Abstract
Rectal cancer (RC) is the third most commonly diagnosed cancer and has a high risk of mortality, although overall survival rates have improved. Preoperative assessments and predictions, including risk stratification, responses to therapy, long-term clinical outcomes, and gene mutation status, are crucial to guide the optimization of personalized treatment strategies. Radiomics is a novel approach that enables the evaluation of the heterogeneity and biological behavior of tumors by quantitative extraction of features from medical imaging. As these extracted features cannot be captured by visual inspection, the field holds significant promise. Recent studies have proved the rapid development of radiomics and validated its diagnostic and predictive efficacy. Nonetheless, existing radiomics research on RC is highly heterogeneous due to challenges in workflow standardization and limitations of objective cohort conditions. Here, we present a summary of existing research based on computed tomography and magnetic resonance imaging. We highlight the most salient issues in the field of radiomics and analyze the most urgent problems that require resolution. Our review provides a cutting-edge view of the use of radiomics to detect and evaluate RC, and will benefit researchers dedicated to using this state-of-the-art technology in the era of precision medicine.
Keywords: Computed tomography, Magnetic resonance imaging, Radiomics, Rectal cancer, Clinical applications, Overall survival
Core Tip: Radiomics has exhibited significant potential for risk stratification of rectal cancer and has yielded excellent performance in response assessment of neoadjuvant radiochemotherapy. While the past 3 years has witnessed an exponential growth of the field, research on radiomics remains in its infancy and is constantly evolving. More rigorous analyses are emerging, and improvements in bias reduction techniques accompanied with multicentric studies will hopefully enable more robust and generalizable models. Here, we review recent updates on the use of radiomics based on computed tomography and magnetic resonance imaging in the detection and evaluation of rectal cancer.
INTRODUCTION
Colorectal cancer (CRC) is ranked third among the most common cancers worldwide and accounts for nearly one-tenth of cancer-related deaths globally[1,2]. Rectal cancer (RC) comprises 27%-58% of all CRCs[3]. The development of treatment strategies and the revision of multidisciplinary treatment approaches such as local excision, total mesorectal excision (TME), and neo-adjuvant radiochemotherapy (nCRT) have decreased local recurrence and distant metastasis rates in recent decades[4,5]. However, accurate pretreatment tumor staging by imaging remains essential for precise decision-making[6].
Traditional medical imaging is routinely used in initial diagnosis of RC and has played a critical role as a non-invasive tool during follow-up[7]. According to the 2018 European Society of Medical Oncology (ESMO) Guidelines, high-resolution magnetic resonance imaging (MRI) is the standard staging modality for RC and has exhibited superior performance for tumor staging when compared with digital rectal examination, computed tomography (CT), and endoscopic ultrasound[8]. CT is mainly used for local lesions due to its inherent traits of low soft-tissue contrast, which limits the accurate approximation of T stage. Even in T4 lesions with gross invasion of adjacent organs, false-positive cases may occur. Therefore, CT is typically employed primarily for the detection of metastases. CT has faster acquisition time and is more practical than MRI, as it is more widely available[9]. However, both CT and MRI have restricted resolution in clinical applications.
Radiomics has emerged with consistently developing methodology and promising results[10-12]. Radiomics is a method that enables quantitative extraction of radiomics features that cannot be captured by visual inspection from available radiological images[13]. In the past few years, an increasing number of studies have evaluated abdominal radiomics models in different oncological scenarios and reported impressive performance for evaluating tumor biological behaviors, prognosis, and prediction of therapeutic responses[14-16]. Radiomics has been validated as a novel approach for improved characterization of tumor subtypes and the lesion microenvironment[17,18]. By making use of the medical images and clinical data, radiomics models have the potential to provide more detailed information to tailor individualized treatment scheme and patient management[19-21].
The purpose of this review is to describe and summarize the recent advances in clinical applications of radiomics based on CT and MRI, to highlight the potential role of radiomics in disease evaluation and clinical decision-making of RC, and to discuss the current limitations and possible optimization directions in the future.
RADIOMICS WORKFLOW AND METHODOLOGICAL ADVANCES IN RC
The concept of radiomics was first developed by Lambin et al[22] in 2012. Radiomics is defined as a research method that includes quantitative data extraction from multimodality medical images, analysis, and modeling of high-dimensional medical image features to explore relationships with clinical outcomes[11,13]. Related research can be divided into five stages: Data acquisition and analysis, medical imaging segmentation, feature extraction and selection, clinical target-oriented modeling, and research quality evaluation. The workflow is presented in Figure 1.
Figure 1.
Workflow of radiomics applied in rectal cancer. US: Ultrasonography; CT: Computed tomography; MRI: Magnetic resonance imaging; PET: Positron emission tomography; ROI: Region of interest; EMVI: Extramural venous invasion; PNI: Perineural invasion.
