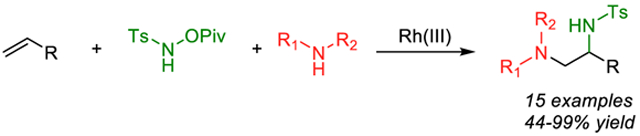
Abstract
We report a three-component diamination of simple unactivated alkenes using an electrophilic nitrene source and amine nucleophiles. The reaction provides rapid access to 1,2-vicinal diamines from terminal alkenes through a one-pot protocol. The transformation proceeds smoothly with excellent tolerance for a broad array of primary and secondary amines, affording the desired product with good yield and regioselectivity. The mechanism is proposed to proceed through a Rh(III)-catalyzed aziridination of alkenes with subsequent ring opening by primary or secondary amines.
Keywords: alkene diamination, Rh(III)-catalysis, One-pot synthesis, nitrene
Graphical Abstract
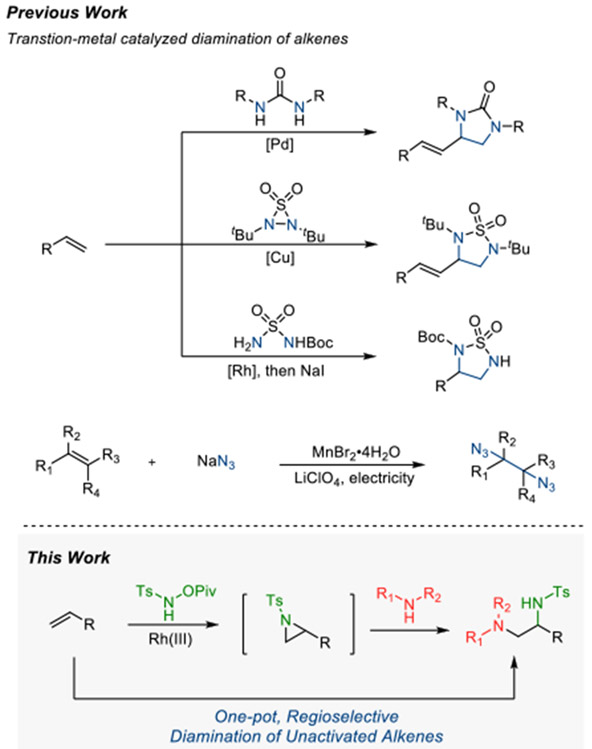
The 1,2-vicinal diamine is a prevalent structural motif in natural products and pharmaceuticals. Moreover, they are commonly utilized as ligands on various metal complexes to catalyze essential organic transformations.1 Owing to the high demand, the development of efficient synthetic strategies for the synthesis of 1,2-diamines has been actively pursued. Several notable synthetic approaches exist including aza-Henry reaction,2 Mannich reaction between α-amino-compounds and imines,3 and the addition of nucleophile into α-amino imines.4 Among the potential synthetic precursors for the synthesis of vicinal amines, alkenes are ideal – they are ubiquitous feedstock materials, and unreactive functionality that is well tolerated in other transformations. Thus, the simultaneous addition of two nitrogen functionalities to the alkene double bond is a straightforward and useful way to access 1,2-vicinal diamines (Scheme 1). However, aside from some prominent successes,5,6 transition-metal-catalyzed 1,2-diamination of alkenes is significantly underdeveloped compared to the much better established dihydroxylation and aminohydroxylation of alkenes. Presumably, this is because 1,2-diamines are generally good ligands and can act as a chelating ligand for the metal complex.
Scheme 1.
Transition metal-catalyzed alkene diaminations
Alternatively, the nucleophilic ring opening of aziridines by amines is an efficient strategy to access diamines from readily available starting materials.7 Recently, we reported a Rh(III)-catalyzed formal [4+1] approach to pyrrolidines from simple unactivated terminal alkenes and nitrene sources.8 Mechanistic investigations led us to propose a Rh-catalyzed intermolecular aziridination with subsequent ring expansion by triflic acid for the synthesis of 5-membered saturated N-heterocycle.
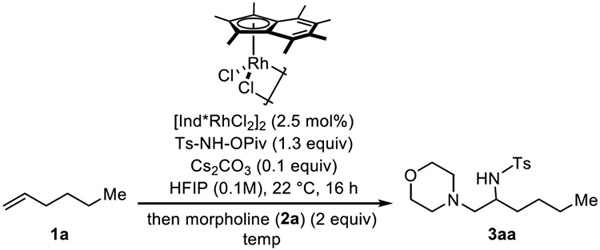
Motivated by the fact that aziridine formation is highly efficient in this system, we envisioned that this intermediate can be utilized for the one-pot synthesis of vicinal diamines in the presence of exogenous nitrogen nucleophiles. In order to test this idea, we added morpholine (2a) as amine nucleophile after the initial aziridination of 1-hexene (1a) and 4-methyl-N-(pivaloyloxy)benzenesulfonamide (Ts-NH-OPiv) in the presence of a catalytic amount of Cs2CO3 (0.1 equiv.) and [Ind*RhCl2]2 catalyst (2.5 mol%) in HFIP.
