Abstract
Video Abstract
BACKGROUND AND OBJECTIVES
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network recently proposed new, severity-based diagnostic criteria for bronchopulmonary dysplasia (BPD). This study provides the first benchmark epidemiological data applying this definition.
METHODS
Retrospective cohort study of infants born from 22 to 29 weeks’ gestation in 2018 at 715 US hospitals in the Vermont Oxford Network. Rates of BPD, major neonatal morbidities, and common respiratory therapies, stratified by BPD severity, were determined.
RESULTS
Among 24 896 infants, 2574 (10.3%) died before 36 weeks’ postmenstrual age (PMA), 12 198 (49.0%) did not develop BPD, 9192 (36.9%) developed grade 1 or 2 BPD, and 932 (3.7%) developed grade 3 BPD. Rates of mortality before 36 weeks’ PMA and grade 3 BPD decreased from 52.7% and 9.9%, respectively, among infants born at 22 weeks’ gestation to 17.3% and 0.8% among infants born at 29 weeks’ gestation. Grade 1 or 2 BPD peaked in incidence (51.8%) among infants born at 25 weeks’ gestation. The frequency of severe intraventricular hemorrhage or cystic periventricular leukomalacia increased from 4.8% among survivors without BPD to 23.4% among survivors with grade 3 BPD. Similar ranges were observed for late onset sepsis (4.8%–31.4%), surgically treated necrotizing enterocolitis (1.4%–17.1%), severe retinopathy of prematurity (1.2%–23.0%), and home oxygen therapy (2.0%–67.5%).
CONCLUSIONS
More than one-half of very preterm infants born in the United States died before 36 weeks’ PMA or developed BPD. Greater BPD severity was associated with more frequent development of major neonatal morbidities, in-hospital mortality, and use of supplemental respiratory support at discharge.
What’s Known on This Subject:
Bronchopulmonary dysplasia (BPD) is among the most consequential morbidities associated with prematurity. The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network recently proposed new, severity-graded diagnostic criteria for BPD; benchmark epidemiological data applying this definition are needed.
What This Study Adds:
Among 24 896 very preterm infants, 10.3% died before 36 weeks postmenstrual age, 36.9% developed grade 1 to 2 BPD, and 3.7% developed grade 3 BPD. The rates of late in-hospital death, neonatal morbidity, and supplemental respiratory support at discharge increased with BPD severity.
Bronchopulmonary dysplasia (BPD) is among the most common and consequential complications of very preterm birth.1–6 Unlike most major neonatal morbidities, however, the diagnostic criteria used to define BPD continue to evolve.7–11 These changes have largely been driven by interval advances in newborn medicine, improved survival among very preterm infants, and changes in the pathophysiology and epidemiology of neonatal respiratory disease.9,12–14 Owing to several limitations of the diagnostic criteria for BPD, investigators from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network proposed a new, evidence-based definition of BPD in 2019.2 Unlike previous definitions, these criteria categorized BPD severity according to the mode of respiratory support administered to very preterm infants at 36 weeks’ postmenstrual age (PMA), irrespective of the use or level of oxygen therapy.2 This new definition, relative to other common diagnostic criteria, was shown to better discriminate between infants who did, compared with did not, develop poor respiratory and neurodevelopmental outcomes in early childhood.2 As of yet, the epidemiology of BPD as defined by these criteria has not been examined at a population level. Such data may further inform the utility and application of these diagnostic criteria.
In this study, we applied the 2019 Neonatal Research Network definition of BPD to a recent cohort of very preterm infants cared for at hospitals in the United States that participate in the Vermont Oxford Network (VON). The VON database encompasses 85% of all very preterm infants born in the United States. Using this large data set, we described the frequency of BPD as well as associated clinical characteristics and in-hospital outcomes among US-born very preterm infants, stratified by BPD severity level. Our goal was to generate a resource for contemporary, benchmark data on the epidemiology of BPD defined according to these new diagnostic criteria.