Data acquisition and analysis
The radiomics workflow begins with the determination of the region of interest (ROI), which depends on the specific clinical problem that requires resolution. Data used in radiomics studies may be retrospective or prospective and single-center or multi-center. ROIs are determined by specific targeted research; therefore, researchers should first define the clinical problems to solve. Here, we consider RC as an example, whereby primary tumors are analyzed and related to existing treatment outcomes such as survival or recurrence. The analysis of lesions and normal tissues may affect the treatment strategies. Via the establishment of a large image database, a large body of imaging data is stored to generate an integrated medical and health care network. Radiologists should be cognizant that imaging protocols may not always be standardized, and variability exists among medical images and institutions. In this regard, the recommendations of the Image Biomarker Standardization Initiative may help to reduce the variability in image pre-processing prior to analysis[23].
ROI segmentation and delineation
Normalizing the original image is essential because data results may be affected by different machines or different parameters during collection. The process of ROI segmentation in radiomics can be performed either manually, semi-automatically, or automatically with software. Each approach has advantages and disadvantages. Manual segmentation is more precise in some occasions (e.g., delineating the RC bed after nCRT) but has lower repeatability. Automatic segmentation depends on algorithms, which are efficient and may help to eliminate subjective errors[24]. To date, a mature automatic segmentation algorithm for RC is lacking. According to our PubMed search results, most radiomics studies on RC applied manual segmentation in which the segmentation is performed by radiologists to annotate the location and precise boundary of the ROI. Itk-snap software (www.itksnap.org) is used extensively for segmentation. Figure 2 shows the segmentation of a rectal tumor using Itk-snap software. Given that subjective bias may occur, segmentation may be inconsistent. At present, several steps are enacted to minimize bias, including the involvement of different medical professionals, multiple segmentation in different respiratory cycles, and adding noise to segmentation[25].
Figure 2.
Segmentation of a rectal tumor with Itk-snap software. A: Example of tumor segmentation using Itk-snap software (www.itksnap.org) on axial plain magnetic resonance image; B-D: Axial (B), reconstructed coronal (C), and sagittal (D) contrast-enhanced magnetic resonance images in the venous phase in a 72-year-old man with rectal cancer.
Feature extraction and selection
Following the evaluation of the consistency of feature calculations across researchers, the initial selection of high-level feature sets is performed, and feature values that are sensitive to subjective factors are ultimately retained. Parmar et al[26] used the “IRR” (evaluator reliability) package in R software to evaluate the consistency of features extracted from ROIs, whereby features with a consistency coefficient less than 0.8 would be deleted.
The key to radiomics is the extraction of high throughput features that are difficult to depict by observers using mathematical algorithms from ROI segmentation directly. These features of essential value can either be directly extracted from original medical images or after applying a transformation or filter method. The process can be performed using different tools (e.g., PyRadiomics, Texrad, MaZda, and others) which are readily available as open-source software. Image filtration enhances the edge qualities and removes interference by noise, thus permitting the collection of more information on spatial locations of features. At present, the main extraction methods are improved based on the method published by Aerts et al[27] in 2014, according to which radiomics features are divided into machine-learning-based features and deep learning features. There are several commonly used subgroups of the former, such as shape features (describing the geometric attributes of the ROIs), histogram-based features (capturing the first-order statistical characteristics of rectal lesions), and texture features (describing the granular textural pattern of the ROIs). Commonly used engineered features according to “order” are presented in Table 1. Recently, a set of 169 standardized radiomics features was published, which enabled the verification and calibration of different radiomics software[23].
Table 1.
Commonly-used radiomics feature categories according to “order”
|
“Order”
|
Paraphrase
|
Categories
|
Description
|
| First-order | Based on an intensity histogram of pixel values, providing no information on neighboring interactions or the spatial distribution | Mean | Average intensity of the pixels |
| Skewness | Asymmetry of histogram | ||
| Kurtosis | Magnitude of pixel distribution | ||
| Entropy | Irregularity of the structure | ||
| Second-order | Considers the spatial relationship between 2 pixels | Grey level co-occurrence matrix (GLCM) | Frequency of specific gray values along a distance or direction |
| Grey level run Length Matrix (GLRLM) | Length of consecutive pixels or voxels with the same grey values in a specific direction | ||
| Grey level size zone matrix (GLSZM) | Length of consecutive pixels or voxels with the same grey values in all directions | ||
| Superior-order | Describes the neighborhood gray difference matrices and the relationship between 3 or more pixels | Neighboring gray tone difference matrix (NGTDM) | Describes the sum and average grey levels of discretized voxels in planes |
| Gray level dependence matrix (GLDM) | Describes the coarseness of overall texture by evaluating the grey levels between a voxel and the neighborhood in 3 dimensions |
In reference to “order”, it is defined as the number of stages required to obtain the quantitative information in a model.
Given that the size of the data sample is relatively small compared with features, this may result in over-fitting and it may be time-consuming to include all features in the classification. Feature selection is a necessary step to obtain features that are closely related to the target results in radiomics analyses. Typically, features extracted by radiomics are high-dimensional in a dataset, and a large proportion of features may not be useful for the task; hence, unstable features should be excluded to retain the most important features and prevent over-fitting. The main methods applied in feature selection can be divided into univariate or multivariate, based on whether the association among features can be considered to contribute towards a specific outcome. In machine-learning, the most commonly used feature selection algorithms include least absolute shrink and selection operator regression, minimum redundancy maximum correlation, recursive feature elimination, and principal component analysis[28].
