Abstract
The overexpression of the human ATP-binding cassette (ABC) drug transporter ABCB1 (P-glycoprotein, P-gp) or ABCG2 (breast cancer resistance protein, BCRP) in cancer cells often contributes significantly to the development of multidrug resistance (MDR) in cancer patients. Previous reports have demonstrated that some epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) could modulate the activity of ABCB1 and/or ABCG2 in human cancer cells, whereas some EGFR TKIs are transport substrates of these transporters. Almonertinib (HS-10296) is a promising, orally available third-generation EGFR TKI for the treatment of EGFR T790M mutation-positive non-small cell lung cancer (NSCLC) in patients who have progressed on or after other EGFR TKI therapies. Additional clinical trials are currently in progress to study almonertinib as monotherapy and in combination with other agents in patients with NSCLC. In the present work, we found that neither ABCB1 nor ABCG2 confers significant resistance to almonertinib. More importantly, we discovered that almonertinib was able to reverse MDR mediated by ABCB1, but not ABCG2, in multidrug-resistant cancer cells at submicromolar concentrations by inhibiting the drug transport activity of ABCB1 without affecting its expression level. These findings are further supported by in silico docking of almonertinib in the drug-binding pocket of ABCB1. In summary, our study revealed an additional activity of almonertinib to re-sensitize ABCB1-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic drugs, which may be beneficial for cancer patients and warrant further investigation.
Keywords: ABCB1, ABCG2, Multidrug resistance, HS-10296, Almonertinib
1. Introduction
Human ABCB1 (MDR1/P-glycoprotein) and ABCG2 (BCRP/MXR/ABCP) are ATP-binding cassette (ABC) transporters that are able to derive energy from ATP hydrolysis to transport a large variety of therapeutic agents across biological membranes [1–3]. Together, these two drug transporters are capable of effluxing key conventional cytotoxic anticancer drugs such as Vinca alkaloids, anthracyclines and taxanes, as well as molecularly targeted therapeutic agents including nilotinib, gefitinib and ricolinostat, out of cancer cells [3–9]. Consequently, the overexpression of ABCB1 and/or ABCG2 is associated with multidrug resistance (MDR) [3, 4] and poor clinical outcome in patients with multiple myeloma (MM) [10–12], chronic lymphocytic leukemia (CLL), acute lymphocytic leukemia (ALL), acute myelogenous leukemia (AML) [13–16] or metastatic breast cancer (MBC) [17].
Considering that cancer MDR remains one of the most serious obstacles in clinical cancer chemotherapy, developing therapeutic agents against the activity of ABCB1 and/or ABCG2 is therefore of great clinical significance [3, 4]. Previous attempts to develop novel synthetic inhibitors against ABCB1 and/or ABCG2 have met with less success, often due to adverse drug-drug interactions or the lack of selectivity [3, 18–21]. Consequently, there are currently no U.S. Food and Drug Administration (FDA)-approved drugs to treat MDR in cancer patients. Interestingly, several studies have reported previously that several human epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) interact strongly with ABCB1 and/or ABCG2, and were able to reverse MDR mediated by ABCB1 and ABCG2 [22–29]. Therefore, rather than synthesizing novel inhibitors, we explore the prospects of repurposing approved EGFR TKIs to resensitize ABCB1- and ABCG2-overexpressing multidrug-resistant cancer cells to conventional cytotoxic anticancer agents.
Almonertinib (HS-10296) is an orally available, third-generation EGFR TKI [30–32]. In 2020, the Chinese National Medical Products Administration (NMPA) approved the use of almonertinib for the treatment of EGFR T790M mutation-positive non-small cell lung cancer (NSCLC) in patients who have progressed on or after other EGFR TKI therapies, based on results from a phase II APOLLO study [33]. Moreover, almonertinib is currently being evaluated as monotherapy in clinical trials for patients with epidermal growth factor receptor mutation-positive, locally advanced or metastatic NSCLC (ClinicalTrials.gov Identifier: NCT03849768 and NCT02981108), EGFR mutation-positive locally advanced or metastatic pulmonary adenosquamous carcinoma (ARISE) (NCT04354961), and as a combination therapy in patients with EGFR-mutant NSCLC (NCT03904823).
In the present work, we examined the interaction between almonertinib and ABCB1 and ABCG2, as well as evaluating whether almonertinib could resensitize ABCB1- and ABCG2-overexpressing multidrug-resistant cancer cells to cytotoxic therapeutic drugs. We found that the overexpression of ABCB1 or ABCG2 had no significant effect on the cytotoxicity of almonertinib in human cancer cell lines. More importantly, we discovered that by selectively inhibiting the transport function of ABCB1, almonertinib was able to resensitize ABCB1-overexpressing cancer cells to drug-induced apoptosis and reverse ABCB1-mediated MDR in human cancer cell lines at submicromolar concentrations. In summary, our data indicate that modulation of ABCB1-mediated transport is an additional mode of action for almonertinib in combination therapies for patients with multidrug-resistant cancers and warrant further studies.
2. Materials and methods
2.1. Chemicals
Rosewell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s Modified Eagle’s Medium (DMEM), Iscove’s Modified Dulbecco’s Medium (IMDM), fetal calf serum (FCS), phosphate-buffered saline (PBS), trypsin-EDTA, penicillin, and streptomycin were obtained from Gibco, Invitrogen (Carlsbad, CA, USA). Fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA, USA). Almonertinib (HS-10296) was purchased from Selleckchem (Houston, TX, USA). Tools Cell Counting (CCK-8) kit was obtained from Biotools Co., Ltd. (Taipei, Taiwan). All other chemicals were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise.
2.2. Cell culture conditions
The human embryonic kidney HEK293 cell lines stably transfected with either empty pcDNA 3.1 vector (pcDNA3.1-HEK293), human ABCB1 (MDR19) [34] or human ABCG2 (482R- 5) [35] were maintained in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 2 mg/mL G418, and 100 units of penicillin/streptomycin/mL. The human ovarian cancer cell line OVCAR-8 and NCI-ADR-RES subline [36]; the human epidermal cancer cell line KB-3-1 and KB-V-1 subline [37], were maintained in DMEM supplemented with 10% FCS, 2 mM L-glutamine, and 100 units of penicillin/streptomycin/mL. NCI-ADR-RES cells and KB-V-1 cells were maintained in media containing 0.85 μM doxorubicin [36] and 1 mg/mL vinblastine [38], respectively. The human non-small cell lung cancer (NSCLC) cell line H460 and H460-MX20 subline [39]; the human NSCLC cell line A549 and A549-Bec150 subline [40], were maintained in RPMI-1640 supplemented with 10% FCS, 2 mM L-glutamine, and 100 units of penicillin/streptomycin/mL. H460-MX20 cells and A549-Bec150 cells were maintained in media containing 20 nM of mitoxantrone [41] or 150 nM of becatecarin [40], respectively. Cell cultures were screened periodically for mycoplasma contamination using TOOLS Mycoplasma Detection Kit. The KB-3-1, KB-V-1, OVCAR-8 and NCI-ADR-RES cell lines were generous gifts from Dr. Michael Gottesman (NCI, NIH, Bethesda, MD, USA). The H460, H460-MX20, A549, A549-Bec150, HEK293 and HEK293 transfected lines were generous gifts from Dr. Susan Bates (NCI, NIH, Bethesda, MD, USA). All cells were cultured at 37 °C in 5% CO2 humidified air and maintained in drug-free medium for 7 days before assay.
2.3. Cell viability assay
The cytotoxicity of almonertinib alone or in combination with other therapeutic drugs was determined by cytotoxic MTT assay [42] or Cell Counting Kit-8 (CCK-8) assay. In brief, cells were seeded in 96-well flat-bottom plates and allowed to attach for 24 h at 37 °C in 5% CO2 humidified air. Different concentrations of almonertinib or drug combinations were added into each plate with 0.5% (v/v) final concentration of DMSO in all wells and incubated for an additional 72 h before processed as described previously [43]. At least three independent experiments were performed to obtain the IC50 values, calculated using the fitted concentration-response curve of each drug regimen. The extent of chemosensitization by a modulator was presented as a fold-reversal (FR) value, determined by adding almonertinib or a reference inhibitor to the cytotoxicity assays as described previously [23].
2.4. Apoptosis assay
The effect of almonertinib on colchicine-induced apoptosis in cancer cells was determined by concurrent staining of annexin V–FITC and propidium iodide (PI) according to the manufacturer’s instructions (BD Pharmingen) and as previously described [44]. In short, KB-3-1 and KB-V-1 cells were treated with DMSO, 5 μM of almonertinib alone, 500 nM of colchicine alone, or the combination of 500 nM of colchicine and 5 μM of almonertinib as indicated for 48 h before stained with annexin V–FITC (1.25 μg/mL) and PI (0.1 mg/mL) for 15 min at room temperature. Labeled cells were analyzed by FACScan equipped with the CellQuest software (Becton-Dickinson Biosciences, San Jose, CA, USA) as described previously [25].
2.5. Fluorescent substrate accumulation assay
The effect of almonertinib on the intracellular accumulation of fluorescent calcein (485 nm excitation and 535 nm emission), a known substrate of ABCB1 [45], was determined according to the method described by Gribar et al. [46]. In brief, cells were first trypsinized and resuspended in IMDM containing 5% FCS before calcein was added to 3 × 105 cells in 4 mL of IMDM in the presence of DMSO (control), 20 μM of almonertinib or tariquidar. The relative fluorescence intensity was detected and analyzed using a FACSort flow cytometer equipped with the CellQuest software (Becton-Dickinson) and the FlowJo software (Tree Star, Inc., Ashland, OR, USA), as described previously [47, 48].
2.6. Immunoblotting
KB-V-1 and NCI-ADR-RES cancer cells were treated with DMSO (control) or almonertinib at 50 nM, 100 nM, 200 nM, or 500 nM for 72 h, harvested and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Membranes were incubated with anti-ABCB1 C219 (1:3000 dilution, #517310, Merck Millipore, Burlington, MA, USA) primary antibody to detect ABCB1 or anti-α-tubulin (1:100000 dilution, #T6199, Sigma-Aldrich, St. Louis, MO, USA) primary antibody to detect the positive loading control tubulin. Membranes were subsequently incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) secondary antibody (1:100000 dilution). Signals were detected using the enhanced chemiluminescence (ECL) kit (Merck Millipore, Billerica, MA, USA).
2.7. Docking analysis
AutoDock Vina [49] was used to dock almonertinib to the recently published cryo-EM structure of ABCB1 bound to taxol (pdb id: 6QEX)[50]. Proteins and ligands were prepared using the MGLtools software package (Scripps Research Institute)[51]. Table 1 shows the parameters used for docking. Analysis of the docked poses was performed using the Pymol molecular graphics system, Version 1.7 (Shrödinger, LLC, NY, USA).
Table 1.