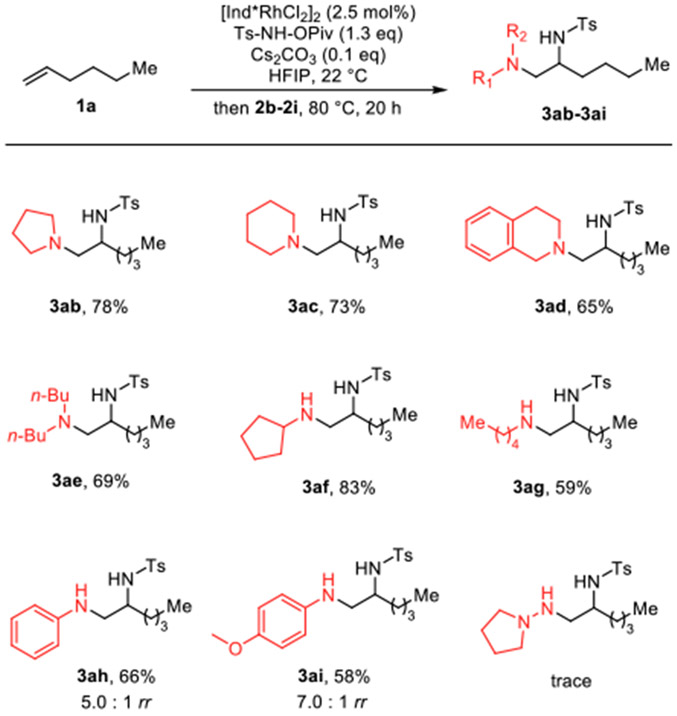
Preliminary results showed that when 2 equiv. of morpholine was added and stirred at room temperature and 40 °C, desired diamination products were formed as a single regioisomer albeit low yield (entry 1, 2). Increasing reaction temperature to 80 °C further improved the reaction yield to 63% (entry 3). Since we observed unreacted aziridine on workup, longer reaction time and higher concentration were applied to complete the reaction, and the yield of 3aa improved to 87% when the reaction was conducted at 80 °C for 16 hours in 0.2 M of HFIP (entry 6). Lewis acid-promoted aziridine ring opening by amines is well-known;7 however, adding AgSbF6 together with morpholine after aziridination gives a lower yield of 3aa (entry 4). Finally, a quantitative yield of the desired product is observed at 0.2 M with 3 equiv. of morpholine (entry 7). Having optimized the reaction conditions, we next sought to explore the scope of this methodology. First, various commercially available primary and secondary amines were examined using 1-hexene as an alkene coupling partner (Scheme 2). Cyclic secondary amines such as pyrrolidine (2b) and piperidine (2c) provide diamination products with good yield (3ab, 3ac). Bicyclic 1,2,3,4-tetrahydroisoquinoline (2d) also works well as a nucleophile giving 65% of corresponding 1,2-diamine (3ad). The reaction with dibutyl amine also delivers 1,2-vicinal diamine product with good yield (3ae). The reaction also proceeds smoothly with primary amines, giving desired products (3af - 3ai) in good yield. When aniline (2h) and 4-methoxyaniline (2i) are used as a nucleophile, the minor regioisomer is also observed in small amounts. Hydrazine-type nucleophile (1-aminopyrrolidine) was also tested but only trace amount of desired product was observed.
Scheme 2.
Amine Nucleophile Substrate Scope a Determined by 1H NMR analysis of unpurified reaction mixture
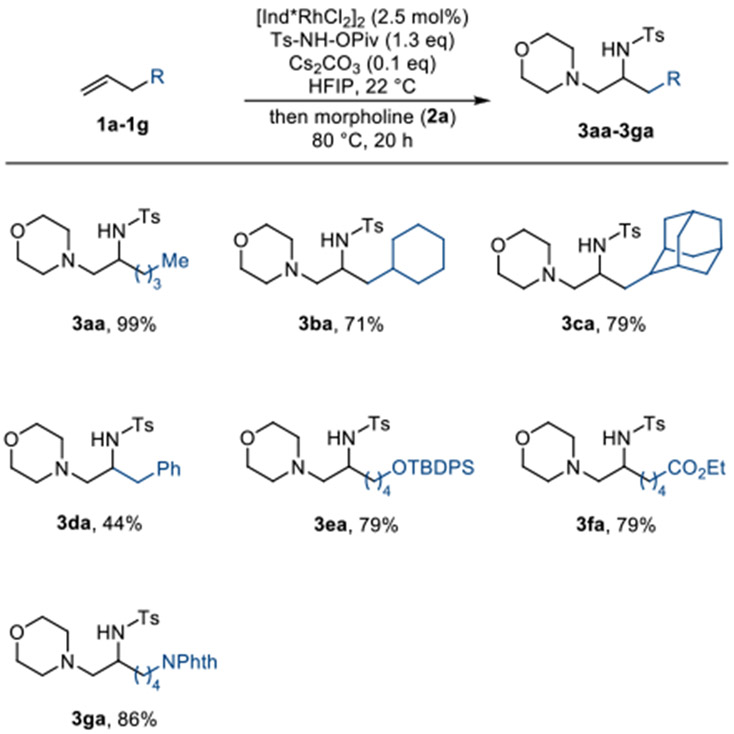
Next, we tested the synthetic utility of this method with a variety of terminal unactivated alkene substrates (Scheme 3). Allyl cyclohexane and 2-allyladamantane are successfully converted to the corresponding diamination products in good yield (3ba, 3ca). A variety of functional groups such as phenyl (3da), tert-butyldiphenylsilyl protected alcohol (3ea), ethyl ester (3fa), and protected amine (3ga) are all well tolerated, giving the desired 1,2-diamination product in good yield.
