Abstract
Background
Ethnic and religious minorities have been disproportionately affected by SARS-CoV-2 worldwide. The UK strictly-Orthodox Jewish community has been severely affected by the pandemic. This group shares characteristics with other ethnic minorities including larger family sizes, higher rates of household crowding and relative socioeconomic deprivation. We studied a UK strictly-Orthodox Jewish population to understand transmission of COVID-19 within this community.
Methods
We performed a household-focused cross-sectional SARS-CoV-2 serosurvey between late-October and early December 2020 prior to the third national lockdown. Randomly-selected households completed a standardised questionnaire and underwent serological testing with a multiplex assay for SARS-CoV-2 IgG antibodies. We report clinical illness and testing before the serosurvey, seroprevalence stratified by age and sex. We used random-effects models to identify factors associated with infection and antibody titres.
Findings
A total of 343 households, consisting of 1,759 individuals, were recruited. Serum was available for 1,242 participants. The overall seroprevalence for SARS-CoV-2 was 64.3% (95% CI 61.6-67.0%). The lowest seroprevalence was 27.6% in children under 5 years and rose to 73.8% in secondary school children and 74% in adults. Antibody titres were higher in symptomatic individuals and declined over time since reported COVID-19 symptoms, with the decline more marked for nucleocapsid titres.
Interpretation
In this tight-knit religious minority population in the UK, we report one of the highest SARS-CoV-2 seroprevalence levels in the world to date, which was markedly higher than the reported 10% seroprevalence in London at the time of the study. In the context of this high force of infection, all age groups experienced a high burden of infection. Actions to reduce the burden of disease in this and other minority populations are urgently required.
Funding
This work was jointly funded by UKRI and NIHR [COV0335; MR/V027956/1], a donation from the LSHTM Alumni COVID-19 response fund, HDR UK, the MRC and the Wellcome Trust.
Research in context.
Evidence before the study
In January 2021, we searched PubMed for articles on rates of SARS-CoV-2 infection amongst ethnic minority groups and amongst the Jewish population. Search teams included “COVID-19”, “SARS-CoV-2”, seroprevalence, “ethnic minority”, and “Jewish” with no language restrictions. We also searched UK government documents on SARS-CoV-2 infection amongst minority groups. By January 2021, a large number of authors had reported that ethnic minority groups experienced higher numbers of cases and increased hospitalisations due to COVID-19. A small number of articles provided evidence that strictly-Orthodox Jewish populations had experienced a high rate of SARS-CoV-2 infection but extremely limited data was available on overall population level rates of infection amongst specific ethnic minority population groups. There was also limited data on rates of infection amongst young children from ethnic minority groups.
Added value of the study
We report findings from a population representative, household survey of SARS-CoV-2 infection amongst a UK strictly-Orthodox Jewish population. We demonstrate an extremely high seroprevalence rate of SARS-CoV-2 in this population which is more than five times the estimated seroprevalence nationally and five times the estimated seroprevalence in London. In addition the large number of children in our survey, reflective of the underlying population structure, allows us to demonstrate that in this setting there is a significant burden of disease in all age groups with secondary school-aged children having an equivalent seroprevalence to adults.
Implications of the available evidence
Our data provide clear evidence of the markedly disproportionate impact of SARS-CoV-2 in minority populations. In this setting infection occurs at high rates across all age groups including pre-school, primary school and secondary school-age children. Contextually appropriate measures to specifically reduce the impact of SARS-CoV-2 amongst minority populations are urgently required.
Alt-text: Unlabelled box
1. Introduction
The UK has been severely affected by the COVID-19 pandemic with 66,197 deaths involving COVID-19 up to the 27th November 2020 [1]. In the UK the pandemic has disproportionately affected minority ethnic populations with relatively higher numbers of positive cases and overrepresentation in admissions to hospitals and Intensive Care Units and deaths [2], [3], [4]. Similar disparities have been observed in other settings and amongst hospitalised patients, mortality has been found to be higher in ethnic minority patients in both the UK and USA [5], [6], [7]. The reasons for this disparity are unclear but are likely multifactorial, reflecting both higher rates of infection resulting from socio-economic factors including deprivation, less ability to work from home, higher use of public transport and larger household sizes, as well as increased severity of illness due to higher rates of comorbidities or delayed access to care [8].
Most population based studies in the UK have focused on the impact experienced by larger minority ethnic groups and not on smaller groups, such as the Jewish community, who compromise approximately 265,000 individuals or 0.5% of the UK's population [9]. In particular, strictly-Orthodox Jewish communities have anecdotally reported high rates of infection, morbidity and hospitalisation during the first wave of the UK pandemic [10]. These anecdotal reports are supported by findings from Public Health England (PHE) which suggests a higher risk from infection in the UK Jewish population and higher rates of death due to COVID-19 in those self identifying as Jewish with an age standardised mortality rates between March and May for Jewish men over 65 years of 759 per 100,000 population, much higher than in other religious groups [11]. This higher rate of infection amongst the Jewish community might reflect socio-demographic differences compared to the general UK population. Strictly-Orthodox Jewish communities have among the highest total fertility rates in the UK, and experience high rates of overcrowded homes and higher levels of socio-economic deprivation [12,13]. However, after adjusting for socio-economic factors the hazard ratio for death in Jewish men remained double that of Christian men suggesting that routine socio-economic factors alone do not explain all of the increased rate of infection seen in this community [11]. During the first wave of the COVID-19 pandemic in Israel, strictly Orthodox Jewish communities also reported higher incidence rates of COVID-19 than other socio-economically similar communities [14,15], or when compared to households in Arab communities with similar sizes and levels of crowding [15].