Clinical target-oriented modeling
Radiomics feature analysis and modeling involve the establishment of a prediction model based on selected imaging characteristics. The model generally encompasses clinical, biological, and occasionally genetic information. Commonly used models include logistic regression, support vector machine (SVM), and random forest[13]. In radiomics analysis, machine-learning algorithms are typically required to establish a classification or prediction model to obtain reliable results instead of relying solely on single factor analysis. The Cox proportional risk model is usually employed as a survival analysis model. Each modeling method has its own limitations. For example, feature independence, feature discretization, and network configuration dependence should be considered in logistic regression, Bayesian networks, and deep learning, respectively.
In the process of model-building, researchers can employ different software tools. R language contains software packages for data-mining and modeling. Clinicians and radiologists can operate various components via a graphical interface, compare different modeling algorithms, and identify the most effective way to resolve clinical issues. SPSS modeler is a commercial data-mining software tool with functions encompassing almost all data-mining procedures. This software possesses a graphical operation interface and automatic modeling function, which permits management of large amounts of data and provision of steady data-mining models. Other software for modeling, data-mining, and analysis, such as Weka (based on JAVA) and B11 (used in combination with Mazda), may also be employed. These software tools contain various modeling algorithms, including artificial neural network, k-nearest neighbor, K-means, hierarchical clustering, and similarity-based clustering methods.
Evaluation of research quality
For a high-quality radiomics study, it is essential to try different types of modeling methods and to choose the best algorithms. Moreover, all the models should be validated internally and externally (multicenter validation would be better if permitted)[29]. Validation is an indispensable part of complete radiomics analysis, because the value of the unverified evaluation model is limited. Except for validation of the model, quality assessment should also be conducted to ensure reproducibility in a radiomics study. A prediction model is appropriate for clinical decision-making only when a standardized evaluation of its performance is accomplished.
Radiomics based on deep learning
Deep learning is defined as a deep neural network structure based on a broad spectrum of algorithms, and the frameworks permit machines to learn highly complex mathematical models for data representation and can subsequently be used to perform accurate data analysis. Radiomics algorithms based on deep learning have developed substantially in recent years[30,31]. Three types of deep learning models are commonly used in medical imaging: Convolutional neural networks (CNNs), generative adversarial networks, and sparse auto encoders. Deep learning-based radiomics performs learning procedures via convolutional operations and CNN structures. Compared with traditional radiomics, convolution operation has strong feature extraction abilities. The neural network structure can flexibly extract different task-related features by changing the convolution kernel and modifying the structure, thereby enabling a more targeted approach. Different features can be obtained by adaptively changing the convolution kernel. For example, convolution and Laplacian kernels can extract high-frequency features, while the Gaussian kernel can propose low-frequency features. For this reason, the deep learning approach exhibits superior feature extraction by extracting and selecting supplementary high-dimensional features through an automatic-learning neural network, and this enables more comprehensive mining of image information. To date, there are six radiomics studies conducted based on deep learning structure in RC. More novel neural network structures are warranted in this field.
RADIOMICS APPLICATIONS IN RC
We searched PubMed (December 17, 2020) for 83 radiomics studies on RC using the terms (“rectal cancer” AND “radiomics”), and identified 78 clinical target-oriented published works. The volume of radiomics-based articles on RC published in medical journals since 2018 has witnessed a steep rise, indicating a trend of growing interest in artificial intelligence-based approaches in the field.
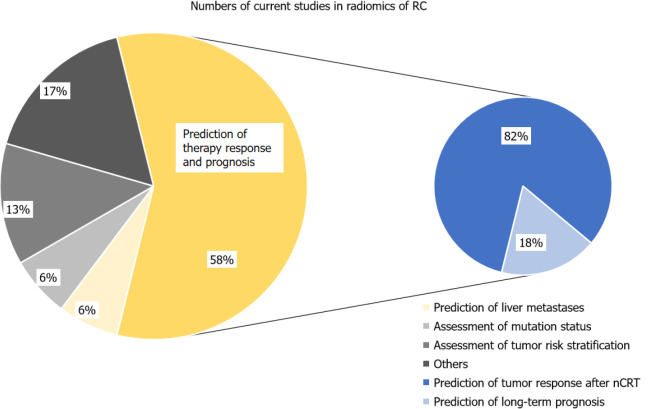
The literature search revealed an exponential growth in RC radiomics studies in the past 3 years. Of the 78 studies, most (61 out of 78) were based on MRI, eight employed CT modality, and nine were based on positron emission tomography/CT or ultrasound images. As of December 2020, most existing studies were performed in a single center with a retrospective cohort, while only seven studies were multi-center. Validation of radiomics models in independent cohorts was performed in all of the studies. However, few studies included external validation cohorts (n = 7). The number of included RC patients ranged widely from 13 to 700. In addition, almost a half of current studies focus on locally advanced RC (LARC) (38 out of 78) or tumor response to nCRT. We suggest that this is mainly because LARC is a major sticking point in the management of RC. Figure 3 shows the distribution of current focus of attention. Here, we discuss recent advances in radiomics applied to CT and MRI for the evaluation of RC.
Figure 3.