Methods
Population
The VON is a voluntary, worldwide community of practice dedicated to improving the quality, safety, and value of care through a coordinated program of data-driven quality improvement, education, and research. In this retrospective analysis, we used data collected prospectively on infants of 22 + 0/7 to 29 + 6/7 weeks’ gestation who were either delivered at a VON member hospital or transferred there within 28 days after birth. All study infants were born in the United States between January 1 and December 31, 2018. Those with a known severe congenital anomaly or genetic syndrome, those who died in the delivery room or within 12 hours of admission to the NICU, and those with missing or implausible values for key study covariates were excluded.
Local staff at each member institution submitted data using uniform definitions for each infant until death, discharge from the reporting hospital, or transfer to another center.15 All data underwent automated checks for quality and completeness at the time of submission. The University of Vermont Committee on Human Research determined that the use of the VON database for this analysis was not human subjects research. All study data were deidentified.
Measures
Infants were categorized by BPD severity according to the highest mode of respiratory support administered at a PMA of 36 + 0/7 weeks by using the diagnostic criteria proposed in 2019 by the NICHD Neonatal Research Network.2 Infants receiving no supplemental respiratory support were classified as no BPD, those treated with nasal cannula (any flow rate) or noninvasive positive airway pressure as grade 1 or 2 BPD, and those treated with invasive mechanical ventilation as grade 3 BPD.2 Grades 1 and 2 BPD were combined into a single severity level because the study data source did not include information on nasal cannula flow rates administered at 36 weeks’ PMA. For infants discharged from the hospital before 36 weeks’ PMA, BPD severity was categorized on the basis of the respiratory support administered at discharge.2
Small for gestational age was calculated as a birth weight <10% for gestational age and sex based on the Fenton growth curves.16 Endotracheal tube ventilation performed during the initial resuscitation immediately after birth only included infants who received assisted ventilation and excluded those for whom the endotracheal tube was placed solely for suctioning. Invasive ventilation after initial resuscitation included conventional or high frequency modes administered by endotracheal tube at any time after leaving the delivery room or initial resuscitation area. Medications and surfactant therapy were reported if administered at least once. Vitamin A only included intramuscular administration. Systemic corticosteroid therapy was only counted if used after birth to prevent or treat BPD or chronic lung disease. Infections required identification using blood or a cerebral spinal fluid culture. Early onset bacterial infection occurred on or before day 3 after birth. Late onset bacterial or fungal infection occurred after day 3. Cultures obtained after day 3 that were positive for coagulase-negative Staphylococcus were counted if accompanied by at least 1 sign of generalized infection and treatment with at least 5 days of intravenous antibiotics. Severe brain injury was defined as the presence of a grade 3 or 4 intraventricular or periventricular hemorrhage and/or cystic periventricular leukomalacia diagnosed on cranial imaging.17 Reported treatments for retinopathy of prematurity (ROP) were retinal cryosurgery, laser surgery, or antivascular endothelial growth factor drug therapy (eg, bevacizumab) to one or both eyes. Monitor use at discharge included an apnea or cardiorespiratory monitor (eg, pulse oximeter). Length of stay was measured as the number of days from the date of admission until the date of hospital discharge.
Analyses
Means with SDs or medians with interquartile ranges were used to summarize continuous study data. The numbers of infants and proportions were used to summarize categorical data. The initial demographic data and BPD rates were summarized for all eligible infants. Values describing clinical therapies, comorbidities, and in-hospital outcomes were reported for infants who survived to discharge from the hospital or remained hospitalized at 1 year of age. All analyses were conducted by using R statistical software version 3.5.3 (R Core Team, Vienna, Austria).