In Spring 2020, a tightly-knit strictly-Orthodox Jewish in the UK community became aware that they appeared to be experiencing a high burden of SARS-CoV-2. In view of this, and national data suggesting a high burden of infection in the Jewish community and a possible disproportionately high burden in the strictly-Orthodox community, we co-developed a cross-sectional serological survey to measure the burden of infection and identify factors associated with transmission and to inform local control efforts.
2. Methods
2.1. Recruitment and survey methodology
Within a strictly-Orthodox Jewish community in the UK we conducted a household-focused seroprevalence survey between late-October and early December 2020 prior to the third national lockdown. We obtained a comprehensive list of all resident households within the community, held by our community collaborators. Each household was assigned a unique identifier and we used simple-random sampling to identify households for recruitment in to the study. A second list of households where laboratory confirmed infections were known to have occured (the ‘enriched’ list) was also included to inform subsequent transmission models. Members of the study team telephoned households to complete a standardised questionnaire, including demographics, comorbidities, report of any previous presumed COVID-19 illness, previous PCR or serological testing for SARS-CoV-2, access to care whilst unwell with a COVID-19 like illness, illness severity, attendance at work, educational or other community locations and travel overseas between February - November 2020. All households were visited within 10 days of completing the questionnaire for collection of a serum sample except where a member of the household reported active symptoms of COVID-19 in which case the visit was scheduled for a minimum of 14 days after symptom onset. All members of the household were eligible to be included without age restrictions.
2.2. Laboratory analysis
Serum samples were analysed for the presence of IgG specific for SARS CoV-2 trimeric spike protein (S), Receptor Binding Domain (RBD) and nucleocapsid (N) antigens using a multiplex chemiluminescence immunoassay (MSD, Rockville, MD) evaluated by our laboratory as previously described [16,17].
2.3. Statistical analysis
For the purpose of this analysis, we considered a positive trimeric-spike response as evidence of a prior SARS-CoV-2 infection as this target was shown to be the most sensitive and specific target in assay validation16. We designed the survey to detect a seroprevalence of anti-trimeric spike antibodies of 10%. We presumed there would be clustering of infections at the household level and used a design-effect of 2 to increase our sample size to adjust for this. We calculated we would therefore need to recruit 1,730 participants. Assuming an average household size of 6 we estimated this would be approximately 300 households.
To obtain unbiased estimates, we restricted our analysis to individuals from randomly selected households. Results related to the ‘enriched’ household dataset will be presented in future analyses. We used cut-off values for the SARS-CoV-2 immunoassay from previous validation studies for antibodies against trimeric-spike, nucleocapsid and receptor binding domain.As assay cut-offs are well defined in adults but less well defined in children, we conducted a sensitivity analysis in which we considered thresholds for the trimeric-spike assay of twice the previously defined limit.
We report the proportion of individuals who had a clinical illness consistent with COVID-19 and the proportion of individuals who had accessed testing prior to this survey. We calculated the seroprevalence of SARS-CoV-2 infection stratified by age and sex and adjusted for clustering at the household level. Age was classified as pre-school (0-4 years), primary education (5-10 years), secondary education (11-18 years), adults and retirement (greater than 67 years). We created a proxy variable which accounted for the number of community events attended during the year (hereinafter ‘community-gatherings’) and a variable reflecting household overcrowding based on the number of household residents and the number of bedrooms [18]. To identify factors associated with SARS-CoV-2 infection we fitted a multivariable random-effects logistic regression model adjusted for clustering at the household level. We hypothesised that age, sex, being unable to work from home, attendance at community events and household overcrowding were a priori likely to be associated with an increased risk of infection. We reported p-values for associations based on likelihood ratio-tests. We assessed relationships between titres of antibodies against each of SARS-CoV-2 spike, receptor binding domain and nucleocapsid in relation to individuals age, sex, the presence or absence of a symptomatic illness, and for symptomatic individuals the timing of their illness using a log-linear random effects model. Analysis was performed in R version 4.02 and random-effects models were fitted using lme4 version 1.1.26
2.4. Ethics
The study was approved by the London School of Hygiene & Tropical Medicine Ethics Committee (Ref 22532). Verbal informed consent was given during the telephone survey and written consent provided prior to phlebotomy. Parents provided written consent for children.
2.5. Role of the funding source
This work was jointly funded by UKRI and NIHR [COV0335; MR/V027956/1], a donation from the LSHTM Alumni COVID-19 response fund, HDR UK, the MRC and the Wellcome Trust.