Parameters used for docking of almonertinib to ABCB1.
| PDBID | 6QEX |
| Flexible residues | L65, M68, M69, F72, Q195, W232, F303, I306, Y307, Y310, F314, F336, L339, I340, F343, Q347, N721, Q725, F728, F732, F759, F770, F938, F942, Q946, M949, Y953, F957, L975, F978, V982, F983, M986, Q990, F993 and F994. |
| Center of the receptor grid | x = 19, y = 53, and z = 3 |
| Inner box dimensions | 44 Å × 44 Å × 44 Å |
| Exhaustiveness | 100 |
2.8. Quantification and statistical analysis
Experimental data are presented as mean ± standard deviation (SD) or as mean ± standard error of the mean (SEM) as indicated from at least three independent experiments. GraphPad Prism software (GraphPad Software, La Jolla, CA, USA) was used for curve plotting. KaleidaGraph software (Synergy Software, Reading, PA, USA) was used for statistical analysis. The difference between mean values of experimental and control or improvement in fit was analyzed by two-tailed Student’s t-test and labeled with asterisks as “statistically significant” if the probability, p, was less than 0.05.
3. Results
3.1. Multidrug-resistant cells overexpressing ABCB1 or ABCG2 are equally sensitive to almonertinib as drug-sensitive cells.
Previous studies have reported that selected EGFR inhibitors are substrates for ABCB1 and/or ABCG2 [52–58]. Therefore, we examined whether cells overexpressing ABCB1 or ABCG2 are less susceptible to almonertinib. The cytotoxicity of almonertinib was determined in HEK293 stably transfected with either empty pcDNA 3.1 vector (pcDNA3.1-HEK293), human ABCB1 (MDR19), or human ABCG2 (482R- 5), as well as in drug-sensitive KB-3-1 human epidermal cancer cell line and its ABCB1-overexpressing multidrug-resistant variant KB-V-1, drug-sensitive OVCAR-8 human ovarian cancer cell line and its ABCB1-overexpressing multidrug-resistant NCI-ADR-RES, drug-sensitive H460 human lung cancer cell line and its ABCG2-overexpressing multidrug-resistant H460-MX20 subline, and drug-sensitive A549 human NSCLC cell line and its ABCG2-overexpressing multidrug-resistant A549-Bec150 subline (Fig. 1). We found that cells overexpressing ABCB1 or ABCG2 are equally sensitive to almonertinib as their corresponding drug-sensitive parental cells (Table 2).
Fig. 1.
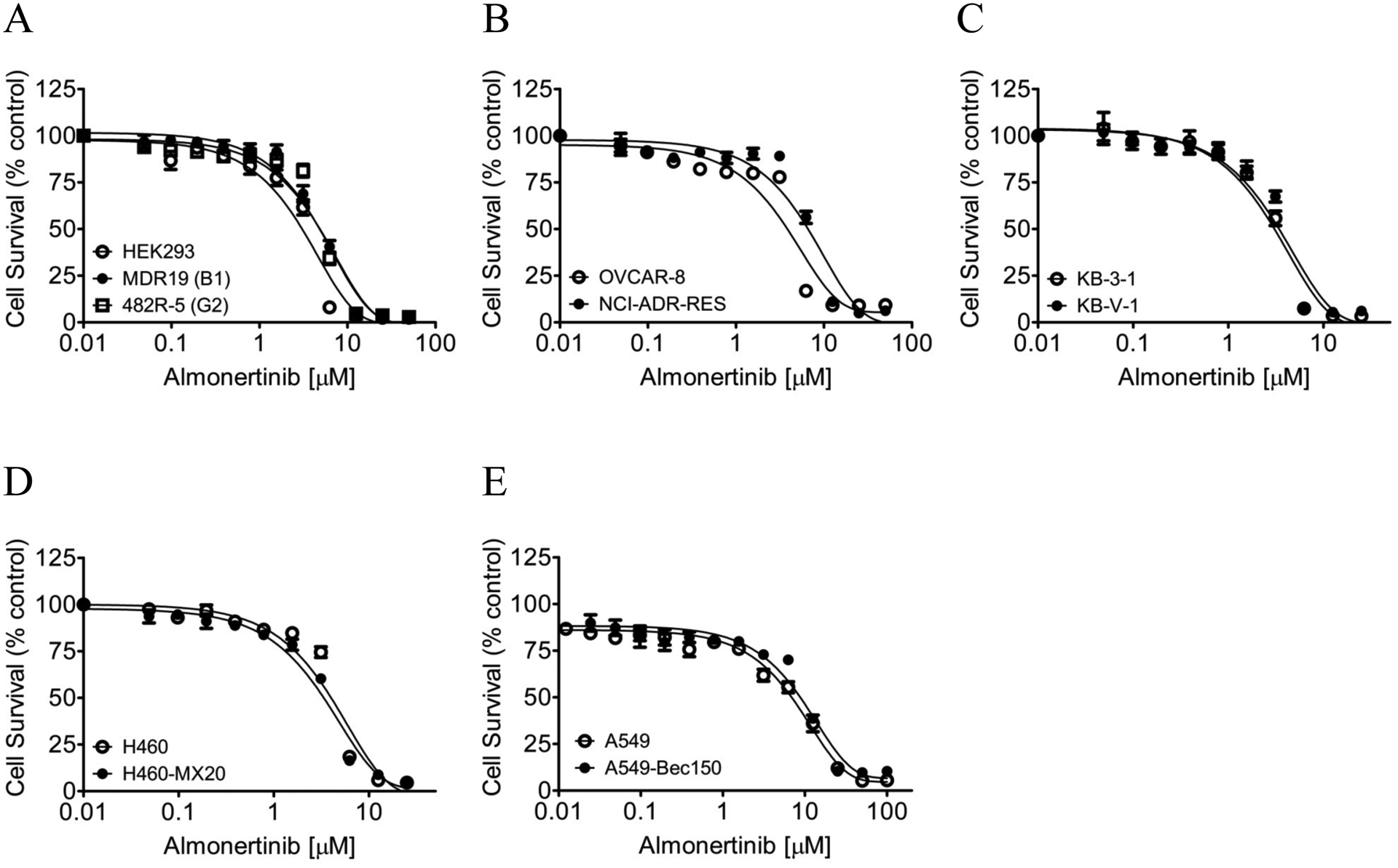
The multidrug-resistant cells overexpressing ABCB1 or ABCG2 are equally sensitive to almonertinib as their respective drug-sensitive parental cells. The cytotoxicity of almonertinib was determined in (A) parental pcDNA-HEK293 cells (open circles) and HEK293 cells transfected with human ABCB1 (MDR19, closed circles) or human ABCG2 (482R-5, open squares), (B) ABCB1-overexpressing multidrug-resistant NCI-ADR-RES human ovarian cancer cell line (closed circles) and its drug-sensitive parental OVCAR-8 cancer cell line (open circles), (C) ABCB1-overexpressing multidrug-resistant KB-V-1 human epidermal cancer cell line (closed circles) and its drug-sensitive parental KB-3-1 cancer cell line (open circles), (D) ABCG2-overexpressing multidrug-resistant H460-MX20 human lung cancer cell line (closed circles) and its drug-sensitive H460 cancer cell line (open circles), as well as (E) ABCG2-overexpressing multidrug-resistant A549-Bec150 human non-small lung cancer cell line (closed circles) and its drug-sensitive parental A549 cancer cell line (open circles). Points, mean values from at least three independent experiments; bars; S.E.M.
Table 2.
Sensitivity of drug-sensitive and multidrug-resistant cells overexpressing ABCB1 or ABCG2 to the epidermal growth factor receptor (EGFR) inhibitor almonertinib.
| Cell line | Type | Transporter expressed | IC50 (μM)† |
|---|---|---|---|
| pcDNA3.1-HEK293 | - | - | 2.76 ± 0.82 |
| MDR19 | - | ABCB1 | 4.19 ± 1.03 |
| 482R-5 | - | ABCG2 | 4.50 ± 1.36 |
| KB-3-1 | epidermal | - | 2.54 ± 0.73 |
| KB-V-1 | epidermal | ABCB1 | 2.94 ± 0.92 |
| OVCAR-8 | ovarian | - | 3.89 ± 1.20 |
| NCI-ADR-RES | ovarian | ABCB1 | 6.37 ± 1.97 |
| H460 | lung | - | 3.57 ± 1.09 |
| H460-MX20 | lung | ABCG2 | 3.06 ± 0.71 |
| A549 | lung | - | 7.75 ± 1.09 |
| A549-Bec150 | lung | ABCG2 | 9.73 ± 1.73 |
IC50 values are mean ± SD calculated from dose-response curves obtained from at least three independent experiments using cytotoxicity assay as described in Materials and methods.
3.2. Almonertinib resensitizes ABCB1-overexpressing cancer cells to therapeutic drugs
Previous reports have demonstrated that some EGFR inhibitors are capable of reversing ABCB1- and/or ABCG2-overexpressing multidrug-resistant cancer cells to conventional cytotoxic anticancer agents [22–29]. Therefore, we examined the potential chemosensitizing of almonertinib in multidrug-resistant cells overexpressing ABCB1 or ABCG2. We found that almonertinib significantly reversed ABCB1-mediated resistance to its substrates paclitaxel (Fig. 2), vincristine and colchicine [59] in ABCB1-overexpressing NCI-ADR-RES, KB-V-1, and MDR19 cell lines at submicromolar concentrations (Table 3). In contrast, we found that almonertinib had no significant effect on ABCG2-mediated resistance to ABCG2 substrates topotecan, SN-38, or mitoxantrone [60, 61] in ABCG2-overexpressing H460-MX20, A549-Bec150 and 482R-5 cell lines (Table 4). Of note, the fold-reversal (FR) value in Table 3 and Table 4 [23] represents the degree of resensitization of a particular cell line to a particular therapeutic drug by almonertinib or by the ABCB1 reference inhibitor verapamil or the ABCG2 reference inhibitor Ko143. Moreover, almonertinib did not significantly affect the proliferation of drug-sensitive parental cells at submicromolar concentrations. It is worth noting that consistent with previous reports illustrating an enhanced cytotoxic effect of vincristine by verapamil at nontoxic concentrations [62, 63], we also observed that verapamil amplified the toxicity of vincristine in drug-sensitive cancer cell lines (Table 3). Our results revealed that almonertinib selectively reversed MDR in ABCB1-overexpressing cancer cells. However, this drug had no effect on ABCG2-mediated drug resistance suggesting that almonertinib is not interacting with ABCG2 in the tested concentration range.
Fig. 2.