Scheme 3.
Terminal Alkene Substrate Scope
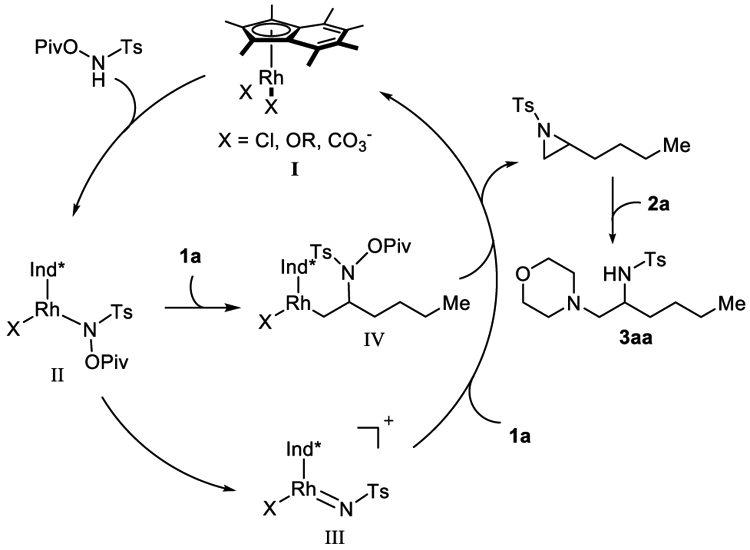
Based on our previous work8 and aziridine ring opening precedent,7 we propose the following mechanism for the reaction. First, [Ind*RhCl2]2 catalyst metalates Ts-NH-OPiv to generate Rh complex II, which undergoes Rh-nitrene formation to yield intermediate III. Subsequent aziridination with an unactivated alkene coupling partner (1a) would give aziridine intermediate with the regeneration of active Rh(III) catalyst. Alternatively, the alkene coupling partner (1a) can coordinate with Rh complex II and undergoes alkene migratory insertion to form complex IV. Subsequent C-N bond formation and N-O bond cleavage would generate aziridine intermediate. Nucleophilic attack of primary and/or secondary amines to sterically less hindered terminal carbon of aziridine produces diamination products at elevated temperature.
In summary, we demonstrate the one-pot synthesis of vicinal diamines from readily available α-olefins through Rh(III)-catalyzed aziridination and subsequent nucleophilic attack by exogenous amines. The reaction exhibits broad functional group tolerance with good yield and regioselectivity.
The experimental section has no title; please leave this line here.
HFIP, Cs2CO3 and 1a, 1b, 1d, 1f, 2a-2i were purchased from Sigma Aldrich and used without further purification. 1c,8 1e,9 1g10 and [Ind*RhCl2]2 catalyst11 were synthesized following literature procedures. 1H and 13C NMR spectra were collected at ambient temperature on Bruker 400 MHz and Bruker Avance III 500 MHz spectrometers. Regioisomeric ratios were measured by integration of 1H NMR spectra of product mixtures prior to purification. Low resolution mass spectra were recorded on a Waters Acquity UPLC-MS or Agilent 5977B GC/MS. Infrared spectra were collected on a Perkin Elmer Spectrum Two FT-IR Spectrometer. Melting point were measured by Stanford Research System MPA160 melting point apparatus.
General Procedures
To a 1 dram vial, [Ind*RhCl2]2 (0.0025 mmol, 2.5 mol%), Ts-NH-OPiv (0.13 mmol, 1.3 equiv) and Cs2CO3 (0.01 mmol, 0.1 equiv) were measured and dissolved in HFIP (0.2 M) and stirred 16 hours at 22 °C. Then, amine (0.3 mmol, 3 equiv) was added to the vial and heated to 80 °C and stirred for additional 16 hours. The mixture was concentrated under reduced pressure and the crude material was purified by flash chromatography either manually on SiliCycle® SilicaFlash® P60 (230-400 mesh) silica gel or automatically using a Teledyne Isco Lumen CombiFlash with RediSep Rf Disposable Flash columns.
4-methyl-N-(1-morpholinohexan-2-yl)benzenesulfonamide (3aa)
Yield: 20.4 mg, 98%, yellow oil.
Rf = 0.35 (EA/Hex 1:1).