The funders had no role in the design, conduct or analysis of the study or the decision to publish.
3. Results
3.1. Enrollment and demographics
A total of 903 randomly selected households were approached, of which 343 households comprising 1,759 individuals were enrolled (Fig. 1). An additional 70 households with known cases of COVID-19 were provided by our community partners (referred to as ‘enriched households’) were approached to participate, of which 28 households comprising 183 individuals were enrolled (Supplementary Figure 3). Serum samples were collected from 1242 individuals (70.6%) from 283 randomly selected households and 137 individuals (74.9%) from 24 ‘enriched households.
Fig. 1.
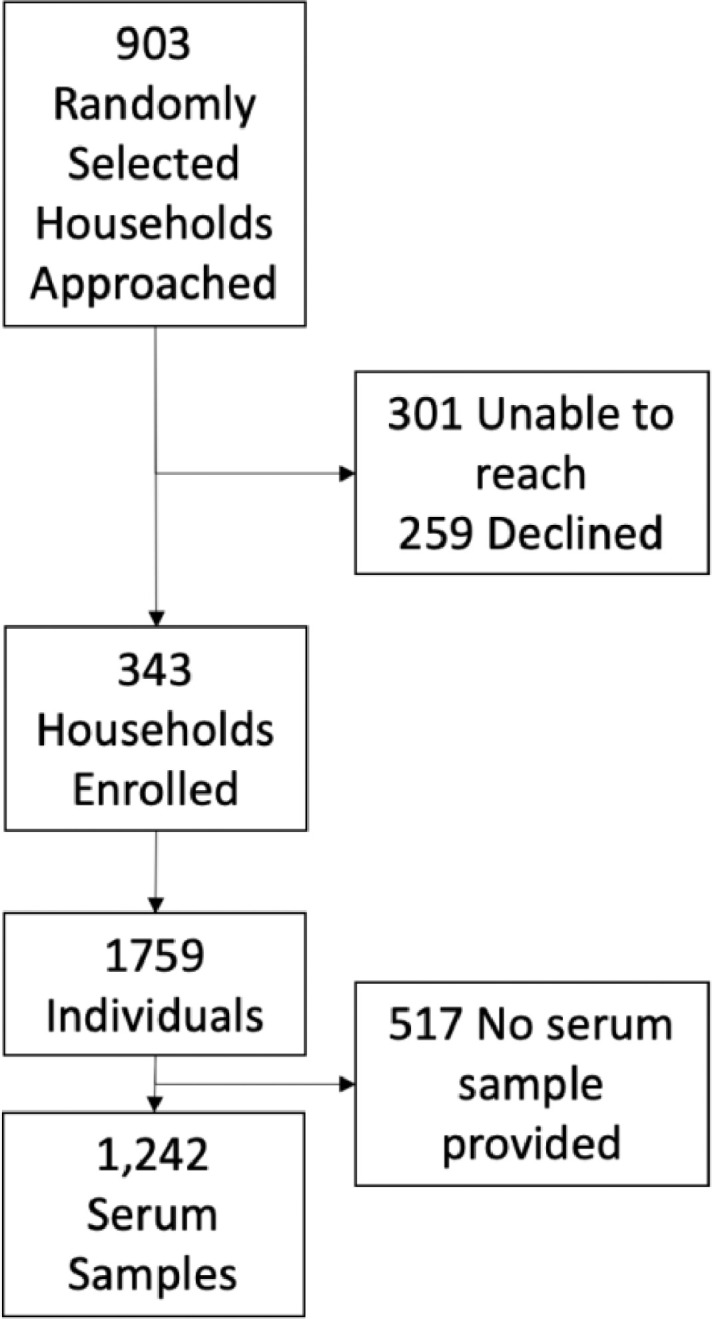
CONSORT diagram for recruitment into the study.
The median household size was 5 (IQR 3-7, Range 1-14 cf. UK median 2) [19] with a median of 3 bedrooms (IQR 2-5). The median age of survey participants was 14 years (IQR 7-33, cd. UK median age 40 years) and 48.6% of participants were male (Supplementary Table 1). Of individuals who gave a serum sample, 48% were male and the median age was 16 years (IQR 9-37).
3.2. Routine testing for SARS-CoV-2
Amongst randomly selected households, a total of 446 individuals (25.4%) had undertaken either PCR or serological testing for SARS-CoV-2 and 182 individuals (10.3%) reported a positive result on at least one test. Reported reasons for testing were because of symptomatic disease or stated as “for other reasons” (Table 1). Individuals who reported testing were older (median 31 IQR 15-46 vs 12 IQR 5-25) and more likely to be male (52.7% vs 47.1%).
Table 1.