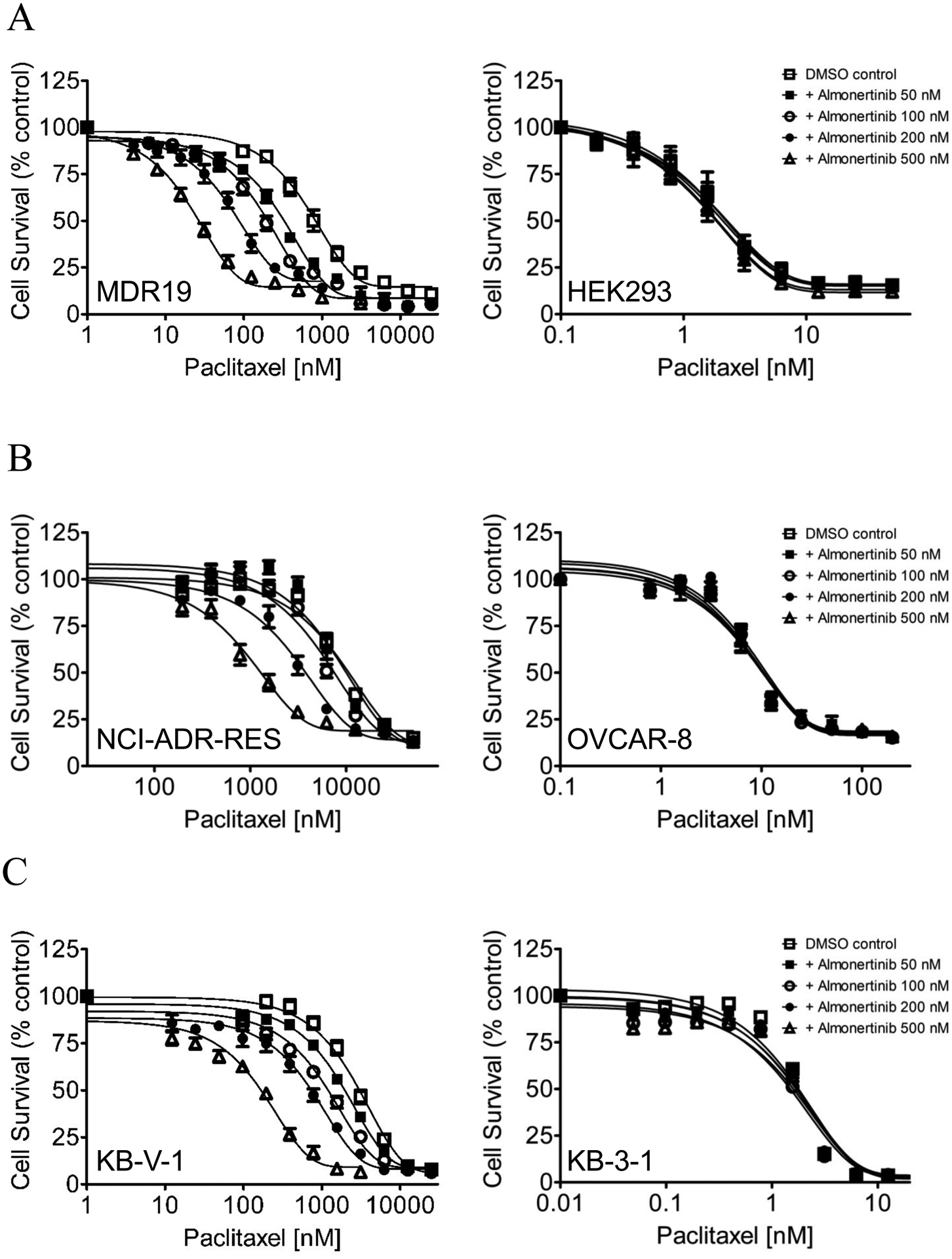
Almonertinib resensitizes ABCB1-overexpressing multidrug-resistant cells to paclitaxel in a concentration-dependent manner. The chemosensitizing effect of almonertinib on paclitaxel resistance mediated by ABCB1 was examined in (A) HEK293 cells transfected with human ABCB1 (MDR1, left panel) and parental HEK293 cells (right panel), (B) ABCB1-overexpressing NCI-ADR-RES cancer cell line (left panel), and its drug-sensitive parental OVCAR-8 cancer cell line (right panel), as well as (C) ABCB1-overexpressing KB-V-1 cancer cell line (left panel) and its drug-sensitive parental KB-3-1 cancer cell line (right panel). Cells were treated with various concentrations of paclitaxel in the presence of DMSO (open squares) or almonertinib at 50 nM (closed squares), 100 nM (open circles), 200 nM (closed circles), or 500 nM (open triangles). Points, mean values from at least three independent experiments; bars; S.E.M.
Table 3.
Chemosensitizing effect of almonertinib on multidrug resistance mediated by ABCB1 in ABCB1-overexpressing human cell lines.
| Mean IC50† ± SD and (FR‡) | |||
|---|---|---|---|
| Treatment | Concentration (nM) |
OVCAR-8 (parental) [nM] |
NCI-ADR-RES (resistant) [μM] |
| Paclitaxel | - | 10.60 ± 2.46 (1.0) | 10.20 ± 1.91 (1.0) |
| + almonertinib | 50 | 10.47 ± 2.52 (1.0) | 8.78 ± 2.21 (1.2) |
| + almonertinib | 100 | 10.04 ± 2.41 (1.1) | 6.76 ± 1.26 (1.5) |
| + almonertinib | 200 | 10.50 ± 3.01 (1.0) | 3.82 ± 0.59** (2.7) |
| + almonertinib | 500 | 10.31 ± 2.74 (1.0) | 1.54 ± 0.35** (6.6) |
| + verapamil | 5000 | 3.85 ± 1.12* (2.8) | 0.39 ± 0.06*** (26.2) |
| [nM] | [μM] | ||
| Vincristine | - | 14.19 ± 1.89 (1.0) | 7.03 ± 1.31 (1.0) |
| + almonertinib | 50 | 12.33 ± 1.47 (1.2) | 6.14 ± 1.20 (1.1) |
| + almonertinib | 100 | 12.24 ± 1.42 (1.2) | 4.66 ± 0.92 (1.5) |
| + almonertinib | 200 | 11.36 ± 1.36 (1.3) | 3.33 ± 0.72* (2.1) |
| + almonertinib | 500 | 12.24 ± 1.75 (1.2) | 1.73 ± 0.43** (4.1) |
| + verapamil | 5000 | 2.91 ± 0.86*** (4.9) | 0.29 ± 0.06*** (24.2) |
| [nM] | [μM] | ||
| Colchicine | - | 20.86 ± 5.87 (1.0) | 3.34 ± 0.82 (1.0) |
| + almonertinib | 50 | 22.33 ± 6.76 (0.9) | 2.88 ± 0.73 (1.2) |
| + almonertinib | 100 | 24.23 ± 8.15 (0.9) | 2.68 ± 0.59 (1.2) |
| + almonertinib | 200 | 23.64 ± 7.04 (0.9) | 1.87 ± 0.55 (1.8) |
| + almonertinib | 500 | 21.91 ± 7.29 (1.0) | 1.12 ± 0.31* (3.0) |
| + verapamil | 5000 | 15.20 ± 3.33 (1.4) | 0.80 ± 0.25** (4.2) |
| Treatment | Concentration (nM) |
KB-3-1 (parental) [nM] |
KB-V-1 (resistant) [μM] |
| Paclitaxel | - | 1.55 ± 0.46 (1.0) | 2.73 ± 0.49 (1.0) |
| + almonertinib | 50 | 1.54 ± 0.42 (1.0) | 1.85 ± 0.18* (1.5) |
| + almonertinib | 100 | 1.42 ± 0.40 (1.1) | 1.18 ± 0.09** (2.3) |
| + almonertinib | 200 | 1.57 ± 0.44 (1.0) | 811.07 ± 93.83** [nM] (3.4) |
| + almonertinib | 500 | 1.55 ± 0.45 (1.0) | 182.60 ± 29.33*** [nM] (15.0) |
| + verapamil | 5000 | 1.31 ± 0.33 (1.2) | 44.21 ± 6.47*** [nM] (61.8) |
| [nM] | [nM] | ||
| Vincristine | - | 0.73 ± 0.21 (1.0) | 871.55 ± 140.69 (1.0) |
| + almonertinib | 50 | 0.79 ± 0.22 (0.9) | 806.07 ± 107.72 (1.1) |
| + almonertinib | 100 | 0.77 ± 0.20 (0.9) | 550.93 ± 80.46* (1.6) |
| + almonertinib | 200 | 0.79 ± 0.26 (0.8) | 182.56 ± 18.08** (4.8) |
| + almonertinib | 500 | 0.76 ± 0.16 (1.0) | 41.39 ± 5.09*** (21.1) |
| + verapamil | 5000 | 0.12 ± 0.03** (6.1) | 12.00 ± 2.11*** (72.6) |
| [nM] | [nM] | ||
| Colchicine | - | 9.97 ± 3.74 (1.0) | 988.30 ± 78.56 (1.0) |
| + almonertinib | 50 | 10.21 ± 3.67 (1.0) | 701.27 ± 98.35* (1.4) |
| + almonertinib | 100 | 10.14 ± 3.75 (1.0) | 598.14 ± 83.54** (1.7) |
| + almonertinib | 200 | 9.92 ± 3.66 (1.0) | 459.00 ± 44.63*** (2.2) |
| + almonertinib | 500 | 9.43 ± 3.41 (1.1) | 274.91 ± 46.86*** (3.6) |
| + verapamil | 5000 | 6.35 ± 2.38 (1.6) | 225.56 ± 41.34*** (4.4) |
| Treatment | Concentration (nM) |
pcDNA3.1-HEK293 (parental) [nM] |
MDR19 (resistant) [nM] |
| Paclitaxel | - | 2.24 ± 0.39 (1.0) | 879.51 ± 107.25 (1.0) |
| + almonertinib | 50 | 2.01 ± 0.36 (1.1) | 345.19 ± 28.18** (2.5) |
| + almonertinib | 100 | 2.06 ± 0.38 (1.1) | 207.16 ± 17.37*** (4.2) |
| + almonertinib | 200 | 1.69 ± 0.32 (1.3) | 98.61 ± 10.58*** (8.9) |
| + almonertinib | 500 | 1.61 ± 0.26 (1.4) | 28.89 ± 3.78*** (30.4) |
| + verapamil | 5000 | 1.57 ± 0.29 (1.4) | 8.68 ± 1.95*** (101.3) |
| [nM] | [nM] | ||
| Vincristine | - | 2.61 ± 0.35 (1.0) | 497.19 ± 79.78 (1.0) |
| + almonertinib | 50 | 2.67 ± 0.48 (1.0) | 228.25 ± 35.92** (2.2) |
| + almonertinib | 100 | 2.14 ± 0.39 (1.2) | 145.99 ± 30.22** (3.4) |
| + almonertinib | 200 | 1.99 ± 0.37 (1.3) | 76.93 ± 12.31*** (6.5) |
| + almonertinib | 500 | 1.61 ± 0.31* (1.6) | 25.81 ± 4.14*** (19.3) |
| + verapamil | 5000 | 0.61 ± 0.14*** (4.3) | 4.30 ± 0.93*** (115.63) |
| [nM] | [nM] | ||
| Colchicine | - | 16.14 ± 3.08 (1.0) | 238.81 ± 31.59 (1.0) |
| + almonertinib | 50 | 14.77 ± 3.28 (1.1) | 171.94 ± 35.83 (1.4) |
| + almonertinib | 100 | 13.66 ± 3.31 (1.2) | 116.51 ± 25.09** (2.0) |
| + almonertinib | 200 | 14.37 ± 3.04 (1.1) | 105.39 ± 21.28** (2.3) |
| + almonertinib | 500 | 13.81 ± 3.36 (1.2) | 65.42 ± 13.46*** (3.7) |
| + verapamil | 5000 | 15.71 ± 3.15 (1.0) | 69.84 ± 13.68** (3.4) |
Abbreviation: FR, fold-reversal.
IC50 values are mean ± SD calculated from dose-response curves obtained from at least three independent experiments using cytotoxicity assay as described in Materials and methods.
FR values were calculated by dividing IC50 values of cells treated with a particular chemotherapeutic drug by IC50 values of cells treated with the same chemotherapeutic drug in the presence of almonertinib or verapamil.
p < 0.05;
p < 0.01;
p < 0.001.
Table 4.
