and Implementation of a UV Disinfection Method for N95 Face Masks. University of Chicago, 2020. Available: https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/5eacab4783c6b418d137baf3/1588374356749/UCMC+Surfacide+Mask+UVGI+Process+Validation+and+Process+v6.pdf [Accessed August 2020].
- 45.Liao L, Xiao W, Zhao M, et al. Can N95 respirators be reused after disinfection? how many times? ACS Nano 2020;14:6348–56. 10.1021/acsnano.0c03597 [DOI] [PubMed] [Google Scholar]
- 46.Mukerji S, MacIntyre CR, Seale H, et al. Cost-effectiveness analysis of N95 respirators and medical masks to protect healthcare workers in China from respiratory infections. BMC Infect Dis 2017;17:464–64. 10.1186/s12879-017-2564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MDS Associates . Disposable face masks: Fluidshield® level 1 sensitive skin covers, 2020. Available: https://www.mdsassociates.com/catalog/p-107720/fluidshield-level-1-sensitive-skin-covers [Accessed June 2021].
- 48.Wigginton KR, Arts PJ, Clack HL, et al. Validation of N95 filtering facepiece respirator decontamination methods available at a large university hospital. Open Forum Infect Dis 2021;8:ofaa610–ofaa10. 10.1093/ofid/ofaa610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Hospital Association . Fast facts on U.S. hospitals, 2020. Available: https://www.aha.org/statistics/fast-facts-us-hospitals [Accessed August 2020].
- 50.Ostriker R. Boston Hospitals getting 'game changer' machine that sterilizes 80,000 protective masks a day. The Boston Globe, 2020. Available: https://www.bostonglobe.com/2020/04/02/metro/boston-hospitals-getting-game-changer-machine-that-sterilizes-80000-protective-masks-day/ [Accessed March 2021].
- 51.The UPS Store . Estimate shipping cost, 2021. Available: https://www.theupsstore.com/tools/estimate-shipping-cost [Accessed March 2021].
- 52.Ou Q, Pei C, Chan Kim S, et al. Evaluation of decontamination methods for commercial and alternative respirator and mask materials - view from filtration aspect. J Aerosol Sci 2020;150:105609–09. 10.1016/j.jaerosci.2020.105609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Robles D, Kramer SW. Improving indoor air quality through the use of ultraviolet technology in commercial buildings. Procedia Engineering 2017;196:888–94. 10.1016/j.proeng.2017.08.021 [DOI] [Google Scholar]
- 54.ADESSO . 3 Ply Disposable Personal Protective Face Mask (50 Masks/Box), 2021. Available: https://www.adesso.com/product/3-ply-disposable-face-mask-with-ear-loop-non-medical-pack-of-50/ [Accessed March 2021].
- 55.Snell K. What’s Inside The Senate’s $2 Trillion Coronavirus Aid Package. NPR, 2020. Available: https://www.npr.org/2020/03/26/821457551/whats-inside-the-senate-s-2-trillion-coronavirus-aid-package2020 [Accessed June 2020].
- 56.3M. get the facts. N95 respirator pricing, 2020. Available: https://multimedia.3m.com/mws/media/1862179O/get-the-facts-n95-respirator-pricing.pdf [Accessed June 2021].
- 57.MSC Industrial Direct Company . 3M series 7500, size L half mask respirator, 2020. Available: https://www.mscdirect.com/product/details/71855167 [Accessed August 2020].
- 58.MSC Industrial Direct Company . Product details 3M p100 filters, series 2000, 2020. Available: https://www.mscdirect.com/product/details/00324533 [Accessed August 2020].
- 59.Chalikonda S, Waltenbaugh H, Angelilli S, et al. Implementation of an elastomeric mask program as a strategy to eliminate disposable N95 mask use and resterilization: results from a large academic medical center. J Am Coll Surg 2020;231:333–8. 10.1016/j.jamcollsurg.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Indiamart . Face mask (3 ply non-woven with ties and full Weld), 2021. Available: https://www.indiamart.com/proddetail/face-mask-3-ply-non-woven-with-ties-and-full-weld-4324828512.html [Accessed March 2021].
- 61.Kortepeter M. Why You’ll Still Need To Wear A Mask Even After Covid-19 Vaccines Arrive Forbes, 2020. Available: https://www.forbes.com/sites/coronavirusfrontlines/2020/10/20/why-youll-still-need-to-wear-a-mask-even-after-covid-19-vaccines-arrive/?sh=600022ab5a42 [Accessed March 2021].
- 62.Dr SJ. Fauci says masks, social distancing will still be needed after a Covid-19 vaccine—here’s why CNBC, 2020. Available: https://www.cnbc.com/2020/11/16/fauci-why-still-need-masks-social-distancing-after-covid-19-vaccine.html [Accessed March 2021].
- 63.BBC News . Covid: Masks and social distancing 'could last years', 2021. Available: https://www.bbc.com/news/uk-56475807 [Accessed March 2021].
- 64.CDC . Recommended guidance for extended use and limited reuse of N95 filtering Facepiece respirators in healthcare settings, 2020. Available: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html [Accessed March 2020].
- 65.Clinical Evidence Assessment . Safety of Extended Use and Reuse of N95 Respirator. ECRI, 2020. Available: https://www.elsevier.com/__data/assets/pdf_file/0006/997863/COVID-ECRI-N95-Respirators_2020-03.pdf [Accessed April 2020].
- 66.Fischer RJ, Morris DH, van Doremalen N, et al. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 virus. Emerg Infect Dis 2020;26:2253–5. 10.3201/eid2609.201524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofheinz E. Environmental Impact of Disposable vs. Reusable Instruments Orthopedics This Week, Spine, 2020. Available: https://ryortho.com/breaking/environmental-impact-of-disposable-vs-reusable-instruments/ [Accessed June 2021].
- 68.Patrício Silva AL, Prata JC, Walker TR, et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem Eng J 2021;405:126683–83. 10.1016/j.cej.2020.126683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robert E. Fischell Institute for biomedical devices. researchers develop rapid deployment mask, University of Maryland, 2020. Available: https://fischellinstitute.umd.edu/news/story/researchers-develop-rapid-deployment-mask [Accessed April 2020].
- 70.U.S. Energy Information Administration . Renewable energy explained: incentives, 2021. Available: https://www.eia.gov/energyexplained/renewable-sources/incentives.php [Accessed March 2020].
- 71.Lobel R, Perakis G. Consumer choice model for forecasting demand and designing incentives for solar technology. SSRN Journal 2011;29. 10.2139/ssrn.1748424 [DOI] [Google Scholar]
- 72.Singh N, Tang Y, Ogunseitan OA. Environmentally sustainable management of used personal protective equipment. Environ Sci Technol 2020;54:8500–2. 10.1021/acs.est.0c03022 [DOI] [PubMed] [Google Scholar]
- 73.United States Census Bureau. U.S. Population . Quick facts, 2019. Available: https://www.census.gov/quickfacts/fact/table/US/PST045219 [Accessed June 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-048687supp001.pdf (48.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data are included in the manuscript and/or supplementary materials, no additional data are available.