1H NMR (500 MHz, CDCl3) δ 7.76 (m, 2H), 7.29 (m, 2H), 3.53 – 3.44 (m, 4H), 3.13 – 3.04 (m, 1H), 2.41 (s, 3H), 2.24 (d, J = 7.4 Hz, 2H), 2.21 – 2.12 (m, 1H), 2.11 – 2.03 (m, 1H), 2.07 (dt, J = 11.0, 4.3 Hz, 2H), 1.67 (ddd, J = 14.4, 9.6, 4.6 Hz, 1H), 1.53 (dddd, J = 14.0, 11.2, 7.3, 4.1 Hz, 1H), 1.29 – 1.18 (m, 3H), 1.17 – 1.08 (m, 1H), 0.84 (t, J = 6.9 Hz, 3H).
13C NMR (126 MHz, CDCl3) δ 143.5, 137.2, 129.7, 127.4, 66.8, 61.1, 53.3, 50.0, 33.1, 26.9, 22.8, 21.6, 14.1.
IR (CDCl3, cm−1) ν 3272, 2954, 2928, 2858, 1598, 1453, 1329, 1302, 1159, 1115, 1092, 815, 665, 550.
LRMS (ESI) m/z [C17/H29N2O3S]+ ([M+H]+ ) calculated 341.2, found 341.1.
4-methyl-N-(1-(pyrrolidin-1-yl)hexan-2-yl)benzenesulfonamide (3ab)
Yield: 25.2 mg, 78%, pale-yellow oil.
Rf = 0.16 (DCM:MeOH 19:1).
1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 8.2 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 5.43 (s, 1H), 3.01 (ddt, J = 9.4, 7.3, 4.6 Hz, 1H), 2.51 (dd, J = 12.3, 9.6 Hz, 1H), 2.41 (s, 3H), 2.32 – 2.21 (m, 3H), 2.16 (td, J = 8.2, 4.4 Hz, 2H), 1.75 – 1.56 (m, 5H), 1.51 (dddd, J = 14.2, 11.3, 7.2, 4.2 Hz, 1H), 1.31 – 1.02 (m, 3H), 0.83 (t, J = 6.9 Hz, 3H).
13C NMR (126 MHz, CDCl3) δ 143.3, 137.3, 129.6, 127.4, 58.3, 54.0, 52.3, 33.2, 27.0, 23.7, 22.8, 21.7, 14.1.
IR (CDCl3, cm−1) ν 3287, 2958, 2928, 1382, 1320, 1159, 1093, 909, 731, 660, 549.
LRMS (ESI) m/z [C17H28N2O2S]+ ([M+H]+) calculated 325.2, found 325.2.
4-methyl-N-(1-(piperidin-1-yl)hexan-2-yl)benzenesulfonamide (3ac)
Yield: 24.8 mg, 73%, yellow oil.
Rf = 0.27 (EA/Hex 1:1).
1H NMR (400 MHz, CDCl3) δ 7.80 – 7.71 (m, 2H), 7.33 – 7.25 (m, 2H), 3.07 (ddt, J = 10.3, 8.2, 4.2 Hz, 1H), 2.41 (s, 3H), 2.28 (dd, J = 12.7, 10.3 Hz, 1H), 2.19 (dd, J = 12.6, 4.7 Hz, 1H), 2.15 – 2.04 (m, 3H), 1.70 (ddt, J = 10.2, 8.8, 4.1, Hz, 1H), 1.56 – 1.46 (m, 1H), 1.45 – 1.32 (m, 6H), 1.32 – 1.16 (m, 6H), 1.15 – 1.05 (m, 1H), 0.84 (t, J = 7.1 Hz, 3H).
13C NMR [101 MHz, CDCl3) δ 143.2, 137.3, 129.6, 127.4, 60.9, 54.2, 50.1, 38.7, 33.2, 27.3, 26.9, 25.7, 24.1, 22.8, 21.6, 14.1.
IR (CDCl3, cm−1) ν 2930, 2859, 1709, 1597, 1553, 1455, 1404, 1332, 1160, 1093, 664, 550.
LRMS (ESI) m/z [C18H31N2O2S]+ ([M+H]+ ) calculated 339.2, found 339.1.
N-(1-(3,4-dihydroisoquinolin-2(1H)-yl)hexan-2-yl)-4-methylbenzenesulfonamide (3ad)
Yield: 25 mg, 65%, yellow solid.
Rf = 0.65 (EA/Hex 1:1).
M.P.= 94 - 98 (°C)
1H NMR (500 MHz, CDCl3) δ 7.73 – 7.67 (m, 2H), 7.22 – 7.17 (m, 2H), 7.17 – 7.04 (m, 3H), 6.78 – 6.73 (m, 1H), 3.28 (d, J = 14.8 Hz, 1H), 3.23 – 3.11 (m, 2H), 2.85 – 2.76 (m, 1H), 2.69 (dt, J = 16.4, 5.4 Hz, 1H), 2.57 (dt, J = 11.2, 5.5 Hz, 1H), 2.50 (ddd, J = 15.3, 8.0, 4.3 Hz, 1H), 2.47 – 2.41 (m, 2H), 2.41 (s, 3H), 1.80 – 1.71 (m, 1H), 1.64 – 1.54 (m, 1H), 1.35 – 1.14 (m, 4H), 0.87 (t, J = 7.0 Hz, 3H).
13C NMR (126 MHz, CDCl3) δ 143.3, 137.0, 134.0, 129.7, 128.7, 127.3, 126.6, 126.4, 125.7, 60.7, 55.5, 51.1, 50.6, 33.2, 29.1, 27.2, 27.0, 22.9, 21.7, 14.1.