Results
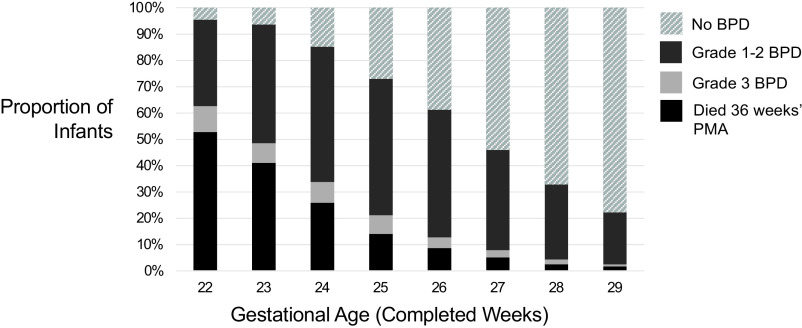
After exclusion of ineligible infants, the study cohort consisted of 24 896 very preterm infants born at 715 US hospitals (Supplemental Fig 2). Of these infants, 2574 (10.3%) died before 36 weeks’ PMA, 12 198 (49.0%) were classified as no BPD, 9192 (36.9%) developed grade 1 or 2 BPD, and 932 (3.7%) developed grade 3 BPD. Table 1 shows the demographic and antenatal characteristics of the study cohort, stratified by BPD severity. On average, the infants who developed a higher severity of BPD were born less mature, at lower birth weights, and more often small for gestational age. When analyzed by completed weeks’ gestation, rates of mortality before 36 weeks’ PMA and of grade 3 BPD were inversely related to the gestational age at birth (Table 1, Fig 1). The proportion of infants who survived to 36 weeks’ PMA and did not develop BPD of any severity increased with gestational age from 4.5% among infants born at 22 weeks’ gestation to 77.8% among those born at 29 weeks’ gestation.
TABLE 1.
Demographic and Antenatal Characteristics
| Characteristic | All Infants (N = 24 896) | No BPD (n = 12 198) | Grade 1–2 BPD (n = 9192) | Grade 3 BPD (n = 932) | Died Before 36 wk PMA (n = 2574) |
|---|---|---|---|---|---|
| Gestational age, wk | |||||
| Mean ± SD | 27.1 ± 2 | 28 ± 1.5 | 26.5 ± 1.8 | 25.7 ± 1.8 | 25 ± 1.8 |
| 22 + 0/7 to 22 + 6/7, n (%) | 313 | 14 (4.5) | 103 (32.9) | 31 (9.9) | 165 (52.7) |
| 23 + 0/7 to 23 + 6/7, n (%) | 1580 | 100 (6.3) | 713 (45.1) | 118 (7.5) | 649 (41.1) |
| 24 + 0/7 to 24 + 6/7, n (%) | 2474 | 365 (14.8) | 1273 (51.5) | 195 (7.9) | 641 (25.9) |
| 25 + 0/7 to 25 + 6/7, n (%) | 2967 | 802 (27.0) | 1538 (51.8) | 210 (7.1) | 417 (14.1) |
| 26 + 0/7 to 26 + 6/7, n (%) | 3292 | 1274 (38.7) | 1596 (48.5) | 136 (4.1) | 286 (8.7) |
| 27 + 0/7 to 27 + 6/7, n (%) | 3940 | 2127 (54.0) | 1503 (38.1) | 110 (2.8) | 200 (5.1) |
| 28 + 0/7 to 28 + 6/7, n (%) | 4853 | 3256 (67.1) | 1387 (28.6) | 89 (1.8) | 121 (2.5) |
| 29 + 0/7 to 29 + 6/7, n (%) | 5477 | 4260 (77.8) | 1079 (19.7) | 43 (0.8) | 95 (1.7) |
| Birth wt, mean ± SD, g | 964 ± 304 | 1112 ± 269 | 866 ± 263 | 738 ± 229 | 691 ± 226 |
| Small for gestational age,a n (%) | 2294 (9.2) | 482 (4.0) | 1102 (12.0) | 199 (21.5) | 511 (20.3) |
| Male sex, n (%) | 12 965 (52.1) | 5953 (48.8) | 4988 (54.3) | 539 (57.8) | 1485 (57.7) |
| Multiple gestation, n (%) | 5660 (22.7) | 2859 (23.4) | 1984 (21.6) | 203 (21.8) | 614 (23.9) |
| Maternal race and/or ethnicity, n (%) | |||||
| Black non-Hispanic | 7966 (32.0) | 4046 (33.5) | 2692 (29.5) | 366 (39.6) | 862 (33.9) |
| White non-Hispanic | 9860 (39.6) | 4639 (38.4) | 3836 (42.1) | 364 (39.4) | 1021 (40.2) |
| Hispanic | 4913 (19.7) | 2435 (20.1) | 1889 (20.7) | 131 (14.2) | 458 (18) |
| Other | 1399 (5.6) | 691 (5.7) | 521 (5.7) | 36 (3.9) | 151 (5.9) |
| Antenatal steroids, n (%) | 21 848 (88.8) | 10 787 (88.8) | 8171 (89.3) | 823 (89.0) | 2067 (80.8) |
| Vaginal delivery, n (%) | 7260 (29.2) | 3726 (30.6) | 2518 (27.4) | 235 (25.2) | 781 (30.4) |
All percentage values were calculated among infants with complete data for the described variable. Data shown in the table were missing in <1% of study infants.