Distribution of the current focus in the industry. Currently 58% of research focuses on tumor response assessment to preoperative neo-adjuvant radiochemotherapy (nCRT) therapies or prediction of the long-term prognosis, of which most studies are about prediction of tumor response after nCRT. RC: Rectal cancer; nCRT: Neo-adjuvant radiochemotherapy.
Response assessment of pre-operative therapy and long-term prognosis prediction in LARC
LARC is of significant concern due to the potential for deterioration. Over the past decade, both disease-free survival and overall survival of LARC have been prolonged, owing to the growing practice of multi-disciplinary treatment and routinization of management, including TME, nCRT, and immunotherapy[3,32]. However, patients tend to present heterogeneous long-term outcomes in clinical practice due to individual responses to preoperative therapy such as short-course preoperative radiotherapy and nCRT[32,33]. Thus, those who are sensitive to chemoradiotherapy may achieve a clinical complete response and employ a watch-and-wait strategy, whereas patients who are resistant to nCRT need more radical measures[34]. Tailoring treatment schemes to a particular patient based on individual probability for achieving a good response is thus essential. The current pre-treatment response prediction approach to nCRT remains indecisive and fails to adequately estimate a patient’s response to a specific therapy. Traditional medical imaging methods including CT, quantified MRI, and functional imaging have limitations in response prediction. In contrast, radiomics with high-dimensional feature extraction applied in RC may facilitate the a priori evaluation of treatment efficacy and selection of optimal therapy.
Almost 60% of the current research focuses on tumor response assessment to preoperative therapies or prediction of long-term prognosis (n = 49), mainly in LARC (38 out of 49). Most studies to date have evaluated T2-weighted imaging (T2WI) and diffusion weighted MRI. Heterogeneous results have been reported in different prediction models, referring to entropy, energy, and kurtosis. Two well-conducted studies reported that none of the T2WI radiomics features were significant predictors of response[35,36]. SVM, RF, and Naïve Bayesian network based on T2WI yielded promising results for complete response (CR) prediction [area under the receiver operating characteristic curve (AUC): 0.71-0.87][35,37,38]. In contrast, apparent diffusion coefficient and intravoxel incoherent motion (IVIM) histogram features did not exhibit predictive value for CR[39]. Similar discrepancies were identified in gray-level co-occurrence matrix (GLCM) dissimilarities for preoperative evaluation of neoadjuvant chemoradiotherapy responses. In this regard, Liu et al[39] proposed that GLCM IVIM parameters independently predicted CR in multivariate analysis. Both the random forest model of Yang et al[40] and logistic regression model of van Griethuysen et al[41] may assist in distinguishing non-insensitive responders to chemoradiotherapy.
Assessment of tumor vascular/perineural invasion (extramural venous invasion/perineural invasion)
Extramural venous invasion (EMVI), which presents in one-third of all patients with RC, is a major factor for higher risk of recurrence and an independent indicator of poorer prognosis[42]. MRI is more suitable to identify the patients with obvious blood vessel invasion beyond the muscularis propria[43]. The verdict of conventional MRI may be affected by surrounding inflammation, edema, and fibrosis caused by nCRT, let alone heterogeneity with image quality, methods, and diagnostic accuracy[44,45]. To obtain more accurate preoperative risk stratification, Yu et al[46] constructed and validated several radiomics models and compared them with quantitative models. All radiomics models outperformed quantitative models for predictive performance in identifying EMVI. Notably, the team compared these models with a perfusion parameter-based model from dynamic contrast-enhanced-MRI and demonstrated that the latter had a weaker predictive value.
The positive perineural invasion (PNI) status at resection can independently predict the local recurrence of RC or progression, indicating that the tumor may be of a more aggressive phenotype. According to the latest ESMO guidelines, PNI is a key factor for determining whether patients with stage II RC would potentially benefit from nCRT and postoperative adjuvant chemotherapy[8]. Unlike EMVI, PNI can only be confirmed in post-resection pathological tests, whereas conventional MRI is inoperative for assessing PNI status, as it is unable to visualize peripheral nerves[47]. Therefore, developing a radiomics prediction model to preoperatively identify PNI and to assist in the selection of patients for adjuvant therapy is crucial. Chen et al[48] developed a MRI-based radiomics predictive model, which yielded favorable performance for individualized PNI prediction in patients with RC. However, this was a single-center retrospective study based only on T2WI sequences. Future studies should explore this issue further with heterogeneous and multi-sequence data.
Prediction of synchronous/metachronous liver metastasis
Approximately 15%-20% of patients with RC have liver metastases at the time of diagnosis, which is defined as synchronous liver metastasis (SLM)[49]. At present, only two studies have explored the use of a radiomics nomogram for predicting SLM derived from primary RC lesions[50,51]. Shu et al[50] extracted a total of 328 radiomics features from the T2WI images and developed a radiomics model composing T-stage and radiomics signatures. Quantified analysis revealed that the Rad score of patients with primary CRC and liver metastasis was significantly higher than that of patients without liver metastasis. Receiver operating characteristic curves based on all 194 patients with RC were plotted for a radiomics nomogram, and the AUC was 0.932. Liu et al[51] subsequently constructed a novel radiomics nomogram and improved the AUC to 0.944, with a sensitivity of 95.83% and specificity of 88.89%, indicating that the radiomics model exhibited good predictive performance for the diagnosis of SLM in patients with RC and may assist physicians in clinical decision-making. The heterogeneity of primary tumors often determines tumor relapse and progression; in contrast, the biological behavior of tumor metastasis and recurrence is closely related to the pathological heterogeneity of the primary tumor. In addition, the radiomics features derived from the primary tumor itself are often more stable in patients with RC. Therefore, radiomics studies examining the prediction of liver metastasis based on primary RC lesions are warranted.