IR (CDCl3, cm−1) ν 3276, 2954, 2926, 2867, 1598, 1454, 1328, 1160, 1093, 903, 813, 741, 666, 550.
LRMS (ESI) m/z [C22H31N2O2S]+ ([M+H]+ ) calculated 387.2, found 387.2.
N-(1-(dibutylamino)hexan-2-yl)-4-methylbenzenesulfonamide (3ae)
Yield: 26.4 mg, 69%, pale-yellow oil.
Rf = 0.31 (DCM:MeOH 19:1).
1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.0 Hz, 2H), 5.39 (s, 1H), 2.99 (tt, J = 9.2, 4.6 Hz, 1H), 2.40 (s, 3H), 2.37 – 2.04 (m, 6H), 1.68 (dtd, J = 12.5, 7.2, 6.4, 3.3 Hz, 1H), 1.52 (dddd, J = 14.0, 11.4, 7.3, 4.1, Hz, 1H), 1.34 – 1.18 (m, 6H), 1.13 (m, 6H), 0.90 – 0.80 (m, 9H).
13C NMR (126 MHz, CDCl3) δ 143.3, 137.3, 129.6, 127.5, 57.2, 53.5, 51.1, 32.8, 28.7, 26.9, 22.9, 21.6, 20.7, 14.2, 14.2.
IR (CDCl3, cm−1) ν 3286, 2976, 2863, 1381, 1349, 1118, 1075, 916, 733, 662, 548.
LRMS (ESI) m/z [C21H38N2O2S]+ ([M+H]+) calculated 383.3, found 383.3.
N-(1-(cyclopentylamino)hexan-2-yl)-4-methylbenzenesulfonamide (3af)
Yield: 28 mg, 83%, clear brown oil
Rf = 0.3 (DCM/MeOH 95:5)
1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.1 Hz, 1H), 7.28 (d, J = 8.1 Hz, 1H), 3.16 (p, J = 6.4 Hz, 1H), 2.85 (p, J = 6.5 Hz, 1H), 2.49 (s, 1H), 2.48 (s, 1H), 2.41 (s, 2H), 1.76 – 1.55 (m, 2H), 1.53 – 1.33 (m, 2H), 1.23 – 1.09 (m, 3H), 0.79 (t, J = 6.9 Hz, 1H).
13C NMR (126 MHz, CDCl3) δ 143.3, 138.0, 129.7, 127.3, 59.6, 53.5, 50.9, 33.0, 32.9, 27.7, 24.0, 23.9, 22.6, 21.6, 14.0.
IR (CDCl3, cm−1) ν 3278, 2953, 2930, 2862, 1598, 1454, 1325, 1157, 1092, 905, 814, 723.
LRMS (ESI) m/z [C18H31N2O2S]+ ([M+H]+ ) calculated 339.2, found 339.2
4-methyl-N-(1-(pentylamino)hexan-2-yl)benzenesulfonamide (3ag)
Yield: 20 mg, 59%, beige solid.
Rf = 0.2 (DCM/MeOH 95:5)
M.P.= 59 - 63 (°C)
1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 8.0 Hz, 2H), 3.20 – 3.10 (m, 1H), 2.49 – 2.45 (m, 2H), 2.42 (s, 3H), 2.40 – 2.26 (m, 2H), 1.52 – 1.37 (m, 2H), 1.36 – 1.26 (m, 4H), 1.24 – 1.12 (m, 6H), 0.89 (t, J = 7.2 Hz, 3H), 0.81 (t, J = 6.8 Hz, 3H).
13C NMR (126 MHz, CDCl3) δ 143.3, 138.0, 129.7, 127.3, 53.3, 52.4, 49.8, 33.4, 29.9, 29.5, 27.6, 22.7, 22.6, 21.6, 14.2, 14.0.
IR (CDCl3, cm−1) ν 3278, 2955, 2926, 2857, 1459, 1326, 1159, 1093, 814, 664.
LRMS (ESI) m/z [C18H31N2O2S]+ ([M+H]+ ) calculated 341.2, found 341.2
4-methyl-N-(1-(phenylamino)hexan-2-yl)benzenesulfonamide (3ah)
Yield: 23.0 mg, 66%, 5.0:1 rr, yellow oil.
Rf = 0.30 (EA/Hex 1:4).
Major isomer: 1H NMR [500 MHz, CDCl3) δ 7.80 – 7.76 (m, 2H), 7.31 – 7.26 (m, 2H), 7.18 – 7.13 (m, 2H), 6.76 – 6.69 (m, 1H), 6.52 – 6.46 (m, 2H), 4.84 (d, J = 7.9 Hz, 1H), 3.45 – 3.36 (m, 1H), 3.17 (dd, J = 12.9, 4.7 Hz, 1H), 3.06 (dd, J = 12.9, 7.3 Hz, 1H), 2.44 (s, 3H), 1.55 – 1.47 (m, 1H), 1.45 – 1.38 (m, 1H), 1.22 – 1.06 (m, 4H), 0.79 (t, J = 7.0 Hz, 3H).