Small for gestational age was defined as a birth wt for gestational age and sex <10% per the Fenton growth curves.16
FIGURE 1.
Rates of death before 36 weeks’ PMA and of BPD, stratified by BPD severity and completed weeks’ gestation.
Of the 22 321 infants who survived to 36 weeks’ PMA and were classified according to BPD severity, 191 (0.9%) died before hospital discharge. Among these late deaths, 119 (62.3%) were diagnosed with grade 3 BPD, 66 (34.6%) with grade 1 or 2 BPD, and 6 (3.1%) with no BPD. In the cohort restricted to infants who survived to discharge, rates of endotracheal tube placement for ventilation during initial resuscitation and treatment with invasive mechanical ventilation at any point after stabilization increased in an incremental, stepwise manner with greater BPD severity (Table 2). A similar trend was observed for the evaluated drug therapies, with the exception of caffeine. Most infants (96.1%) received caffeine at some point during their hospitalization, with minimal variation in the rates of use by BPD severity level (Table 2).
TABLE 2.
Delivery Room and Respiratory Focused Therapies Among Survivors to Discharge
| Therapy | All Infants (N = 22 131) | No BPD (n = 12 192) | Grade 1–2 BPD (n = 9126) | Grade 3 BPD (n = 813) |
|---|---|---|---|---|
| Endotracheal tube ventilation during initial resuscitation | 11 124 (50.3) | 4544 (37.3) | 5923 (65.0) | 657 (81.0) |
| Epinephrine or cardiac compressions during initial resuscitation | 316 (1.4) | 104 (0.9) | 183 (2.0) | 29 (3.6) |
| Invasive ventilation after initial resuscitation | 15 255 (68.9) | 6550 (53.7) | 7892 (86.5) | 813 (100) |
| Surfactant | 15 930 (72.0) | 7321 (60.1) | 7850 (86.1) | 759 (93.5) |
| Inhaled nitric oxide | 1087 (4.9) | 145 (1.2) | 708 (7.8) | 234 (28.9) |
| Systemic corticosteroids for chronic lung disease | 4009 (18.1) | 456 (3.7) | 3033 (33.2) | 520 (64.0) |
| Caffeine | 21 246 (96.1) | 11 502 (94.4) | 8952 (98.2) | 792 (97.4) |
| Intramuscular Vitamin A | 1772 (8.0) | 663 (5.4) | 1013 (11.1) | 96 (11.8) |
Data shown in the table are number of infants (%). All percentage values were calculated among infants with complete data for the described variable. Data shown in the table were missing in <1% of study infants.
The frequency of all evaluated major neonatal morbidities increased in a stepwise manner as BPD severity increased (Table 3). In most cases, these adverse outcomes were more than twice as common among those who developed grade 3 BPD as those who developed grade 1 or 2 BPD and >4 times more common among those with grade 3 BPD as those with no BPD (Table 3). Hospital lengths of stay and use of durable medical equipment at discharge varied by BPD severity level (Table 4). Few infants (2.0%) without BPD were treated with supplemental oxygen at discharge, whereas 43.3% of those with grade 1 or 2 BPD and 67.5% of those with grade 3 BPD received oxygen therapy at the time of discharge from a VON hospital. Although only 1% of all infants in the study cohort underwent tracheostomy, 18.3% of those who developed grade 3 BPD received this intervention.
TABLE 3.