Metachronous liver metastasis (MLM) is defined as the absence of evidence of metastatic disease at initial diagnosis but the presence of liver metastasis after baseline staging and treatment[52,53]. MLM is thought to evolve from occult and micro metastases[54]. Approximately 26.5% of patients with RC develop MLM within 5 years of follow-up[55]. Based on machine-learning algorithms and imaging sequences, noninvasive radiomics models constructed on baseline rectal MRI presented good potential for MLM prediction in patients with RC. The most optimal model employed a logistic regression algorithm, incorporating both T2WI and venous phase radiomics features[56]. To date, reports on MLM prediction based on primary rectal tumors are lacking.
Assessment of genetic mutational status
With the development of targeted therapies such as epidermal growth factor receptor (EGFR)-targeted monoclonal antibody treatment, genetic profiling for mutations is recommended when metastases are diagnosed in patients with RC[8,57]. Previous studies have demonstrated that the mutation statuses of rat sarcoma viral oncogene homolog and v-raf murine sarcoma viral oncogene homolog B1 are critical biomarkers in the prognosis of RC, especially for patients with suspected or proven metastases[58]. Kirsten rat sarcoma (KRAS) mutations, which occur in approximately 27%-43% of patients with RC, have been identified as a critical factor, as they indicate a lack of response to EGFR-targeted therapy[59]. Pathologic tests are the current gold standard of genotyping diagnosis in clinical practice, although results can only be obtained after invasive procedures and may not always be available or reliable. Thus, personalized treatment strategies are warranted to develop a non-invasive and more feasible, timely, and cost-effective surrogate biomarker to evaluate mutation status. With the advent of artificial intelligence (AI) approaches, non-invasive prediction of genetic status and efficacy of CRT using radiomics analysis has become a highlight in the field.
We identified a limited number of studies that used modality-based radiomics models to predict KRAS mutation status or microsatellite instability in RC[60], or to evaluate the relative diagnostic potential of radiomics features for predicting mutational status[59,61-64]. Relationships between radiomics features extracted from CT or multiparametric MRI and KRAS mutations have been confirmed in several studies. Meng et al[60] and Xu et al[61] reported that radiomics features based on MRI predicted KRAS mutations. Oh et al[62] reported that MR-based texture analysis differentiated KRAS mutation status with an accuracy of 81.7%. Furthermore, Cui et al[63] used a two-center cohort to predict KRAS mutations in RC and observed that the model obtained with the SVM classifier exhibited the best predictive value. Notably, most of these MRI-derived radiomics models demonstrated moderate predictive ability for KRAS mutations. Further, they were underscored by small sample sizes and lack of independent/external validation. As such, further research is warranted.
FUTURE CHALLENGES AND OPPORTUNITIES
Extant literature has highlighted the potential of radiomics analysis for prediction of nCRT response and prognosis, tumor risk stratification, and evaluation of gene mutation status. Nevertheless, some limitations of radiomics applied for RC should be acknowledged. Current issues arise due to the reliance of results on high-quality data sets, which may bias comparisons of efficacy among radiomics studies due to differences in scanning parameters, feature extraction, software, and vendors/modalities. Hence, there is an urgent need to establish unified data acquisition and processing standards. In addition, radiomics is sensitive to accurate ROI segmentation in the acquisition process. Currently, the process is predominantly conducted manually by radiologists or physicians. Manual segmentation is laborious, and the results may be affected by the observer’s subjectivity. The use of semi-automatic or fully automatic methods for segmentation may resolve these issues and should be pursued in future studies. In this regard, accurate and automatic labeling procedures should be developed and promoted to address current technical limitations, and there is still a long way to go. In addition, most existing radiomics studies are retrospective in nature and lack independent external validation across races or populations from various geographical sources, which may limit the reliability and applicability of results. Therefore, further multi-center and prospective studies with standardized acquisition, segmentation, and imaging postprocessing are required to ensure the generalizability and validation of radiomics findings. With regard to mechanistic investigations of biological substrates and their relationships with pathological underpinnings, AI algorithms with improved accuracy and interpretability will facilitate broader translation and clinical adoption. Moreover, radiogenomics for RC is burgeoning, which will bridge the gap between AI-aided prognostics and precision medicine.
As we have discussed above, radiomics models based on deep learning have proven superior. Nevertheless, several issues remain to be optimized; although more parameters exist in the whole connection layer, the gradient of the input end can easily dissipate during network training. A possible solution is to reduce the complexity of the model by reducing network parameters and optimizing the gradient flow, such as VGg, concept, residual network, and dense net.