Major isomer: 13C NMR (126 MHz, CDCl3) δ 147.7, 143.5, 137.6, 129.7, 129.2, 127.2, 117.7, 112.9, 53.5, 48.1, 33.3, 27.6, 22.4, 21.5, 13.8.
IR (CDCl3, cm−1) ν 3400, 3280, 2954, 2930, 2861, 1602, 1508, 1322, 1157, 1091, 749, 665, 550.
LRMS (ESI) m/z [C19H27N2O2S]+ ([M+H]+ ) calculated 347.2, found 347.1.
N-(1-((4-methoxyphenyl)amino)hexan-2-yl)-4-methylbenzenesulfonamide (3ai)
Yield: 22.0 mg, 58%, 7.0:1 rr, yellow oil.
Rf = 0.77 (EA/Hex 1:1).
Major isomer:
1H NMR (500 MHz, CDCl3) δ 7.76 – 7.72 (m, 2H), 7.28 – 7.24 (m, 2H), 6.78 – 6.70 (m, 2H), 6.46 – 6.41 (m, 2H), 4.79 (d, J = 7.8 Hz, 1H), 3.74 (s, 4H), 3.40 – 3.32 (m, 1H), 3.09 (dd, J = 12.8, 4.6 Hz, 1H), 2.97 (dd, J = 12.8, 7.2 Hz, 1H), 2.42 (s, 3H), 1.53 – 1.33 (m, 2H), 1.28 – 1.20 (m, 1H), 1.20 – 1.02 (m, 4H), 0.77 (t, J = 7.0 Hz, 3H).
Major isomer:
13C NMR (126 MHz, CDCl3) δ 152.5, 144.4, 142.0, 137.8, 129.8, 127.3, 114.9, 113.8, 55.9, 52.8, 50.1, 35.1, 27.7, 22.5, 20.2, 12.3.
IR (CDCl3, cm−1) ν 3278, 2953 2930, 2860, 1512, 1463, 1322, 1236, 1157, 1091, 1037, 903, 817, 725, 664.
LRMS (ESI) m/z [C20H29N2O3S]+ ([M+H]+ ) calculated 377.2, found 377.2.
N-(1-cyclohexyl-3-morpholinopropan-2-yl)-4-methylbenzenesulfonamide (3ba)
Yield: 26 mg, 71%, colorless oil
Rf = 0.30 (EA/Hex 6:4)
1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 5.21 (bs, 1H), 3.54 – 3.45 (m, 4H), 3.15 (ddt, J = 9.9, 8.1, 5.1 Hz, 1H), 2.42 (s, 3H), 2.29 (dd, J = 12.7, 5.0 Hz, 1H), 2.22 – 2.15 (m, 3H), 2.07 (m, 2H), 1.76 – 1.48 (m, 4H), 1.34 – 1.07 (m, 5H), 0.95 – 0.79 (m, 3H).
13C NMR (126 MHz, CDCl3) δ 143.5, 137.2, 129.7, 127.4, 66.9, 61.9, 53.5, 48.1, 41.7, 34.0, 34.0, 33.1, 29.8, 26.5, 26.2, 21.6.
IR (CDCl3, cm−1) ν 3273, 2921, 2851, 1598, 1448, 1327, 1160, 1117, 1072, 1009, 815.
LRMS (ESI) m/z [C20H33N2O3S]+ ([M+H]+ ) calculated 381.2, found 381.2
N-((1-adamantan-2-yl)-3-morpholinopropan-2-yl)-4-methylbenzenesulfonamide (3ca)
Yield: 34 mg, 79%, colorless oil
Rf = 0.4 (EA/Hex 6:4)
1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.1 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 3.51 (t, J = 4.7 Hz, 4H), 3.10 (ddt, J = 9.8, 8.2, 5.0 Hz, 1H), 2.41 (s, 3H), 2.30 (dd, J = 12.7, 5.1 Hz, 1H), 2.26 – 2.15 (m, 3H), 2.10 (dt, J = 11.2, 4.6 Hz, 2H), 2.01 (ddd, J = 13.7, 8.3, 4.9 Hz, 1H), 1.91 (dd, J = 12.6, 2.8 Hz, 1H), 1.79 (tdt, J = 14.5, 5.4, 3.0 Hz, 5H), 1.70 (d, J = 3.3 Hz, 2H), 1.66 – 1.41 (m, 9H).
13C NMR (126 MHz, CDCl3) δ 143.5, 137.2, 129.7, 127.4, 66.9, 61.8, 53.5, 48.7, 40.4, 39.2, 38.3, 37.1, 32.8, 31.6, 31.6, 31.5, 28.2, 28.0, 21.6.
IR (CDCl3, cm−1) ν 3270, 2904, 2851, 1452, 1402, 1329, 1160, 1116, 1092, 906, 727.
LRMS (ESI) m/z [C24H37N2O3S]+ ([M+H]+) calculated 433.3, found 433.3
4-methyl-N-(1-morpholino-3-phenylpropan-2-yl)benzenesulfonamide (3da)
Yield: 16.4 mg, 44%, yellow oil.
Rf = 0.36 (EA/Hex 1:1).