Major Nonrespiratory Morbidities Among Survivors to Discharge
| Outcome | All Infants (N = 22 131) | No BPD (n = 12 192) | Grade 1–2 BPD (n = 9126) | Grade 3 BPD (n = 813) |
|---|---|---|---|---|
| Early onset bacterial sepsis or meningitisa | 279 (1.3) | 134 (1.1) | 125 (1.4) | 20 (2.5) |
| Late onset bacterial or fungal sepsis or meningitisa | 2209 (10.0) | 582 (4.8) | 1372 (15.0) | 255 (31.4) |
| Severe brain injury on cranial imagingb | 1934 (8.9) | 569 (4.8) | 1177 (13.1) | 188 (23.4) |
| Surgical or interventional closure of a PDA | 1122 (5.1) | 96 (0.8) | 859 (9.4) | 167 (20.6) |
| Surgery for confirmed or suspected NEC or bowel perforation | 786 (3.6) | 175 (1.4) | 472 (5.2) | 139 (17.1) |
| Surgery or anti-VEGF therapy for ROP | 1210 (5.5) | 141 (1.2) | 882 (9.7) | 187 (23.0) |
Data shown in the table are number of infants (%). All percentage values were calculated among infants with complete data for the described variable. Data shown in the table were missing in <1% of study infants, except for severe brain injury on cranial imaging, which was missing for 2.1% of the cohort. NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; VEGF, vascular endothelial growth factor.
Early onset infection occurred on or before day 3 after birth; late onset infection occurred after day 3. All infections were identified by using a culture of blood or cerebral spinal fluid.
Defined as a grade 3 or 4 intraventricular or periventricular hemorrhage and/or cystic periventricular leukomalacia.
TABLE 4.
In-Hospital Outcomes Among Survivors to Discharge
| Outcome | All Infants (N = 22 131) | No BPD (n = 12 192) | Grade 1–2 BPD (n = 9126) | Grade 3 BPD (n = 813) |
|---|---|---|---|---|
| Supplemental oxygen at discharge, n (%) | 4238 (20.4) | 236 (2.0) | 3613 (43.3) | 389 (67.5) |
| Tracheostomy, n (%) | 224 (1.0) | 2 (0.0) | 73 (0.8) | 149 (18.3) |
| Cardiorespiratory monitor at discharge, n (%) | 4421 (21.3) | 854 (7.2) | 3226 (38.7) | 341 (59.1) |
| Length of stay, median (IQR), d | 80 (62–106) | 66 (53–80) | 103 (84–127) | 163 (122–233) |
| PMA at discharge, median (IQR), wk | 39 (37–41.6) | 37.4 (36.3–39) | 41 (39.1–43.9) | 48.9 (43.1–58.4) |
All percentage values shown in the table were calculated among infants with complete data for the described variable. Data for supplemental oxygen and cardiorespiratory monitor use at discharge were missing for 6.1% of infants. Data for the length of stay and PMA at discharge were missing for 1.1% infants. Data for tracheostomy were missing for <0.1% of infants. IQR, interquartile range.
Discussion
Using a cohort that represents 85% of all very preterm infants born in the United States, we provide the first benchmark data on the epidemiology of BPD defined according to the severity-based diagnostic criteria proposed in 2019 by the NICHD Neonatal Research Network.2 In total, 10% of infants born with gestational ages of 22 to 29 weeks died before 36 weeks’ PMA, and approximately one-half developed BPD. The majority of infants with BPD were classified with a disease severity of grade 1 or 2; 3.7% developed grade 3 BPD. Rates of survival without BPD increased with greater gestational age, whereas rates of death before 36 weeks’ PMA and rates of grade 3 BPD occurred at the highest frequencies among infants born the least mature.
The frequencies of most evaluated outcome measures were reassuring among infants who survived without BPD. However, the rates of major neonatal morbidities and mortality, in-hospital treatment with respiratory drug therapies, and use of supplemental oxygen at hospital discharge all rose in an incremental, stepwise manner among infants with greater BPD severity. It is uncertain whether BPD played a causal role in these observed associations or is simply a marker of illness acuity. Regardless of the etiology of these findings, they agree with several previous studies showing that BPD occurs along a spectrum of disease severity that strongly correlates with the risk of developing multiple, prognostically important adverse outcomes.2,18–20 Our study adds to this growing body of literature and supports routine severity-based classification of BPD when reporting outcomes in very preterm infants.