CONCLUSION
Radiomics is an emerging quantitative technique that has witnessed exponential growth in application of RC management. In this review, we discuss the current utility of radiomics in RC research and describe its potential applications for precision diagnostics and cancer treatment. Thus far, radiomics has shown promise in the diagnosis, treatment evaluation, and prediction of prognosis in RC. Nevertheless, further multi-center and prospective validation studies are required to validate its clinical utility, especially in prognosis-related targets. The purpose of this review is to help radiologists, endoscopists, and oncologists better understand and harness radiomics for tailoring personalized treatments and to encourage their collaboration with AI scientists to promote the translation of research into clinical practice.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests related to this manuscript.
Manuscript source: Invited manuscript
Peer-review started: January 27, 2021
First decision: May 2, 2021
Article in press: May 21, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mirnezami R S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Yuan YY
Contributor Information
Min Hou, Department of Radiology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China.
Ji-Hong Sun, Department of Radiology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China. sunjihong@zju.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Bouvier AM, Foschi R, Hackl M, Larsen IK, Lemmens V, Mangone L, Francisci S EUROCARE Working Group. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131:1649–1658. doi: 10.1002/ijc.26192. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID, de Wilt JHW, Verhoeven RHA. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758–2766. doi: 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 6.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33:1797–1808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curvo-Semedo L. Rectal Cancer: Staging. Magn Reson Imaging Clin N Am. 2020;28:105–115. doi: 10.1016/j.mric.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. doi: 10.1093/annonc/mdy161. [DOI] [PubMed] [Google Scholar]
- 9.Balyasnikova S, Brown G. Optimal Imaging Strategies for Rectal Cancer Staging and Ongoing Management. Curr Treat Options Oncol. 2016;17:32. doi: 10.1007/s11864-016-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Yang X, Shi Z, Yang Z, Du X, Zhao Z, Cheng X. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2019;29:1211–1220. doi: 10.1007/s00330-018-5683-9. [DOI] [PubMed] [Google Scholar]
- 11.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomaszewski MR, Gillies RJ. The Biological Meaning of Radiomic Features. Radiology. 2021;298:505–516. doi: 10.1148/radiol.2021202553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595–3605. doi: 10.1007/s00330-018-5985-y. [DOI] [PubMed] [Google Scholar]
- 15.Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology. 2020;294:568–579. doi: 10.1148/radiol.2020191470. [DOI] [PubMed] [Google Scholar]
- 16.Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, Sala E, Garcia-Aguilar J, Gollub MJ, Petkovska I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology. 2018;287:833–843. doi: 10.1148/radiol.2018172300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino M. Perinodular Radiomic Features to Assess Nodule Microenvironment: Does It Help to Distinguish Malignant versus Benign Lung Nodules? Radiology. 2019;290:793–795. doi: 10.1148/radiol.2018182619. [DOI] [PubMed] [Google Scholar]
- 18.Colen RR, Hassan I, Elshafeey N, Zinn PO. Shedding Light on the 2016 World Health Organization Classification of Tumors of the Central Nervous System in the Era of Radiomics and Radiogenomics. Magn Reson Imaging Clin N Am. 2016;24:741–749. doi: 10.1016/j.mric.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhu HT, Zhang XY, Shi YJ, Li XT, Sun YS. A Deep Learning Model to Predict the Response to Neoadjuvant Chemoradiotherapy by the Pretreatment Apparent Diffusion Coefficient Images of Locally Advanced Rectal Cancer. Front Oncol. 2020;10:574337. doi: 10.3389/fonc.2020.574337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Liu X, Hu B, Gao Y, Chen J, Li J. Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. Abdom Radiol (NY) 2020 doi: 10.1007/s00261-020-02846-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Meng X, Zhang H, Li Z, Liu J, Sun K, Meng Y, Dai W, Xie P, Ding Y, Wang M, Cai G, Tian J. Predicting distant metastasis and chemotherapy benefit in locally advanced rectal cancer. Nat Commun. 2020;11:4308. doi: 10.1038/s41467-020-18162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, El Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Götz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegård A, Maier-Hein KH, Morin O, Müller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers RJHM, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Löck S. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology. 2020;295:328–338. doi: 10.1148/radiol.2020191145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berenguer R, Pastor-Juan MDR, Canales-Vázquez J, Castro-García M, Villas MV, Mansilla Legorburo F, Sabater S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology. 2018;288:407–415. doi: 10.1148/radiol.2018172361. [DOI] [PubMed] [Google Scholar]