1H NMR [500 MHz, CDCl3) δ 7.86 – 7.74 (m, 2H), 7.33 – 7.29 (m, 2H), 7.28 – 7.23 [m, 2H), 7.23 – 7.18 (m, 1H), 7.17 – 7.11 (m, 2H), 3.51 – 3.40 (m, 4H), 3.34 – 3.24 (m, 1H), 3.17 (dd, J = 13.7, 3.8 Hz, 1H), 2.79 (dd, J = 13.7, 8.1, Hz, 1H), 2.42 (s, 3H), 2.24 – 2.13 (m, 2H), 2.13 – 2.02 (m, 2H), 2.02 – 1.92 (m, 2H).
13C NMR (126 MHz, CDCl3) δ 143.67, 137.06, 136.76, 129.83, 129.80, 128.53, 127.39, 126.70, 66.80, 60.39, 53.15, 50.95, 39.72, 21.64. ff
IR (CDCl3, cm−1) ν 3261, 3060, 3027, 2922, 2854, 2814, 1598, 1453, 1332, 1160, 1115, 1089, 702, 665, 551.
LRMS (ESI) m/z [C20H27N2O3S]+ ([M+H]+ ) calculated 375.2, found 375.2.
N-(6-((tert-butyldiphenylsilyl)oxy)-1-morpholinohexan-2-yl)-4-methylbenzenesulfonamide (3ea)
Yield: 47.1 mg, 79%, colorless oil.
Rf = 0.40 (EA/Hex 2:3).
1H NMR (500 MHz, CDCl3) δ 7.81 – 7.75 (m, 2H), 7.72 – 7.64 (m, 4H), 7.48 – 7.37 (m, 6H), 7.33 – 7.26 (m, 2H), 5.28 (s, 1H), 3.70 – 3.59 (m, 2H), 3.58 – 3.47 (m, 4H), 3.11 (qd, J = 7.4, 4.1 Hz, 1H), 2.42 (s, 3H), 2.25 (d, J = 7.3 Hz, 2H), 2.22 – 2.14 (m, 2H), 2.14 – 2.05 (m, 2H), 1.77 – 1.68 (m, 1H), 1.60 – 1.48 (m, 3H), 1.41 – 1.27 (m, 2H), 1.07 (s, 9H).
13C NMR (126 MHz, CDCl3) δ 143.4, 137.0, 135.6, 134.0, 129.6, 127.6, 127.2, 66.7, 63.6, 60.9, 53.2, 49.9, 33.0, 32.5, 26.9, 21.5, 21.0, 19.3.
IR (CDCl3, cm−1) ν 3267, 2930, 2857, 1427, 1330, 1160, 1109, 1093, 816, 703, 550, 504.
LRMS (ESI) m/z [C33H47N2O4SSi]+ ([M+H]+ ) calculated 595.3, found 595.3.
ethyl 6-((4-methylphenyl)sulfonamido)-7-morpholinoheptanoate (3fa)
Yield: 32.5 mg, 79%, yellow oil.
Rf = 0.43 (DCM:MeOH 19:1).
1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 4.12 (q, J = 7.1 Hz, 2H), 3.50 (dt, J = 12.3, 6.1 Hz, 4H), 3.09 (qd, J = 7.1, 4.6 Hz, 1H), 2.42 (s, 3H), 2.29 – 2.21 (m, 4H), 2.11 (m, 4H), 1.74 – 1.50 (m, 4H), 1.35 (m, 1H), 1.25 (t, J = 7.1 Hz, 3H), 1.21 (m, 1H).
13C NMR (126 MHz, CDCl3) δ 173.6, 143.5, 137.0, 129.7, 127.3, 66.7, 60.9, 60.3, 53.3, 49.7, 34.1, 32.9, 24.9, 24.1, 21.6, 14.3.
IR (CDCl3, cm−1) ν 3268, 2930, 2858, 1729, 1329, 1302, 1158, 1116, 1092, 666, 551.
LRMS (ESI) m/z [C20H32N2O5S]+ ([M+H]+) calculated 413.2, found 413.2.
N-(6-(1,3-dioxoisoindolin-2-yl)-1-morpholinohexan-2-yl)-4-methylbenzenesulfonamide (3ga)
Yield: 42.0 mg, 86%, yellow oil.
Rf = 0.25 (EA/Hex 2:1).
1H NMR (400 MHz, CDCl3) δ 7.88 – 7.83 (m, 2H), 7.79 – 7.71 (m, 4H), 7.32 – 7.27 (m, 2H), 5.13 (brs, 1H), 3.70 – 3.60 (m, 2H), 3.56 – 3.46 (m, 4H), 3.17 – 3.08 (m, 1H), 2.42 (s, 3H), 2.28 – 2.14 (m, 4H), 2.14 – 2.05 (m, 2H), 1.76 – 1.67 (m, 1H), 1.66 – 1.56 (m, 3H), 1.40 – 1.20 (m, 2H).
13C NMR (101 MHz, CDCl3) δ 168.4, 143.4, 137.1, 133.9, 132.1, 129.6, 127.2, 123.2, 66.7, 61.0, 53.2, 49.7, 37.6, 32.6, 28.5, 21.8, 21.5.