Characteristics of the tests undertaken, stratified by presence of symptoms.
| Tested for any reason |
Tested due to symptomatic illness |
Tested for any other reasons |
||||
|---|---|---|---|---|---|---|
| Tested | Positive Result | Tested | Positive Result | Tested | Positive Result | |
| PCR | 364 (20.7%) | 133 (36.5%) | 191 (10.9%) | 113 (59.2%) | 181 (10.3%) | 20 (11.0%) |
| Serology | 128 (7.3%) | 55 (43.0%) | 47 (2.7%) | 31 (66.0%) | 83 (4.7%) | 25 (30.1%) |
| Total | 446 (25.4%) | 182 (40.8%) | 228 (13.0%) | 141 (61.8%) | 238 (13.5%) | 44 (18.5%) |
Overall 228 individuals (13%) underwent either PCR and/or serological testing because of symptomatic illness. A total of 191 symptomatic individuals reported providing a sample for PCR with a swab positivity rate of 59% (n = 113). By comparison 20 of 181 PCR tests done for other reasons were positive (11%). Overall 182 individuals (10.3%) reported already having received a positive test for SARS-CoV-2 prior to the survey.
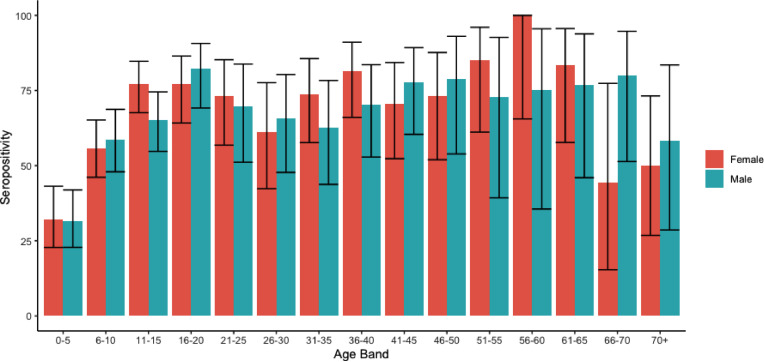
Serology on this cohort revealed an overall seroprevalence for SARS-CoV-2 of 64.3% (95% CI 61.6-67.0%, 799/1,242). The seroprevalence varied by age between 27.6% (95% confidence interval (CI) 20.8 - 35.6%) for children aged under 5 years of age to 73.8% (95% CI 68.2 - 78.8%) amongst secondary school children and 74% (95% CI 70.0 -77.6%) adults (Fig. 2) (Supplementary Table 2). Seroprevalence was significantly higher amongst men (68.8%, 95% CI 64.9 - 72.5%) than women (59.7%, 95% CI 55.8 - 63.5%) (p = 0.001). Only three individuals (2%) reported a previous positive PCR result but did not have detectable anti-spike antibodies. All three individuals had a positive PCR in October and therefore a negative serological test might reflect that they had not yet seroconverted at the time of sample collection. In a multivariable random-effects logistic regression model, seropositivity was associated with increasing age, male sex, household density and whether and individual was was working or in education, but not associated with attendance at community gatherings (Table 2). No pre-existing comorbidities were associated with seropositivity.
Fig. 2.
Age specific seroprevalence in participants in the study. Colours indicate male and female participants.
Table 2.
Association between seropositivity and demographic and behavioural variables. *Likelihood ratio test
| Unadjusted Analysis |
Adjusted Analysis |
||||||
|---|---|---|---|---|---|---|---|
| Marginal Probability | OR (95% CI) | p-value* | Marginal Probability | aOR (95% CI) | p-value* | ||
| Age | Young Children (0-4 years) | 29.7% | 0.07 (0.04 - 0.12) | <0.001 | 31.5% | 0.10 (0.05-0.20) | <0.001 |
| Primary Education (5-10 Years) | 57.6% | 0.36 (0.24-0.54) | 64.2% | 0.77 (0.37-1.61) | |||