- 25.Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol. 2017;90:20160665. doi: 10.1259/bjr.20160665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmar C, Rios Velazquez E, Leijenaar R, Jermoumi M, Carvalho S, Mak RH, Mitra S, Shankar BU, Kikinis R, Haibe-Kains B, Lambin P, Aerts HJ. Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One. 2014;9:e102107. doi: 10.1371/journal.pone.0102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJWL. Machine Learning methods for Quantitative Radiomic Biomarkers. Sci Rep. 2015;5:13087. doi: 10.1038/srep13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers AJ. Prediction models: revolutionary in principle, but do they do more good than harm? J Clin Oncol. 2011;29:2951–2952. doi: 10.1200/JCO.2011.36.1329. [DOI] [PubMed] [Google Scholar]
- 30.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kermany DS, Goldbaum M, Cai W, Valentim CCS, Liang H, Baxter SL, McKeown A, Yang G, Wu X, Yan F, Dong J, Prasadha MK, Pei J, Ting MYL, Zhu J, Li C, Hewett S, Ziyar I, Shi A, Zhang R, Zheng L, Hou R, Shi W, Fu X, Duan Y, Huu VAN, Wen C, Zhang ED, Zhang CL, Li O, Wang X, Singer MA, Sun X, Xu J, Tafreshi A, Lewis MA, Xia H, Zhang K. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell. 2018;172:1122–1131.e9. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 33.Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, Sun W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2030097. doi: 10.1001/jamanetworkopen.2020.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O'Dwyer ST. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–183. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 35.Antunes JT, Ofshteyn A, Bera K, Wang EY, Brady JT, Willis JE, Friedman KA, Marderstein EL, Kalady MF, Stein SL, Purysko AS, Paspulati R, Gollamudi J, Madabhushi A, Viswanath SE. Radiomic Features of Primary Rectal Cancers on Baseline T2 -Weighted MRI Are Associated With Pathologic Complete Response to Neoadjuvant Chemoradiation: A Multisite Study. J Magn Reson Imaging. 2020;52:1531–1541. doi: 10.1002/jmri.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H, Kim KA, Jung JH, Rhie J, Choi SY. MRI features and texture analysis for the early prediction of therapeutic response to neoadjuvant chemoradiotherapy and tumor recurrence of locally advanced rectal cancer. Eur Radiol. 2020;30:4201–4211. doi: 10.1007/s00330-020-06835-4. [DOI] [PubMed] [Google Scholar]
- 37.Petkovska I, Tixier F, Ortiz EJ, Golia Pernicka JS, Paroder V, Bates DD, Horvat N, Fuqua J, Schilsky J, Gollub MJ, Garcia-Aguilar J, Veeraraghavan H. Clinical utility of radiomics at baseline rectal MRI to predict complete response of rectal cancer after chemoradiation therapy. Abdom Radiol (NY) 2020;45:3608–3617. doi: 10.1007/s00261-020-02502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi X, Pei Q, Zhang Y, Zhu H, Wang Z, Chen C, Li Q, Long X, Tan F, Zhou Z, Liu W, Li C, Zhou Y, Song X, Li Y, Liao W, Li X, Sun L, Pei H, Zee C, Chen BT. MRI-Based Radiomics Predicts Tumor Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Front Oncol. 2019;9:552. doi: 10.3389/fonc.2019.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Wen L, Hou J, Nie S, Zhou J, Cao F, Lu Q, Qin Y, Fu Y, Yu X. Predicting the pathological response to chemoradiotherapy of non-mucinous rectal cancer using pretreatment texture features based on intravoxel incoherent motion diffusion-weighted imaging. Abdom Radiol (NY) 2019;44:2689–2698. doi: 10.1007/s00261-019-02032-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, Jiang ZK, Liu LH, Zeng MS. Pre-treatment ADC image-based random forest classifier for identifying resistant rectal adenocarcinoma to neoadjuvant chemoradiotherapy. Int J Colorectal Dis. 2020;35:101–107. doi: 10.1007/s00384-019-03455-3. [DOI] [PubMed] [Google Scholar]
- 41.van Griethuysen JJM, Lambregts DMJ, Trebeschi S, Lahaye MJ, Bakers FCH, Vliegen RFA, Beets GL, Aerts HJWL, Beets-Tan RGH. Radiomics performs comparable to morphologic assessment by expert radiologists for prediction of response to neoadjuvant chemoradiotherapy on baseline staging MRI in rectal cancer. Abdom Radiol (NY) 2020;45:632–643. doi: 10.1007/s00261-019-02321-8. [DOI] [PubMed] [Google Scholar]
- 42.Jhaveri KS, Hosseini-Nik H, Thipphavong S, Assarzadegan N, Menezes RJ, Kennedy ED, Kirsch R. MRI Detection of Extramural Venous Invasion in Rectal Cancer: Correlation With Histopathology Using Elastin Stain. AJR Am J Roentgenol. 2016;206:747–755. doi: 10.2214/AJR.15.15568. [DOI] [PubMed] [Google Scholar]