Chemosensitizing effect of almonertinib on multidrug resistance mediated by ABCG2 in ABCG2-overexpressing human cell lines.
| Mean IC50† ± SD and (FR‡) | |||
|---|---|---|---|
| Treatment | Concentration (nM) |
H460 (parental) [nM] |
H460-MX20 (resistant) [nM] |
| Topotecan | - | 58.55 ± 7.77 (1.0) | 1648.90 ± 465.88 (1.0) |
| + almonertinib | 50 | 54.50 ± 6.71 (1.1) | 1551.00 ± 433.33 (1.1) |
| + almonertinib | 100 | 54.10 ± 6.30 (1.1) | 1800.21 ± 503.43 (0.9) |
| + almonertinib | 200 | 50.22 ± 6.38 (1.2) | 1613.53 ± 451.17 (1.0) |
| + almonertinib | 500 | 46.73 ± 6.29 (1.3) | 1219.52 ± 331.87 (1.4) |
| + Ko143 | 1000 | 27.85 ± 5.03** (2.1) | 121.54 ± 40.90** (13.6) |
| [nM] | [nM] | ||
| SN-38 | - | 16.30 ± 2.03 (1.0) | 524.35 ± 137.66 (1.0) |
| + almonertinib | 50 | 16.91 ± 2.14 (1.0) | 454.85 ± 105.21 (1.2) |
| + almonertinib | 100 | 17.23 ± 2.39 (0.9) | 454.36 ± 108.72 (1.2) |
| + almonertinib | 200 | 16.47 ± 2.07 (1.0) | 372.35 ± 85.17 (1.4) |
| + almonertinib | 500 | 15.17 ± 2.14 (1.1) | 319.94 ± 84.68 (1.6) |
| + Ko143 | 1000 | 4.85 ± 1.08*** (3.4) | 12.34 ± 4.68** (42.5) |
| [nM] | [nM] | ||
| Mitoxantrone | - | 38.83 ± 8.37 (1.0) | 851.68 ± 113.26 (1.0) |
| + almonertinib | 50 | 41.02 ± 7.87 (0.9) | 937.54 ± 90.37 (0.9) |
| + almonertinib | 100 | 37.80 ± 7.53 (1.0) | 890.69 ± 116.69 (1.0) |
| + almonertinib | 200 | 34.67 ± 6.04 (1.1) | 726.15 ± 127.91 (1.2) |
| + almonertinib | 500 | 28.28 ± 5.21 (1.4) | 815.60 ± 168.21 (1.0) |
| + Ko143 | 1000 | 27.69 ± 7.59 (1.4) | 109.17 ± 43.52*** (7.8) |
| Treatment | Concentration (nM) |
A549 (parental) [μM] |
A549-Bec150 (resistant) [μM] |
| Topotecan | - | 0.53 ± 0.12 (1.0) | 1.79 ± 0.26 (1.0) |
| + almonertinib | 50 | 0.69 ± 0.18 (0.8) | 1.80 ± 0.30 (1.0) |
| + almonertinib | 100 | 0.61 ± 0.16 (0.9) | 1.52 ± 0.25 (1.2) |
| + almonertinib | 200 | 0.58 ± 0.15 (0.9) | 1.34 ± 0.23 (1.3) |
| + almonertinib | 500 | 0.71 ± 0.21 (0.7) | 1.42 ± 0.23 (1.3) |
| + Ko143 | 1000 | 0.28 ± 0.08* (1.9) | 0.12 ± 0.02*** (14.9) |
| [nM] | [nM] | ||
| SN-38 | - | 22.51 ± 6.36 (1.0) | 200.14 ± 44.67 (1.0) |
| + almonertinib | 50 | 22.92 ± 6.03 (1.0) | 220.71 ± 48.70 (0.9) |
| + almonertinib | 100 | 22.26 ± 6.19 (1.0) | 213.95 ± 48.30 (0.9) |
| + almonertinib | 200 | 24.24 ± 7.08 (0.9) | 200.75 ± 46.44 (1.0) |
| + almonertinib | 500 | 19.11 ± 5.22 (1.2) | 145.86 ± 33.70 (1.4) |
| + Ko143 | 1000 | 14.73 ± 4.23 (1.5) | 6.44 ± 1.80** (31.1) |
| [nM] | [nM] | ||
| Mitoxantrone | - | 7.07 ± 1.93 (1.0) | 471.92 ± 111.19 (1.0) |
| + almonertinib | 50 | 6.74 ± 1.93 (1.0) | 528.13 ± 116.32 (0.9) |
| + almonertinib | 100 | 6.81 ± 2.09 (1.0) | 430.80 ± 87.59 (1.1) |
| + almonertinib | 200 | 7.14 ± 2.17 (1.0) | 324.75 ± 68.12 (1.5) |
| + almonertinib | 500 | 6.61 ± 2.11 (1.1) | 336.82 ± 66.32 (1.4) |
| + Ko143 | 1000 | 5.01 ± 1.51 (1.4) | 46.15 ± 10.30** (10.2) |
| Treatment | Concentration (nM) |
pcDNA3.1-HEK293 (parental) [nM] |
482R-5 (resistant) [nM] |
| Topotecan | - | 30.06 ± 5.27 (1.0) | 672.58 ± 182.46 (1.0) |
| + almonertinib | 50 | 32.66 ± 5.61 (0.9) | 1010.80 ± 314.06 (0.7) |
| + almonertinib | 100 | 33.64 ± 6.86 (0.9) | 829.73 ± 321.37 (0.8) |
| + almonertinib | 200 | 30.81 ± 6.04 (1.0) | 537.47 ± 170.83 (1.3) |
| + almonertinib | 500 | 31.98 ± 6.07 (0.9) | 402.33 ± 174.25 (1.7) |
| + Ko143 | 1000 | 30.42 ± 5.81 (1.0) | 70.43 ± 9.31** (9.5) |
| [nM] | [nM] | ||
| SN-38 | - | 2.30 ± 0.51 (1.0) | 66.52 ± 10.34 (1.0) |
| + almonertinib | 50 | 2.79 ± 0.58 (0.8) | 65.17 ± 10.10 (1.0) |
| + almonertinib | 100 | 2.92 ± 0.76 (0.8) | 70.73 ± 8.39 (0.9) |
| + almonertinib | 200 | 3.41 ± 0.88 (0.7) | 56.77 ± 4.70 (1.2) |
| + almonertinib | 500 | 3.42 ± 1.08 (0.7) | 57.59 ± 5.95 (1.2) |
| + Ko143 | 1000 | 2.52 ± 0.60 (0.9) | 3.48 ± 0.83*** (19.1) |
| [nM] | [nM] | ||
| Mitoxantrone | - | 1.77 ± 0.21 (1.0) | 36.12 ± 6.15 (1.0) |
| + almonertinib | 50 | 1.37 ± 0.22 (1.3) | 37.04 ± 6.85 (1.0) |
| + almonertinib | 100 | 1.36 ± 0.18 (1.3) | 31.91 ± 6.33 (1.1) |
| + almonertinib | 200 | 1.41 ± 0.23 (1.3) | 32.42 ± 6.57 (1.1) |
| + almonertinib | 500 | 2.15 ± 0.27 (0.8) | 25.83 ± 7.07 (1.4) |
| + Ko143 | 1000 | 2.08 ± 0.27 (0.9) | 3.86 ± 1.34*** (9.4) |
Abbreviation: FR, fold-reversal.
IC50 values are mean ± SD calculated from dose-response curves obtained from at least three independent experiments using cytotoxicity assay as described in Materials and methods.
FR values were calculated by dividing IC50 values of cells treated with a particular chemotherapeutic drug by IC50 values of cells treated with the same chemotherapeutic drug in the presence of almonertinib or Ko143.
p < 0.05;
p < 0.01;
p < 0.001.
3.3. Almonertinib preserves the effect of drug-induced apoptosis in ABCB1-overexpressing multidrug-resistant cancer cells
Growth retardation initiated by almonertinib could also lead to the seemingly resensitization of ABCB1-overexpressing cancer cells. Therefore, in order to confirm that almonertinib reverses ABCB1-mediated MDR was by restoring the cytotoxicity of therapeutic drugs in ABCB1-overexpressing cancer cells, we examined the effect of almonertinib on drug-induced apoptosis. Parental KB-3-1 and ABCB1-expressing KB-V-1 cancer cells were treated with 0.5 μM of colchicine, a known inducer of apoptosis [64] and a substrate of ABCB1 [59], 1 μM of tariquidar, a known inhibitor of ABCB1 [65], or 5 μM of almonertinib alone or in combinations for 48 h before processed as detailed in Materials and methods. As shown in Fig. 3, the extent of total apoptosis induced by colchicine increased substantially from 6% basal to approximately 51% in KB-3-1 cells, in contrast to an increase of merely 2% in ABCB1-overexpressing KB-V-1 cells. More importantly, we found that without inducing apoptosis itself, the colchicine-induced apoptosis was significantly restored in KB-V-1 cancer cells by tariquidar (from 8% basal to approximately 56% total apoptosis) and almonertinib (from 8% basal to approximately 51% total apoptosis).
Fig. 3.
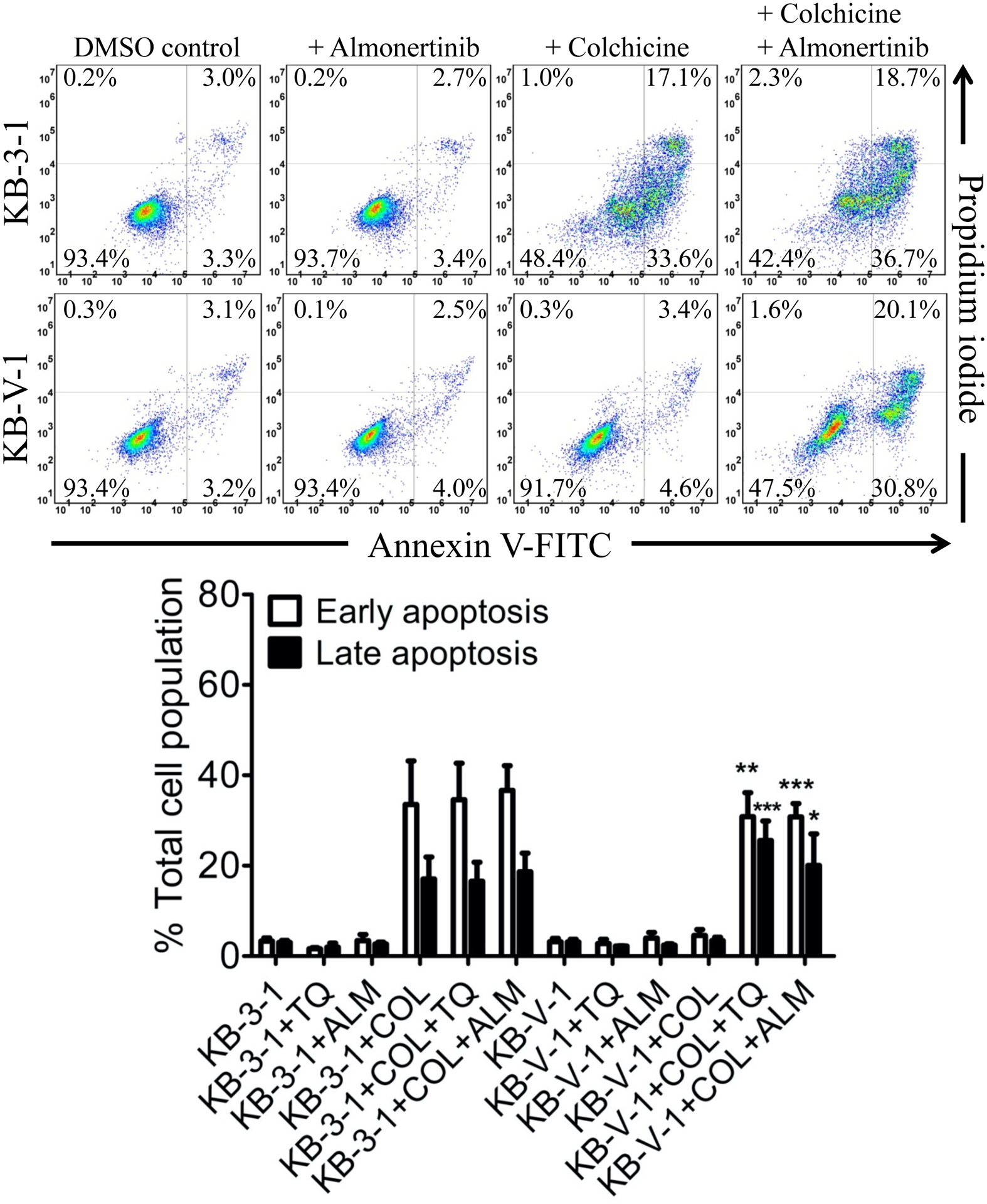
Almonertinib resensitizes ABCB1-overexpressing multidrug-resistant KB-V-1 cancer cells to drug-induced apoptosis. Drug-sensitive KB-3-1 and multidrug-resistant KB-V-1 cancer cells were treated with either DMSO (control), 5 μM of almonertinib (+ALM), 500 nM of colchicine (+COL), or a combination of 500 nM of colchicine with 5 μM of almonertinib (+COL +ALM) and analyzed by flow cytometry as described in Materials and methods. The quantification data are presented as mean ± SD calculated from at least three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001, versus the same treatment in the absence of tariquidar or almonertinib.