| Secondary Education (11-18 Years) | 73.4% | 0.87 (0.58-1.31) | 77.4% | 1.70 (0.89-3.27) | |||
| Working age Adults (19-67) | 74.9% | 1 | 70.1% | 1 | |||
| Retirement age adults (67+ Years) | 58.3% | 0.32 (0.13-0.80) | 54.7% | 0.35 (0.14-0.91) | |||
| Sex | Female | 63.4% | 1 | 0.009 | 62.1% | 1 | 0.002 |
| Male | 69.2% | 1.46 (1.10 - 1.93) | 69.0% | 1.62 (1.18-2.24) | |||
| Housing | Not overcrowded | 67.2% | 1 | 0.498 | 63.9% | 1 | 0.412 |
| Overcrowded | 64.3% | 0.86 (0.55 - 1.34) | 67.2% | 1.23 (0.74-2.06) | |||
| Employment and Education Status | Neither Working nor in Education | 68.5% | 1 | <0.001 | 69.7% | 1 | 0.015 |
| In Education | 56.4% | 0.49 (0.35 - 0.67) | 58.6% | 0.45 (0.25-0.81) | |||
| Working From Home | 77.8% | 1.78 (1.1 -2.87) | 74.7% | 1.40 (0.82-2.38) | |||
| Working Outside Home | 72.1% | 1.25 (0.68-2.3) | 66.3% | 0.81 (0.41-1.58) | |||
| Number of Community Gatherings Attended | 1 = 66.2% | 1.02 (0.98-1.06) | 0.666 | 1 = 65.2% | 1.04 (0.84-1.28) | 0.702 | |
| 3 = 66.1% | 3 = 65.4% | ||||||
| 5= 66.1% | 5 = 65.5% | ||||||
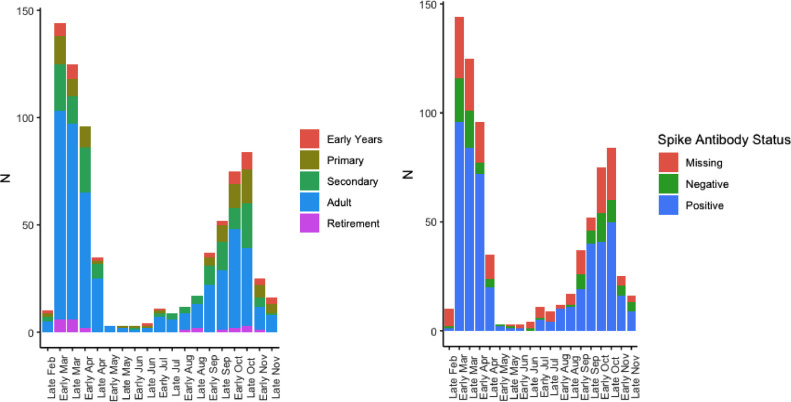
Overall, 697 (37.5%) individuals reported an illness they thought was consistent with COVID-19. There were clear peaks in reported illness consistent with the first and second waves of COVID-19 in the UK (Fig. 3). Of individuals reporting a suspected illness 49.8% were male and the median age was 28 (IQR 14-41). A total of 16 (0.9%) individuals reported hospitalisation for COVID-19 and a further three individuals were reported to have died of COVID-19.
Fig. 3.
Self-reported COVID-19-like illness in participants in the study stratified by age and antibody status. The reduced number of self-reported cases in November is an artefact reflecting the timing of the survey. Missing Anti-Spike antibody status occured when participants did not participate in phlebotomy after completing the survey. No phlebotomy samples gave an inconclusive result.
Overall 81.9 % of individuals who reported an illness consistent with COVID-19 were sero-positive and 53.7% of asymptomatic individuals were sero-positive. Of cardinal symptoms of COVID-19, self-reported cough (OR 3.0, 95% CI: 2.1-4.2), fever (OR 2.3, 95% CI: 1.6 -3.4) and loss of smell or taste (OR 11.8, 95% CI: 6.8 -20.6) were all associated with seropositivity. In the overall population self-reported loss of smell or taste had a positive predictive value of 94.5% for positive serology (Supplementary Table 3).
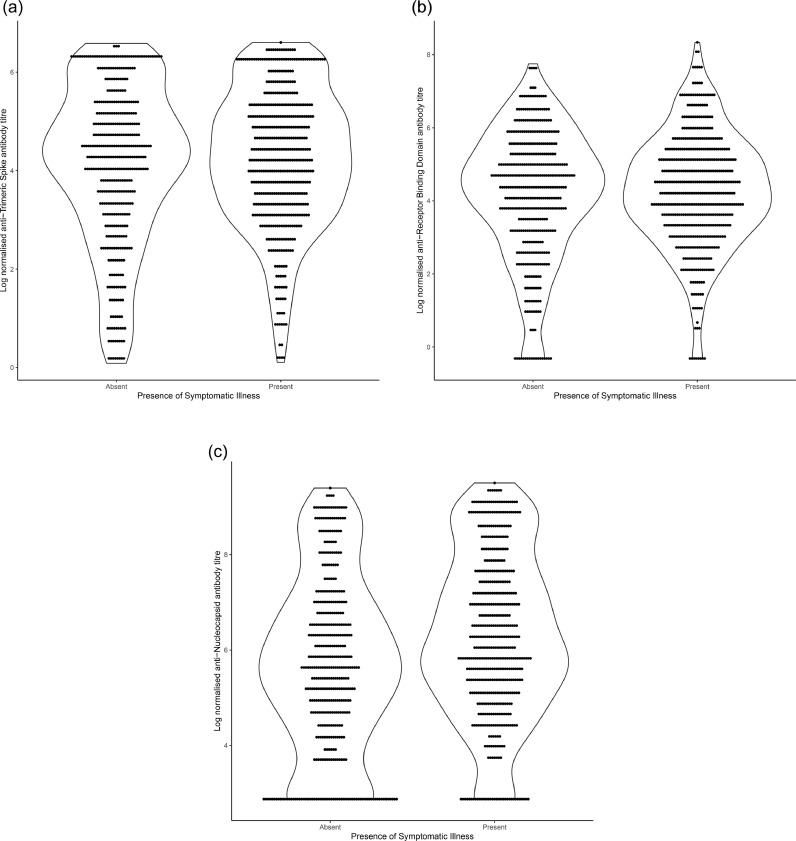
Titres of spike, receptor binding domain and nucleocapsid antibodies were higher amongst individuals who reported a symptomatic illness (Fig. 4) (Table 3). Amongst symptomatic individuals titres declined following time since reported symptomatic illness which was more marked for nucleocapsid than for other targets (Supplementary Figure 1).