The rates of BPD observed in the VON database are lower than those reported by the NICHD Neonatal Research Network.2 In that previous cohort, 71% of survivors to 36 weeks’ PMA developed BPD, with a 9% rate of grade 3 BPD.2 Among infants in the current study who survived to 36 weeks’ PMA, 45.3% developed BPD and 4.2% grade 3 BPD. These differences might be explained by the lower average gestational ages among infants evaluated by the Neonatal Research Network. In total, 89% of infants in the Neonatal Research Network study were born with gestational ages of <27 weeks, compared with 59% of infants in the present cohort.2 Restricting our data set to infants who were born at <27 weeks’ gestation and survived to 36 weeks’ PMA revealed similar BPD rates between the two studies. In this subset of infants in the VON database, 69.8% developed BPD of any severity level, and 8.1% developed grade 3 BPD.
Several of our findings may have implications for future efforts aimed at preventing lung injury and ameliorating BPD severity in very preterm infants. Although there is general consensus that avoidance of invasive ventilation is an evidence-based strategy to reduce the risk of BPD, one-half of the study infants who survived to hospital discharge received mechanical ventilation in the delivery room, and more than two-thirds were treated with invasive respiratory support and surfactant during their hospitalization.21,22 These numbers are similar to the rates of mechanical ventilation observed among infants randomly assigned to nasal continuous positive airway pressure (nCPAP) in the large clinical trials of prophylactic nCPAP conducted more than a decade ago.23–25 Together, these results highlight the ongoing need to develop novel therapies that will enable sustained avoidance of invasive ventilation in high-risk infants. Notably, however, more than one-half of the infants who were breathing in room air at 36 weeks’ PMA were exposed to invasive mechanical ventilation for some duration. Understanding how the characteristics of these infants differ from those who were ventilated and developed BPD may inform the design of new, lung-protective interventions.
We noted differences in the use of caffeine, intramuscular vitamin A, and systemic corticosteroids, 3 drug therapies shown in randomized controlled trials to lower the risk of developing BPD.26–29 In particular, there were stark discrepancies in the rates of caffeine and vitamin A use, 2 drugs that are typically administered beginning in the early postnatal period. The overwhelming majority of infants received caffeine with minimal variability in the rates of use between BPD severity levels. In contrast, only 8.0% of infants received vitamin A, including a low treatment rate of 5.4% among those who did not develop BPD. It is uncertain why clinicians have largely abandoned the use of vitamin A in very preterm infants, particularly because there are few evidence-based drug therapies shown to prevent BPD.30,31 There was a national shortage of the injectable form of vitamin A from 2010 to 2014, but the drug is now widely available, albeit at a much higher purchase price.32,33 It is possible that the elevated cost, coupled with the need to inject the drug intramuscularly, and questions about the effectiveness of vitamin A to prevent BPD among contemporary very preterm infants contribute to the low treatment rates.32,33 Nonetheless, it is noteworthy that this medication is used so infrequently, particularly because the trial data indicate that vitamin A may be as or even more effective than prophylactic nCPAP at reducing BPD risk.22,26 The most recent Cochrane systematic reviews on the prevention of BPD with these 2 therapies report a relative risk of 0.85 (95% confidence interval: 0.74–0.98) with intramuscular vitamin A and 0.89 (0.79–0.99) with prophylactic nCPAP versus invasive assisted ventilation.22,26
Of the 3 evaluated drug therapies shown to prevent BPD, the use of systemic postnatal corticosteroids varied the greatest between BPD severity levels. Use increased from 3.7% among infants who did not develop BPD to 64.0% of those who developed grade 3 BPD. Approximately one-third of infants who developed grade 1 or 2 BPD received systemic corticosteroid therapy. Although the data from randomized controlled trials raise concern for possible long-term neurologic harm with certain corticosteroid regimens, they also suggest the possibility of net benefit among infants at high risk of developing BPD.27,28,34,35 The relatively large proportion (64%) of infants in this cohort who developed BPD but did not receive corticosteroids calls into question whether some of these infants represent a missed opportunity to reduce lung disease severity and improve long-term outcomes with the use of corticosteroid therapy.