3.4. Almonertinib inhibits the drug efflux function of ABCB1 without affecting its expression in cancer cells
Two of the most common mechanisms for reversing ABCB1-mediated MDR in cancer cells are transiently inhibiting the transport function of ABCB1 and down-regulating the protein expression of ABCB1 in cancer cells [66–68]. Therefore, we examined the effect of almonertinib on ABCB1-mediated drug efflux and the protein expression of this transporter in KB-V-1 cancer cells. ABCB1-overexpressing KB-V-1 and NCI-ADR-RES cancer cells, as well as ABCB1-transfected MDR19 cells, were treated with calcein-AM in the absence or presence of almonertinib or verapamil and processed as described in Materials and methods. We found that 20 μM of almonertinib completely inhibited ABCB1-mediated efflux of calcein-AM and increased the intracellular accumulation of calcein, a fluorescent product of an ABCB1 substrate calcein-AM [45], in ABCB1-overexpressing MDR19 (Fig. 4A), NCI-ADR-RES (Fig. 4B) and KB-V-1 (Fig. 4C) cells, and in a concentration-dependent manner (Fig. 4D), with the IC50 values of approximately 2, 4 and 8 μM, respectively. Of note, 20 μM of almonertinib and 20 μM of verapamil alone had no significant effect on the intracellular accumulation of calcein in parental cell lines (Figs. 4A – C, right panels). Next, we examined the effect of almonertinib on ABCB1 protein expression by treating NCI-ADR-RES and KB-V-1 cancer cells with increasing concentrations of almonertinib (0 – 500 nM) for 72 h followed by Western blot analysis as described in Materials and methods. We found that the protein expression of ABCB1 was not significantly affected by almonertinib over a period of 72 h in NCI-ADR-RES (Fig. 5A) and KB-V-1 (Fig. 5B) cancer cells.
Fig. 4.
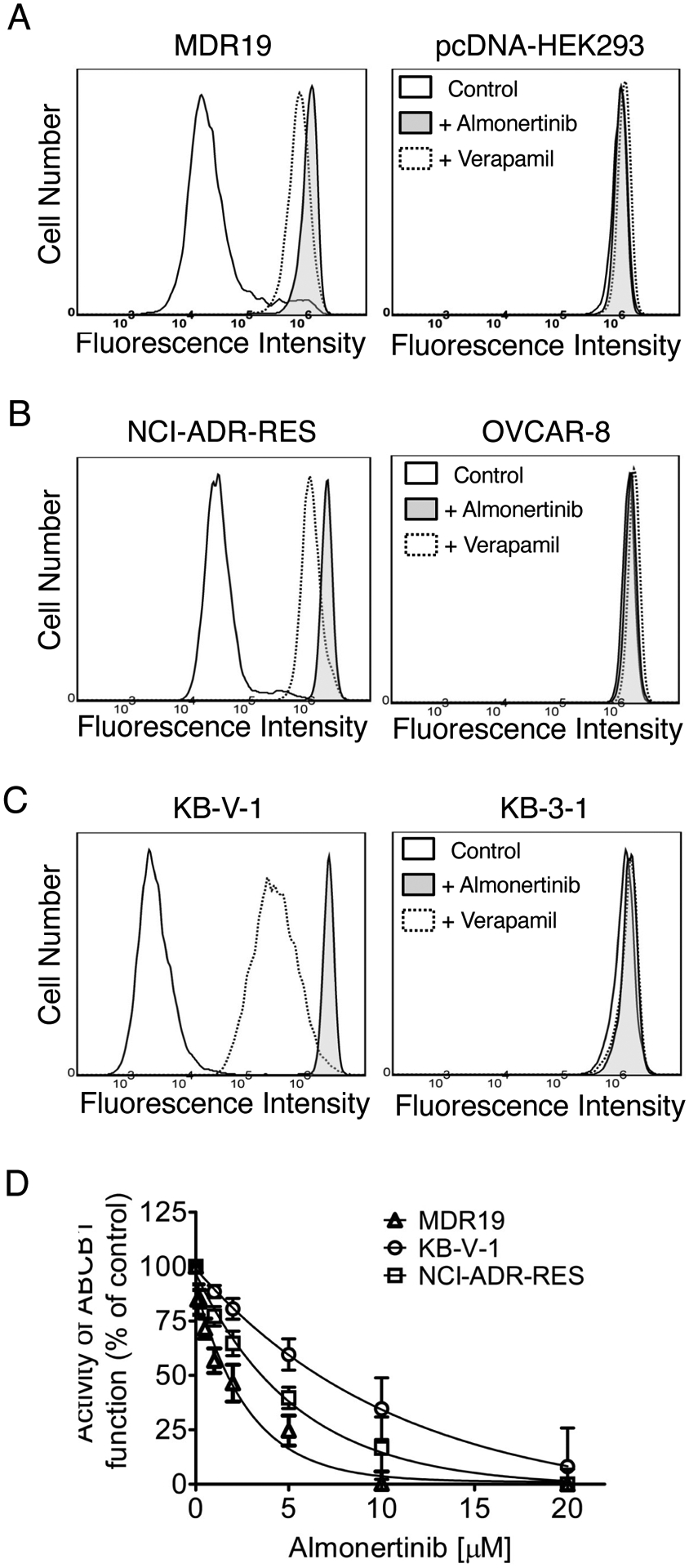
Almonertinib inhibits ABCB1-mediated transport of calcein-AM. (A) HEK293 cells transfected with human ABCB1 (MDR19, left panel), (B) ABCB1-overexpressing NCI-ADR-RES cancer cells (left panel), and (C) ABCB1-overexpressing KB-V-1 cancer cells (left panel), as well as in respective parental cells (A-C, right panels). Cells were treated with 0.25 μM calcein-AM and DMSO (A-C, solid line), 20 μM of almonertinib (A-C, filled solid line), or 20 μM of verapamil (A-C, dotted line) as a positive control for ABCB1. Representative histograms of at least three independent experiments are shown. (D) Effect of increasing concentrations (0 – 20 μM) of almonertinib on ABCB1-mediated calcein-AM efflux in MDR19 (open triangles), KB-V-1 (open circles), and NCI-ADR-RES (open squares) cells. Points, mean values from at least three independent experiments; bars; S.D.
Fig. 5.
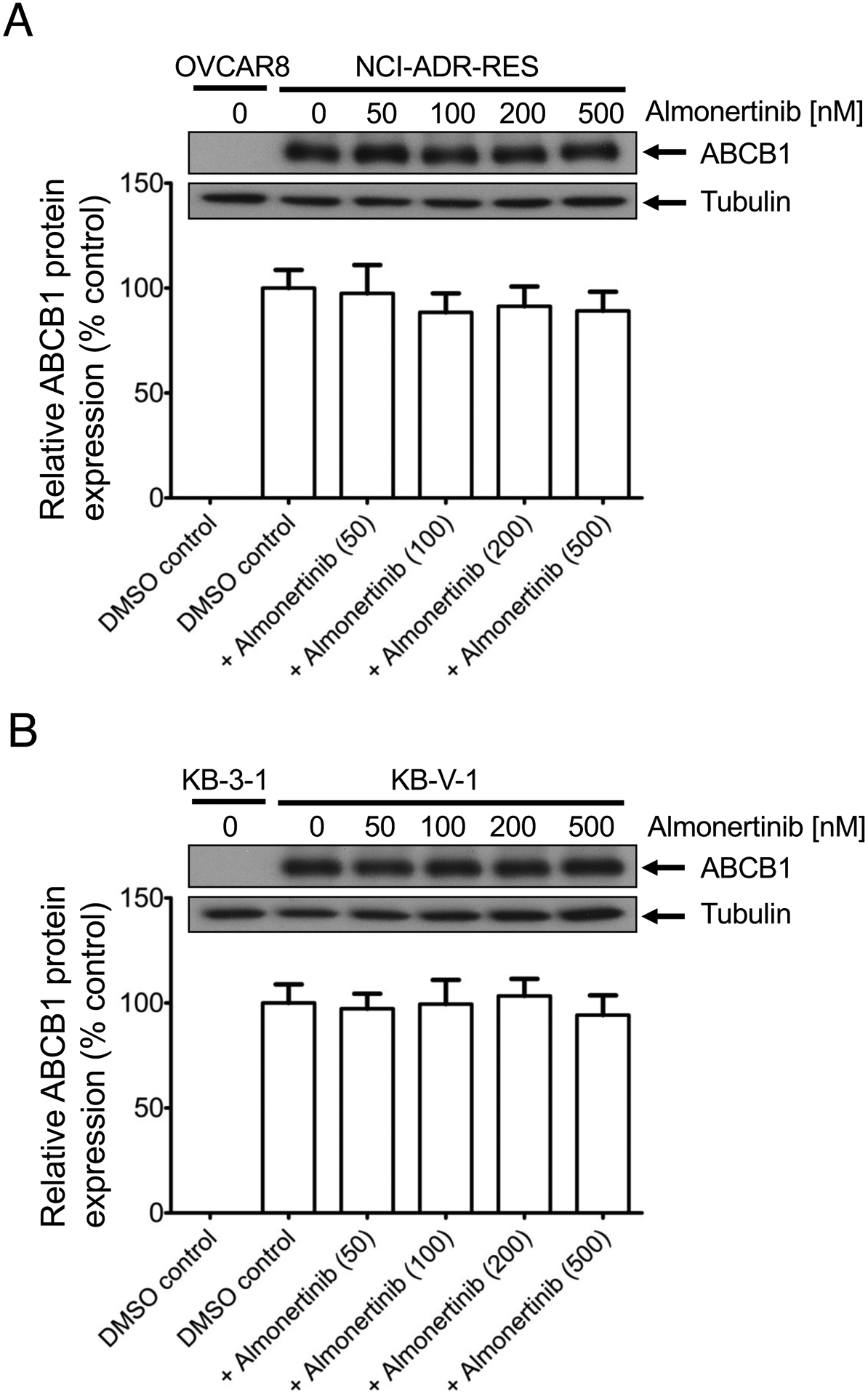
Almonertinib does not significantly affect the protein expression of ABCB1 in human (A) NCI-ADR-RES or (B) KB-V-1 cancer cells. Cells were treated with DMSO (vehicle control) or almonertinib at 50 nM, 100 nM, 200 nM, or 500 nM for 72 h and processed for Western blotting as described in Materials and methods. The representative immunoblots (upper panel) and the corresponding quantification (lower panel) human ABCB1 protein are shown. α-Tubulin was used as an internal loading control. Values are presented as mean ± S.D. calculated from at least three independent experiments.