Fig. 4.
Log normalised antibody titres for anti-Spike, anti-Receptor Binding Domain and anti-Nucleocapsid. Each panel is stratified by reporting of COVID-19-like symptoms by the participant. The shape shows the density of the distribution of samples.
Table 3.
Association between log-transformed antibody titres and participant characteristics.
| Anti-Spike Antibody Titre |
Anti-Receptor Binding Domain Titre |
Anti-Nucleocapsid Antibody Titre |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value | |
| All Patients | |||||||||
| Male Sex | 0.383 | 0.142-0.623 | 0.002 | 0.412 | 0.193-0.640 | <0.0013 | 0.314 | 0.120-0.509 | 0.002 |
| Age (years) | 0.006 | -0.001-0.013 | 0.095 | 0.008 | 0.001-0.014 | 0.031 | 0.010 | 0.004-0.0160 | 0.001 |
| Symptomatic Illness | 1.25 | 0.975 - 1.532 | <0.001 | 1.029 | 0.772-1.286 | <0.001 | 1.020 | 0.796 - 1.244 | <0.001 |
| Symptomatic Individuals | |||||||||
| Male Sex | 0.137 | -0.202 - 0.479 | 0.448 | 0.1411 | -0.173 - 0.457 | 0.381 | 0.079 | -0.212 - 0.372 | 0.612 |
| Age (years) | 0.023 | 0.013-0.033 | <0.0001 | 0.021 | 0.012 - 0.030 | <0.001 | 0.028 | 0.020 - 0.037 | <0.001 |
| Time since reported illness (days) | -0.002 | -0.004 - 0.000 | 0.041 | -0.002 | -0.004 - 0.000 | 0.022 | 0.005 | -0.007 - -0.003 | <0.001 |
4. Discussion
We found an extremely high seroprevalence of SARS-CoV-2 antibodies in a strictly-Orthodox Jewish community in the UK. Our estimate of 65% population seroprevalence is markedly higher than recent estimates of 6.9% (95%CI 6.3-7.4%) nationally and 10.8% (95% CI 9.3-12.5%) in London by random sampling in October by the Office for National Statistics (ONS) [20]. Rapid declines in self-reported illness followed the introduction and adherence to lockdown in March, demonstrating that even in this highly connected community such measures are effective at reducing transmission. However over the course of 2020, the overall seroprevalence in this tightly knit religious community reached levels similar to those seen in Manaus, Brazil where a seroprevalence of more than 65% has been reported in adults [21]. As our survey was completed by early December 2020, prior to the subsequent winter case surge in London, it is likely that the overall burden of infection in this community is now even higher.
Our estimates are amongst the highest sero-prevalence of SARS-CoV-2 described anywhere in the world to date. In the UK other studies have also reported a higher seroprevalence of SARS-CoV-2 amongst ethnic minority individuals. In the nationally-representative REACT-2 study in September 2020, seroprevalence amongst adults from an ethnic minority was 7.9% compared to 3.6% for the white population21. Whilst direct comparison with our study population is limited by the absence of equivalently detailed sampling of other populations, the overall pattern suggests a particularly high seroprevalence in our study population compared with ethnic minority groups across the UK. The precise reasons why the burden of SARS-CoV-2 has been so high in this population are unclear. In Israel, strictly-Orthodox Jewish communities had markedly higher SARS-CoV-2 PCR swab positive incidence rates compared to other socio-economically similar communities during the first wave of the Israeli epidemic [14,15], whilst seroprevalence following the first wave of COVID-19 was above 30% in many strictly-Orthodox communities the United States [22]. Data from other sources suggest that lower socio-economic status, ongoing need to travel to work and a greater burden of pre-existing comorbidities, may all contribute to increased risk of acquiring SARS-CoV-2.
In our study there was high seroprevalence in all age groups, with the highest in working-age adults and older children where it reached 74%. A strength of our study is the extremely high number of children recruited, which reflects the higher total fertility rates amongst strictly-Orthodox Jewish women compared to the UK average [12,13]. Seroprevalence in the youngest children was lower at 27% but rose rapidly to more than 50% amongst primary school-aged children. The seroprevalence found in this group is approximately four times that reported in a UK multicentre study on healthcare worker children aged 2-15 years demonstrating the high rates of infection in all age groups in the current study [23].
Seroprevalence was higher amongst men than women in our study. Higher SARS-CoV-2 attack rates in men compared to women have also been reported in some but not all otherstudies [24,25]. The higher rate of infection amongst men might reflect biological differences, differences in comorbidities, differences in social mixing patterns or other behaviours. As these differences might vary between different ethnic minority groups it is possible that association between sex and infection may not be consistent across all communities. We did not find any significant associations with reported attendance at community events, workplace or household overcrowding in this population which may be because of a true absence of effect, or that due to the extremely high seroprevalence, the ability to detect risk factors is limited. Further modelling work is planned to investigate this in more detail.