The available data indicate that infants who develop grade 3 BPD are at high risk for poor outcomes throughout early childhood.2,19 Data from the NICHD Neonatal Research Network showed that more than three-quarters of very preterm infants with grade 3 BPD died in the first years of life or developed significant neurocognitive delay.2 Our findings confirm that infants with grade 3 BPD experience a high burden of prognostically important neonatal comorbidities, which may represent important antecedent events that contribute to poor developmental outcomes. In the present cohort, nearly one-third of those who developed grade 3 BPD were diagnosed with a culture-confirmed bacterial or fungal infection, and almost one-quarter were diagnosed with severe brain injury or ROP.3,36 Nearly 20% underwent surgery for necrotizing enterocolitis or bowel perforation.37 In most cases, these event rates are an order of magnitude higher than those observed among the survivors without BPD. More than two-thirds of study infants with grade 3 BPD continued to receive supplemental oxygen at hospital discharge, and the median lengths of hospital stay were almost 100 days longer among those with grade 3 BPD, compared with those without BPD. The frequent presence of multiple complications of prematurity observed among infants with grade 3 BPD underscores the difficult task of improving respiratory and developmental outcomes in this high-risk population.
The key strength of this study is the use of a large and diverse population of very preterm infants with data collected prospectively according to standardized definitions. Our primary limitation is the inability to differentiate between and report stratified outcome rates for infants who developed grade 1 versus grade 2 BPD. Existing data suggest that grade 1 BPD is the most common severity level and infants with grade 2 BPD, on average, experience worse in-hospital outcomes than those with grade 1 BPD.2 The VON database also does not include information on the timing of most interventions and onset of the evaluated morbidities, preventing assessment of whether these occurred before or after the diagnosis of BPD. Information on interventions and outcomes, other than mortality, may be undercounted in some infants who were transferred from a VON center to one that does not participate in the data repository. Finally, this descriptive analysis does not establish causation between BPD and the evaluated patient characteristics or outcomes.
Conclusions
This study provides the first benchmark epidemiological data using the diagnostic criteria for BPD proposed in 2019 by the NICHD Neonatal Research Network.2 Our study data confirm that despite ongoing advances in neonatal care, BPD remains one of the most common and significant complications of premature birth. We found that more than one-half of infants born with gestational ages of 22 to 29 weeks in the United States died before 36 weeks’ PMA or met criteria for BPD according to this new definition. Moreover, greater BPD severity was associated with stepwise increases in the rates of multiple major neonatal morbidities and mortality and more frequent use of respiratory drug therapies and supplemental respiratory support, including at the time of hospital discharge. These data emphasize the ongoing need to develop new strategies to prevent and treat BPD in very preterm infants. Furthermore, the stark differences in outcomes and rates of health care use observed across BPD severity levels strongly suggest that future studies should report rates of BPD stratified by severity level rather than by the presence or absence of BPD alone.
Acknowledgments
We are indebted to our colleagues who submit data to VON on behalf of infants and their families. The list of centers contributing data to this study are shown in Supplemental Table 5.
Glossary
- BPD
bronchopulmonary dysplasia
- nCPAP
nasal continuous positive airway pressure
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- PMA
postmenstrual age
- ROP
retinopathy of prematurity
- VON
Vermont Oxford Network
Footnotes
Dr Jensen conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Edwards and Ms Greenberg assisted with the study design, coordinated and supervised data collection, performed the statistical analyses, and critically revised the manuscript; Drs Soll, Ehret, and Horbar assisted with the study design, oversaw development of the data collection instruments, and critically revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Drs Horbar and Soll and Ms Greenberg are employees of the Vermont Oxford Network. Drs Edwards and Ehret receive salary support from the Vermont Oxford Network; and Dr Jensen has indicated he has no financial relationships relevant to this article to disclose.