3.5. Docking of almonertinib in the drug-binding pocket of ABCB1
Knowing that almonertinib blocks ABCB1-mediated drug efflux (Fig. 4), we performed docking studies of almonertinib to ABCB1 to identify the potential site(s) where almonertinib interacts with the drug-binding pocket of ABCB1. The most energy favorable docking pose of almonertinib with human ABCB1 protein structure (pdb.6QEX) is shown in Fig. 6. The nine lowest energy poses had scores ranging from −10.0 to −10.4 kcal/mol. This inward-open structure of ABCB1 is the conformation with the binding of substrate Taxol (paclitaxel) in the drug-binding pocket. These in silico results indicated several hydrophobic and aromatic interactions between almonertinib and the residues within the transmembrane domain of ABCB1.
Fig. 6.
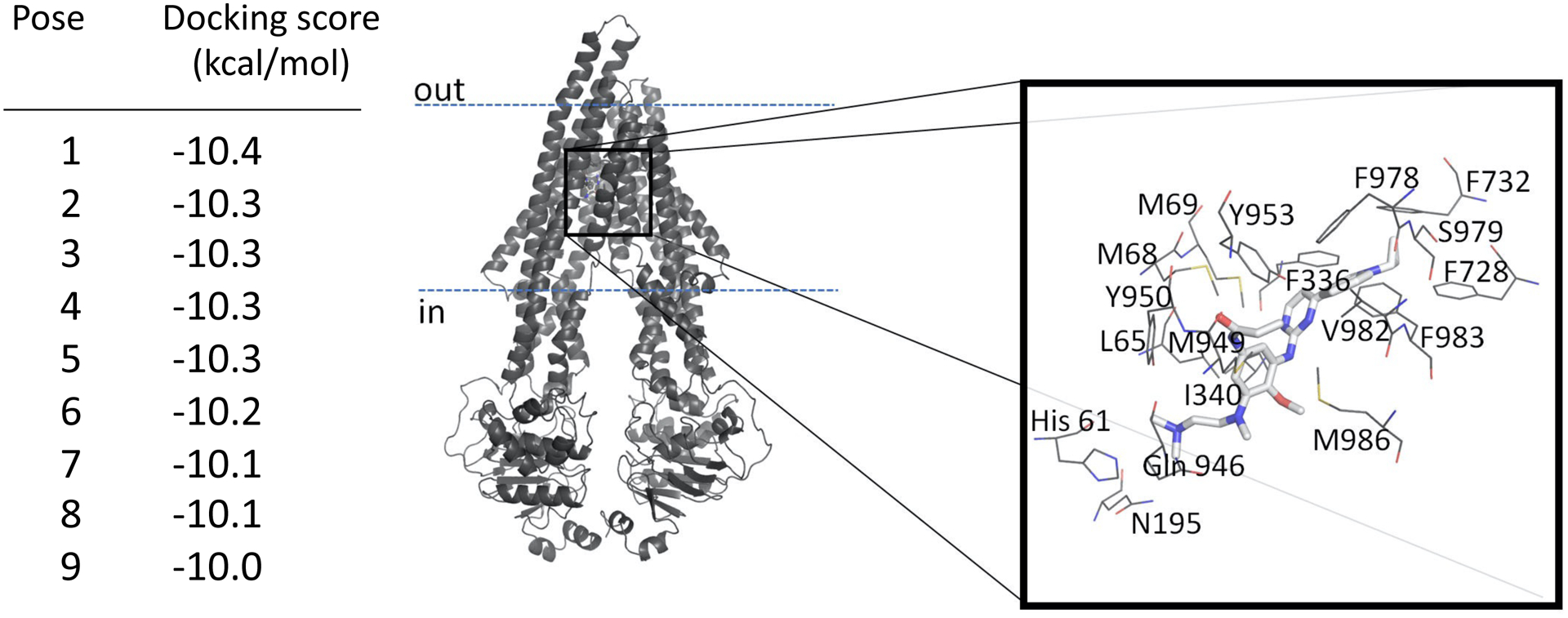
Docking of almonertinib in the drug-binding pocket of ABCB1. The AutoDock Vina software was used to dock almonertinib in the substrate-binding site of ABCB1 as described in the methods section. Cryo-EM structure of ligand-bound ABCB1 in the inward-open conformation (pdb id.6QEX) was used for the docking. The lowest energy pose of almonertinib bound to ABCB1 is presented in stick representation. Residues within 4.5Å of the ligand are shown in line representation. Colors as follow carbon-gray, nitrogen-blue, oxygen-red, hydrogen-white, sulfur-yellow. The energy values of the lowest nine dockings are presented on the left. The figure was prepared using Pymol molecular graphics system.
4. Discussion
The development of MDR against cytotoxic anticancer drugs in cancer cells is often mediated by the overexpression of ABCB1, a well-known factor that is associated with poor therapeutic response in patients receiving conventional anticancer drugs [3, 69]. Despite tremendous efforts in developing novel synthetic inhibitors of ABCB1, MDR mediated by ABCB1 remains a substantial obstacle in cancer chemotherapy due to the lack of clinically safe and effective modulators [3, 21, 69–71]. Alternatively, we and others have discovered that numerous TKIs, including EGFR inhibitors, could reverse ABCB1-mediated MDR in cancer cells through inhibition of its drug efflux function [25, 28, 72–76]. Interestingly, studies have suggested that the co-administration of FDA-approved TKIs could be beneficial for cancer patients undergoing conventional chemotherapy. For instance, in trials comparing the outcome of monotherapy to combination-therapy, the combination-therapy of gemcitabine with erlotinib was superior to monotherapy with gemcitabine in patients with advanced pancreatic cancer [77, 78], while the combination-therapy of capecitabine with lapatinib was superior to monotherapy with capecitabine in patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer [79, 80]. Moreover, promising results from a more recent phase I trial of using doxorubicin with nilotinib as a co-adjuvant treatment to inhibit the drug efflux function of ABCB1 in patients with sarcomas [81] signify the importance of using combination therapies against multidrug-resistant cancers. Collectively, these findings prompted us to investigate the interaction between almonertinib and the MDR-linked ABC drug transporters ABCB1 and ABCG2.
Knowing that some of the EGFR inhibitors, such as gefitinib [52, 53], afatinib [55] and osimertinib [56], are substrates of ABCB1 and/or ABCG2 [57], we compared the chemosensitivity of multidrug-resistant cells overexpressing ABCB1 or ABCG2 and respective drug-sensitive parental cells to almonertinib. We discovered that cells overexpressing ABCB1 or ABCG2 are equally sensitive to almonertinib (Fig. 1). Although the effect of prolonged treatment with almonertinib on ABCB1 and ABCG2 in patients remains to be determined in clinical studies, our results here suggest that almonertinib is not rapidly effluxed by ABCB1 or ABCG2, and both transporters are unlikely to contribute significantly to the development of almonertinib resistance in cancer patients. Moreover, studies have also reported that some EGFR inhibitors, such as dacomitinib, olmutinib, and rociletinib, could inhibit the drug efflux function of ABCB1 and ABCG2, thus re-sensitize ABCB1- and ABCG2-overexpressing cancer cells to various cytotoxic drugs [27–29]. Similar to those findings, we discovered that at subtoxic concentrations (< 1 μM), almonertinib was able to reverse MDR mediated by ABCB1 in multidrug-resistant cancer cells overexpressing this transporter and cells transfected with human ABCB1 (MDR19-HEK293) in a concentration-dependent manner (Table 3). However, almonertinib did not resensitize ABCG2-overexpressing multidrug-resistant cancer cells or cells transfected with human ABCG2 (482R- 5-HEK293) to known drug substrates of ABCG2 (Table 4), indicating that almonertinib is selective to ABCB1 relative to ABCG2. Interestingly, Cmax values of 522 ng/mL and 492 ng/mL were reported for 220 mg and 260 mg single dose of almonertinib, respectively [32], suggesting that almonertinib (50 – 500 nM) reversed ABCB1-mediated MDR at physiologically relevant concentrations. Furthermore, our data showed that almonertinib inhibits the drug efflux function of ABCB1 (Fig. 4), and consequently restores the susceptibility of ABCB1-overexpressing cancer cells to drug-induced apoptosis (Fig. 3) and resensitizes ABCB1-overexpressing cancer cells to cytotoxic drugs (Table 3). These findings are further supported by the in silico docking analysis of almonertinib binding to the drug-binding pocket of ABCB1 in the inward-open conformation (Fig. 6), indicating that almonertinib could outcompete another drug substrate for binding to the transmembrane domain of ABCB1 (Fig. 7).
Fig. 7.