- 43.Moreno CC, Sullivan PS, Mittal PK. MRI Evaluation of Rectal Cancer: Staging and Restaging. Curr Probl Diagn Radiol. 2017;46:234–241. doi: 10.1067/j.cpradiol.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Bae JS, Kim SH, Hur BY, Chang W, Park J, Park HE, Kim JH, Kang HJ, Yu MH, Han JK. Prognostic value of MRI in assessing extramural venous invasion in rectal cancer: multi-readers' diagnostic performance. Eur Radiol. 2019;29:4379–4388. doi: 10.1007/s00330-018-5926-9. [DOI] [PubMed] [Google Scholar]
- 45.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X, Song W, Guo D, Liu H, Zhang H, He X, Song J, Zhou J, Liu X. Preoperative Prediction of Extramural Venous Invasion in Rectal Cancer: Comparison of the Diagnostic Efficacy of Radiomics Models and Quantitative Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Front Oncol. 2020;10:459. doi: 10.3389/fonc.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lino-Silva LS, Salcedo-Hernández RA, España-Ferrufino A, Ruiz-García EB, Ruiz-Campos M, León-Takahashi AM, Meneses-García A. Extramural perineural invasion in pT3 and pT4 rectal adenocarcinoma as prognostic factor after preoperative chemoradiotherapy. Hum Pathol. 2017;65:107–112. doi: 10.1016/j.humpath.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Chen Y, Zheng D, Pang P, Zhang H, Zheng X, Liao J. Pretreatment MR-based radiomics nomogram as potential imaging biomarker for individualized assessment of perineural invasion status in rectal cancer. Abdom Radiol (NY) 2021;46:847–857. doi: 10.1007/s00261-020-02710-4. [DOI] [PubMed] [Google Scholar]
- 49.Butte JM, Gonen M, Ding P, Goodman KA, Allen PJ, Nash GM, Guillem J, Paty PB, Saltz LB, Kemeny NE, Dematteo RP, Fong Y, Jarnagin WR, Weiser MR, D'Angelica MI. Patterns of failure in patients with early onset (synchronous) resectable liver metastases from rectal cancer. Cancer. 2012;118:5414–5423. doi: 10.1002/cncr.27567. [DOI] [PubMed] [Google Scholar]
- 50.Shu Z, Fang S, Ding Z, Mao D, Cai R, Chen Y, Pang P, Gong X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci Rep. 2019;9:3374. doi: 10.1038/s41598-019-39651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Ma X, Shen F, Xia Y, Jia Y, Lu J. MRI-based radiomics nomogram to predict synchronous liver metastasis in primary rectal cancer patients. Cancer Med. 2020;9:5155–5163. doi: 10.1002/cam4.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckers RCJ, Lambregts DMJ, Schnerr RS, Maas M, Rao SX, Kessels AGH, Thywissen T, Beets GL, Trebeschi S, Houwers JB, Dejong CH, Verhoef C, Beets-Tan RGH. Whole liver CT texture analysis to predict the development of colorectal liver metastases-A multicentre study. Eur J Radiol. 2017;92:64–71. doi: 10.1016/j.ejrad.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clément O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556–561. doi: 10.1148/radiology.218.2.r01fe10556. [DOI] [PubMed] [Google Scholar]
- 55.Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20:6113–6122. doi: 10.3748/wjg.v20.i20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang M, Cai Z, Zhang H, Huang C, Meng Y, Zhao L, Li D, Ma X, Zhao X. Machine Learning-based Analysis of Rectal Cancer MRI Radiomics for Prediction of Metachronous Liver Metastasis. Acad Radiol. 2019;26:1495–1504. doi: 10.1016/j.acra.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouché O, Landi B, Louvet C, André T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 58.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 59.Guo XF, Yang WQ, Yang Q, Yuan ZL, Liu YL, Niu XH, Xu HB. Feasibility of MRI Radiomics for Predicting KRAS Mutation in Rectal Cancer. Curr Med Sci. 2020;40:1156–1160. doi: 10.1007/s11596-020-2298-6. [DOI] [PubMed] [Google Scholar]
- 60.Meng X, Xia W, Xie P, Zhang R, Li W, Wang M, Xiong F, Liu Y, Fan X, Xie Y, Wan X, Zhu K, Shan H, Wang L, Gao X. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019;29:3200–3209. doi: 10.1007/s00330-018-5763-x. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y, Xu Q, Sun H, Liu T, Shi K, Wang W. Could IVIM and ADC help in predicting the KRAS status in patients with rectal cancer? Eur Radiol. 2018;28:3059–3065. doi: 10.1007/s00330-018-5329-y. [DOI] [PubMed] [Google Scholar]
- 62.Oh JE, Kim MJ, Lee J, Hur BY, Kim B, Kim DY, Baek JY, Chang HJ, Park SC, Oh JH, Cho SA, Sohn DK. Magnetic Resonance-Based Texture Analysis Differentiating KRAS Mutation Status in Rectal Cancer. Cancer Res Treat. 2020;52:51–59. doi: 10.4143/crt.2019.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Y, Liu H, Ren J, Du X, Xin L, Li D, Yang X, Wang D. Development and validation of a MRI-based radiomics signature for prediction of KRAS mutation in rectal cancer. Eur Radiol. 2020;30:1948–1958. doi: 10.1007/s00330-019-06572-3. [DOI] [PubMed] [Google Scholar]
- 64.Taguchi N, Oda S, Yokota Y, Yamamura S, Imuta M, Tsuchigame T, Nagayama Y, Kidoh M, Nakaura T, Shiraishi S, Funama Y, Shinriki S, Miyamoto Y, Baba H, Yamashita Y. CT texture analysis for the prediction of KRAS mutation status in colorectal cancer via a machine learning approach. Eur J Radiol. 2019;118:38–43. doi: 10.1016/j.ejrad.2019.06.028. [DOI] [PubMed] [Google Scholar]