The majority of reported symptomatic illness occurred during the first and second waves of the COVID-19 pandemic mirroring case reporting patterns seen across London and the UK. Just over 10% of participants reported at least one swab test due to symptomatic illness since March, with an overall swab positivity rate of 59%. In line with other studies, children were significantly less likely to report a symptomatic illness [26,27] and this likely explains the higher rates of routine testing amongst adults we observed in our study.
We found that antibody titres against spike and nucleocapsid proteins declined at different rates, and in line with other studies [16,28], that anti-nucleocapsid declined more quickly. If we had used only anti-nucleocapsid antibodies as a marker of previous infection our estimated seroprevalence would have been 42.8%, significantly under-estimating the likely size of the epidemic that has occurred in this population. Declines in anti-spike antibody titre were less marked over time but it is still possible that we have under-estimated the true seroprevalence in this population. Titres of both spike and nucleocapsid antibodies were higher amongst individuals who reported symptomatic illness.
Our study has a number of limitations. We recruited 38% of households that were approached and obtained serum samples from 70% of study participants. This enrollment rate is similar to other national COVID-19 household surveillance studies, such as the ONS COVD-19 Infection survey [29], suggesting it is unlikely to be a major source of bias. Individuals who gave serum samples were slightly older than those from whom serum was unavailable which may result in an over-estimation of the overall population seroprevalence. We relied on self-report of presumed COVID-19 illness which may be unreliable. However the timings of self reported illness match well to national surveillance data and self-reported illness was strongly associated with the presence of anti-spike antibodies suggesting that in this population this was a reliable metric. Whilst household income in strictly-Orthodox Jewish communities is below the national average this is partly offset by the wide network of community charities and support networks [30]. We did not collect detailed data on socio-economic status beyond household size and employment status and so are not able to directly assess the importance of these factors on seroprevalence in the community.
Although our study was conducted in a strictly-Orthodox Jewish community, these communities share many characteristics with other ethnic and religious minority groups including larger family sizes, increased population density, children attending select schools, regular attendance at communal events and gatherings, and English as a second language. As such our findings are likely relevant to other tightly-knit ethnic and religious minority groups in the UK and elsewhere. Our work, conducted in direct collaboration with the community, should be a model for understanding risk in minority populations where there are no similar published data currently.
In conclusion, we found evidence for an extremely high rate of SARS-CoV-2 infection in this specific community affecting individuals of all ages. This provides further evidence that minority communities in the UK and elsewhere are disproportionately affected by the COVID-19 pandemic. The reasons for this remain unclear, although are likely to be a complex interplay of socio-economic and behavioural factors. Further studies to better understand drivers of transmission in ethnic and religious minority populations, conducted wherever possible in partnership with communities themselves, are urgently needed to reduce health disparities and improve outcomes for these populations.
Supplementary data
1. Supplementary Appendix
2. STROBE Checklist
Author Contributions
MM, RME, ChR, DG conceived of the study. KG and DL co-ordinated the survey. KG, MJ, VG conducted lab work. SL, WW, BK, TC, NS contributed to the design of the study. MM, KG, WW, RME verified the underlying data. All authors contributed to the analysis and writing of the manuscript.
Data sharing
Data available on request to the corresponding author:
Dr Michael Marks
Clinical Research Department
Faculty of Infectious and Tropical Diseases
London School of Hygiene & Tropical Medicine
London
WC1E 7HT
United Kingdom
michael.marks@lshtm.ac.uk
Declaration of Competing Interests
RME reports funding from HDR UK (grant: MR/S003975/1), MRC (grant: MC_PC 19065), NIHR (grant: NIHR200908). RME is a member of UK government COVID-19 working groups and a WHO working group. MM MM, KG, WW and RME report funding from UKRI and NIHR (application COV0335, grant: MR/V027956/1). KG reports funding from the Wellcome Trust (grant: 210830/Z/18/Z). WW reports funding from the Chief Scientific Officer of Scotland and the Foundation for Innovative New Diagnostics. All other authors declare no competing interests. This research was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation and the NIHR HPRU in Modelling and Economics at London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE. The funders had no role in the design, conduct or analysis of the study or the decision to publish. The authors have no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We thank Elizabeth Miller for helpful discussions on study design, and Daniel Grint for statistical queries.
Footnotes
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.lanepe.2021.100127.
Appendix. Supplementary materials
References
- 1.Deaths registered weekly in England and Wales, provisional - Office for National Statistics.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredweeklyinenglandandwalesprovisional/weekending1january2021. Accessed Jan 14, 2021.