FUNDING: Dr Jensen received grant support from the National Heart, Lung, and Blood Institute (grant K23HL136843) and the American Lung Association (Clinical Patient Care Research grant 512247). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6): 1019–1026 [DOI] [PubMed] [Google Scholar]
- 2. Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. 2019;200(6):751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt B, Roberts RS, Davis PG, et al. ; Caffeine for Apnea of Prematurity (CAP) Trial Investigators; Caffeine for Apnea of Prematurity CAP Trial Investigators . Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167(5):982–986.e2 [DOI] [PubMed] [Google Scholar]
- 4. Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev Med Child Neurol. 2016;58(6):554–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel RM, Kandefer S, Walsh MC, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H; Trial Indomethacin Prophylaxis in Preterms Investigators . Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140(5):555–560 [DOI] [PubMed] [Google Scholar]
- 8. Hines D, Modi N, Lee SK, et al. ; International Network for Evaluating Outcomes (iNeo) of Neonates . Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr. 2017;106(3):366–374 [DOI] [PubMed] [Google Scholar]
- 9. Isayama T, Lee SK, Yang J, et al. ; Canadian Neonatal Network and Canadian Neonatal Follow-Up Network Investigators . Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017;171(3):271–279 [DOI] [PubMed] [Google Scholar]
- 10. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 11. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–532 [PubMed] [Google Scholar]
- 12. Jobe AH, Steinhorn R. Can we define bronchopulmonary dysplasia? J Pediatr. 2017;188:19–23 [DOI] [PubMed] [Google Scholar]
- 13. Poindexter BB, Feng R, Schmidt B, et al. ; Prematurity and Respiratory Outcomes Program . Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12(12):1822–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steinhorn R, Davis JM, Göpel W, et al. ; International Neonatal Consortium . Chronic pulmonary insufficiency of prematurity: developing optimal endpoints for drug development. J Pediatr. 2017;191:15–21.e1 [DOI] [PubMed] [Google Scholar]
- 15. Vermont Oxford Network . 2018 Manual of Operations: Part 2 Data Definitions & Infant Data Forms. Vol 22. Burlington, VT: Vermont Oxford Network; 2017 [Google Scholar]
- 16. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 18. Ehrenkranz RA, Walsh MC, Vohr BR, et al. ; National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 19. Brumbaugh JE, Bell EF, Grey SF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Behavior profiles at 2 years for children born extremely preterm with bronchopulmonary dysplasia. J Pediatr. 2020;219:152–159.e532008764 [Google Scholar]
- 20. Short EJ, Kirchner HL, Asaad GR, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161(11): 1082–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Committee on Fetus and Newborn; American Academy of Pediatrics . Respiratory support in preterm infants at birth. Pediatrics. 2014;133(1):171–174 [DOI] [PubMed] [Google Scholar]
- 22. Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016; (6):CD001243. [DOI] [PubMed] [Google Scholar]
- 23. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700–708 [DOI] [PubMed] [Google Scholar]
- 24. Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group . Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1069 [DOI] [PubMed] [Google Scholar]
- 25. Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants [published correction appears in N Engl J Med. 2010;362(23):2235]. N Engl J Med. 2010;362(21):1970–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2016;(8):CD000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;(10):CD001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;(10):CD001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group . Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121 [DOI] [PubMed] [Google Scholar]
- 30. Jensen EA, Foglia EE, Schmidt B. Evidence-based pharmacologic therapies for prevention of bronchopulmonary dysplasia: application of the Grading of Recommendations Assessment, Development, and Evaluation methodology. Clin Perinatol. 2015;42(4):755–779 [DOI] [PubMed] [Google Scholar]
- 31. Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F285–F291 [DOI] [PubMed] [Google Scholar]
- 32. Couroucli XI, Placencia JL, Cates LA, Suresh GK. Should we still use vitamin A to prevent bronchopulmonary dysplasia? J Perinatol. 2016;36(8):581–585 [DOI] [PubMed] [Google Scholar]
- 33. Tolia VN, Murthy K, McKinley PS, Bennett MM, Clark RH. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth-weight infants. JAMA Pediatr. 2014;168(11):1039–1044 [DOI] [PubMed] [Google Scholar]
- 34. Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr. 2014;165(6):1258–1260 [DOI] [PubMed] [Google Scholar]
- 35. Watterberg KL; American Academy of Pediatrics. Committee on Fetus and Newborn . Policy statement–postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126(4):800–808 [DOI] [PubMed] [Google Scholar]
- 36. Mitha A, Foix-L’Hélias L, Arnaud C, et al. ; EPIPAGE Study Group . Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2). Available at: www.pediatrics.org/cgi/content/full/132/2/e372 [DOI] [PubMed] [Google Scholar]
- 37. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F193–F198 [DOI] [PMC free article] [PubMed] [Google Scholar]