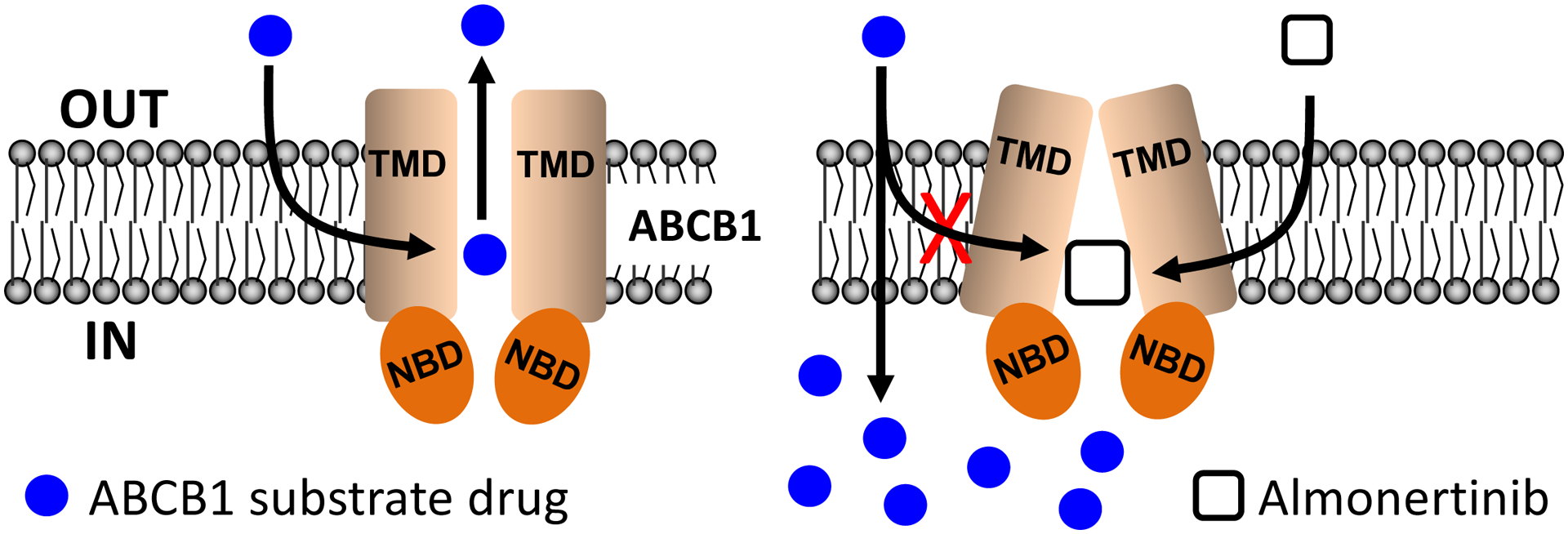
Schematic diagram showing almonertinib resensitizing ABCB1-overexpressing multidrug-resistant cancer cells to conventional anticancer drugs. The efficacy of ABCB1 substrate anticancer drugs (blue circles) is reduced by ABCB1-mediated drug transport in ABCB1-expressing cancer cells (left). In the presence of almonertinib (white square), almonertinib reduces ABCB1-mediated drug efflux by outcompeting the binding of ABCB1 substrate anticancer drugs to the drug-binding pocket of ABCB1, thus restoring the efficacy of these drugs in ABCB1-expressing cancer cells.
In summary, despite the potential adverse drug reactions that may occur in combination therapies and the presence of other mechanisms that may also contribute to MDR [3, 70, 71, 82], our data indicate that the third-generation EGFR TKI almonertinib is capable of modulating the drug efflux function of ABCB1 and reversing ABCB1-mediated MDR, and can potentially be used to battle against multidrug-resistant cancers associated with the overexpression of ABCB1. Concomitant administration of almonertinib with ABCB1 substrate cytotoxic anticancer drugs warrants further investigation.
Acknowledgments
This work was supported by the Ministry of Science and Technology of Taiwan (MOST-108-2320-B-182-035) and Chang Gung Medical Research Program (BMRPC17 and CMRPD1K0391). LS and SVA were supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations:
- ABC
ATP-binding cassette
- MDR
multidrug resistance
- EGFR
epidermal growth factor receptor
- Vi
sodium orthovanadate
- FR
fold-reversal
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- [1].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Targeting multidrug resistance in cancer, Nature reviews 5(3) (2006) 219–34. [DOI] [PubMed] [Google Scholar]
- [2].Wu C-P, Hsieh C-H, Wu Y-S, The Emergence of Drug Transporter-Mediated Multidrug Resistance to Cancer Chemotherapy, Molecular Pharmaceutics 8(6) (2011) 1996–2011. [DOI] [PubMed] [Google Scholar]
- [3].Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM, Revisiting the role of ABC transporters in multidrug-resistant cancer, Nat Rev Cancer 18(7) (2018) 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gottesman M, Ambudkar SV, Overview: ABC transporters and human disease., J Bioenerg Biomembr 33(6) (2001) 453–8. [DOI] [PubMed] [Google Scholar]
- [5].Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF, Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux, J Pharmacol Exp Ther 334(1) (2010) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shukla S, Skoumbourdis AP, Walsh MJ, Hartz AM, Fung KL, Wu CP, Gottesman MM, Bauer B, Thomas CJ, Ambudkar SV, Synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna (nilotinib): evidence for transport of Tasigna and its fluorescent derivative by ABC drug transporters, Mol Pharm 8(4) (2011) 1292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brozik A, Hegedus C, Erdei Z, Hegedus T, Ozvegy-Laczka C, Szakacs G, Sarkadi B, Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: substrates, chemosensitizers or inducers of acquired multidrug resistance?, Expert Opin Drug Metab Toxicol 7(5) (2011) 623–42. [DOI] [PubMed] [Google Scholar]
- [8].Wu CP, Hsieh YJ, Hsiao SH, Su CY, Li YQ, Huang YH, Huang CW, Hsieh CH, Yu JS, Wu YS, Human ATP-Binding Cassette Transporter ABCG2 Confers Resistance to CUDC-907, a Dual Inhibitor of Histone Deacetylase and Phosphatidylinositol 3-Kinase, Mol Pharm 13(3) (2016) 784–94. [DOI] [PubMed] [Google Scholar]
- [9].Wu CP, Hsieh YJ, Murakami M, Vahedi S, Hsiao SH, Yeh N, Chou AW, Li YQ, Wu YS, Yu JS, Ambudkar SV, Human ATP-binding cassette transporters ABCB1 and ABCG2 confer resistance to histone deacetylase 6 inhibitor ricolinostat (ACY-1215) in cancer cell lines, Biochem Pharmacol 155 (2018) 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwarzenbach H, Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma, Medical oncology 19(2) (2002) 87–104. [DOI] [PubMed] [Google Scholar]
- [11].Tsubaki M, Satou T, Itoh T, Imano M, Komai M, Nishinobo M, Yamashita M, Yanae M, Yamazoe Y, Nishida S, Overexpression of MDR1 and survivin, and decreased Bim expression mediate multidrug-resistance in multiple myeloma cells, Leuk Res 36(10) (2012) 1315–22. [DOI] [PubMed] [Google Scholar]
- [12].Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM, ABCG2 expression, function, and promoter methylation in human multiple myeloma, Blood 108(12) (2006) 3881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ross DD, Karp JE, Chen TT, Doyle LA, Expression of breast cancer resistance protein in blast cells from patients with acute leukemia, Blood 96(1) (2000) 365–8. [PubMed] [Google Scholar]
- [14].Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A, BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia, Leukemia 16(8) (2002) 1443–7. [DOI] [PubMed] [Google Scholar]
- [15].Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, Tidefelt U, BCRP mRNA expression v. clinical outcome in 40 adult AML patients, Leuk Res 29(2) (2005) 141–6. [DOI] [PubMed] [Google Scholar]
- [16].Matthews C, Catherwood MA, Larkin AM, Clynes M, Morris TC, Alexander HD, MDR-1, but not MDR-3 gene expression, is associated with unmutated IgVH genes and poor prognosis chromosomal aberrations in chronic lymphocytic leukemia, Leuk Lymphoma 47(11) (2006) 2308–13. [DOI] [PubMed] [Google Scholar]
- [17].Kovalev AA, Tsvetaeva DA, Grudinskaja TV, Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer, Experimental oncology 35(4) (2013) 287–90. [PubMed] [Google Scholar]
- [18].Kannan P, Telu S, Shukla S, Ambudkar SV, Pike VW, Halldin C, Gottesman MM, Innis RB, Hall MD, The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2), ACS Chem Neurosci 2(2) (2011) 82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB, Hall MD, The Inhibitor Ko143 Is Not Specific for ABCG2, J Pharmacol Exp Ther 354(3) (2015) 384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toyoda Y, Takada T, Suzuki H, Inhibitors of Human ABCG2: From Technical Background to Recent Updates With Clinical Implications, Frontiers in pharmacology 10 (2019) 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dong J, Qin Z, Zhang WD, Cheng G, Yehuda AG, Ashby CR Jr., Chen ZS, Cheng XD, Qin JJ, Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update, Drug Resist Updat 49 (2020) 100681. [DOI] [PubMed] [Google Scholar]
- [22].Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR Jr., Fu LW, Ambudkar SV, Chen ZS, Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance, Cancer Res 67(22) (2007) 11012–20. [DOI] [PubMed] [Google Scholar]
- [23].Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr., Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW, Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2, Cancer Res 68(19) (2008) 7905–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang H, Wang YJ, Zhang YK, Wang DS, Kathawala RJ, Patel A, Talele TT, Chen ZS, Fu LW, AST1306, a potent EGFR inhibitor, antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance, Cancer Lett 350(1–2) (2014) 61–8. [DOI] [PubMed] [Google Scholar]
- [25].Hsiao SH, Lu YJ, Li YQ, Huang YH, Hsieh CH, Wu CP, Osimertinib (AZD9291) Attenuates the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCB1 in Vitro, Mol Pharm (2016). [DOI] [PubMed] [Google Scholar]
- [26].Wu CP, Hsiao SH, Murakami M, Lu MJ, Li YQ, Hsieh CH, Ambudkar SV, Wu YS, Tyrphostin RG14620 selectively reverses ABCG2-mediated multidrug resistance in cancer cell lines, Cancer Lett 409 (2017) 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang Z, Guo X, To KKW, Chen Z, Fang X, Luo M, Ma C, Xu J, Yan S, Fu L, Olmutinib (HM61713) reversed multidrug resistance by inhibiting the activity of ATP-binding cassette subfamily G member 2 in vitro and in vivo, Acta pharmaceutica Sinica. B 8(4) (2018) 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fan YF, Zhang W, Zeng L, Lei ZN, Cai CY, Gupta P, Yang DH, Cui Q, Qin ZD, Chen ZS, Trombetta LD, Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters, Cancer Lett 421 (2018) 186–198. [DOI] [PubMed] [Google Scholar]
- [29].Zeng F, Wang F, Zheng Z, Chen Z, Wah To KK, Zhang H, Han Q, Fu L, Rociletinib (CO-1686) enhanced the efficacy of chemotherapeutic agents in ABCG2-overexpressing cancer cells in vitro and in vivo, Acta pharmaceutica Sinica. B 10(5) (2020) 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sullivan I, Planchard D, Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line, Frontiers in medicine 3 (2016) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu SG, Shih JY, Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer, Molecular cancer 17(1) (2018) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chih-Hsin Yang J, Camidge DR, Yang CT, Zhou J, Guo R, Chiu CH, Chang GC, Shiah HS, Chen Y, Wang CC, Berz D, Su WC, Yang N, Wang Z, Fang J, Chen J, Nikolinakos P, Lu Y, Pan H, Maniam A, Bazhenova L, Shirai K, Jahanzeb M, Willis M, Masood N, Chowhan N, Hsia TC, Jian H, Lu S, Safety, Efficacy and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients with EGFR-mutated Advanced NSCLC: a Multicenter, Open-label, Phase I Trial, Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer (2020). [DOI] [PubMed] [Google Scholar]
- [33].Lu, Q. SW; Zhang G; Dong X; Yang C; Song Y; Chang G; Lu Y; Pan H; Chiu C; Wang Z; et al. , The Third Generation EGFR Inhibitor (EGFR-TKI) HS-10296 in Advanced NSCLC Patients with Resistance to First Generation EGFR-TKI, Journal of Thoracic Oncology 14(10) (2019) S208–S209. [Google Scholar]
- [34].Robey RW, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, Fojo T, Bates SE, Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1((R)), Biochem Pharmacol 75(6) (2008) 1302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE, Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity, Br J Cancer 89(10) (2003) 1971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roschke AV, Tonon G, Gehlhaus KS, McTyre N, Bussey KJ, Lababidi S, Scudiero DA, Weinstein JN, Kirsch IR, Karyotypic complexity of the NCI-60 drug-screening panel, Cancer Res 63(24) (2003) 8634–47. [PubMed] [Google Scholar]
- [37].Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM, Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins, J. Biol. Chem 261(17) (1986) 7762–70. [PubMed] [Google Scholar]