- 2.Mathur R, Bear L, Khunti K, Eggo RM. Urgent actions and policies needed to address COVID-19 among UK ethnic minorities. The Lancet. 2020;396:1866–1868. doi: 10.1016/S0140-6736(20)32465-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison EM, Docherty AB, Barr B. Social Science Research Network; Rochester, NY: 2020. Ethnicity and Outcomes from COVID-19: The ISARIC CCP-UK Prospective Observational Cohort Study of Hospitalised Patients. [DOI] [Google Scholar]
- 5.Perez-Guzman PN, Daunt A, Mukherjee S. Clinical characteristics and predictors of outcomes of hospitalized patients with coronavirus disease 2019 in a multiethnic London National Health Service Trust: a retrospective cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1091. published online Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apea VJ, Wan YI, Dhairyawan R. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. medRxiv. 2020 doi: 10.1136/bmjopen-2020-042140. 2020.06.10.20127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and Racial/Ethnic Disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drivers of the higher COVID-19 incidence, morbidity and mortality among minority ethnic groups, 23 September 2020. GOV.UK.https://www.gov.uk/government/publications/drivers-of-the-higher-covid-19-incidence-morbidity-and-mortality-among-minority-ethnic-groups-23-september-2020. Accessed Jan 19, 2021.
- 9.Office for National Statistics. National Records of Scotland. Northern Ireland Statistics and Research Agency . Edition: June 2016. UK Data Service; 2016. 2011 Census aggregate data. https://census.ukdataservice.ac.uk/use-data/citing-data.aspx. Accessed Jan 14, 2021. [DOI] [Google Scholar]
- 10.Staetsky LD, Paltiel A. Institute for Jewish Policy Research; 2020. COVID-19 mortality and Jews: A global overview of the first wave of the coronavirus pandemic, March to May 2020.https://archive.jpr.org.uk/object-1431 Accessed Jan 19, 2021. [Google Scholar]
- 11.Coronavirus (COVID-19) related deaths by religious group, England and Wales - Office for National Statistics.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronaviruscovid19relateddeathsbyreligiousgroupenglandandwales/2marchto15may2020. Accessed Jan 14, 2021.
- 12.Staetsky LD, Boyd J. Institute for Jewish Policy Research; 2015. Strictly Orthodox rising: What the demography of British Jews tells us about the future of the community.https://archive.jpr.org.uk/object-uk285 accessed Jan 14, 2021. [Google Scholar]
- 13.Ben Kasstan. Making Bodies Kosher: The Politics of Reproduction among Haredi Jews in England. Berghahn Bookshttps://www.berghahnbooks.com/title/KasstanMaking/recommend. Accessed Jan 14, 2021.
- 14.Schattner A, Klepfish A. Orthodox Judaism as a Risk Factor of Covid-19 in Israel. Am J Med Sci. 2020;360:304. doi: 10.1016/j.amjms.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saban M, Shachar T, Miron O, Wilf-Miron R. Effect of socioeconomic and ethnic characteristics on COVID-19 infection: The case of the Ultra-Orthodox and the Arab communities in Israel. medRxiv. 2020 doi: 10.1007/s40615-021-00991-z. 2020.05.25.20111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson M, Wagstaffe HR, Gilmour KC. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J Clin Virol. 2020;130 doi: 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldblatt D, Johnson M, Falup-Pecurariu O. Cross sectional prevalence of SARS-CoV-2 antibodies in health care workers in paediatric facilities in eight countries. J Hosp Infect. 2021 doi: 10.1016/j.jhin.2020.12.019. published online Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cable N, Sacker A. Validating overcrowding measures using the UK Household Longitudinal Study. SSM - Popul Health. 2019;8 doi: 10.1016/j.ssmph.2019.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Families and households in the UK - Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/families/bulletins/familiesandhouseholds/2017. Accessed Jan 15, 2021.
- 20.Coronavirus (COVID-19) Infection Survey: characteristics of people testing positive for COVID-19 in England and antibody data for the UK - Office for National Statistics.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/november2020. Accessed Jan 25, 2021.
- 21.Buss LF, Prete CA, Abrahim CMM. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2020 doi: 10.1126/science.abe9728. published online Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zyskind I, Rosenberg AZ, Zimmerman J. SARS-CoV-2 seroprevalence and symptom onset in culturally linked orthodox Jewish communities across multiple regions in the United States. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterfield T, Watson C, Moore R. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320558. published online Nov 10. [DOI] [PubMed] [Google Scholar]
- 24.Ward H, Cooke G, Atchison C. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020 2020.10.26.20219725. [Google Scholar]
- 25.Lavezzo E, Franchin E, Ciavarella C. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- 26.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manisty C, Treibel TA, Jensen M. Characterising heterogeneity and sero-reversion in antibody responses to mild SARS⍰CoV-2 infection: a cohort study using time series analysis and mechanistic modelling. medRxiv. 2020 doi: 10.1016/j.ebiom.2021.103259. 2020.11.04.20225920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pouwels KB, House T, Pritchard E. Community prevalence of SARS-CoV-2 in England from April to November, 2020: results from the ONS Coronavirus Infection Survey. Lancet Public Health. 2021;6:e30–e38. doi: 10.1016/S2468-2667(20)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlomit FA. Springer International Publishing; 2020. Spatial Behavior in Haredi Jewish Communities in Great Britain. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.