IR (CDCl3, cm−1) ν 3274, 2938, 2859, 2814, 1708, 1397, 1331, 1159, 1116, 721.
LRMS (ESI) m/z [C25H32N3O5S]+ ([M+H]+) calculated 486.2, found 486.1.
Supplementary Material
Scheme 4.
Proposed Mechanism
Table 1.
Optimization Table
 | |||||
|---|---|---|---|---|---|
| entry | conc. (M) | morpholine (equiv) | temp.(°C) | reaction time (h) | yield (%)a |
| 1 | 0.1 | 2 | 22 | 8 | < 5 |
| 2 | 0.1 | 2 | 40 | 8 | 19 |
| 3 | 0.1 | 2 | 80 | 8 | 63 |
| 4b | 0.1 | 2 | 80 | 8 | 28 |
| 5 | 0.1 | 2 | 80 | 20 | 78 |
| 6 | 0.2 | 2 | 80 | 20 | 87 |
| 7 | 0.2 | 3 | 80 | 24 | 99 |
Determined by 1H NMR analysis of unpurified reaction mixture
AgSbF6 (1 equiv.) was added.
Acknowledgments
Funding Information
We thank the National Institute of General Medical Sciences (NIGMS, Grant No. GM80442) for support.
Footnotes
Supporting Information
YES (this text will be updated with links prior to publication)
Primary Data
NO (this text will be deleted prior to publication)
Dedicated to Professor Mark Lautens on the occasion of his 70th birthday
Reference
- (1) (a).Saibabu Kotti SRS; Timmons C; Li G Chem. Biol. Drug Des 2006, 67, 101. [DOI] [PubMed] [Google Scholar]; (b) Lucet D; Le Gall T; Mioskowski C Angew. Chem. Int. Ed 1998, 37, 2580. [DOI] [PubMed] [Google Scholar]; (c) Cardona F; Goti A Nat. Chem 2009, 1, 269. [DOI] [PubMed] [Google Scholar]
- (2) (a).Handa S; Gnanadesikan V; Matsunaga S; Shibasaki MJ Am. Chem. Soc 2010, 132, 4925. [DOI] [PubMed] [Google Scholar]; (b) Rampalakos C; Wulff WD Adv. Synth. Catal 2008, 350, 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Anderson JC; Howell GP; Lawrence RM; Wilson CS J. Org. Chem 2005, 70, 5665. [DOI] [PubMed] [Google Scholar]
- (3) (a).Bandar JS; Lambert TH J. Am. Chem. Soc 2013, 135, 11799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kano T; Sakamoto R; Akakura M; Maruoka KJ Am. Chem. Soc 2012, 134, 7516. [DOI] [PubMed] [Google Scholar]
- (4).Reetz MT; Jaeger R; Drewlies R; Hübel M Angew. Chem. Int. Ed 1991, 30, 103. [Google Scholar]
- (5) (a).Chong AO; Oshima K; Sharpless KB J. Am. Chem. Soc 1977, 99, 3420. [Google Scholar]; (b) Bäckvall J-E Tetrahedron Lett. 1978, 19, 163. [Google Scholar]; (c) Becker PN; White MA; Bergman RG J. Am. Chem. Soc 1980, 102, 5676. [Google Scholar]
- (6) (a).Streuff J; Hövelmann CH; Nieger M; Muñiz K J. Am. Chem. Soc 2005, 127, 14586. [DOI] [PubMed] [Google Scholar]; (b) Bar GLJ; Lloyd-Jones GC; Booker-Milburn KI J. Am. Chem. Soc 2005, 127, 7308. [DOI] [PubMed] [Google Scholar]; (c) Du H; Zhao B; Shi Y J. Am. Chem. Soc 2007, 129, 762. [DOI] [PubMed] [Google Scholar]; (d) Khoder ZM; Wong CE; Chemler SR ACS Catal. 2017, 7, 4775. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Olson DE; Su JY; Roberts DA; Du Bois J J. Am. Chem. Soc 2014, 136, 13506. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Fu N; Sauer GS; Saha A; Loo A; Lin S Science 2017, 357, 575. [DOI] [PubMed] [Google Scholar]
- (7) (a).McCoull W; Davis FA Synthesis 2000, 1347. [Google Scholar]; (b) Hu XE Tetrahedron 2004, 60, 2701. [Google Scholar]; (b) Pineschi M Eur. J. Org. Chem 2006, 2006, 4979. [Google Scholar]; (c) Schneider C Angew. Chem. Int. Ed 2009, 48, 2082. [DOI] [PubMed] [Google Scholar]
- (8).Lee S; Lei H; Rovis T J. Am. Chem. Soc 2019, 141, 12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Itoh T; Matsueda T; Shimizu Y; Kanai M Chem.– A Eur. J 2015, 21, 15955. [DOI] [PubMed] [Google Scholar]
- (10).Kitagawa T; Nishino J; Inomata T; Ozawa T; Funahashi Y; Masuda H Chem. Commun 2016, 52, 4780. [DOI] [PubMed] [Google Scholar]
- (11).Semakul N; Jackson KE; Paton RS; Rovis T Chem. Sci 2017, 8, 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.