- [38].Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, Gottesman MM, Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification, Science 232(4750) (1986) 643–645. [DOI] [PubMed] [Google Scholar]
- [39].Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, Dean M, McMahon JB, New inhibitors of ABCG2 identified by high-throughput screening, Molecular cancer therapeutics 6(12 Pt 1) (2007) 3271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robey RW, Obrzut T, Shukla S, Polgar O, Macalou S, Bahr JC, Di Pietro A, Ambudkar SV, Bates SE, Becatecarin (rebeccamycin analog, NSC 655649) is a transport substrate and induces expression of the ATP-binding cassette transporter, ABCG2, in lung carcinoma cells, Cancer Chemother Pharmacol 64(3) (2009) 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Henrich CJ, Bokesch HR, Dean M, Bates SE, Robey RW, Goncharova EI, Wilson JA, McMahon JB, A high-throughput cell-based assay for inhibitors of ABCG2 activity, Journal of biomolecular screening 11(2) (2006) 176–83. [DOI] [PubMed] [Google Scholar]
- [42].Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K, A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet, Biol Pharm Bull 19(11) (1996) 1518–20. [DOI] [PubMed] [Google Scholar]
- [43].Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV, Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter, Mol Cancer Ther 6(12 Pt 1) (2007) 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E, Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells, Nature immunology 4(1) (2003) 87–91. [DOI] [PubMed] [Google Scholar]
- [45].Hollo Z, Homolya L, Davis CW, Sarkadi B, Calcein accumulation as a fluorometric functional assay of the multidrug transporter, Biochimica et biophysica acta 1191(2) (1994) 384–8. [DOI] [PubMed] [Google Scholar]
- [46].Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV, Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system, J Membr Biol 173(3) (2000) 203–14. [DOI] [PubMed] [Google Scholar]
- [47].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res 64(4) (2004) 1242–1246. [DOI] [PubMed] [Google Scholar]
- [48].Wu CP, Hsiao SH, Sim HM, Luo SY, Tuo WC, Cheng HW, Li YQ, Huang YH, Ambudkar SV, Human ABCB1 (P-glycoprotein) and ABCG2 mediate resistance to BI 2536, a potent and selective inhibitor of Polo-like kinase 1, Biochem Pharmacol 86(7) (2013) 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Trott O, Olson AJ, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, Journal of computational chemistry 31(2) (2010) 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alam A, Kowal J, Broude E, Roninson I, Locher KP, Structural insight into substrate and inhibitor discrimination by human P-glycoprotein, Science 363(6428) (2019) 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sanner MF, Olson AJ, Spehner JC, Reduced surface: an efficient way to compute molecular surfaces, Biopolymers 38(3) (1996) 305–20. [DOI] [PubMed] [Google Scholar]
- [52].Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A, Baker SD, Pharmacogenetics of ABCG2 and adverse reactions to gefitinib, J Natl Cancer Inst 98(23) (2006) 1739–42. [DOI] [PubMed] [Google Scholar]
- [53].Li J, Cusatis G, Brahmer J, Sparreboom A, Robey RW, Bates SE, Hidalgo M, Baker SD, Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients, Cancer Biol Ther 6(3) (2007) 432–8. [DOI] [PubMed] [Google Scholar]
- [54].Wu CP, Hsiao SH, Su CY, Luo SY, Li YQ, Huang YH, Hsieh CH, Huang CW, Human ATP-Binding Cassette transporters ABCB1 and ABCG2 confer resistance to CUDC-101, a multi-acting inhibitor of histone deacetylase, epidermal growth factor receptor and human epidermal growth factor receptor 2, Biochem Pharmacol 92(4) (2014) 567–76. [DOI] [PubMed] [Google Scholar]
- [55].van Hoppe S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH, Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-gp/ABCB1) transport afatinib and restrict its oral availability and brain accumulation, Pharmacol Res 120 (2017) 43–50. [DOI] [PubMed] [Google Scholar]
- [56].van Hoppe S, Jamalpoor A, Rood JJM, Wagenaar E, Sparidans RW, Beijnen JH, Schinkel AH, Brain accumulation of osimertinib and its active metabolite AZ5104 is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein), Pharmacol Res 146 (2019) 104297. [DOI] [PubMed] [Google Scholar]
- [57].Kim M, Laramy JK, Mohammad AS, Talele S, Fisher J, Sarkaria JN, Elmquist WF, Brain Distribution of a Panel of Epidermal Growth Factor Receptor Inhibitors Using Cassette Dosing in Wild-Type and Abcb1/Abcg2-Deficient Mice, Drug Metab Dispos 47(4) (2019) 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Karbownik A, Sobanska K, Plotek W, Grabowski T, Klupczynska A, Plewa S, Grzeskowiak E, Szalek E, The influence of the coadministration of the p-glycoprotein modulator elacridar on the pharmacokinetics of lapatinib and its distribution in the brain and cerebrospinal fluid, Invest New Drugs 38(3) (2020) 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kartner N, Riordan JR, Ling V, Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines, Science 221(4617) (1983) 1285–8. [DOI] [PubMed] [Google Scholar]
- [60].Bates SE, Medina-Perez WY, Kohlhagen G, Antony S, Nadjem T, Robey RW, Pommier Y, ABCG2 mediates differential resistance to SN-38 (7-ethyl-10-hydroxycamptothecin) and homocamptothecins, J Pharmacol Exp Ther 310(2) (2004) 836–42. [DOI] [PubMed] [Google Scholar]
- [61].Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH, Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line, Cancer Res. 59(18) (1999) 4559–63. [PubMed] [Google Scholar]
- [62].Tsuruo T, Iida H, Naganuma K, Tsukagoshi S, Sakurai Y, Promotion by verapamil of vincristine responsiveness in tumor cell lines inherently resistant to the drug, Cancer Res 43(2) (1983) 808–13. [PubMed] [Google Scholar]
- [63].Tsuruo T, Iida H, Yamashiro M, Tsukagoshi S, Sakurai Y, Enhancement of vincristine- and adriamycin-induced cytotoxicity by verapamil in P388 leukemia and its sublines resistant to vincristine and adriamycin, Biochem Pharmacol 31(19) (1982) 3138–40. [DOI] [PubMed] [Google Scholar]
- [64].Riordan JR, Ling V, Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability, J Biol Chem 254(24) (1979) 12701–5. [PubMed] [Google Scholar]
- [65].Agrawal M, Abraham J, Balis FM, Edgerly M, Stein WD, Bates S, Fojo T, Chen CC, Increased 99mTc-sestamibi accumulation in normal liver and drug-resistant tumors after the administration of the glycoprotein inhibitor, XR9576, Clin Cancer Res 9(2) (2003) 650–6. [PubMed] [Google Scholar]
- [66].Cuestas ML, Castillo AI, Sosnik A, Mathet VL, Downregulation of mdr1 and abcg2 genes is a mechanism of inhibition of efflux pumps mediated by polymeric amphiphiles, Bioorg Med Chem Lett 22(21) (2012) 6577–9. [DOI] [PubMed] [Google Scholar]
- [67].Natarajan K, Bhullar J, Shukla S, Burcu M, Chen ZS, Ambudkar SV, Baer MR, The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms, Biochem Pharmacol 85(4) (2013) 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang SQ, Liu ST, Zhao BX, Yang FH, Wang YT, Liang QY, Sun YB, Liu Y, Song ZH, Cai Y, Li GF, Afatinib reverses multidrug resistance in ovarian cancer via dually inhibiting ATP binding cassette subfamily B member 1, Oncotarget 6(28) (2015) 26142–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gillet JP, Gottesman MM, Mechanisms of multidrug resistance in cancer, Methods Mol Biol 596 (2010) 47–76. [DOI] [PubMed] [Google Scholar]
- [70].Libby E, Hromas R, Dismounting the MDR horse, Blood 116(20) (2010) 4037–8. [DOI] [PubMed] [Google Scholar]
- [71].Cripe LD, Uno H, Paietta EM, Litzow MR, Ketterling RP, Bennett JM, Rowe JM, Lazarus HM, Luger S, Tallman MS, Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999, Blood 116(20) (2010) 4077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu S, Fu L, Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells, Molecular cancer 17(1) (2018) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wu ZX, Teng QX, Cai CY, Wang JQ, Lei ZN, Yang Y, Fan YF, Zhang JY, Li J, Chen ZS, Tepotinib reverses ABCB1-mediated multidrug resistance in cancer cells, Biochem Pharmacol 166 (2019) 120–127. [DOI] [PubMed] [Google Scholar]
- [74].Wu CP, Lusvarghi S, Wang JC, Hsiao SH, Huang YH, Hung TH, Ambudkar SV, Avapritinib: A Selective Inhibitor of KIT and PDGFRalpha that Reverses ABCB1 and ABCG2-Mediated Multidrug Resistance in Cancer Cell Lines, Mol Pharm 16(7) (2019) 3040–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wu CP, Hsiao SH, Huang YH, Hung LC, Yu YJ, Chang YT, Hung TH, Wu YS, Sitravatinib Sensitizes ABCB1- and ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs, Cancers 12(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wu CP, Hung TH, Hsiao SH, Huang YH, Hung LC, Yu YJ, Chang YT, Wang SP, Wu YS, Erdafitinib Resensitizes ABCB1-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer Drugs, Cancers 12(6) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National G Cancer Institute of Canada Clinical Trials, Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group, J Clin Oncol 25(15) (2007) 1960–6. [DOI] [PubMed] [Google Scholar]
- [78].Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen JZ, Ding H, Wu XY, Huang YF, Mao C, Tang JL, Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with meta-analysis, PLoS One 8(3) (2013) e57528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D, Lapatinib plus capecitabine for HER2-positive advanced breast cancer, N Engl J Med 355(26) (2006) 2733–43. [DOI] [PubMed] [Google Scholar]
- [80].Cetin B, Benekli M, Turker I, Koral L, Ulas A, Dane F, Oksuzoglu B, Kaplan MA, Koca D, Boruban C, Yilmaz B, Sevinc A, Berk V, Uncu D, Harputluoglu H, Coskun U, Buyukberber S, Lapatinib plus capecitabine for HER2-positive advanced breast cancer: a multicentre study of Anatolian Society of Medical Oncology (ASMO), Journal of chemotherapy 26(5) (2014) 300–5. [DOI] [PubMed] [Google Scholar]
- [81].Alemany R, Moura DS, Redondo A, Martinez-Trufero J, Calabuig S, Saus C, Obrador-Hevia A, Ramos RF, Villar VH, Valverde C, Vaz MA, Medina J, Felipe-Abrio I, Hindi N, Taron M, Martin-Broto J, Nilotinib as co-adjuvant treatment with doxorubicin in patients with sarcomas: A phase I trial of the Spanish Group for Research on Sarcoma, Clin Cancer Res (2018). [DOI] [PubMed] [Google Scholar]
- [82].Shukla S, Wu CP, Ambudkar SV, Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges, Expert Opin Drug Metab Toxicol 4(2) (2008) 205–223. [DOI] [PubMed] [Google Scholar]


